19 Basic principles of pharmacology
Additive Effect: Occurs when a second drug with properties similar to the first is added to produce an effect equal to the algebraic sum of the effects of the two individual drugs. Shorthand often used is 1 + 1 = 2.
Agonists: Drugs such as dopamine that attach and activate specific receptors.
Antagonists: Drugs such as naloxone (Narcan) that attach to a specific receptor and do not activate the receptor, but prevent an agonist or body chemical such as a neurotransmitter from stimulating the receptor.
Competitive Antagonist: When the concentration of the antagonist is higher than the agonist concentration resulting in reversal or antagonism of the agonist. Examples include naloxone (Narcan) reversing fentanyl or flumazenil (Romazicon) reversing midazolam (Versed). Shorthand often used is 1 + 1 = 0.
Cross Tolerance: Tolerance to a drug because of an existing tolerance to a similar drug. An example of cross tolerance is a patient who has developed a tolerance to morphine due to repeated administration will also require higher doses of all other opioids as well.
Efficacy of a Drug: Refers to the maximum effect that can be produced by a drug.
Hyperreactivity: An abnormal reaction to an unusually low dose of a drug. For example, patients with Addison disease, myxedema, or dystrophia myotonica have hyperreactivity to unusually low doses of barbiturates.
Hypersensitivity (Anaphylaxis): A drug-induced antigen-antibody reaction. The particular hypersensitivity reaction can be either an immediate (anaphylactic) or a delayed reaction. Hypersensitivity reactions can occur with succinylcholine, antibiotics, and many other drugs that are administered in the PACU (see Chapter 18).
Hyporeactivity: An indication that a person needs excessively large doses of a drug to obtain a therapeutic or desired effect.
Idiosyncrasy: An adverse drug reaction that occurs in a small number of persons and has no correlation to dosage or of type of therapy. Postoperative liver dysfunction following halothane administration is an example.
Pharmacodynamics: The study of the mechanisms of action of drugs and other biochemical and physiologic effects on the body.
Pharmacokinetics: The study of the movement of drugs throughout the body, including the processes of absorption, distribution, biotransformation or metabolism, and excretion.
Potency of a Drug: The dose necessary of a particular drug to produce a specific effect that is designated as the effective dose (ED). When that effect is achieved in a particular percentage of patients, it is quantified as ED50 for 50% of the patients and ED95 for 95% of the patients who show an effect to the drug.
Potentiation: The enhancement of the action of one drug by a second drug that has no detectable action of its own. Shorthand commonly used is 1 + 0 = 3.
Receptors: The portion on or in a cell, usually a protein complex, at which attachment of drugs leads to a physiologic response. The receptors are selective in that they recognize and bind only to specific pharmacologic or physiologic agents.
Synergistic Effect: Addition of a second drug to a drug with properties similar to the first that results in an effect greater than the algebraic sum of the effects of the two individual drugs. Shorthand often used is 1 + 1 = 3.
Tachyphylaxis: An acute drug tolerance—for example, succinylcholine administered by intravenous drip. Over time, a higher drip rate is needed to achieve the necessary response.
Tolerance: A type of hyporeactivity that is acquired during chronic exposure to a drug in which unusually large doses are needed to reach a desired effect. A prime example is a person who has become dependent on opioids and needs larger than normal doses to elicit the desired therapeutic response.
A thorough understanding of the pharmacology of the drugs used in perianesthesia care is necessary to ensure the best outcomes in surgical patients. Anesthesia care continues to evolve, and the judicious use of a number of selective, potent drugs in various combinations represents the cornerstone of current practice. Consequently, a comprehensive review of the principles and concepts of pharmacology is presented in this chapter. The specific actions and uses of drugs related to perianesthesia care are discussed in the physiology chapters in Section II, as are the concepts of anesthetic agents in the chapters in Section III. The pharmacology of individual drugs can be best understood in relation to the physiologic functions they affect and their common clinical applications.
A significant portion of this chapter is dedicated to an overview of drug interactions, because modern anesthesia care requires balancing the administration of multiple drugs throughout the perianesthesia period. These drugs include anesthesia-related agents, the patient’s existing medications, including herbal agents, and other over-the-counter preparations.1 Clinically, at least 10% of the patients for perianesthesia are taking some form of herbal preparation.2 Consequently, knowledge of the principles of pharmacology becomes a meaningful and useful tool in the delivery of nursing care to the patient in the postanesthesia care unit (PACU).
Drug responses
Pharmacokinetic actions
Systemic absorption by various routes of administration
Oral route of administration
When a drug is administered orally, it is absorbed in the small intestine, which has a large surface area. A drug must be lipid soluble to cross the gastrointestinal lining. After such absorption, the drug passes to the liver by way of the portal veins before it can enter the systemic circulation. The liver extracts and metabolizes some of the drug in a process termed the first-pass hepatic effect. The drugs that are particularly subject to this effect are agents such as propranolol, metoprolol, verapamil, chlorpromazine, and morphine; therefore these drugs are administered in much higher doses orally than intravenously.3
Sublingual route of administration
The sublingual route of administration has several important advantages over the oral route, because the sublingual route bypasses the first-pass hepatic effect. This route can be particularly favorable in the PACU for drugs such as nitroglycerin. A nitroglycerin tablet can be placed sublingually in an intubated as well as a cooperative awake patient.4
Subcutaneous and intramuscular routes of administration
These routes of administration require simple diffusion from the site of injection into the systemic circulation and are dependent on blood flow to the injection site. Consequently, these administration routes can result in variations in absorption particularly in the PACU when patients are hypothermic and hypotensive and usually have some peripheral vasoconstriction. Subcutaneous absorption is slow and is reserved for drugs such as insulin or hormones, for which a slow, continuous absorption is advantageous. Intramuscular injections produce a more rapid action and are a common practice in the PACU. In addition, if a patient with a hypothermic condition in the PACU is administered a drug either subcutaneously or intramuscularly, absorption can be delayed. However, when the patient undergoes rewarming, a significant amount of the drug can be rapidly liberated from the injection site, thus causing a large concentration of the drug in the systemic circulation and an exaggerated effect.3
Intravenous route of administration
This route of administration facilitates the delivery of a desired concentration of a drug in a rapid and precise fashion by single bolus injection or continuous infusion. In the PACU, because patients have an intravenous line, it is the most popular means of drug administration.
Aerosolized medications to the respiratory tract
The aerosolized route of administration facilitates direct delivery from inhaled aerosols to a targeted organ, which reduces the systematic drug exposure and side effects. Many aerosol delivery devices can be used for administration of bronchoactive inhaled aerosols. The most commonly used method of administration in the PACU is the aerosolized nebulizer, in which a specific amount of drug is administered in a solution of normal saline and is nebulized with a ventilator or oxygen delivery devices. Other devices for delivery of the drug either orally or nasally via inhalation are the metered dose inhaler (MDI) with or without a holding chamber or spacer device, the small volume nebulizer (SVN), and the dry powder inhaler (DPI). The MDI, SVN, and the DPI devices deliver approximately the same percentage of the drug to the target organ, the lungs, with the MDI increasing the patient flow rates better than the SVN and the DPI.4
Drug distribution
A drug’s distribution in the body is envisioned as entering various defined compartments. A compartment represents a theoretic space. A mathematic model can be used to describe the pharmacokinetics of the disposition of a drug. A two-compartment model is usually used in depiction of a central compartment and a peripheral compartment. The central compartment includes plasma and blood cells and highly perfused tissues such as the heart, lungs, brain, liver, and kidneys. The peripheral compartment represents all other fluids and tissues in the body. With this two-compartment model, a drug can be introduced into the central compartment, move into the peripheral compartment, and then return to the central compartment where removal from the body occurs. The two-compartment model is constructed with the serum concentration versus time and is called the plasma concentration curve. From this curve, the distribution and elimination half-times and other kinetic parameters of the drug can be calculated. A drug like propofol will rapidly distribute into the brain when given intravenously, causing a rapid onset of sedation. It also quickly redistributes from the tissues into the blood, resulting in a short duration of action.5
Metabolism and elimination
The major mechanisms for elimination of a drug from the body are hepatic and renal clearance. The half-life (t½) of a drug is the time at which 50% of the total amount of the drug has been eliminated from the body. The elimination half-life (t½B) is the time that the plasma concentration is at 50% of the elimination phase; the t½B is directly proportional to the volume distribution of the drug and inversely proportional to the drug clearance. Consequently, when the t½B for a particular drug is known, a large initial dose called a loading dose of a drug can be given to achieve a therapeutic concentration. The drug can then be given via infusion or in multiple doses at calculated intervals, based on the t½B, for a steady-state plasma concentration. In addition, the time necessary for elimination of a particular dose of a drug can be predicted with the t½B. Usually, 95% of the drug can be eliminated in five half-lives.6
Removal of drugs from the systemic circulation
Drugs are principally cleared from the systemic circulation via the hepatic, biliary, and renal systems. The hepatic system has a high blood flow and can extract many lipid-soluble drugs from the systemic circulation. In the liver, drugs undergo biotransformation and for the most part become pharmacologically inactive. Enzyme induction or a decrease in protein binding enhances the hepatic clearance of some drugs (see Chapter 16). After they have been metabolized in the liver, drugs can be transported to the biliary system or the kidney for excretion. Some drugs, such as the glucuronides, are actively transported to the bile and excreted in an inactive form.6
The kidneys secrete many water-soluble drugs in their unchanged form or as hepatic metabolites. Renal excretion of drugs depends on the following major physiologic processes that occur in the kidneys: glomerular filtration, active tubular secretion, and passive tubular reabsorption. Appropriate renal function is needed for facilitation of many of the drugs administered in the perioperative period. Consequently, concern about renal function should merit creatinine clearance or serum creatinine level tests, because these laboratory tests correlate well with renal drug elimination.6
Effects of physiologic dysfunction on pharmacokinetic action
Renal disease
Kidney disease reduces the effectiveness of drug clearance and results in a prolongation in the action of the drugs that rely on the kidneys for their removal. In renal drug clearance, the laboratory test of creatinine clearance, for measurement of glomerular filtration (see Chapter 13), can be used for prediction of the degree of a drug’s renal clearance. In patients who are anephric or in patients with severe kidney disease, the elimination clearance is decreased and the t½B is increased, thus prolonging the effects of the drugs, especially with repeated administrations.7
Hepatic disease
Patients with hepatic diseases such as cirrhosis may have difficulty clearing some anesthetic drugs from the body. Liver function testing is unreliable for predicting the level of impairment in hepatic clearance of drugs. Any patient with documented liver disease should be considered at risk for decreased clearance of drugs. Therefore, in patients with documented hepatic disease, all drugs administered in the PACU should be titrated to desired effect for an appropriate pharmacologic outcome.8
Cardiovascular disease
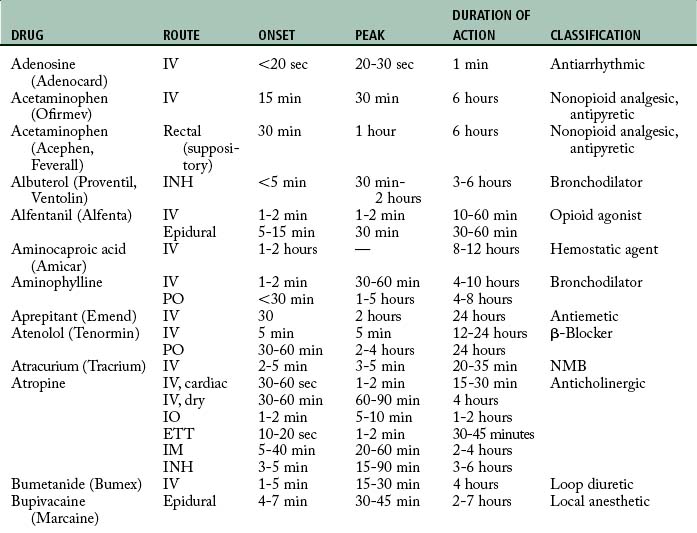
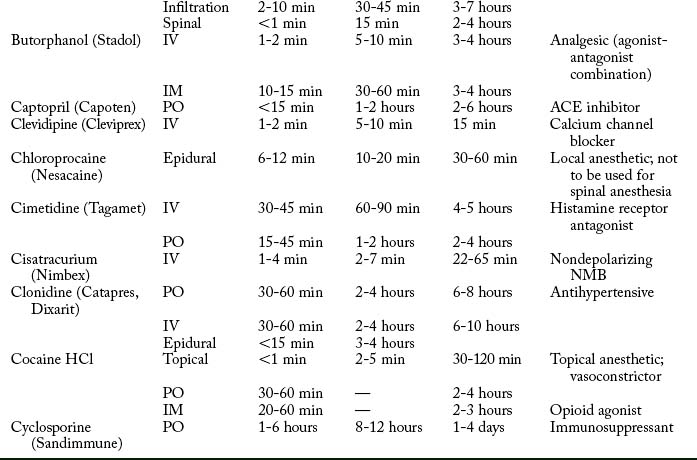
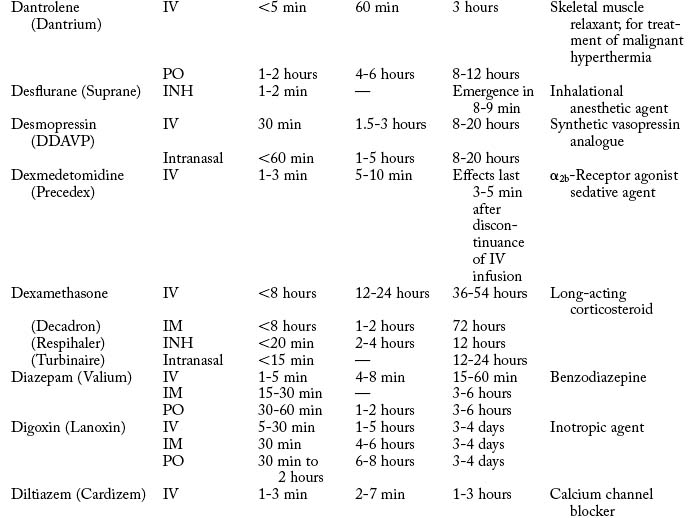
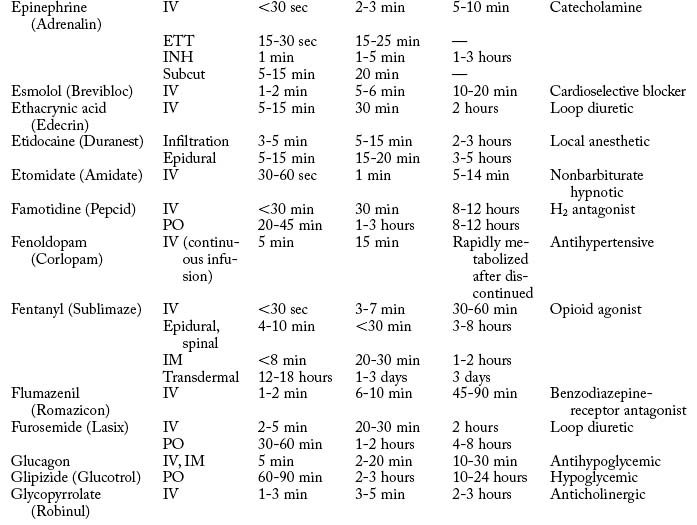
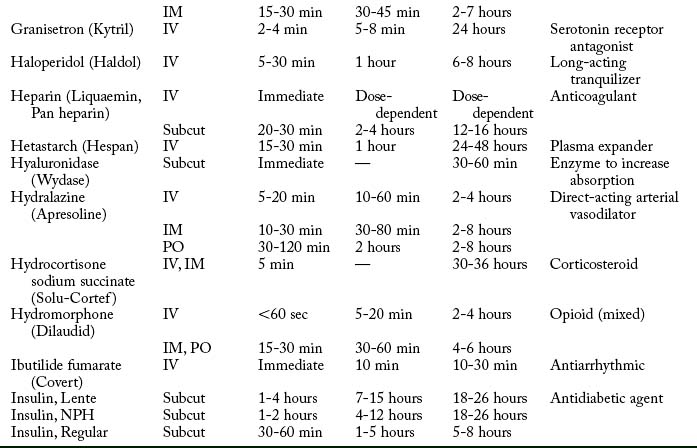
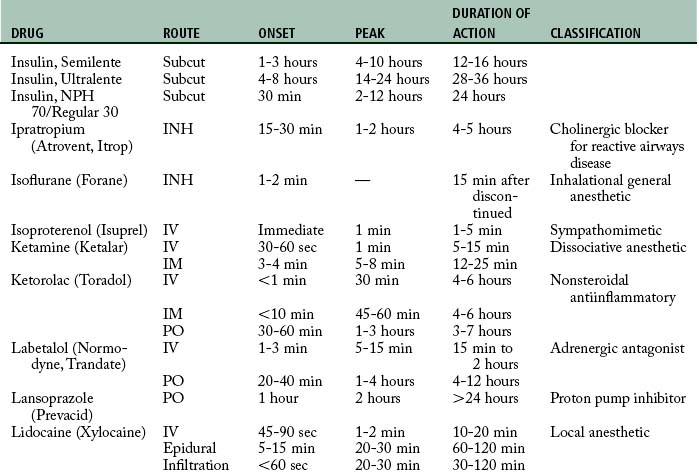
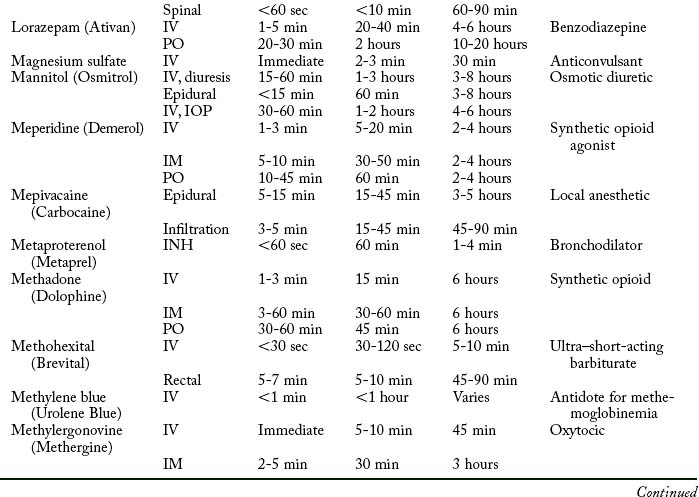
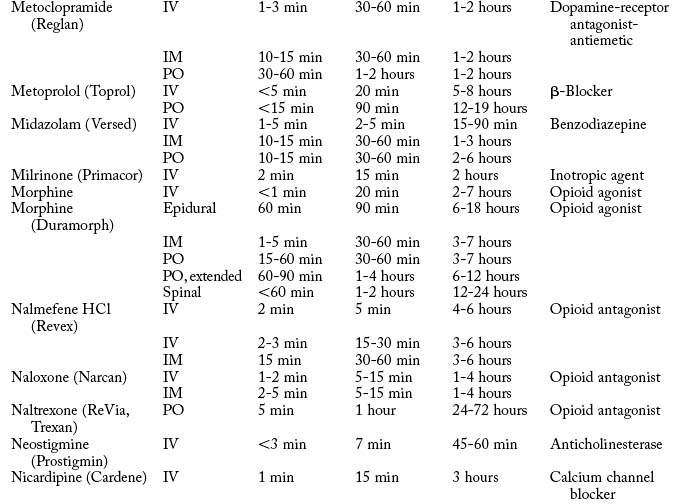
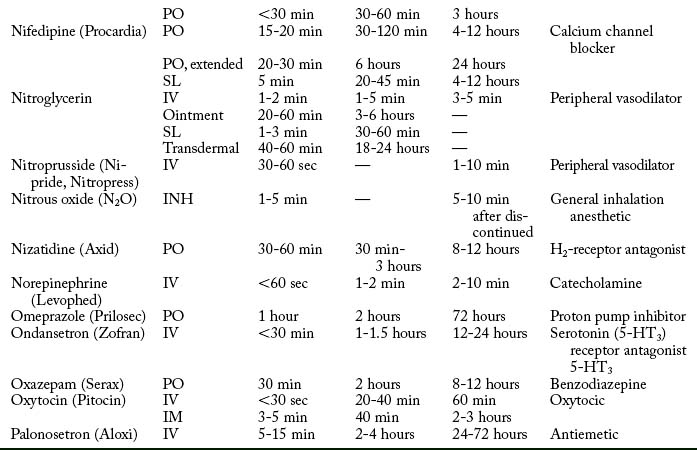
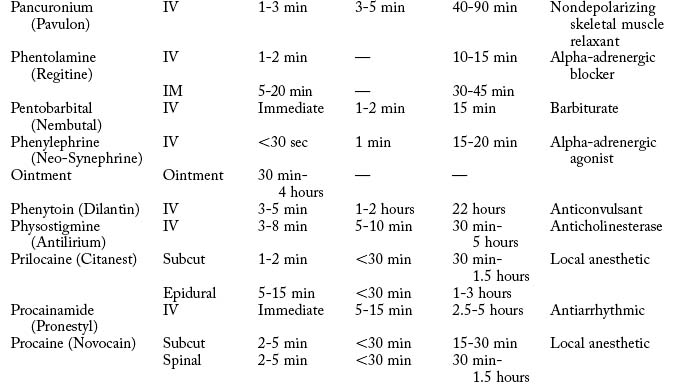
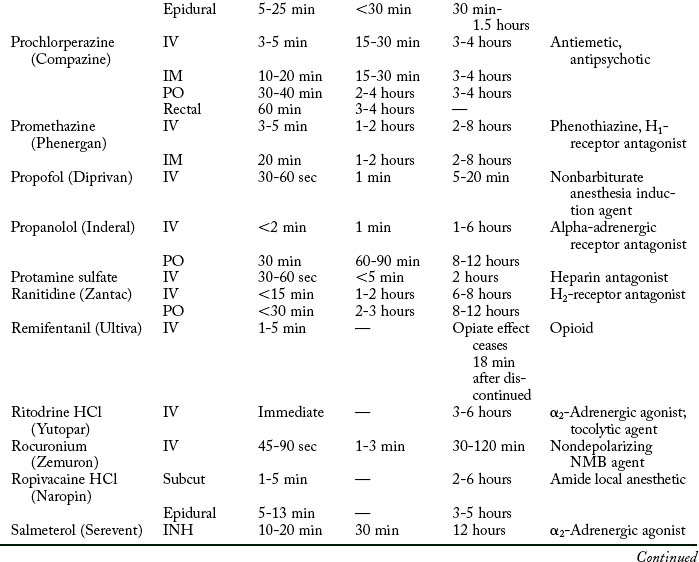
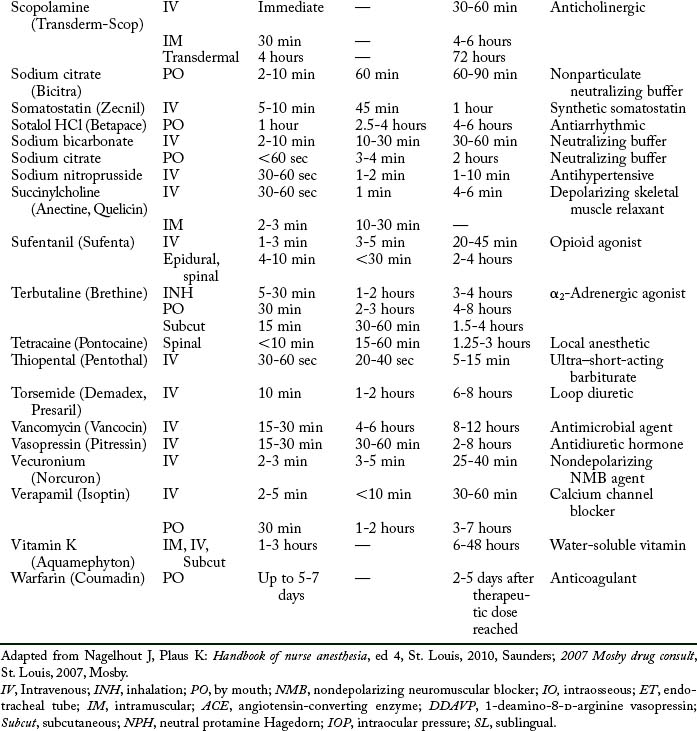
Cardiovascular diseases that cause a reduction in tissue perfusion have a significant effect on drug distribution and clearance. For example, when lidocaine is administered to patients with congestive heart failure, the dose should be reduced by half because of changes in volume distribution and clearance. Patients who have undergone cardiopulmonary bypass surgery can have a hemodilution of drugs. Patients with cardiac disease should have their medications given at lower doses over longer dosing interval with careful monitoring.9 Drugs used in the PACU are listed in Table 19-1.
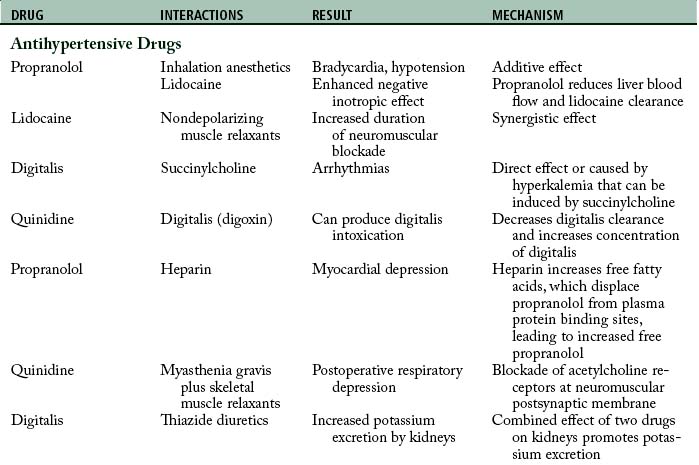
Drug-drug interactions
When a patient simultaneously receives two or more drugs, the drugs might interact to cause a different action than expected including an adverse outcome. Patients are often given drugs other than the ones associated with anesthesia and surgery. As a result, the potential for a drug-drug interaction is present in these patients (Table 19-2). These interactions are divided into two broad categories: pharmacokinetic and pharmacodynamic.
Pharmacokinetic interactions
Absorption
The absorption of one drug can be enhanced or inhibited by another drug. With the addition of epinephrine to solutions of local anesthetics, the absorption of the anesthetic is prolonged through the vasoconstrictive action of epinephrine. The local vasoconstriction produced by epinephrine delays the systemic absorption of the local anesthetic, and the effect of the local anesthetic ultimately is prolonged. When a patient has been administered a preoperative aluminum-containing antacid and then is administered tetracycline in the late postoperative period, absorption of the tetracycline is reduced.10
Distribution
Pharmaceutical incompatibility is one type of pharmacokinetic distribution interaction. This situation occurs, for example, when one drug reacts chemically with another. In this situation, when one drug (e.g., aspirin) displaces another drug (e.g., phenytoin) from plasma protein-binding sites, the blood concentration of the free drug is increased, which can result in altered blood levels.10
Biotransformation
The drug cimetidine (Tagamet) is sometimes administered before surgery for a reduction in the amount of gastric secretion and for a possible increase in the gastric pH. Cimetidine is a potent inhibitor of drug metabolism and can slow the elimination of antipyrine, warfarin, diazepam, and propranolol. This effect results in an increased drug concentration and enhanced pharmacologic effect of the latter drugs.11
Excretion
The pharmacokinetic parameters of concern in excretion relate to one drug facilitating or hindering the excretion of another. An example occurs when probenecid is administered together with penicillin. The outcome of this interaction is that the pharmacologic actions of penicillin are prolonged because probenecid delays the excretion of penicillin. This effect can be considered a desirable drug-drug interaction.10
Pharmacodynamic interactions
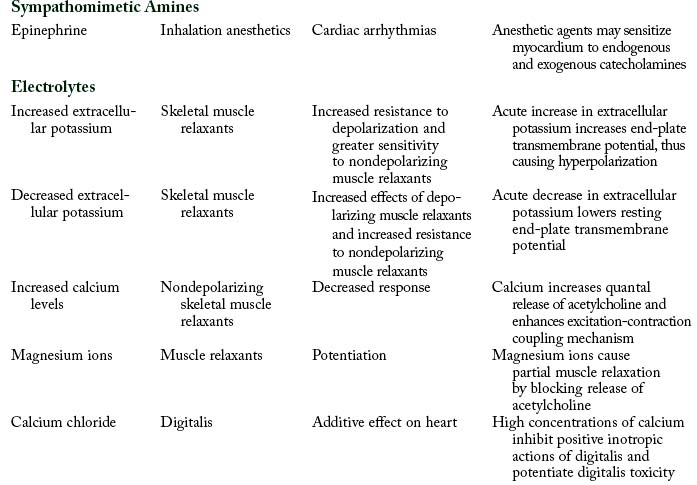
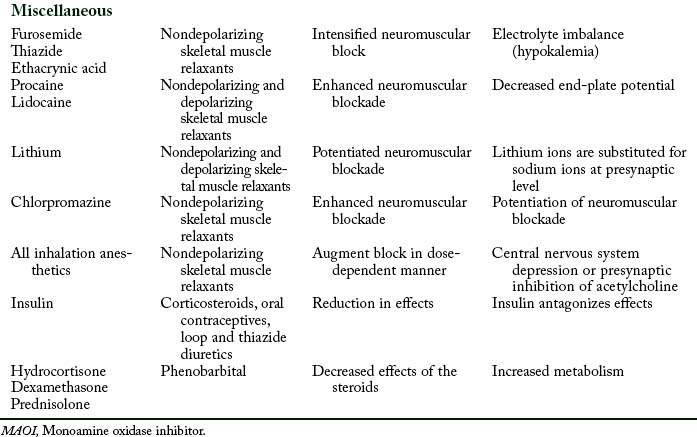
Pharmacodynamic interactions occur when one drug alters the pharmacologic effects of another drug. For example, when a patient is treated with an antibiotic such as an aminoglycoside or polymyxin and receives a skeletal muscle relaxant such as rocuronium, a prolonged neuromuscular blockage can result. Another example is a patient who receives thiazide diuretic therapy and has resultant hypokalemia. If the patient is administered digitalis, digitalis toxicity can result.12
Drug-drug interactions and the PACU
Antibiotics
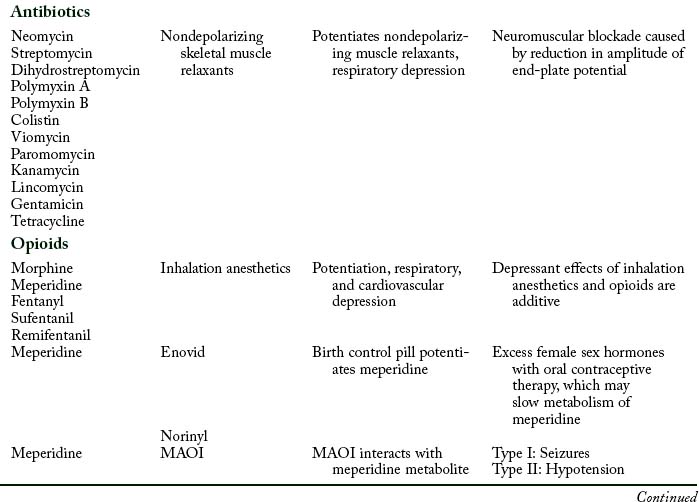
Aminoglycoside and polymyxin antibiotics have been reported to interact with some anesthetic agents and with skeletal muscle relaxants. Streptomycin and the other aminoglycoside antibiotics gentamicin, tobramycin, amikacin, neomycin, kanamycin, and paromomycin can produce a partial neuromuscular blockade by inhibiting the release of acetylcholine from the presynaptic membrane and by altering calcium mobility in muscles. When a nondepolarizing skeletal muscle relaxant such as rocuronium is administered to a patient who receives an aminoglycoside antibiotic, the neuromuscular blockade is intensified and pharmacologic reversal is difficult.9
Opioids
If the two drugs have interacted in this way, an opioid antagonist, such as naloxone (Narcan), can be administered to reverse the respiratory depression produced by the opioid. However, naloxone does not reverse respiratory depression produced by inhalation anesthetic agents such as sevoflurane, desflurane, or isoflurane.13
Steroids
If these symptoms appear in a patient in the PACU who did not receive this steroid coverage, the preferred treatment is hydrocortisone (see Chapter 15).14 Major drug groups and intended outcomes in respiratory care are listed in Table 19-3.
Table 19-3 Major Drug Groups and Intended Outcome in Respiratory Care
| DRUG GROUP | INTENDED PHYSIOLOGIC RESPONSE | GENERIC AGENT (TRADE NAME) |
|---|---|---|
| Adrenergic agents | Alpha-adrenergic stimulation produces bronchial relaxation to reduce airway resistance and improve flow rates in patients with obstructive lung disease | Epinephrine, isoproterenol (Isuprel), isoetharine (Bronkosol), terbutaline (Brethine), metaproterenol (Metaprel), albuterol (Ventolin), pirbuterol (Maxair), bitolterol (Tornalate), salmeterol (Serevent), procaterol (Mescalcin) |
| Alpha-adrenergic stimulation produces bronchial relaxation, peripheral vascular vasoconstriction, and nasal decongestion | Ephedrine, phenylephrine (Neo-Synephrine) | |
| Anticholinergic agents | Relaxation of cholinergic (vagal)-induced bronchoconstriction to improve flow rates in patients with obstructive lung disease | Ipratropium (Atrovent) |
| Mucoactive agents | Modification of properties of respiratory tract mucus to facilitate clearance of secretions | Acetylcystine (Mucomist), dornase alfa (Pulmozyme) |
| Corticosteroids | Reduce inflammation in respiratory tract | Dexamethasone (Decadron), beclomethasone (Vanceril), triamcinolone (Kenalog), flunisolide (Aerobid), fluticasone (Flonase/Flovent), budesonide (Rhinocort) |
| Antiasthmatic agents | Inhibits chemical mediators of inflammation to help prevent onset of asthma attack | Cromolyn (Intal), nedocromil (Tilade), zafirlukast (Accolate), zileuton (Zyflo), montelukast (Singulair) |
| Antiinfective agents | Inhibits or stops specific infective agents, such as Pneumocystis carinii (pentamidine) or respiratory syncytial virus (ribavirin), or for management of Pseudomonas aeruginosa in cystic fibrosis (tobramycin) | Pentamidine, ribavirin (Rebetron), tobramycin (TOBI) |
| Exogenous surfactants | Enhances lung compliance by reducing surface tension; approved for direct intratracheal instillation | Colfosceril (Exosurf), beractant (Survanta) |
Adapted from Rau J: Recent developments in respiratory care pharmacology, J Perianesth Nurs 13:362, 1998.
Special considerations in pharmacology associated with perianesthesia care
Sedative drugs for PACU patients
Patients in the PACU sometimes need sedation for a variety of reasons, most commonly fear, pain, loss of control, confusion, noise, lights, and alarms. These stimuli can cause a stress response that is experienced by some patients in the PACU; sedative drugs are sometimes used to prevent the adverse physiologic effects of stress, such as increased oxygen consumption, tachycardia, hypertension, and exacerbated hyperglycemia. The drugs that are most commonly used for sedation in the PACU are listed in Box 19-1. The institution of drugs to promote sedation is a difficult task in the PACU because the patient may have residual effects of the intraoperative anesthetic agents still active in the body. A bedside sedation scoring system is helpful in determining the degree of sedation and predicting when concerns about oversedation should be realized. Although many sedation scoring systems are available, the Ramsay Sedation Scoring System appears to be appropriate for assessing drug-induced sedation. The Ramsay Scale consists of six scoring levels. The first three levels (Table 19-4) are usually administered while the patient is awake, and levels 4 through 6 are assessed during varying degrees of sleep. Because oversedation is associated with increased risk of edema, thromboemboli, gastric regurgitation, and aspiration, to name a few, patients in the PACU should have Ramsay Sedation levels in the range of 3 or less.15,16
| RAMSAY SCORE | CLINICAL PARAMETERS FOR BEDSIDE ASSESSMENT OF SEDATION | GLOBAL DEGREE OF SEDATION |
|---|---|---|
| 1 | Anxious, restless, perhaps agitated | |
| 2 | Cooperative and oriented | Varying degrees of awake state |
| 3 | Easily arousable, responds appropriately | |
| 4 | Brisk response to light glabellar tap or loud auditory stimulus | |
| 5 | Sluggish response to glabellar tap or auditory stimulus | Varying degrees of asleep state |
| 6 | Asleep, does not respond to previous stimuli |
From Prielipp R, Young C: Current drugs for sedation of critically ill patients, Semin Anesthesia Perioperative Med Pain 20: 85-94, 2001.
Herbal medicines
During the past few years, the use of herbal products has greatly increased, and many patients receiving care in the PACU are taking one or more of these preparations. Research into the effect of herbal medications during the perioperative period is lacking, particularly in the area of the effect on postoperative outcomes. In response, the American Society of Anesthesiologists has issued a statement cautioning patients who take herbal products to refrain from those medications at least 2 weeks before surgery.17 Because of the possible negative perianesthesia outcome caused by herbal products, a brief overview of the more popular herbal products is presented in Table 19-5.
Summary
The intent of this chapter is to provide the perianesthesia nurse with a review of the drugs that are currently used in the perianesthesia period. An overview of the pharmacodynamics and pharmacokinetics was presented, and the common drugs were presented in table form along with the drug-drug interactions and an overview of the drugs used in sedation. Finally, because many people take some form of herbal drugs, a brief overview is present on this area of pharmacology.
1. Lee A, et al. Incidence and risk of adverse perioperative events among surgical patients taking traditional Chinese herbal medicines. Anesthesiology. 2006;105:454–461.
2. Moss J, Yuan C. Herbal medicines and perioperative care. Anesthesiology. 2006;105:441–442.
3. Bardal SK, et al. Applied pharmacology. St. Louis: Saunders; 2011.
4. Chisholm-Burns MA, et al. Pharmacotherapy: principles and practice. ed 2. New York: McGraw-Hill; 2010.
5. Katzung BG, et al. Basic and clinical pharmacology, ed 11. New York: McGraw Hill; 2009.
6. Brunton L. Goodman & Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill; 2011.
7. Rang HP, et al. Rang and Dale’s pharmacology, ed 6. London: Churchill Livingstone; 2007.
8. Hall JE. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
9. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2010.
10. Dipiro JT, et al. Pharmacotherapy: a pathophysiologic approach. ed 7. New York: McGraw-Hill; 2008.
11. Miller RD, et al. Miller’s anesthesia, ed 7. Philadelphia: Churchill Livingstone; 2009.
12. Barasch PG, et al. Clinical anesthesia, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2009.
13. Longnecker DE, et al. Anesthesiology. New York: McGraw-Hill; 2008.
14. Elisha S. Case studies in nurse anesthesia. Sudbury, Mass: Jones and Bartlett; 2011.
15. Atlee JL. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
16. Fleisher LA. Anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2006.
17. Kaye AD, et al. Perioperative anesthesia clinical considerations of alternative medicines. Anesthesiol Clin North America. 2004;22(1):125–139.