Chapter 39
Basic Life Support and Advanced Cardiac Life Support
Vissia S. Pinili, MSN, RN, CPAN, CCRN, Nicole R. Keller, PharmD, BCNSP and Glenn N. Levine, MD, FACC, FAHA
1. Why was the A-B-C (airway, breathing, compression) sequence of cardiopulmonary resuscitation (CPR) changed to C-A-B (compression, airway, breathing)?
2. What factors constitute “high-quality CPR”?
Components of high-quality CPR include:
 Chest compressions at least 100 times per minute
Chest compressions at least 100 times per minute
 Depth of chest compressions for adults of at least 2 inches (5 cm), for children compressions of 2 inches, and for infants compressions of 1.5 inches (4 cm)
Depth of chest compressions for adults of at least 2 inches (5 cm), for children compressions of 2 inches, and for infants compressions of 1.5 inches (4 cm)
 Allowing for complete chest recoil after each compression
Allowing for complete chest recoil after each compression
3. What is your first step if you are alone and come across an unresponsive adult victim with no signs of breathing?
Figure 39-1 is an algorithm for adult basic life support (BLS) decisions and actions. A summary of key BLS components for treating adults, children, and infants is given in Table 39-1.
TABLE 39-1
SUMMARY OF KEY BASIC LIFE SUPPORT COMPONENTS FOR ADULTS, CHILDREN, AND INFANTS
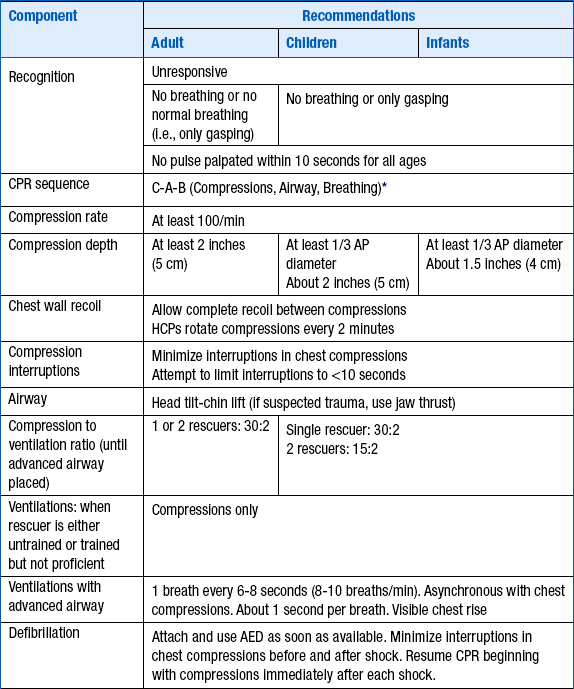
∗Excluding the newly born, in whom the etiology of an arrest is nearly always asphyxia.
From American Heart Association: Highlights of the 2010 American Heart Association Guidelines for CPR and ECC. Available at : http://www.heart.org/idc/groups/heart-public/@wcm/@ecc/documents/downloadable/ucm_317350.pdf. Accessed March 26, 2013.
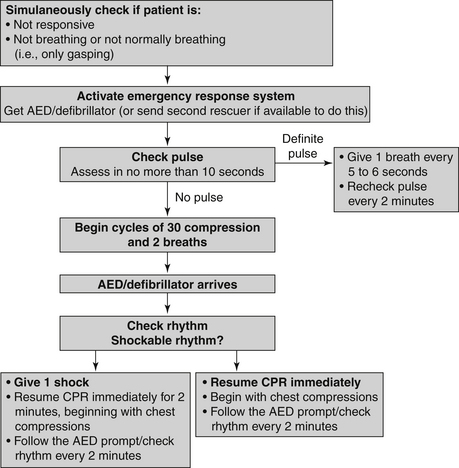
Figure 39-1 Adult basic life support (BLS) algorithm. AED, Automated external defibrillator; CPR, cardiopulmonary resuscitation.
4. For patients with respiratory arrest (but with a perfusing heart rhythm), how often should breaths be delivered?
5. What is the most common cause of airway obstruction in the unconscious adult patient?
6. How many joules of energy are indicated for the treatment of ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT) when using a biphasic defibrillator?
Biphasic defibrillators are now used at many institutions, replacing the older monophasic defibrillators. The amount of energy will often be device specific, ranging from 120 to 200 J. If the appropriate setting is unknown, use 200 J. Additional shocks should be equivalent or higher energy. This contrasts to the older monophasic defibrillators, for which a setting of 360 J is recommended.
7. In order, what are the preferred routes of drug administration?
 Intravenous (IV) is the preferred route of drug administration. The guidelines emphasize that resuscitation attempts should not be delayed by trying to achieve central IV access when peripheral IV access can be easily achieved.
Intravenous (IV) is the preferred route of drug administration. The guidelines emphasize that resuscitation attempts should not be delayed by trying to achieve central IV access when peripheral IV access can be easily achieved.
 When IV access is not possible, intraosseous (IO) is preferred over endotracheal (ET). The IO route can be used in both children and adults.
When IV access is not possible, intraosseous (IO) is preferred over endotracheal (ET). The IO route can be used in both children and adults.
 Absorption of drugs given via the ET route is considered to be less reliable and predictable. In addition, the optimal dose of most drugs given via the ET route is unknown; the typical dose of drugs administered via the ET route is reported to be 2 to 2.5 times that of the IV route. Drugs delivered via the ET route should be diluted in 5 to 10 mL of water or normal saline. Advanced cardiac life support (ACLS) drugs that can be given via the ET route are naloxone, atropine, vasopressin, epinephrine, and lidocaine (NAVEL).
Absorption of drugs given via the ET route is considered to be less reliable and predictable. In addition, the optimal dose of most drugs given via the ET route is unknown; the typical dose of drugs administered via the ET route is reported to be 2 to 2.5 times that of the IV route. Drugs delivered via the ET route should be diluted in 5 to 10 mL of water or normal saline. Advanced cardiac life support (ACLS) drugs that can be given via the ET route are naloxone, atropine, vasopressin, epinephrine, and lidocaine (NAVEL).
8. After the first unsuccessful shock of a patient with VF or pulseless VT, which two drugs should be considered?
 Epinephrine 1 mg IV or IO is the traditional medication to be considered. Additional similar doses of epinephrine can be administered every 3 to 5 minutes. If ET administration is necessary, the suggested dose is epinephrine 2 to 2.5 mg diluted in 5 to 10 mL of water or normal saline, injected directly into the ET tube. Although a mainstay of the AHA’s ACLS VT/VF algorithm, it is acknowledged that there is actually very little data to support the use of epinephrine in this situation.
Epinephrine 1 mg IV or IO is the traditional medication to be considered. Additional similar doses of epinephrine can be administered every 3 to 5 minutes. If ET administration is necessary, the suggested dose is epinephrine 2 to 2.5 mg diluted in 5 to 10 mL of water or normal saline, injected directly into the ET tube. Although a mainstay of the AHA’s ACLS VT/VF algorithm, it is acknowledged that there is actually very little data to support the use of epinephrine in this situation.
 One dose of vasopressin 40 units IV or IO can be used to replace the first or second dose of epinephrine. Vasopressin is a nonadrenergic peripheral vasoconstrictor that causes coronary and renal vasoconstriction.
One dose of vasopressin 40 units IV or IO can be used to replace the first or second dose of epinephrine. Vasopressin is a nonadrenergic peripheral vasoconstrictor that causes coronary and renal vasoconstriction.
9. After several unsuccessful shocks, and treatment with epinephrine or vasopressin, what other drugs (and their doses) should be considered?
 The dose of amiodarone is 300 mg IV or IO once, with consideration of a subsequent 150 mg IV or IO, if indicated, 3 to 5 minutes after the first 300-mg dose.
The dose of amiodarone is 300 mg IV or IO once, with consideration of a subsequent 150 mg IV or IO, if indicated, 3 to 5 minutes after the first 300-mg dose.
 The dose of lidocaine is 1 to 1.5 mg/kg IV or IO, with subsequent doses of 0.5 to 0.75 mg/kg IV or IO given over 5- to 10-minute intervals, to a maximum total lidocaine dose of 3 mg/kg.
The dose of lidocaine is 1 to 1.5 mg/kg IV or IO, with subsequent doses of 0.5 to 0.75 mg/kg IV or IO given over 5- to 10-minute intervals, to a maximum total lidocaine dose of 3 mg/kg.
10. If the patient is in torsades de pointes, in addition to defibrillation, what medication can be considered?
11. After administering a drug via a peripheral IV, what steps should be taken to promote delivery of the drug to the central circulation?
12. Can one shock a hypothermic patient who is in VF or VT?
13. If a person who was in VF or VT is successfully defibrillated, and amiodarone is to be started to prevent further VF or VT, what is the dosing (assuming he or she has not already received any amiodarone)?
14. What are the treatable causes of pulseless electrical activity?
Because pulseless electrical activity (PEA) has such a poor prognosis unless a reversible cause of the rhythm is quickly identified and addressed, it is important to commit to memory the treatable causes of PEA. The H’s and T’s of PEA are given in Table 39-2. Hypovolemia and hypoxemia are reported to be the two most common and easily reversible causes of PEA.
TABLE 39-2
POTENTIALLY TREATABLE CAUSES OF PULSELESS ELECTRICAL ACTIVITY
| H’s | T’s |
| Hypovolemia | Toxins |
| Hypoxia | Tamponade (cardiac) |
| Hydrogen ion (acidosis) | Tension pneumothorax |
| Hyper- or hypokalemia | Thrombosis (coronary and pulmonary) |
| Hypoglycemia | Trauma |
| Hypothermia |
Modified from American Heart Association: Advanced cardiovascular life support provider manual, Dallas, 2011, American Heart Association.
An algorithm for ACLS of adult cardiac arrest (including patients exhibiting PEA) is given in Figure 39-2.
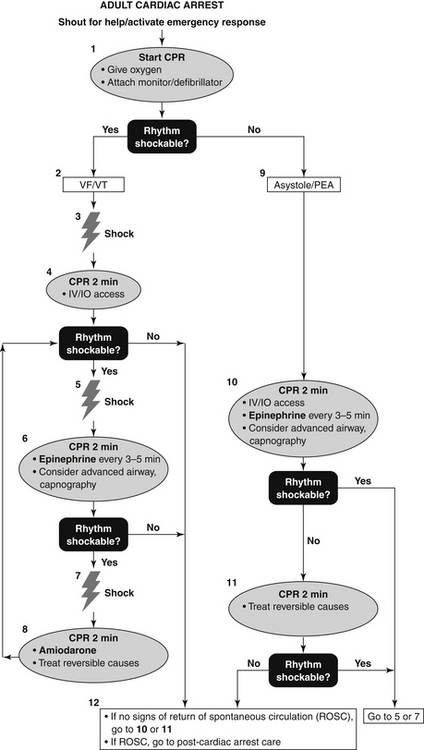
Figure 39-2 Advanced cardiac life support (ACLS) adult cardiac arrest algorithm. CPR, Cardiopulmonary resuscitation; IO, intraosseous; IV, intravenous; PEA, pulseless electrical activity; VF, ventricular fibrillation; VT, ventricular tachycardia.
15. What drugs can be considered in a patient with PEA?
Epinephrine and vasopressin can be used, as described earlier for the treatment of VF or VT, although no vasopressors has been shown to increase survival in PEA. Atropine is no longer recommended for asystole or PEA.
16. In bradycardic patients, such as those with hears block, what are the primary treatments if they are symptomatic and suffering from poor perfusion?
 Atropine 0.5 mg IV, which may be repeated every 3 to 5 minutes to a total dose of 3 mg. The use of atropine should not be relied on in patients with Mobitz II second-degree atrioventricular (AV) block or third-degree (complete) AV block. Some have considered the use of atropine in Mobitz II or third-degree heart block (“infrahisian” heart block) contraindicated, although this is not explicitly stated in the latest ACLS guidelines.
Atropine 0.5 mg IV, which may be repeated every 3 to 5 minutes to a total dose of 3 mg. The use of atropine should not be relied on in patients with Mobitz II second-degree atrioventricular (AV) block or third-degree (complete) AV block. Some have considered the use of atropine in Mobitz II or third-degree heart block (“infrahisian” heart block) contraindicated, although this is not explicitly stated in the latest ACLS guidelines.
17. Is transcutaneous pacing recommended for the treatment of a patient in asystole?
18. In a symptomatic yet stable patient with a regular narrow complex tachyarrhythmia, what drug is recommended as a first-line agent?
Adenosine has become the drug of choice for a symptomatic yet stable patient with a narrow complex tachyarrhythmia. The distinction between stable and unstable is subjective, but the symptomatic yet stable patient might be described as one who is slightly lightheaded (systolic blood pressure [SBP] of approximately 80 mm Hg) or having mild shortness of breath or chest discomfort. Patients experiencing more severe symptoms would be those with altered mentation because of low blood pressure, or with moderate to severe shortness or breath or chest discomfort. Note that although adenosine, which blocks AV conduction, may terminate some narrow complex tachycardias, such as AV nodal reentrant tachycardia (AVNRT) or AV reentrant tachycardia (AVRT), it will not terminate rhythms such as atrial flutter or atrial tachycardia (although it may lead to a transient decrease in conduction through the AV node and slower ventricular response rate, allowing identification of the rhythm). Adenosine would not be expected to terminate an irregular narrow complex tachycardia, because the genesis of these rhythms does not involve the AV node.
19. What is the dosing regimen for adenosine, and what are its primary side effects?
The adenosine dosing regimen is a 6 mg rapid IV push, followed (if no rhythm conversion) by a 12 mg rapid IV push. The half-life of adenosine is only several seconds, so every effort must be made to administer it quickly and ensure its quick delivery to the central circulation (see Question 11). The effects of adenosine may be potentiated by dipyridamole or carbamazepine (consider a starting dose of 3 mg) and blocked by theophylline or caffeine. Adenosine may cause the patient to experience flushing, shortness of breath, or chest discomfort. Because it can cause bronchospasm, it should be avoided in patients with significant reactive airway disease. The practitioner should also be aware that because of its profound effects on AV nodal conduction, it may result in asystole for several seconds or more, an often disconcerting occurrence to the practitioner watching the telemetry monitor. Prolonged asystole has been reported in patients with transplanted hearts and following central venous administration; a lower dose of 3 mg should be considered in such situations.
20. After return of spontaneous circulation (ROSC), what intervention has been shown to improve neurologic recovery in comatose patients?






