Chapter 4. Basic science in orthopaedics
By the end of this chapter you should be able to:
• Understand the general principles of joint lubrication and biomechanics.
• Understand the different types of implant used to fix fractures and orthopaedic conditions and their complications.
• Understand the different types of metals used.
• Have a clear understanding of how fractures heal.
Biomechanics
An understanding of the way the body works as a machine is essential to an orthopaedic surgeon but mechanical considerations must always come second to the clinical assessment of the individual patient. The temptation to regard the patient as a machine must be resisted; people are not machines, even if the body is.
Joint loading
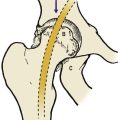
The loads imposed on the joints and the direction of the forces in which they act are not always obvious from gross anatomy (Fig. 4.1a). The load across the hip, for example, is the resultant of the patient’s weight acting downwards and muscles pulling the femur medially and upwards at an angle of about 16° from the vertical. This should not be a surprise because the bone trabeculae in the femoral neck and ilium are oriented in the same direction (Fig. 4.1b). According to Wolff’s law, which states that the position of the trabeculae is dictated by the forces acting on the bone, the trabeculae are the ‘materialized trajectory of the force’.
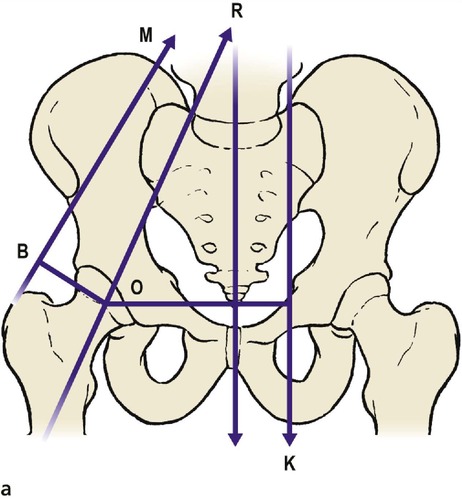 |
| Fig. 4.1a
Vectors acting at the hip joint.
|
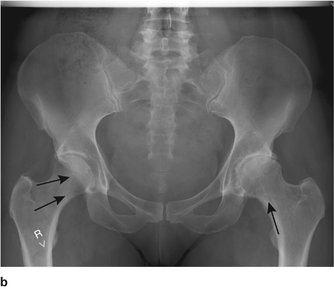 |
| Fig. 4.1b
Radiograph of the pelvis to show that the trabeculae are arranged according to the line of weight transmission and follow Wolff’s law.
|
When standing on one leg, the abductors act at a mechanical disadvantage because they are nearer the centre of the hip joint than the patient’s centre of gravity (L1). In consequence, they have to lift about three times body weight and the load across the hip joint is correspondingly increased. Because loading a diseased hip is painful, it is useful to reduce the load across the hip by allowing the abductor muscles to work at a greater advantage. Patients need no biomechanical knowledge to walk with an antalgic (pain-relieving) gait and quickly find that leaning the body to the side of the affected hip is less painful than walking upright because it brings the centre of gravity closer to the fulcrum and reduces the load across the joint (Fig. 4.2).
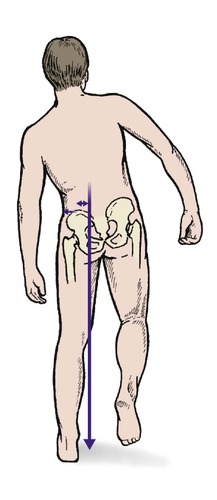 |
| Fig. 4.2
Antalgic gait tilting the pelvis reduces the load on the joint and thus the pain (see also Fig. 2.35).
|
Muscles act at a mechanical disadvantage in other joints as well. The elbow and knee both have flexors and extensors so close to the axis of rotation that they must contract with a force several times greater than the weight they are lifting.
Joint lubrication
Calculations of the shear stresses on a joint surface show, among other things, that the patella has a greater shear stress imposed upon it than any other bone – seven times body weight.
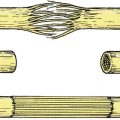
Joint lubrication is complex and depends on three mechanisms (Fig. 4.3).
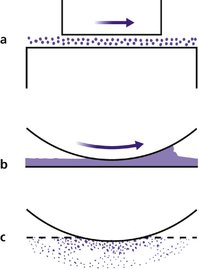 |
| Fig. 4.3
Different types of lubrication: (a) boundary lubrication; (b) surface lubrication; (c) boosted lubrication.
|
Boundary lubrication
Boundary lubrication depends on molecules sliding over each other and is best for heavy loads and slow movements. Graphite is a boundary lubricant. The large hyaluronic acid-based proteoglycan molecules in synovial fluid function in the same way.
Surface lubrication
Compounds which flow between surfaces, such as oils, are the best known lubricants. Synovial fluid acts as a surface lubricant and its efficiency is increased by the ‘wedge effect’. The joint surfaces are not a perfect fit and the irregularities create a wedge of fluid at the point of contact, a little like a car ‘hydroplaning’ on a wet road.
‘Boosted’ lubrication
To improve lubrication still further, fluid from the articular cartilage is squeezed out when it is compressed. This is ‘boosted’ lubrication.
Implants
It is often necessary to insert plates, screws, prostheses and other devices in the body and much effort goes into implant materials and design. It was not always so; in the early days of internal fixation, ordinary wood screws were used to fix fractures but these were specifically designed for timber and proved unsuitable for bone. Not only did the screws rust but a wood screw, which is tapered and ideal for a fibrous material like wood, cannot be used on a dense material like cortical bone. Different types of screws, threads and bone are dealt with on page 133.
Stress risers
Bones are more flexible than metal plates. Screwing a metal plate to bone stiffens it and produces a ‘stress riser’ at each end, which can cause a fracture at the end of the plate (Fig. 4.4).
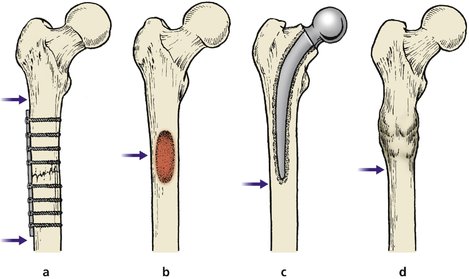 |
| Fig. 4.4
Stress risers. Bones may break at the junction of areas of differing stiffness: (a) upper and lower limits of internal fixation device; (b) tumour; (c) lower limit of hip prosthesis; (d) above or below a united fracture.
|
Similar problems arise around joint prostheses. Not only do fractures occur immediately below the tip of the femoral component of a total hip replacement ( THR), but the different stiffnesses of the bone and implant mean that the interface between the two is under strain. Attempts have been made to overcome this problem by developing ‘isoelastic’ implants with the same elasticity as bone.
Holes
Drilling a hole through bone also produces a stress riser by weakening the bone, but a hole filled with a screw weakens it much less. These considerations only concern orthopaedic surgeons but it is important to appreciate that there is more to reconstructive surgery than the layman supposes.
Materials
Many materials have been used for implants, and most have proved unsatisfactory (Fig. 4.5). The salts of the constituent metals are slowly leached out of the implant over the years and some are toxic or allergenic. The ideal material must be insoluble, strong, non-toxic, non-carcinogenic, and also non-irritant in particulate form.
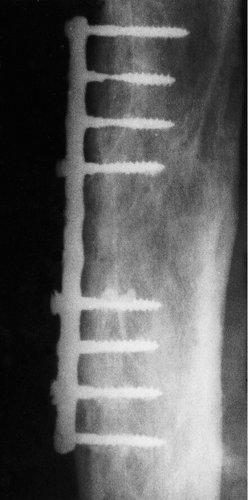 |
| Fig. 4.5
An early implant. The metal was not truly inert and the screws have corroded.
|
Metals
Stainless steel, perhaps the simplest implant material, contains a cocktail of different elements and is generally satisfactory, although not as strong as chrome, cobalt and molybdenum alloys of which many implants are made (Table 4.1). Prostheses are also made of almost pure titanium. Precious metals such as silver and gold are satisfactory implant materials but lack the strength required of a prosthesis, quite apart from their cost.
| Stainless steel (%) | Chrome/cobalt (%) | Titanium alloy (%) | |
|---|---|---|---|
| Iron | 62.2 | ||
| Chromium | 21.5 | 28 | |
| Nickel | 9 | ||
| Manganese | 4 | ||
| Molybdenum | 2.6 | 5 | |
| Niobium | 0.3 | ||
| Nitrogen | 0.4 | ||
| Silicon | 0.75 | ||
| Cobalt | 65 | ||
| Titanium | 90 | ||
| Vanadium | 4 | ||
| Aluminium | 6 |
Plastic
Most artificial joints consist of a metal and plastic articulation. The plastic usually used is high density polyethylene (HDP), but some early prostheses were made of polytetrafluoroethylene (PTFE, Teflon), which was satisfactory until its wear particles provoked a vigorous foreign body reaction that eroded bone. Particles of HDP also produce an inflammatory response, but it is much milder than that caused by PTFE.
Ceramics (aluminium oxide)
Ceramics are also used in prostheses but they are brittle and their wear particles are irritant.
Fixation
Many patients – and some doctors – believe that once an implant is in position it will stay fixed to bone for ever. This is not so; bone implants do not behave like dental fillings. Teeth have no ‘turnover’ and do not change throughout life. Bones do not have this advantage and turn over regularly so that after about a year an implant is attached to a different bone from the one in which it was placed. Although the body reproduces itself fairly accurately it does not do so precisely and this leads to loosening, wear particles, a foreign body reaction, more loosening, etc. This is a particular problem in cancellous bone because the trabeculae change shape in response to load.
Implants are fixed to the skeleton in four ways (Fig. 4.6):
1. By being a ‘tight fit’.
2. Mechanically with screws.
3. With bone cement.
4. By bone ingrowth.
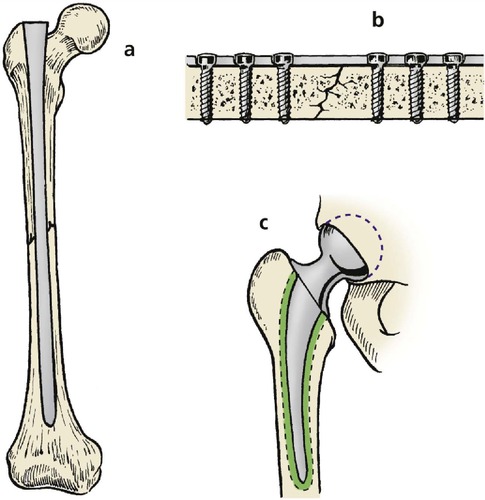 |
| Fig. 4.6
Methods of attaching implants to bone: (a) tight interference fit; (b) screws; (c) bone cement.
|
Interference fit
An interference fit is nothing more than a tight fit like a cork in a bottle or a nail in wood. Some implants have fins and flanges to make them fit more firmly at operation, but sound immediate fixation does not prevent the implant loosening with time.
Screws
Screws produce sound fixation of a plate to bone but are themselves only held by an interference fit and can loosen.
Bone cements
Bone cements are not adhesives; they do not ‘stick’ to bone but fill up spaces to produce a better mechanical fit. In builders’ language, bone cement is a grout, like the material used to fill the cracks between tiles.
The most commonly used is acrylic cement, which is polymethylmethacrylate and chemically the same as Perspex. The cement is prepared during operation by mixing a liquid which contains the monomer (monomethylmethacrylate) and a stabilizer to prevent it polymerizing, with a powder that includes a catalyst to initiate polymerization, a filler consisting of polymethylmethacrylate powder, a radiopaque material such as barium sulphate, and sometimes an antibiotic. The mixture forms a dough-like material which can be forced into the medullary cavity around the implant, where it sets solid. Low viscosity cement with the consistency of thick cream is also available. Although more difficult to handle, low viscosity cement can be forced into cancellous bone, hopefully to achieve more secure fixation.
Acrylic cement is the best available at present but has many disadvantages. While strong in compression, it is weak in tension and breaks when twisted. Once fractured, the two surfaces rub together and produce wear particles which are irritant to bone. A brisk foreign body reaction results, more bone is resorbed, more acrylic cement loosens, more particles are formed and a vicious circle is established, leading to irreversible loosening of the implant and bone destruction.
Bone ingrowth
One way of overcoming the problem of bone fixation is to coat the prosthesis with a porous surface of sintered metal granules, so that bone may grow into the minute passages that cover the prosthesis and thus fix it to the bone. However, even if the bone does grow into the prosthesis, loosening and fracture may still occur unless the prosthesis has the same elastic properties as the bone. A coating of hydroxyapatite to the component can also be used. This again allows the bone to grow up to the edge of the component and integrate with the coating. Ligament prostheses can be fixed to bone by making a bone/prosthesis/bone sandwich so that the cancellous bone grows through the weave of the prosthetic material. Fixation of fabric prostheses occurs readily in soft tissues, both in arterial grafts and hernia repairs.
Tissue healing
Bone
When a bone is fractured, it normally heals with bone. Bone is the only solid tissue in the body that can replace itself in this way. The rest heal with fibrous tissue that leaves a scar.
Bone healing is simple when it occurs smoothly, complicated when it does not. In ideal circumstances the haematoma that forms around the bone ends coagulates, the clot is invaded by cells which form a hard mass, or callus, that resembles cartilage, and the callus is gradually converted to bone (Fig. 4.7). Fortunately, haematomas in other situations do not normally become converted to bone, the process being initiated by stimuli from the bone itself. These stimuli are to be found in the bone marrow, surrounding osteoblasts and the periosteum, but their exact nature is not known.
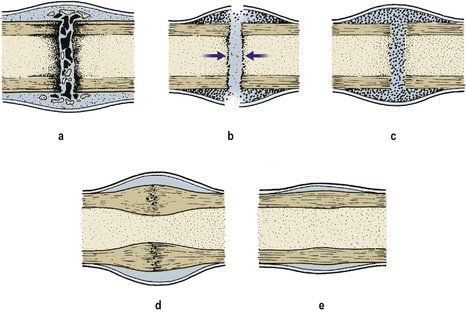 |
| Fig. 4.7
Bone healing: (a) first 2 weeks, blood clot and macrophages form around the fracture; (b) 2–6 weeks, sharp edges are removed by osteoclasts and callus forms within the haematoma and medullary cavity; (c) 6–12 weeks, bone forms within the callus and bridges the gap between the fragments; (d) 6–12 months, the cortical gap is bridged with bone; (e) 1–2 years, remodelling occurs and normal architecture returns.
|
Sound bone healing does not always occur, and the bone ends are then joined by either a mat of fibrous tissue or a false joint ( pseudarthrosis), neither of which is mechanically satisfactory. Fractures through cancellous bone, with a good blood supply, surrounded by muscle and without associated soft tissue trauma, have an excellent chance of healing whatever is done to them, but fractures at the middle of the shaft of long bones, particularly with extensive soft tissue damage, have a high incidence of non-union (p. 106).
Bone gradually gains in strength and the bone is ‘united’ when it is strong enough for normal use. This is a variable criterion because a weight-bearing lower limb bone carries more load than a non-weight-bearing upper limb bone and therefore takes longer to ‘unite’, even though the rate of healing and the strength of the bone may be the same.
Stages of bone healing
1. For the first 2 weeks, bone healing follows the same pattern as the healing of skin or any other wound. The site of the wound is filled with blood and the broken ends of the bone become necrotic.
2. The blood clot is invaded by macrophages and osteoclasts, which remove dead bone, and osteoblasts, which produce bone, instead of the fibroblasts which form fibrous tissue in soft tissue injuries.
3. Between 2 and 6 weeks after injury osteoid tissue develops and forms a firm mass, or callus, around the fracture and ossification of the osteoid begins. Callus forms both outside the bone as subperiosteal callus, and inside as endosteal callus. The pH of the tissues increases at this stage and calcium is deposited.
4. Between 6 and 12 weeks, ossification occurs, a solid bony bridge crosses the gap and the bone regains some mechanical strength.
5. Between 12 and 26 weeks, the callus matures.
6. Between 6 and 12 months, the gaps between the cortical ends are bridged.
7. Between 1 and 2 years, remodelling occurs, bony prominences become smooth and normal bone architecture is restored.
The timing is very variable and is much faster in children, in whom callus can be seen at 2 weeks.
Remodelling
In children, remodelling will correct some, but not all, bone deformities. Up to 30° of malalignment in the plane of movement of the joint can be expected to correct itself by remodelling in the young child. Rotational deformities and malalignments in other planes do not do so well (Fig. 4.8 and Fig. 4.9). A further blessing is that, in children, a broken limb will grow faster than normal and a loss of length of 1–1.5 cm can be accepted under the age of 12 years.
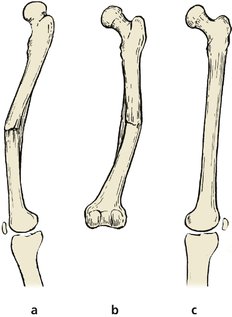 |
| Fig. 4.8
Remodelling occurs: (a) well in the plane of flexion and extension; (b) partly in a plane at right-angles to flexion and extension; (c) not at all in rotation.
|
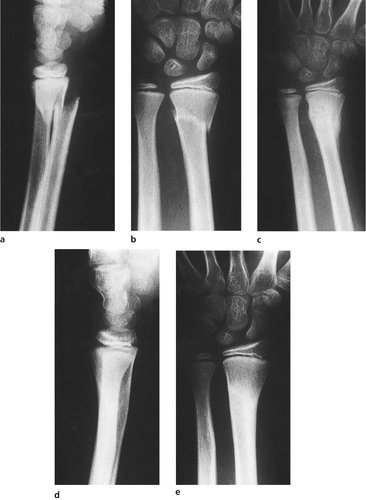 |
| Fig. 4.9
Fracture remodelling in a child: (a), (b) a fracture of the lower end of the radius; (c) remodelling has begun 6 weeks later; (d), (e) the final position 6 months later. The radial epiphysis on the lateral view has regained its normal forward angulation, but the anteroposterior position has not altered.
|
The electrical activity of bone
Bone has piezoelectric properties and produces a small electric current when it is bent, like a crystal in an old-fashioned record player pick-up head. The convex side of the bent bone, which is under tension, has a positive charge relative to the concave side, which is under compression (Fig. 4.10). Bone is formed on the negative concave side and, if a potential difference is applied to the two sides of a bone, bone will form around the negative cathode and be eroded around the positive anode. This suggests that electricity is responsible for bone formation and resorption, an idea supported by the observation that, if screws and plates are made of dissimilar metals, bone resorption occurs between them as a result of the small current that flows between the two different metals.
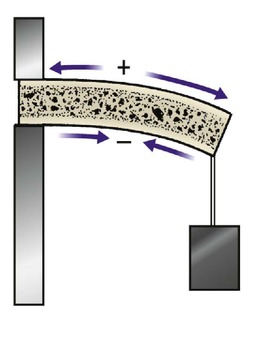 |
| Fig. 4.10
Piezoelectric effect in bone. When a bone is bent, the tension side has a positive charge relative to the compression side.
|
Against this must be weighed the fact that almost anything has some piezoelectric activity, even a dead twig, and despite many efforts to apply a potential difference to broken bones there is still little convincing evidence that it induces more rapid healing or makes an non-united fracture unite with bone.
Articular cartilage
Hyaline cartilage does not regenerate in the adult. Although superficial damage can heal in the very young, for all practical purposes injuries to hyaline cartilage heal with fibrocartilage and fibrous tissue with inferior weight-bearing properties (Fig. 4.11).
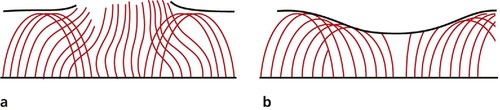 |
| Fig. 4.11
Healing of articular cartilage: (a) the collagen arcades are disrupted; (b) the defect is filled with fibrocartilage but the collagen arcades do not regenerate.
|
Skin
Unlike bone, skin cannot reproduce itself and heals with a fibrous scar. This can be a special problem to the orthopaedic surgeon because any scar, even a surgical scar, that crosses a joint can contract and restrict movement (Fig. 4.12).
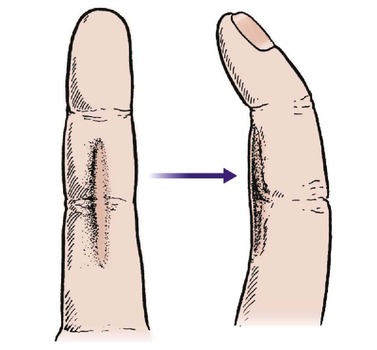 |
| Fig. 4.12
Scars that cross flexion creases may contract to pull the joint into flexion.
|
To operate upon a joint and create a scar which limits joint movement is not helpful and the site of incisions around joints must be carefully selected. As a general rule, incisions should never cross skin creases on the flexor surface of joints.
Stages of skin healing
1. The wound edges bleed, the space fills with clot and the surrounding vessels dilate. White blood cells invade the clot.
2. During the first 2–3 days, the wound margins fill with macrophages, which remove dead tissue. Fibroblasts and capillary buds appear and the clot is replaced with granulation tissue.
3. Between 3 and 14 days, the fibroblasts form fibrous tissue, vascularity diminishes and the scar contracts to 80% of its original size. After 14 days the wound is healed soundly enough to withstand normal stresses but does not regain its full strength until 3 months.
4. Between 2 weeks and 2 years, the fibrous tissue contracts further. The wound, a dull purplish colour at first, gradually becomes pale. Scars on the flexor aspects of joints tend to produce tight contractures but those on the extensor aspect stretch and leave ugly wide scars.
Nerves
Nerves are often severed in limb trauma and small cutaneous nerves may be divided during operation. When a nerve is cut, changes are seen in the cell body and the axon cylinder distal to the cut degenerates.
Fine fibrils from the proximal end enter the distal sheath and grow down it at the rate of 1 mm per day. Healing of the nerves depends upon the two cut ends of the nerve being so close that the axon can cross from the proximal end of the nerve to the distal and grow down its own axonal tube to the end plate. Although individual nerve fibres may heal well and nerves can be approximated surgically using a microscope so that they appear anatomically normal, there is no guarantee that each individual neurone will find its correct destination. A telephone cable provides a useful analogy: to cut across a large telephone cable and hold the two ends together with tape is unlikely to make all the right connections (Fig. 4.13).
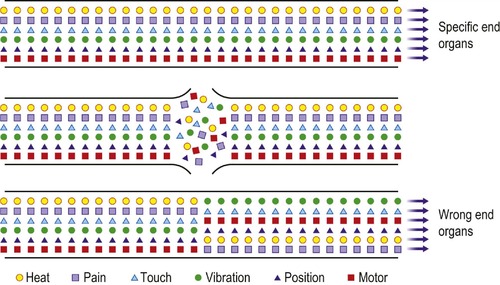 |
| Fig. 4.13
Healing of nerves. The nerve fibres carrying different types of sensibility do not heal correctly after division and produce dysaesthesiae in the extremities.
|
Because the nerve ends cannot be accurately apposed even with the help of a microscope, nerves do sometimes grow down the wrong axonal tube and reach the wrong end organ. The result of this can be that heat or light touch are experienced as pain and the incorrectly innervated skin is hypersensitive. If the cut ends are not approximated, a neuroma will form at the end of the nerve and this also leads to altered sensibility, which can be distressing.
Muscle
Muscle, like skin, heals with fibrous tissue and a cut muscle never regains its full bulk or power, even if it does all that is asked of it in normal use. A few multinucleate muscle cells may be seen. These contribute little to the function of the injured muscle. Areas of ischaemic muscle following arterial damage, compartment syndromes or crushing injuries are replaced with a mass of fibrous tissue that contracts and limits joint movement (Fig. 4.14). In severe cases, the contracture will pull the limb down into a position of extreme flexion, as in Volkmann’s ischaemic contracture of the forearm.
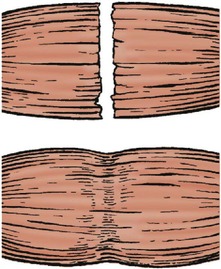 |
| Fig. 4.14
Healing of muscle. Muscle heals with fibrous tissue, which produces a contracture within the muscle belly.
|
Immunology
Bone, like other tissues, evokes an immune response but it is weaker than in other tissues. Some hospitals maintain banks of cadaveric bone to fill large bone defects but the bone is never as good as an autograft, which has excellent osteogenic properties, no antigenic potential and will not transmit AIDS or other diseases.
Exposing bone to very low temperatures, in the region of − 20°C, diminishes, but does not eliminate, the antigenicity of the transplanted bone. Because of this, deep-frozen allograft ‘bank bone’ is better than refrigerated bone and will act as a scaffold for gradual replacement with the patient’s own bone by creeping substitution, although it is affected by a cell-mediated immune response just like other tissue.
Freeze-dried tissues can also be used and there is some evidence that the HIV does not survive freeze-drying.
Articular cartilage can also be moved from one patient to another with a limited immune reaction, but muscle, nerve and other musculoskeletal tissues evoke too great an immune response to permit useful transplantation.