6 Basic science
Xeomin®
Summary and Key Features
• IncobotulinumtoxinA (NT 201, Xeomin®, Bocouture®) is an effective and well-tolerated treatment for facial wrinkles and is approved for the treatment of glabellar frown lines in many countries
• IncobotulinumtoxinA differs from other commercially available botulinum toxin type A products as it is free from complexing proteins and other impurities
• Complexing proteins have no therapeutic effect and do not prevent the active neurotoxin from diffusing and may lead to an increased risk of antibody formation
• IncobotulinumtoxinA is highly stable and can be stored at room temperature
• IncobotulinumtoxinA has a higher specific biological activity than other available botulinum toxin type A preparations, such as onabotulinumtoxinA (Botox® / Vistabel) or abobotulinumtoxinA (Dysport® / Azzalure®)
• In practice, the clinical equipotency of incobotulinumtoxinA and onabotulinumtoxinA has been proven when a dose conversion ratio of 1 : 1 is used. Multiple small dose injections result in a precise and predictable outcome
• The best results are achieved when the face is treated as a whole, since treating some muscles or areas in isolation can draw attention to the untreated areas
Properties of incobotulinumtoxinA
Purity
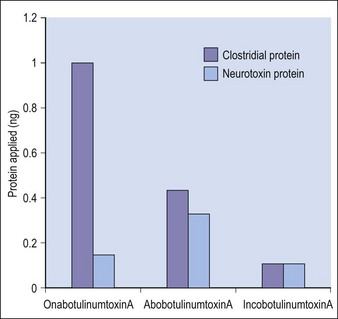
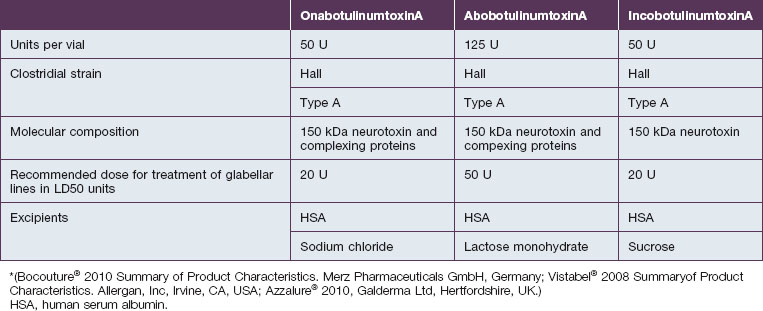
Botulinum toxin is produced by anaerobic fermentation of the bacterium Clostridium botulinum type A (Hall strain). During the manufacture of incobotulinumtoxinA, following fermentation and extraction of the toxin, the complexing proteins are removed by chromatography. Thus incobotulinumtoxinA contains markedly less clostridial protein than do onabotulinumtoxinA and abobotulinumtoxinA (Fig. 6.1). In addition, incobotulinumtoxinA has the highest specific biological activity of all three products. A high-sensitivity sandwich ELISA was used in a study by Frevert to measure the amount of botulinum toxin type A per 100 U of onabotulinumtoxinA, incobotulinumtoxinA and abobotulinumtoxinA. The results were 0.73 ng, 0.44 ng and 0.65 ng, respectively, giving incobotulinumtoxinA the highest specific biological activity (U/ng neurotoxin) at 227 U/ng compared with 137 U/ng for onabotulinumtoxinA and 154 U/ng for abobotulinumtoxinA (but it must be noted that the units of abobotulinumtoxinA are different from those of onabotulinumtoxinA and incobotulinumtoxinA). This suggests that, in addition to containing complexing proteins, onabotulinumtoxinA may also contain denatured / inactivated neurotoxin, unlike incobotulinumtoxinA (see Fig. 6.1). Like the other botulinum toxin type A products, incobotulinumtoxinA contains human serum albumin as an excipient but, whereas incobotulinumtoxinA contains sucrose, abobotulinumtoxinA contains lactose, and onabotulinumtoxinA contains neither of these sugars (Table 6.1).
Clinical performance of incobotulinumtoxinA
Clinical experience with incobotulinumtoxinA
The clinical development program for incobotulinumtoxinA has demonstrated excellent efficacy in the treatment of glabellar frown lines and periorbital lines, and it is well tolerated. In addition, the characteristics of incobotulinumtoxinA: the lack of complexing proteins, high specific biological activity, predictability of results, and good tolerability, recommend it as a precise and effective tool in the field of aesthetic medicine. When making the decision of which product to use, physicians have to consider factors such as therapeutic efficacy, patient satisfaction, safety, price, and handling. IncobotulinumtoxinA has shown comparable efficacy, patient satisfaction, and safety to onabotulinumtoxinA using equal doses, and has the benefit of having no need for cold storage. Along with the emergence of such an efficient product, injection techniques have improved over time so that treatment has become more sophisticated, with more subtle results and avoidance of the ‘frozen’ look. With multiple small doses and a sound awareness of facial anatomy (Fig. 6.2), incobotulinumtoxinA gives very predictable results.
 The total face approach with incobotulinumtoxinA
The total face approach with incobotulinumtoxinA
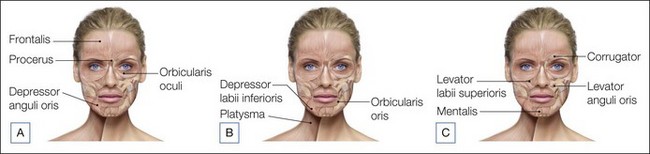
To obtain the most effective improvement in facial wrinkles, treatment of the whole face, as described below, is recommended. The glabellar region can display single and double vertical lines and can be treated with one or two injections into the superior region of the procerus along the median line (total dose of 4–6 U), along with further medial injections into the central part of the muscle (total dose of 2–4 U). In addition, treatment should include one medial injection (1–2 U) in the direction of the fibers of the corrugator supercilii and one superficial injection into the medial aspect of the orbicularis oculi (2–4 U; Fig. 6.3A). Treatment of the eyebrow includes four injections in the part of the eyebrow above the orbital rim: superficial injections in fibers of the orbicularis and deep injections in caudal fibers of the frontalis (Fig. 6.3B). The mephisto appearance should be treated at the point of maximum contraction, with 1 U incobotulinumtoxinA injected into the lateral part of the frontalis (Fig. 6.3C). For lateral canthal lines, lateral intracutaneous injections and deep subcutaneous injections above the lateral orbital rim (1–2 U per injection) can be performed into the orbicularis oculi (Fig. 6.3D). There may be no need to treat the center of the frontalis as this can enhance brow ptosis and a ‘tired’ look. Horizontal forehead lines form part of normal expressions of surprise and interest and hence do not necessarily have negative connotations. It is therefore advisable to delay treatment of the frontalis until approximately 4 weeks after treatment of the glabellar region if it is still deemed necessary. The forehead can be treated by five deep muscle injections of 1–2 U each administered in a V-shape configuration in the medial area of the frontalis and multiple superficial injections of (0.5–1 U lateral to the midline (Fig. 6.3E)).
In cases of fine wrinkles in the area of the lower eyelid four intradermal injections of 0.5 U incobotulinumtoxinA, in a volume of approximately 0.04 mL (i.e. a more diluted preparation of incobotulinumtoxinA), can be used treat each lower eyelid. In order to open the eye, a single superficial pretarsal injection of 1–2 U in the orbicularis oculi along the mid-pupillary line can also be administered (Fig. 6.3F, G). Treatment of bunny lines can be achieved by one or two superficial injections of 1–2 U in the medial, superior part of the nasal wing (Fig. 6.3H). A single deep injection of 1 U in part of the levator labii superioris at the base of the nasolabial fold can treat a ‘gummy’ smile (Fig. 6.3I). An injection of 0.5 U between the vermilion border and the white portion of the lip can improve the appearance of the upper and lower lips (Fig. 6.3J), and a deep injection (1–2 U) lateral to the marionette line will cover the superior parts of the platysma muscle and the depressor anguli oris to treat marionette lines. Care should be taken to ensure that the distance to the corner of the mouth is at least 1 cm in the inferior direction to prevent diffusion into the orbicularis oris (Fig. 6.3K). To treat the chin, both the left and right mentalis muscles should be injected. This should preferably be done at the top of the corner of the chin in the direction of the neck to prevent treatment of the orbicularis oris (Fig. 6.3L). Finally the platysma muscle can be treated by multiple injections of 1–2 U along the hypertrophic bands, 2–3 cm apart (Fig. 6.3M).
Conclusion
Botulinum toxins have been used for aesthetic purposes for almost 25 years and are an effective treatment of facial wrinkles. According to the cosmetic surgery national data bank statistics of the American Society for Aesthetic Plastic Surgery (found at www.surgery.com), botulinum toxin treatment was the most common non-surgical aesthetic procedure performed in the USA in 2010. IncobotulinumtoxinA (NT 201, Xeomin®, Bocouture®) is a highly purified botulinum toxin type A that is free from complexing proteins, potent and well tolerated, with proven efficacy in neurological and aesthetic indications. Studies have shown that incobotulinumtoxinA is clinically equipotent to onabotulinumtoxinA in the treatment of cervical dystonia, blepharospasm, and glabellar frown lines and these two products are routinely used in a 1 : 1 dose conversion ratio. With accurate injection of small doses of incobotulinumtoxinA, unwanted effects can be avoided, leaving the patient with a predictable, subtle and softer look.
anon. Azzalure®. Summary of Product Characteristics. Berkshire, UK: Ipsen Ltd; 2010.
anon. Bocouture®. Summary of Product Characteristics. Germany: Merz Pharmaceuticals GmbH; 2010.
anon. Vistabel®. Summary of Product Characteristics. Irvine, CA, USA: Allergan, Inc; 2008.
anon. Xeomin®. Summary of Product Characteristics. Germany: Merz Pharmaceuticals GmbH; 2011.
Benecke R, Jost WH, Kanovsky P, et al. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology. 2005;64:1949–1951.
Blümel J, Frevert J, Schwaier A. Comparative antigenicity of three preparations on boutlinum neurotoxin A in the rabbit. Neurotoxicity Research. 2006;9:238.
Borodic G. Immunologic resistance after repeated botulinum toxin type A injections for facial rhytides. Ophthalmic Plastic and Reconstructive Surgery. 2006;22:239–240.
Carli L, Montecucco C, Rossetto O. Assay of diffusion of different botulinum neurotoxin type A formulations injected in the mouse leg. Muscle Nerve. 2009;40:374–380.
Carruthers A, Carruthers J. Botulinum toxin products overview. Skin Therapy Letter. 2008;2013:1–4.
Dressler D, Adib Saberi F. New formulation of Botox: complete antibody-induced treatment failure in cervical dystonia. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:108–109.
Dressler D, Wohlfahrt K, Meyer-Rogge E, et al. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatologic Surgery. 2010;36(suppl 4):2182–2187.
Eisele K-H, Fink K, Vey M, et al. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57:555–565.
Frevert J. Content of botulinum neurotoxin in Botox® / Vistabel®, Dysport® / Azzalure®, and Xeomin® / Bocouture®. Drugs R D. 2010;2010(10):67–73.
Grein S, Mander GJ, Fink K. Stability of botulinum neurotoxin type A, devoid of complexing proteins. Botulinum Journal. 2011;2:49–58.
Imhof M, Kuhne U. A Phase III study of incobotulinumtoxinA in the treatment of glabellar frown lines. Journal of Clinical and Aesthetic Dermatology. 2011;4:28–34.
Jost WH, Kohl A, Brinkmann S, et al. Efficacy and tolerability of a botulinum toxin type A free of complexing proteins (NT 201) compared with commercially available botulinum toxin type A (BOTOX) in healthy volunteers. Journal of Neural Transmission. 2005;112:905–913.
Kanovsky P, Slawek J, Denes Z, et al. Efficacy and Safety of Botulinum Neurotoxin NT 201 in Poststroke Upper Limb Spasticity. Clinical Neuropharmacology. 2009;35:259–265.
Kerscher M, Roll S, Becker A, et al. Comparison of the spread of three botulinum toxin type A preparations. Archives of Dermatological Research. 2012;304(2):155–161.
Lee S-K. Antibody-induced failure of botulinum toxin type A therapy in a patient with masseteric hypertrophy. Dermatologic Surgery. 2007;33:S105–S110.
Prager W, Wissmüller E, Kollhorst B, et al. Treatment of crow’s feet with two different botulinum toxin type A preparations in split-face technique. Hautarzt. 2011;62:375–379.
Prager W, Wissmüller E, Kollhorst B, et al. Comparison of two botulinum toxin type A preparations for treating crow’s feet: A split-face, double-blind, proof-of-concept study. Dermatologic Surgery. 2010;36:2155–2160.
Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. Journal of Neural Transmission. 2006;113:303–312.
Sattler G, Callander M, Grablowitz D, et al. Noninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatologic Surgery. 2010;36:2146–2154.
Stengel G, Bee K. Antibody-induced secondary treatment failure in a patient treated with botulinum toxin type A for glabellar frown lines. Journal of Clinical Interventions in Aging. 2011;6:281–284.
Wohlfarth K, Muller C, Sassin I, et al. Neurophysiological double-blind trial of a botulinum neurotoxin type a free of complexing proteins. Clinical Neuropharmacology. 2007;30:86–94.