7 Basic science
Myobloc®
Summary and Key Features
• Myobloc® is the only botulinum toxin product that is based on serotype B
• Botulinum toxin B (BoNT-B) has its own unique mechanism of action, binds specific serotype receptors, and targets specific intracellular proteins
• Myobloc® requires no reconstitution before injecting as it is available in a liquid formulation
• Myobloc® is FDA approved for the treatment of abnormal head position and neck pain that accompanies cervical dystonia in adults
• BoNT-B is an important replacement whenever it comes to immunoresistance in patients treated with BoNT-A
• Myobloc® has a higher rate of adverse effects and the injection can be more painful compared with other BoNT products
• BoNT-B has a rapid onset of effect (within 48 hours after injection). The duration is limited to a maximum of 10–12 weeks
• BoNT-B affects a larger area around the injection point associated with a smoother clinical effect
• The most common side effects when used for the treatment of facial rhytides are xerostomia and headaches as well as dry eyes and brow ptosis
Pharmacology of botulinum toxin B
Botulinum toxin B affects the nervous system in a similar way to all other botulinum toxins in a series of cellular actions including binding, internalization, and inhibition of acetylcholine release. The heavy chain of the complex contributes to the irreversible binding of the toxin to the serotype-specific protein within the soluble N-ethyl maleimide-sensitive factor attachment protein receptor (SNARE) complex, which is involved in release of acetylcholine-containing vesicles from the presynaptic neuron. The light chain mediates proteolysis of SNARE proteins and subsequent inhibition of synaptic vesicle fusion to the presynaptic membrane of the human motor neurons. This leads to flaccid paralysis associated with botulism. Each serotype cleaves a specific residue on one of the N-ethylmaleimide sensitive factor (NSF) attachment proteins. Botulinum toxin B cleaves the vesicle-associated membrane protein (VAMP) also known as synaptobrevin. VAMP is cleaved by the serotypes F, G, and D too, but at differing locations (Table 7.1). The high specificity of the BoNT light chain cleavage is attributed to the light chain recognizing long SNARE sequences with 30–50 residues indicated by the specific serotype.
Table 7.1 Pharmacology of botulinum toxin serotypes by target / cleavage sizes
| Serotype | Target size | Cleaves at |
|---|---|---|
| A | SNAP-25* | Gln197–Arg198 |
| B | VAMP† | Glu76–Phe77 |
| C | Syntaxin | Lys253–Ala254 |
| Lys252–Ala252 | ||
| SNAP-25* | Arg198–Ala199 | |
| D | VAMP† | Ala67–Asp68 |
| Lys59–Leu60 | ||
| E | SNAP-25* | Arg180–Ile181 |
| F | VAMP† | Gln58–Lys59 |
| G | VAMP† | Ala81–Ala82 |
* SNAP-25 is a soluble NSF attachment protein of 25 000 kDa.
† VAMP = vesicle-associated membrane protein (synaptobrevin).
Myobloc® in the aesthetics practice
Efficacy in the treatment of hyperkinetic rhytides
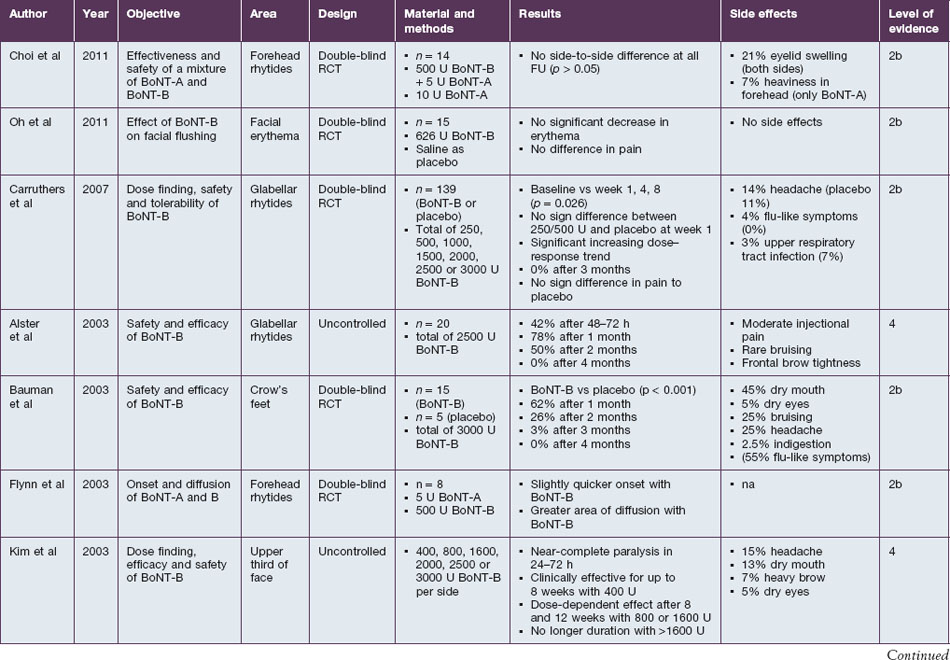
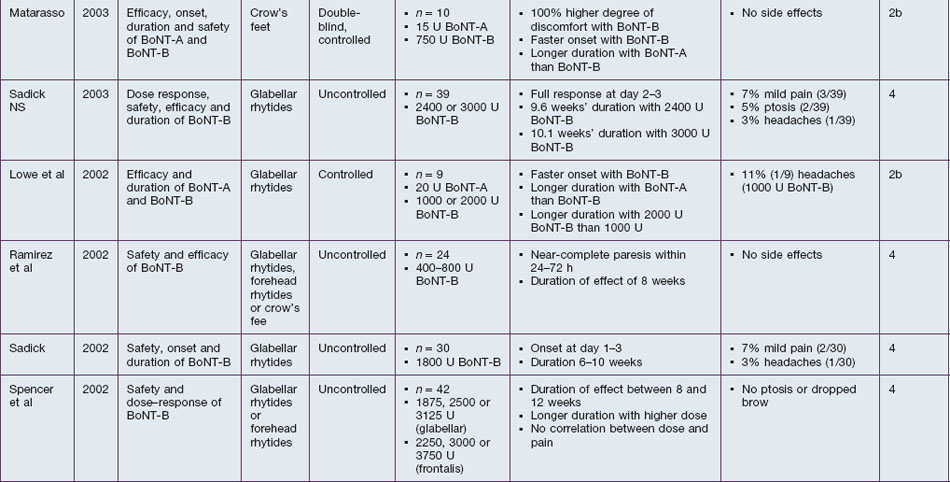
BoNT-B is shown to be efficient in the treatment of hyperkinetic rhytides in the upper third of the face. Studies confirm the denervating effect on orbicularis oculi, frontalis, and corrugator supercilii (Table 7.2). The described efficacy with 2500–3000 U per treatment side is 42–100% after 24–72 hours, 62–78% after 4 weeks, 26–50% after 8 weeks and <5% after 12 weeks. Rhytides in the lower two-thirds of the face have not yet been assessed. Referring to the study by Carruthers et al, a dose of at least 1500 U BoNT-B seems to be ideal for a complete paresis of the frontalis muscle. Direct comparisons with BoNT-A in a 1 : 50 ratio show similar efficacy in the first 4 weeks after injections, but an inferiority of BoNT-B at later timepoints.
Onset and duration
Onset and duration of effect of BoNT-B have been assessed in several studies. The agent is described as taking effect rapidly after injection. Near-complete paresis of the treated muscle is usually seen within 24–72 hours after injection. In direct comparison to BoNT-A, the onset of BoNT-B is quicker and the clinical result is smoother. The duration of effect depends on the dosage used, but seems to be limited to a maximum of 10–12 weeks. Sadick (in 2003) specified the duration of effect with 2400 U BoNT-B to be 9.6 weeks, while it was 10.1 weeks with 3000 U. Bauman et al found an average wear-off time of 7 weeks after injection of 1500 U BoNT-B into each orbicularis oculi. While studies by Comella et al and Tintner et al show a superior duration of BoNT-A in patients treated for cervical dystonia, a study by Pappert et al shows no difference. The non-inferiority is supported by an animal study by Arezzo, which shows a consistent duration of the direct effects on the treated muscle when equivalent doses of BoNT-A or BoNT-B were injected.
Myobloc® in the treatment of hyperhidrosis
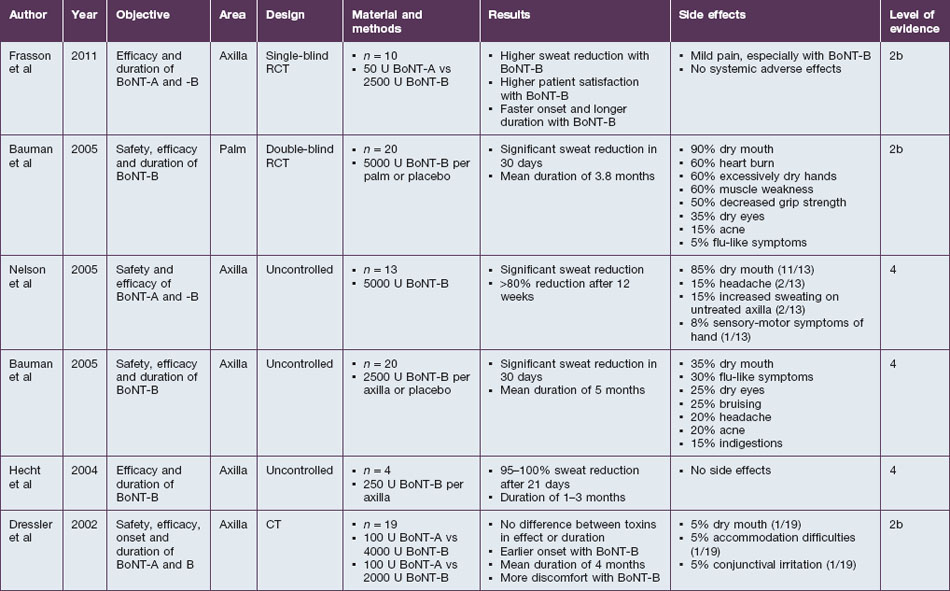
Beside its use for the treatment of hyperkinetic facial lines, BoNT-B is effective for the treatment of hyperhidrosis. The eccrine sweat glands are innervated by sympathetic nerves that use acetylcholine. As botulinum toxin has the ability to block the release of this neurotransmitter in the postganglionic sympathetic fibers of the glands, it can be used to stop the excessive sweat production for several weeks (Table 7.3).
Treatment considerations for Myobloc®
A good rule of thumb to produce Myobloc® results comparable to BoNT-A is to use the established total of Botox® / Xeomin® units and multiply it by 100 to find the number of Myobloc® units needed. As the clinical effect of Myobloc® is wider than that of BoNT-A, the number of injection points can be reduced in most cases (Table 7.4). According to this rule, to eradicate glabellar lines the procerus complex and corrugators supercilii muscles have to be treated with 2000–3000 U Myobloc® in three or four injection sites instead of 20–30 U Botox® / Xeomin® units in five injection sites. The injection of the frontalis utilizes 1500–3000 U of Myobloc®, distributed evenly over three or four injection sites to provide satisfactory results. A dose of 1500 U of Myobloc® has to be injected on each side of the face, into the corrugator supercilii, the procerus complex, and the medial portion of the orbicularis oculi muscle, to achieve effective results in the lifting of the brow. It is found that, for the treatment of crow’s feet, 1000–2000 U of Myobloc® per side have to be injected into two to three sites of each orbicularis oculi muscle.
Table 7.4 Provisional dosing guidelines for botulinum toxin B injections for facial rhytids
| Muscle site | BoNT-B (Myobloc®) | |
|---|---|---|
| Units | No. of injections | |
| Glabella | 2000–3000 | 3 |
| Frontalis | 1500–3000 | 3–4 |
| Brow lift | 1500 total | 2–4 per side |
| Periorbital | 1000–2000 per side | 2–3 per side |
Alster TS, Lupton JR. Botulinum toxin type B for dynamic glabellar rhytides refractory to botulinum toxin type A. Dermatologic Surgery. 2003;29(5):516–518.
Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. European Journal of Neurology. 2001;8(suppl 5):21–29.
Arezzo JC. NeuroBloc / Myobloc: unique features and findings. Toxicon. 2009;54(5):690–696.
Baumann L, Slezinger A, Halem M, et al. Pilot study of the safety and efficacy of Myobloc (botulinum toxin type B) for treatment of axillary hyperhidrosis. International Journal of Dermatology. 2005;44(5):418–424.
Baumann L, Slezinger A, Halem M, et al. Double-blind, randomized, placebo-controlled pilot study of the safety and efficacy of Myobloc (botulinum toxin type B) for the treatment of palmar hyperhidrosis. Dermatologic Surgery. 2005;31(3):263–270.
Baumann L, Slezinger A, Vujevich J, et al. A double-blinded, randomized, placebo-controlled pilot study of the safety and efficacy of Myobloc (botulinum toxin type B)-purified neurotoxin complex for the treatment of crow’s feet: a double-blinded, placebo-controlled trial. Dermatologic Surgery. 2003;29(5):508–515.
Berman B, Seeberger L, Kumar R. Long-term safety, efficacy, dosing, and development of resistance with botulinum toxin type B in cervical dystonia. Movement Disorders. 2005;20(2):233–237.
Callaway JE. Botulinum toxin type B (Myobloc): pharmacology and biochemistry. Clinical Dermatology. 2004;22(1):23–28.
Carruthers A, Carruthers J, Flynn TC, et al. Dose-finding, safety, and tolerability study of botulinum toxin type B for the treatment of hyperfunctional glabellar lines. Dermatologic Surgery. 2007;33(1 spec no.):S60–S68.
Chapman MA, Barron R, Tanis DC, et al. Comparison of botulinum neurotoxin preparations for the treatment of cervical dystonia. Clinical Therapeutics. 2007;29(7):1325–1337.
Choi J-W, Youn C-S, An H-T, et al. Combined use of botulinum toxin type A and B for forehead rhytides: a randomized, double-blind, split-face study. Journal of Dermatological Treatment. 2011. Online. Available http://www.ncbi.nlm.nih.gov/pubmed/21801115 20 October 2011 [Epub ahead of print]
Comella CL, Jankovic J, Shannon KM, et al. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology. 2005;65(9):1423–1429.
Dressler D, Adib Saberi F, Benecke R. Botulinum toxin type B for treatment of axillar hyperhidrosis. Journal of Neurology. 2002;249(12):1729–1732.
Flynn TC, Clark RE, 2nd. Botulinum toxin type B (MYOBLOC) versus botulinum toxin type A (BOTOX) frontalis study: rate of onset and radius of diffusion. Dermatologic Surgery. 2003;29(5):519–522. discussion 522
Frasson E, Brigo F, Acler M, et al. Botulinum toxin type A vs type B for axillary hyperhidrosis in a case series of patients observed for 6 months. Archives of Dermatology. 2011;147(1):122–123.
Halpern JL, Smith LA, Seamon KB, et al. Sequence homology between tetanus and botulinum toxins detected by an antipeptide antibody. Infection and Immunity. 1989;57(1):18–22.
Hecht MJ, Birklein F, Winterholler M. Successful treatment of axillary hyperhidrosis with very low doses of botulinum toxin B: a pilot study. Archives of Dermatological Research. 2004;295(8-9):318–319.
Jankovic J, Hunter C, Dolimbek BZ, et al. Clinico-immunologic aspects of botulinum toxin type B treatment of cervical dystonia. Neurology. 2006;67(12):2233–2235.
Kim EJ, Ramirez AL, Reeck JB, et al. The role of botulinum toxin type B (Myobloc) in the treatment of hyperkinetic facial lines. Plastic and Reconstructive Surgery. 2003;112(5 suppl):88S–93S. discussion 94S–97S
Kranz G, Paul A, Voller B, et al. Long-term efficacy and respective potencies of botulinum toxin A and B: a randomized, double-blind study. British Journal of Dermatology. 2011;164(1):176–181.
Lowe NJ, Yamauchi PS, Lask GP, et al. Botulinum toxins types A and B for brow furrows: preliminary experiences with type B toxin dosing. Journal of Cosmetic and Laser Therapy. 2002;4(1):15–18.
Matarasso SL. Comparison of botulinum toxin types A and B: a bilateral and double-blind randomized evaluation in the treatment of canthal rhytides. Dermatologic Surgery. 2003;29(1):7–13. discussion 13
Nelson L, Bachoo P, Holmes J. Botulinum toxin type B: a new therapy for axillary hyperhidrosis. British Journal of Plastic Surgery. 2005;58(2):228–232.
Oh YJ, Lee NY, Suh DH, et al. A split-face study using botulinum toxin type B to decrease facial erythema index. Journal of Cosmetic and Laser Therapy. 2011;13(5):243–248.
Pappert EJ, Germanson T. Botulinum toxin type B vs. type A in toxin-naïve patients with cervical dystonia: randomized, double-blind, noninferiority trial. Movement Disorders. 2008;23(4):510–517.
Ramirez AL, Reeck J, Maas CS. Botulinum toxin type B (MyoBloc) in the management of hyperkinetic facial lines. Otolaryngology Head and Neck Surgery. 2002;126(5):459–467.
Sadick NS. Prospective open-label study of botulinum toxin type B (Myobloc) at doses of 2,400 and 3,000 U for the treatment of glabellar wrinkles. Dermatologic Surgery. 2003;29(5):501–507. discussion 507
Sadick NS. Botulinum toxin type B for glabellar wrinkles: a prospective open-label response study. Dermatologic Surgery. 2002;28(9):817–821.
Spencer JM, Gordon M, Goldberg DJ. Botulinum B treatment of the glabellar and frontalis regions: a dose response analysis. Journal of Cosmetic and Laser Therapy. 2002;4(1):19–23.
Tintner R, Gross R, Winzer UF, et al. Autonomic function after botulinum toxin type A or B: a double-blind, randomized trial. Neurology. 2005;65(5):765–767.