5 Basic science
Abobotulinum toxin A
Summary and Key Features
• Abobotulinum toxin is a botulinum toxin-A with very similar features to the other type A botulinum toxins
• The accessory proteins that surround the neurotoxin protein are already dissociated in the vial
• Is ‘diffusion’ an issue? Clinical studies supporting both issues are presented
• Equivalency between abo- and obobotulinum toxins is approximately 2.5; a true number is impossible to define as the units – Speywood units versus Botox units – are measured differently
• Because the clinical products Dysport® and Azzalure® are identical, except for the number of Speywood units per vial, all clinical data for one product can be considered applicable to the other
• Clinical trials in the United States and Europe confirm both efficacy and safety for glabellar injection for frown lines
• Other features evaluated include time of onset, efficacy, and safety with re-injection and dose equivalency to glabellar muscle mass
• Reviews of the international consensus board for dosage and treatment technique gave recommendations for injections at both upper and lower facial sites
• Parameters for safe injections with abobotulinum toxin are reviewed
• Dosage and dilution are important determinant points for efficacy, duration, and field of effect.
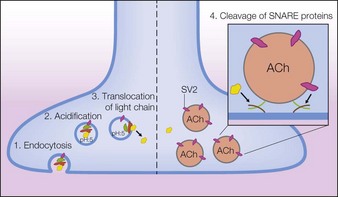
Before the BoNT-A neuroprotein can become active, the NAPS must release the active BoNT-A 150 kDa neuroprotein from the progenitor complex. This occurs with a change in environment to a physiological pH. The issue arises as to when this occurs. Earlier pilot in vitro studies by Eisele & Taylor showed the timescale of release varied from immediate with pH change to a delay from minutes to hours, depending on the product serotype with BoNT type B taking longest. A recent study by Merz Pharmaceuticals, reported by Eisele et al in 2011, found that the naked neuroprotein was released from its associated complex in less than 1 minute with a change to physiological pH; this occurs with both onabotA and abobotA. The investigation implied that release of the naked neuroprotein probably occurs in the vial during reconstitution, well before injection and tissue spread. However, definitive studies on complex dissociation by the same group were also published in 2011 and clearly demonstrated that all the BoNT type A products existed as free neurotoxin within the vials even before reconstitution. In other words, the manufacturing processes for the products all released the free BoNT neurotoxin during manufacture.
These biochemical studies do have implications for clinical usage in understanding efficacy and safety of each product. With the neurotoxin protein of 150 kDa released before injection, the active toxins are stoichiometrically similar and one would not expect a difference in diffusion since complex size is now shown to be irrelevant. It is the authors’ opinion that the difference seen with the products has to do with dosage-unit differences and volume reconstitution. The relative kinetics of dissociation versus diffusion have implications for the safety profiles of the various formulations of BoNT-A in current or future clinical use, and therefore these remain contentious issues for their respective manufacturers, as reported by Pickett in 2009.
Pearl 5
Dilution, dosage, and injection points are most important for proper usage of abobotulinum toxin.
Clinical studies performed for abobotulinumtoxinA
Aesthetic studies for abobotulinum toxin (Dysport®) by Rzany et al and Ascher et al followed a similar pattern to those performed for Botox® by Allergan Inc. a few years earlier (reported by Carruthers et al in 2004). The first European studies for aesthetic usage of abobotulinum toxin (Dysport®) were performed by Ascher and colleagues in the later 90s, and these were followed by Ascher and Rzany in 2004 and 2006. A ‘dose-finding’ study by Vandenbergh & Lison was first performed on 119 patients using placebo, 25, 50, and 75 s.U in a double-blinded control. The subjects were measured for efficacy as a responder at 1 month and then assessed for safety and duration. A responder rate of over 80% was found for all three groups and at 6 months two-thirds of the treated patients were still responders. There was a favorable safety profile of 7% mild adverse events, with headache the most common. There were no reported cases of blepharoptosis or diplopia. The result suggested 50 units was the optimal dosage for the glabella with 10 units injected into each of five glabellar sites.
AbobotA has been used worldwide for aesthetic needs for over 10 years. Both glabella and crow’s feet have been well studied by Ascher with results comparable to onabotA. AbobotA has been used in clinical cosmetic practice worldwide over the last 10 years with similar injection points and techniques as onabotA, but with different dosages. An international consensus conference held in Paris in January 2009 provided general guidelines for effective and safe use of abobotA on the generally useful yet off-label sites for injection, as reported by Ascher and colleagues. These have included the common upper face sites: the glabella, forehead, brow, crows’ feet, and eyelid; and other less common facial sites including: bunny lines, depressor anguli oris, orbicularis oris, mentalis, and platysma. These recommendations gave guidelines to beginning dosage and range for each site (see Table 5.1) as well as injection points and technique.
| Indications | Total usual dose (Dysport® / Speywood units) | Dose range (Dysport® / Speywood units) |
|---|---|---|
| Glabella | 50 | 30–70 |
| Forehead | 40–50 | 40–70 |
| Crows’ feet | 30 × 2 | 20–50 × 2 |
| Lateral eyebrow lift | 20 × 2 | 20–40 × 2 |
| Glabella and forehead | 90–100 | 70–140 |
| Glabella and lateral eyebrow lift | 90 | 50–110 |
| Complete upper third face | 150 | 110–240 |
Treatment of the upper face
The site-specific dosage recommendations for the upper face are shown in Table 5.1.
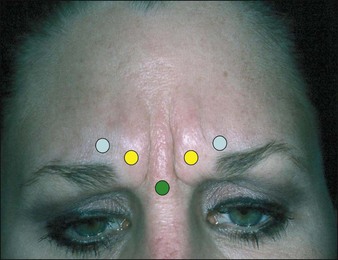
Glabellar lines
Injection of the glabella is the original and by far most common cosmetic usage of BoNT-A. The standard 5-injection-point approach is appropriate for most patients with two injections in each corrugator and one injection in the procerus as shown in Figure 5.1. The procerus and medial corrugators are injected deeply and directly into the bodies of the muscles, which are easily identified at maximal frown in most patients. The lateral corrugators and orbicularis oculi muscle fibers are injected more superficially as it inserts into the dermis medial to the mid-pupillary line. All injections should be 1.0 cm (approximately one finger-breadth) above the orbital rim to limit the risk of eyelid ptosis from the spread of toxin (at the time of injection) to the levator palpebrae muscle. The measurement should be made from the orbital rim and not from the brow itself as a dropped brow may mislead the physician to inject the toxin below the orbital rim. Injecting too high on the forehead – above 1 cm. at the lateral glabellar site – can also cause ptosis in those individual who recruit eyelid and eyebrow elevation from the frontalis muscle.
Forehead lines
The author commonly uses a V-shaped configuration for injections in women (Fig. 5.2). While often desirable in females, this should be avoided in male patients. This approach can also produce an excessively arched brow (the mephisto sign or ‘Mr Spock look’). This is prevented with a high lateral forehead injection above the tail of the brow. If an excessive arched brow occurs, it can be corrected with a small dose of additional toxin 1–2 cm superior to the apex of the arched brow.
Lateral eyebrow lift
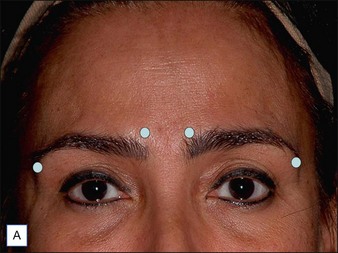
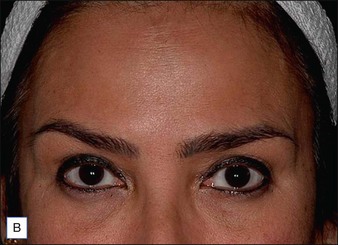
The vertical fibers of the lateral orbicularis oculi function to depress the lateral brow. When injected at the tail of the brow the lateral brow will be elevated. For this chemical brow lift, the author typically injects 10 s.U of abobotA intradermally at the lateral tail of the eyebrow 5–7 mm superolateral to the orbital rim (Fig. 5.3A). If performed only for brow lift and not to correct frown lines, an additional 5–10 s.U are injected into each corrugator body. In combination with medial and central frontalis denervation, which tends to lower the medial brow, a pleasingly arched female pattern eyebrow can be shaped (Fig. 5.3B).
Crow’s feet
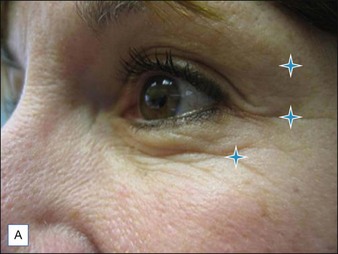
Lateral periorbital wrinkling is effectively treated with neurotoxin. Crow’s feet are typically treated with three equal injections of 10–15 s.U evenly spaced along an arc at the external orbital rim (Fig. 5.4A, B). The middle injection is placed in line with the lateral canthus. Injections flanking this point at 8–10 mm are then placed, but their exact positioning depends on the width of the individual’s canthal lines.
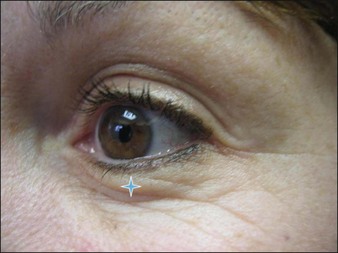
An additional 2 s.U microinjection can be judiciously placed in the lower lid at the mid-pupillary line 2 mm below the tarsal plate (Fig. 5.5). This will flatten the bulging muscle and create an image of an ‘open eye’. Overaggressive treatment may, though, create an unwanted ectropion.
Treatment of the lower face
Treatment of the lower face should be performed with more precaution as over-dosage leads to significant dysfunction of the orbicularis oris, creating an asymmetric smile and oral aperture. Dosage recommendations in the lower face are shown in Table 5.2. As with onabotA treatment, the physician must have a thorough understanding of facial musculature. This is especially important in the lower face as injections in or near many perioral muscles can cause facial asymmetry with expression. Because the field of effect may be influenced by volume, one may consider the 1.5 mL reconstitution is recommended to limit abobotA effect to the muscles injected.
| Indications | Total usual dose (Dysport® / Speywood units) | Dose range (Dysport® / Speywood units) |
|---|---|---|
| Obicularis oris | 2.5 per injection point | 10–15 units total |
| Depressor angularis oris | 5–10 units per site | 10–20 units |
| Mentalis | 5–10 units per site | 10–20 units |
| Platysma | 5–10 units per injection point | 20–40 units Maximum total dosage 50/side |
Alam M, Dover JS, Arndt KA. Pain associated with injection of botulinum a exotoxin reconstituted using isotonic sodium chloride with and without preservative: a double-blind, randomized controlled trial. Archives of Dermatology. 2002;138(4):510–514.
Allen SB, Goldenberg NA. Pain difference associated with injection of abobotulinumtoxina reconstituted with preserved saline and preservative-free saline: a prospective, randomized, side-by-side, double-blind study. Dermatologic Surgery. 2012. Jan 23; doi: 10.1111/j.1524-4725.2011.02284.x. [Epub ahead of print]
Allergan, Inc. Safety analysis (data on file). Online. Available http://www.botoxcosmetic.com/botox_physician_info/clinical_information.aspx, 2010. 10 January 2010
Ascher B, Talarico S, Cassuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit) – part I: upper facial wrinkles. Journal of the European Academy of Dermatology and Venereology. 2010;24:1278–1284.
Ascher B, Talarico S, Cassuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit) – Part II: Wrinkles on the middle and lower face, neck and chest. Journal of the European Academy of Dermatology and Venereology. 2010;24(11):1285–1295.
Ascher B, Rzany BJ, Grover R. Efficacy and safety of botulinum toxin type A in the treatment of lateral crow’s feet: double-blind, placebo-controlled, dose-ranging study. Dermatologic Surgery. 2009;35:1478–1486.
Ascher B, Zakine B, Kestemont P, et al. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin type A in the treatment of glabellar lines. Journal of the American Academy of Dermatology. 2004;51(2):223–233.
Carruthers A, Carruthers J, Lowe NJ, et al. One-year, randomized, multicenter, two-period study of the safety and efficacy of repeated treatments with botulinum toxin type A in patients with glabellar lines. for the BOTOX Glabellar Lines I & II Study Groups. Journal of Clinical Research. 2004;7:1–20.
Carruthers J, Lowe NJ, Mentor MA, et al. Double-blind placebo-controlled study of the safety and efficacy of Botulinum toxin type A for patients with glabellar lines. Plastic and Reconstructive Surgery. 2003;112:1089–1098.
Carruthers J, Fagien S, Matarasso SL. Consensus recommendations on the use of botulinum toxin type A in facial aesthetics. Plastic and Reconstructive Surgery. 2004;114(suppl 1):S1–S22.
Dressler D, Wohlfahrt K, Meyer-Rogge E, et al. Antibody-induced failure of botulinum toxin A therapy in cosmetic indications. Dermatologic Surgery. 2010;36(s4):2182–2187.
Eisele KH 2009 Is there a role for complexing proteins in pharmaceutical neurotoxin formulations? Presented at the International Masters Course on Aging Skin, 8-11 January 2009, Paris, France
Eisele KH, Taylor HV. Dissociation of the 900 kDa neurotoxin complex from C. botulinum under physiological conditions. Toxicon. 2008;51(suppl 1):10.
Eisele KH, Fink K, Vey M, et al. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57(4):555–565.
Hambleton P, Pickett AM. Potency equivalence of botulinum toxin preparations. Journal of the Royal Society of Medicine. 1994;87(11):719.
Hexsel D, Dal’Forno T, Hexsel C, et al. A randomized pilot study comparing the action halos of two commercial preparations of botulinum toxin type A. Dermatologic Surgery. 2008;34:52–59.
Inoue K, Fujinaga Y, Watanabe T, et al. Molecular composition of Clostridium botulinum type A progenitor toxins. Infection and Immunity. 1996;64(5):1589–1594.
Kane MA, Brandt F, Rohrich RJ, et al. Reloxin Investigational Group. Evaluation of variable-dose treatment with a new U.S. botulinum toxin type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plastic and Reconstructive Surgery. 2009;124(5):1619–1629.
Karsai S, Adrian R, Hammes S, et al. A randomized double-blind study of the effect of botox and dysport/reloxin on forehead wrinkles and electromyographic activity. Archives of Dermatology. 2007;143(11):1447–1449.
Kessler KR, Skutta M, Benecke R. Long-term treatment of cervical dystonia with botulinum toxin A: efficacy, safety, and antibody frequency. German Dystonia Study Group. Journal of Neurology. 1999;246(4):265–274.
Kerscher M, Roll S, Becker A, et al. Comparison of the spread of three botulinum toxin type A preparations. Archives of Dermatological Research. 2012;304(2):155–161.
Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nature Structural Biology. 2003;10(1):13–18.
Lawrence I, Moy R. An evaluation of neutralizing antibody induction during treatment of glabellar lines with a new US formulation of botulinum neurotoxin type A. Aesthetic Surgery Journal. 2009;29(6):S66–S71.
Lee S-K. Antibody-induced failure of botulinum toxin type A therapy in a patient with masseteric hypertrophy. Dermatologic Surgery. 2007;33(suppl 1):S105–S110.
Lietzow MA, Gielow ET, Le D, et al. Subunit stoichiometry of the Clostridium botulinum type A neurotoxin complex determined using denaturing capillary electrophoresis. Protein Journal. 2008;27(7-8):420–425.
Lowe P, Patnaik R, Lowe N. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: A double-blind, randomized study. Journal of the American Academy of Dermatology. 2006;55(6):975–980.
Monheit G, Carruthers A, Brandt F, et al. A randomized, double blind, placebo controlled study of botulinum toxin A for the treatment of glabellar lines: Determination of optimal dose. Dermatologic Surgery. 2007;33:51–59.
Monheit G, Cohen J, Reloxin Investigational Group. Long-term safety of repeated administration of a new formulation of botulinum toxin type A in the treatment of glabellar lines: Interim analysis from an open-label extension study. Journal of the American Academy of Dermatology. 2009;61(3):421–425.
Nestor MS, Ablon GR. Comparing the clinical attributes of abobotulinumtoxinA and onabotulinumtoxinA utilizing a novel contralateral frontalis model and the frontalis activity measurement standard. Journal of Drugs in Dermatology. 2011;10(10):1148–1157.
Nettar KD, Yu KC, Bapna S, et al. An internally controlled, double-blind comparison of the efficacy of onabotulinumtoxinA and abobotulinumtoxinA. Archives of Facial Plastic Surgery. 2011;13(6):380–386.
Pickett A 2009 BoNT-A: myths and realities. Presented at the International Masters Course on Aging Skin, 8-11 January 2009, Paris, France
Pickett A. Dysport(r): Pharmacological properties and factors that influence toxin action. Toxicon. 2009;54(5):683–689.
Rubin M, Dover J, Glogau R, et al. The efficacy and safety of a new US botulinum toxin type A in the retreatment of glabellar lines following open-label treatment. Journal of Drugs in Dermatology. 2009;8(5):439–444.
Rzany B, Ascher B, Monheit G. Treatment of glabellar lines with botulinum toxin type A (Speywood Unit): a clinical overview. Journal of the European Academy of Dermatology and Venereology. 2010;24(suppl 1):1–14.
Sampaio C, Costa J, Ferreira JJ. Clinical comparability of marketed formulations of botulinum toxin. Movement Disorders. 2004;19(suppl 8):S129–S136.
Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiological Reviews. 2000;80(2):717–766.
Trindade de Almeida AR, Marques E, de Almeida J, et al. Pilot study comparing the diffusion of two formulations of botulinum toxin type A in patients with forehead hyperhidrosis. Dermatologic Surgery. 2007;33(1 spec. no.):S37–S43.
Vandenbergh PYK, Lison DF. Dose standardization of botulinum toxin. Advances in Neurology. 1998;78:231–235.
Wohlfarth K, Schwandt I, Wegner F, et al. Biological activity of two botulinum toxin type A complexes (Dysport(r) and Botox(r)) in volunteers: a double-blind, randomized, dose-ranging study. Journal of Neurology. 2008;255(12):1932–1939.

 centimeter more medially. The results, though, for efficacy and safety were similar for both studies.
centimeter more medially. The results, though, for efficacy and safety were similar for both studies.