4 Basic science
BOTOX® Cosmetic
Summary and Key Features
• The introduction of BOTOX® Cosmetic (onabotulinumtoxinA) into aesthetic dermatology has revolutionized the management of facial lines
• Botulinum neurotoxins are biological products, synthesized by bacteria, then purified, formulated, and packaged into minute quantities for medical use
• Seven different serotypes of botulinum toxins occur in nature (types A through G), although most clinical products, including onabotulinumtoxinA, are based on the A serotype
• Botulinum toxin type A has a well-defined mechanism of action; at the neuromuscular junction, it reduces acetylcholine release from motor nerves and inhibits muscular contractions
• Clinical and preclinical data suggest that onabotulinumtoxinA may also act on nociceptive neurons
• Each commercially available botulinum toxin product is a unique biological therapeutic, with a distinct structure, formulation, unit strength and clinical profile
• As biologics, the doses of botulinum neurotoxins are expressed in units of biological activity that are not interchangeable or convertible among different products
• Recent analyses demonstrate that the onset of effect of onabotulinumtoxinA in the management of glabellar lines occurs within 24 hours and that benefits last at least 4 months
• Although all botulinum toxin products may stimulate antibody formation, the immunogenicity profile of onabotulinumtoxinA is well characterized
• The clinical efficacy and safety profile of onabotulinumtoxinA in facial lines are well understood by skilled practitioners
Introduction
The introduction of botulinum toxin type A into the field of aesthetic dermatology has profoundly impacted the clinical management of undesirable facial lines. Botulinum toxins are injected into discrete facial muscles where they act locally to reduce muscle contractions that produce skin creases, either with facial animation or at rest. The pattern of injections can be tailored to individual needs and the results in glabella have been demonstrated by Carruthers and colleagues in 2004 to last an average of 4 months.
Serotypes and structure
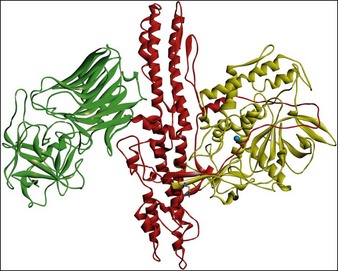
All botulinum toxins are produced by bacteria as protein complexes consisting of a core neurotoxin molecule with a molecular mass of approximately 150 kDa and one or more associated proteins. This protein contains three distinct functional domains. The binding domain is responsible for the docking of the molecule with its specific cell surface receptors, the translocation domain is critical in allowing the catalytic domain to access the neuronal cytosol, and the catalytic domain is responsible for the enzymatic activity that ultimately interferes with neurotransmitter release (Fig. 4.1).
Mechanism of action
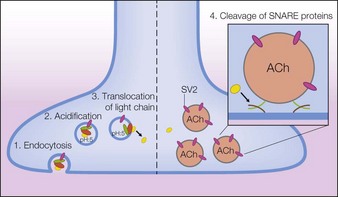
Following intramuscular injection, botulinum neurotoxins inhibit the release of acetylcholine (ACh) from motor nerve terminals, resulting in reduced muscular contractions. This inhibition occurs in multiple steps referred to as binding, internalization, translocation, and cleavage. Through a similar process, botulinum neurotoxins also inhibit acetylcholine release from autonomic nerve terminals that innervate smooth muscle or glands. Further studies have found that botulinum toxin type A exerts selective effects on the nociceptive system. These actions are described in the following text, which for onabotulinumtoxinA begins with the dissociation of the 150 kDa neurotoxin from the NAPs (Fig. 4.2).
Clinical pharmacology of onabotulinumtoxinA in aesthetics
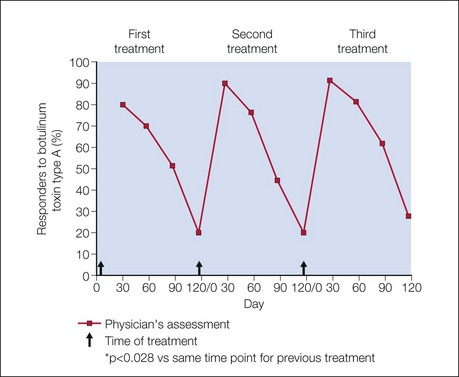
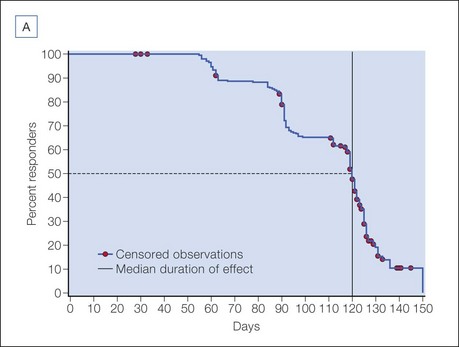
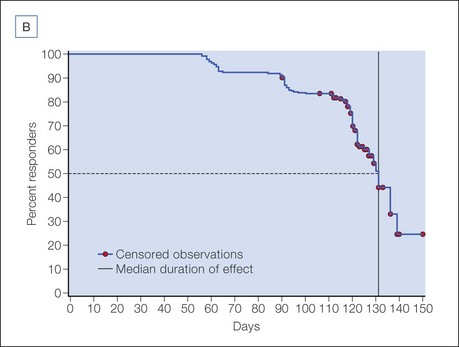
The efficacy and safety of onabotulinumtoxinA in glabellar lines has been studied in rigorous double-blind, placebo-controlled registration trials in North America by Carruthers et al and in Asia by Harii & Kawashima and Wu et al. Responder rates in these trials were uniformly high, with more than 80% of subjects exhibiting significant clinical efficacy on dynamic glabellar lines with a 20 U dose of onabotulinumtoxinA, according to a priori responder criteria (Fig. 4.3). Based on these trials, a median duration of efficacy of 120 days or 4 months may be expected in those exhibiting a clinical response. The duration of efficacy on resting glabellar lines in these trials was found to be longer than that on dynamic rhytides, with a median duration of 131 days (Fig. 4.4).
The safety of onabotulinumtoxinA in the management of facial lines has been evaluated in a systematic meta-analysis by Brin et al. published in 2009. This analysis was based on nine manufacturer-sponsored clinical trials of onabotulinumtoxinA (two on lateral canthal lines and seven on glabellar lines), and included 1678 subjects who received 3–18 U per side for lateral canthal lines or 10 U or 20 U total dose for glabellar lines. In these studies, the overall incidence of adverse events was not different between the onabotulinumtoxinA- and placebo-treated groups. Eyelid ptosis (1.8%) and eyelid sensory disorder (characterized by feelings of tightness, pressure, or a heaviness to the eyelids, 2.5%) were the only adverse events that occurred at a significantly higher rate in the onabotulinumtoxinA than in the placebo group, and this was found only in the glabellar lines population. In the assessment of treatment-related adverse events, eyelid edema was also significantly more frequent in the onabotulinumtoxinA- than the placebo-treated group. As the number of treatment cycles with onabotulinumtoxinA increased, the incidence of all three of these adverse events decreased.
Allergan, Inc., Corporate statement on animal testing, Online. Available http://www.allergan.com/responsibility/product_safety_and_animal_testing.htm 6 February 2011
Allergan, Inc. Botox(r) (onabotulinumtoxinA) Prescribing information. Irvine, CA: Allergan, Inc.; 2011.
Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26(5):785–793.
Beer KR, Boyd C, Patel RK, et al. Rapid onset of response and patient-reported outcomes after onabotulinumtoxinA treatment of moderate-to-severe glabellar lines. Journal of Drugs in Dermatology. 2011;10(1):39–44.
Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. Journal of the American Academy of Dermatology. 2009;61(6):961–970.
Carruthers A, Carruthers J, Lowe NJ, et al. One-year, randomised, multicenter, two-period study of the safety and efficacy of repeated treatments with botulinum toxin type A in patients with glabellar lines. for the BOTOX(r) Glabellar Lines I & II, Groups S. Journal of Clinical Research. 2004;7:20.
Carruthers A, Carruthers J, Said S. Dose-ranging study of botulinum toxin type A in the treatment of glabellar rhytids in females. Dermatol Surgery. 2005;31(4):414–422. discussion 422
Chen F, Kuziemko GM, Stevens RC. Biophysical characterization of the stability of the 150-kilodalton botulinum toxin, the nontoxic component, and the 900-kilodalton botulinum toxin complex species. Infection and Immunity. 1998;66(6):2420–2425.
Dailey RA, Philip A, Tardie G. Long-term treatment of glabellar rhytides using onabotulinumtoxina. Dermatol Surgery. 2011;37(7):918–928.
Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. Journal of Urology. 2010;184(6):2416–2422.
Eisele KH, Fink K, Vey M, et al. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57(4):555–565.
Goschel H, Wohlfarth K, Frevert J, et al. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies – therapeutic consequences. Experimental Neurology. 1997;147(1):96–102.
Grumelli C, Verderio C, Pozzi D, et al. Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology. 2005;26(5):761–767.
Hambleton P. Clostridium botulinum toxins: a general review of involvement in disease, structure, mode of action and preparation for clinical use. Journal of Neurology. 1992;239(1):16–20.
Harii K, Kawashima M. A double-blind, randomized, placebo-controlled, two-dose comparative study of botulinum toxin type A for treating glabellar lines in Japanese subjects. Aesthetic Plastic Surgery. 2008;32(5):724–730.
Hunt T, Clarke K. Potency evaluation of a formulated drug product containing 150-kd botulinum neurotoxin type A. Clinical Neuropharmacology. 2009;32(1):28–31.
Kawashima M, Harii K. An open-label, randomized, 64-week study repeating 10- and 20-U doses of botulinum toxin type A for treatment of glabellar lines in Japanese subjects. International Journal of Dermatology. 2009;48(7):768–776.
Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nature Structural Biology. 2003;10(1):13–18.
Lowe NJ, Glaser DA, Eadie N, et al. Botulinum toxin type A in the treatment of primary axillary hyperhidrosis: a 52-week multicenter double-blind, randomized, placebo-controlled study of efficacy and safety. Journal of the American Academy of Dermatology. 2007;56(4):604–611.
Merz. Xeomin(r) (incobotulinumtoxinA) 2010 Prescribing information. LLC: Merz Pharmaceuticals; 2010.
Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, et al. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. Journal of Biological Chemistry. 2004;279(24):25665–25672.
Naumann M, Carruthers A, Carruthers J, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (BOTOX(R)) across multiple indications. Movement Disorders. 2010;25(13):2211–2218.
Sakaguchi G, Kozaki SIO. Structure and function of botulinum toxins. In: AIouf JE, ed. Bacterial protein toxins. London: Academic Press; 1984:435–443.
Simpson LL, Maksymowych AB, et al. The role of exoproteases in governing intraneuronal metabolism of botulinum toxin. Protein Journal. 2005;24(3):155–165.
United States Food and Drug Administration, Update of safety review of onabotulinumtoxinA (marketed as Botox/Botox Cosmetic), abobotulinumtoxinA (marketed as Dysport) and rimabotulinumtoxinB (marketed as Myobloc), Online. Available http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm174959.htm 5 February 2011
Verderio C, Rossetto O, Grumelli C, et al. Entering neurons: botulinum toxins and synaptic vesicle recycling. EMBO Reports. 2006;7(10):995–999.