Basal ganglia disorders
After reading this chapter the student or therapist will be able to:
1. Describe the circuitry of the basal ganglia.
2. Relate the anatomy and physiology of the basal ganglia to its roles in sensorimotor and cognitive processes.
3. Use the information on anatomy, physiology, and pharmacology to explain the signs and symptoms seen in classic disease states—for example, Parkinson disease, Huntington disease, and dystonia.
4. Develop an evaluation plan for patients with diseases of the basal ganglia.
5. Develop an intervention plan for patients, with the rationale for treatment methods.
6. Determine treatment effectiveness, especially in the case of degenerative disease.
7. Integrate the information in this chapter with the information provided in Section I of this book to develop treatment plans for patients with metabolic or toxic disorders.
This chapter considers the degenerative, metabolic, hereditary, and genetic disorders that typically have their onset in adulthood, including Parkinson disease, parkinsonian syndromes, Huntington chorea, Wilson disease, dystonias, heavy metal poisoning, and drug intoxication. Because of the wide variety of diseases with their wide variety of causes, the concentration is on understanding the clinical problems and commonalities that exist within this grouping. In general, the practice parameter of the diseases discussed in this chapter is the physical therapy diagnostic parameter 5E: Impaired motor and sensory integrity associated with progressive disorders of the central nervous system, from the Guide to Physical Therapist Practice.1 Although the occupational therapy guide does not classify practice parameters in that manner, the concepts and clinical reasoning process can be used by both professionals. The predominant area of the brain affected by these disorders is the basal ganglia: this group of central nervous system (CNS) structures is therefore discussed in some detail.
The basal ganglia
The most commonly seen disorders affecting the basal ganglia include Parkinson disease, Huntington chorea, and dystonias, including drug-induced dyskinesias. All of these medical diagnoses involve impairments in muscle tone, movement coordination and motor control, and postural stability and the presence of extraneous movement. Taken together, these disorders now affect approximately 1 million people in the United States.2–4
Anatomy
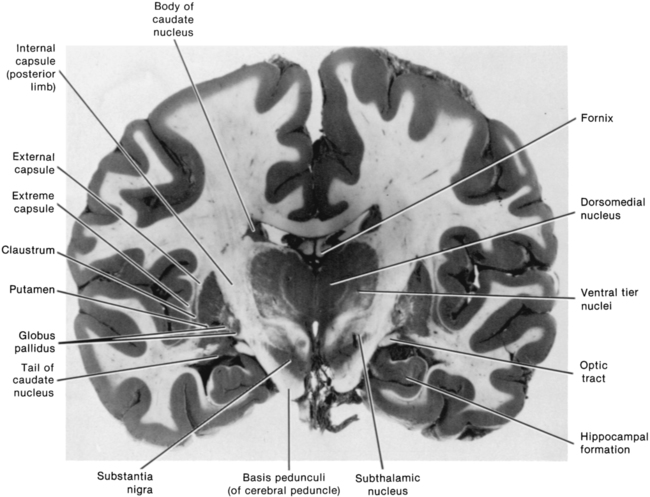
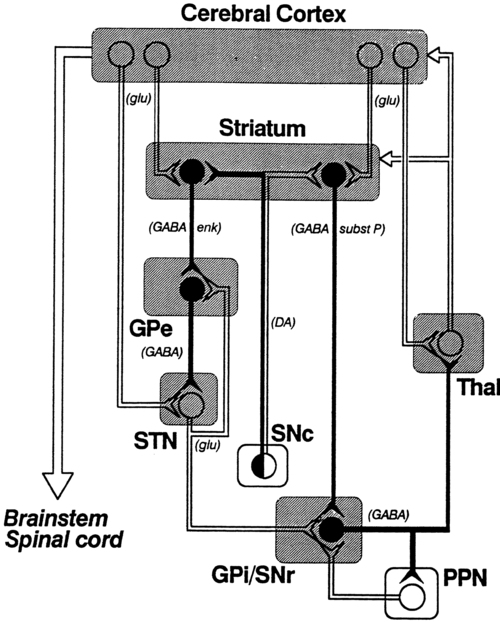
The dorsal or sensorimotor basal ganglia are composed of three nuclei located at the base of the cerebral cortex—hence their name. These nuclei are the caudate nucleus, the putamen, and the globus pallidus. Two brain stem nuclei, the substantia nigra and the subthalamic nucleus, are included as part of the basal ganglia because they have a close functional relation to the forebrain nuclei. In addition, connections between the basal ganglia and the pedunculopontine nucleus (PPN) are important in regulating underlying tone. Other parts of the basal ganglia, the ventral basal ganglia, are intimately related to the limbic system and are discussed in Chapter 5. The anatomical location of the various parts of the basal ganglia is shown in Figure 20-1.
Afferent pathways
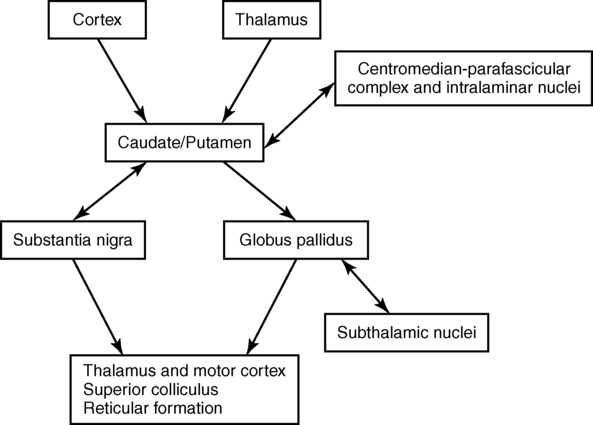
Functionally, the basal ganglia can be divided into an afferent portion and an efferent portion (Figure 20-2). The afferent structures are the caudate and putamen. They receive input from the entire cerebral cortex, the intralaminar thalamic nuclei, and the centromedian-parafascicular complex of the thalamus as well as from the substantia nigra and the dorsal raphe nucleus, both located within the brain stem. The projections from the cortex are systematically arranged so that the frontal cortex projects to the head of the caudate and putamen and the visual cortex projects to the tail. In addition, the prefrontal cortex projects mainly to the caudate, whereas the sensorimotor cortex projects mainly to the putamen.5–8 Projections from the cortical regions that represent the proximal musculature, and those from the premotor regions may be bilateral.6,9–11 These close and profuse connections between the cortex and the basal ganglia suggest a close interfunctional relationship. The projections from the thalamus to the caudate-putamen are also somatotopically arranged. The heaviest projections are from the centromedian nucleus, and these nuclei also receive massive input from the motor cortex.7–10
The somatotopic arrangement of the cortico-striatal–thalamic-cortical pathways is maintained throughout the loop. This finding has led to an important functional hypothesis that the basal ganglia form parallel pathways subserving specific sensorimotor and associative functions.5 The putamen is linked to the sensorimotor functions and the caudate to the associative, including cognitive functions.9,12
As knowledge of the circuitry of the basal ganglia has advanced, so has the knowledge regarding the microscopic structure. The caudate-putamen looks somewhat homogeneous because of the predominance of one cell type. Careful analysis using precise staining methods has demonstrated the appearance of patches within these nuclei. It is hypothesized that this organization is important for the ability of the basal ganglia to modulate ongoing sensory input and choose the appropriate motor response.12 The intrinsic structure of the caudate-putamen also suggests that at least nigral input occurs in a way that could immediately modulate the input coming from the cortex.13,14
Efferent pathways
The input that has been processed in the caudate-putamen is sent to the globus pallidus (pallidum) and substantia nigra (nigra), which constitute the efferent portion of the basal ganglia. The globus pallidus and substantia nigra are each divided into two regions. The globus pallidus has an external and an internal region; the substantia nigra consists of the dorsal pars compacta and the ventral pars reticulata. Embryologically and microscopically, the internal segment of the globus pallidus and the pars reticulata of the substantia nigra are similar. These two regions are the primary efferent structures for the basal ganglia. The projections from the caudate and putamen to the pallidum and nigra maintain a somatotopic arrangement.10,15,16 From these structures the information is transmitted to the thalamus and then to the cortex, still maintaining somatotopy. The superior colliculus, the PPN, and other, less defined brain stem structures (perhaps the reticular formation) also receive pallidal and nigral output. All output of the basal ganglia has then been processed through the globus pallidus and/or the substantia nigra before proceeding to other areas of the brain (see Figure 20-2).
Pathways to the motor system
Information processed in the basal ganglia can influence the motor system in several ways, but no direct pathway to the alpha or gamma motor neurons of the spinal cord exists. The first route is the projection to the ventroanterior and ventrolateral nuclei of the thalamus, which then project predominantly to the premotor cortex. Another pathway is through the superior colliculus and then to the tectospinal tract. Pathways exist from the globus pallidus and substantia nigra that terminate in areas of the reticular formation (e.g., the PPN) and thus may influence the motor system through the reticulospinal pathways. Anatomically the basal ganglia are therefore in good position to affect the motor system at many levels. Many of these connections are also areas that receive cerebellar input, and thus these two regions of the brain have ample opportunity to further integrate movement responses.17
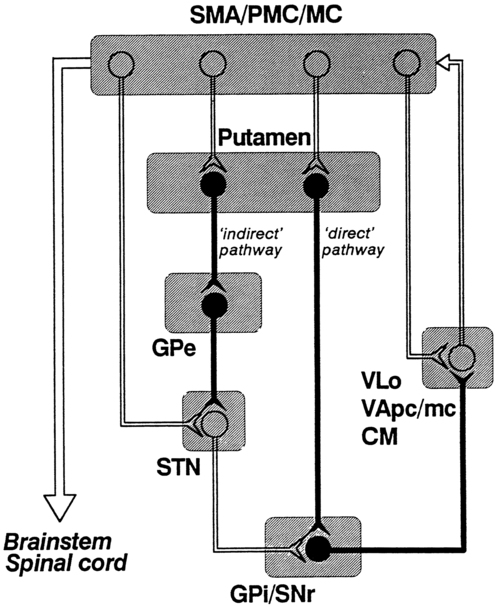
The basic circuitry of the basal ganglia comprises two loops.7 The loops for the sensorimotor system are shown in Figure 20-3. The direct loop is the loop that begins in the motor regions of the cortex and projects to the putamen and then directly to the globus pallidus, the internal segment, and on to the thalamus. The indirect pathway adds the subthalamic nucleus between the globus pallidus, external segment, and internal segment before sending the signal on to the thalamus. The subthalamic nucleus also receives direct input from the premotor and motor cortex as well as from the pallidum.18,19 The darkened neurons represent inhibitory connections, and the open neurons represent excitatory connections. In general, the direct pathway, by disinhibition, activates the thalamocortical pathway; the indirect pathway inhibits the thalamocortical system. The role of these loops in normal and diseased states is clarified in the discussion of the physiology and pharmacology of the basal ganglia.
Physiology
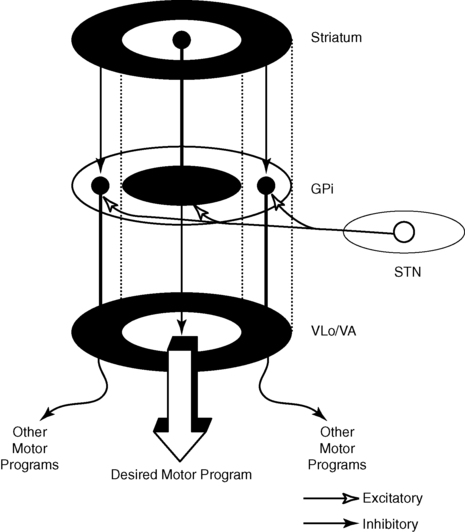
The caudate and putamen are composed of neurons that fire slowly; the globus pallidus neurons fire tonically at high rates. The low firing rates of the caudate-putamen are partially a result of the nature of thalamic inputs. Input from the cortex seems to have priority over input from the thalamus and substantia nigra. These data indicate that the cortex is instrumental in regulating the responsiveness of caudate and putamen neurons.20 In turn, basal ganglia stimulation may prepare the cortex for subsequent inputs; this might be especially important when a response must be withheld until an appropriate stimulus occurs, such as keeping the foot on the brake until the light turns green.20–23 Mink hypothesized that basal ganglia inputs to the cortex activate only the most necessary pathways and inhibit all unnecessary pathways (Figure 20-4).24
The pattern of neuronal firing in the direct and indirect pathways also suggests that the basal ganglia modify input to the cortex. The neurons of the efferent portion of the basal ganglia respond with either phasic increases or phasic decreases in activity, which in turn will affect the activity in the thalamus and hence the cortex. A decrease in activity of the internal segment of the globus pallidus removes inhibition to the thalamus and thus enables cortical activation. Whether the two pathways are activated concurrently or whether different activities activate the two pathways separately is not yet known; either way, the basal ganglia would have a role in cortical activation and modulation. One of the current views in relationship to disease processes is that an underactive direct pathway and/or an overactive indirect pathway would lead to decreased activation of the cortex and hence bradykinesia and akinesia, whereas an overactive direct pathway and/or underactive indirect pathway would lead to the presence of extraneous movements (see Figure 20-3).6,25
How do these pathways relate to everyday function? Rigidity could be explained by too much muscle activity (through the pathways from the basal ganglia to the PPN and on to the spinal cord). Akinesia and bradykinesia typical of individuals with Parkinson disease are caused by insufficient excitation or too many conflicting patterns of movement. Increased extraneous movements are characteristic of basal ganglia diseases and can be attributed to the dysfunctions within these pathways. If the amount of muscle activity and the sequence and timing of activation are inappropriate, the individual will have difficulty in selecting the environmentally appropriate behavior.24–27 Aldridge and colleagues found that the basal ganglia were modulated dependent on the purpose of the impending movement.27
Relationship of the basal ganglia to movement and posture
Automatic movement
The earliest view of the basal ganglia came from Willis in 1664. He hypothesized that the corpus striatum received “the notion of spontaneous localized movements in ascending tracts . . . . Conversely, from here tendencies are dispatched to enact notions without reflection [automatic movements] over descending pathways” (p. 7).28 Willis possessed great insights in the discussion of the signs and symptoms of basal ganglia disease. Magendie in 1841 demonstrated that removal of the striatum bilaterally produced compulsive movements, whereas removal of only one striatum produced no visible effect.29 Studies by Nothnagel30 demonstrated that lesions of the nigra tended to produce immobility. With the advent of the use of electrical stimulation in the late nineteenth century, further information on the function of the basal ganglia was gathered. Stimulation of the caudate nucleus did not (and does not) produce movement of muscles or limbs as occurs with stimulation of the motor cortex. However, at higher levels of current, total body patterns and postures were usually evoked. The earliest stimulation of the caudate nucleus produced an increase of flexion of the head, trunk, and limbs and tonic contraction of the facial muscles.31 These early studies are mentioned because of the insights they provide for the symptoms of the disorders of today.
Motor problems in animals
Contemporary experiments using lesion paradigms show a wide variety of motor problems in a variety of animals. Hypokinesia, a decrease or poverty of movements, a decreased amount of exploration of novel environments, and a tendency to assume a fixed posture are the most common problems after a lesion in the basal ganglia. These motoric dysfunctions are seen regardless of the method by which the lesion is made: pharmacologic, surgical, or by stimulation. In essence, movements are altered in scale (related to the gain), take longer for completion, and take place under altered conditions of antagonistic muscle interactions (e.g., contraction).32–40
Movement initiation and preparation
The hypothesis that the basal ganglia are involved in movement initiation and preparation is an area of some research disagreement. A “readiness potential,” recorded from the scalp of human beings before movement and thought to reflect basal ganglia activity, is more apparent in complex than in simple movements, for example, before dorsiflexion with gait but not before dorsiflexion when sitting.41–44
Neuronal recordings from awake, behaving animals found that units in the basal ganglia alter their activity before changes in the electromyographic activity of the prime movers of the task.45–51 Studies recording from multiple units in animals moving freely in their home environment suggest that neurons in the caudate-putamen and in the substantia nigra are activated in sequential, purposeful movements.27
Postural adjustments
The basal ganglia have been implicated in the process of posture and postural adjustments. People with diseases of the basal ganglia assume flexed or other fixed postures as the disease progresses (Figure 20-5). In addition, these individuals have decreased postural stability and are therefore at risk for falls. Animal experiments indicate that a deficit exists in determining response based on one’s own body position, or “egocentric localization.”52–54 This deficit decreases the ability of a person with basal ganglia disease to modify a postural response to the precise environmental demands.
Martin,55 in his extensive studies of individuals with Parkinson disease, was the first to describe severe disturbances in posture, especially when vision was occluded. Melnick and colleagues56 showed that a decrease in static postural adjustments in persons with Parkinson disease could be seen early in the disease process.57 Bloem and colleagues58–60 and Visser and colleagues61,62 meticulously studied the reflexes involved in postural adjustments and described deficits in the longer loop reflexes but not in the short latency reflex associated with the stretch reflex.
Others have investigated the interactions of the sensory systems involved in balance in those with Parkinson disease.62–66 Bloem and colleagues60 and Visser and colleagues62 concluded that postural instability was caused by a decrease in proprioception. In a recent review of proprioception and postural stability and motor control, Nicola and colleagues also describe the kinesthetic and proprioceptive deficits in people with Parkinson disease. Nicola and colleagues concluded that there was a “failure” in the body map similar to the failure in egocentric localization described previously.54 A decrease in the ability to use proprioceptive and kinesthetic information to properly scale the input and response also contributes to a loss of balance reactions.
Perceptual and cognitive functions
The basal ganglia are not solely motor systems. The previous paragraphs demonstrate the role of the basal ganglia in sensory integration. The basal ganglia are also involved in cognitive functions and responses associated with reward.36,37,48,50,67–70 Researchers have found that learned movements are more affected by basal ganglia lesions than reflexes, that neurons in the basal ganglia are responsive to some sensory input, especially proprioceptive input, and that neurons in other parts of the basal ganglia are responsive to reward and anticipation of the reward.26,71,72 Klockgether and Dichgans73 as well as Jobst and colleagues74 found that patients with Parkinson disease likewise had impairments in kinesthesia and that as a person moved a limb further from the body’s center, kinesthetic sense decreased. Schneider and colleagues75 found that animals that developed parkinsonian symptoms from a neurotoxin had deficits in operantly conditioned behavior. They suggested that the decrease in performance resulted from a “defect in the linkage” between a stimulus and the motor output centers. These sensory difficulties may be important factors in evaluation and treatment of basal ganglia diseases, especially those associated with dystonia.
The basal ganglia appear to be involved in the process of withholding a response until it is appropriate.76 A deficit in alternation of response may be the result of a tendency toward perseveration of a previously reinforced cue.77 Additional deficits exist in remembering or relearning tasks requiring a temporal sequence.78 Graybiel26 integrated the behavioral findings with information from her anatomical and chemical studies to suggest that the basal ganglia are important in providing behavioral flexibility. She hypothesizes that the basal ganglia are involved in procedural learning that leads to the development of habits. These habits become routine and are easily performed without conscious effort. Because these activities can proceed without thought, we are free to react to new events in our environment and to think. She and colleagues have performed electrophysiological experiments that explain this learning process, and these studies demonstrate great plasticity in basal ganglia networks.79 This enables the individual to select the proper movements in the proper environmental context. An elegant study by Brown and colleagues80 demonstrates a model of the basal ganglia that can reflect these cognitive and learning activities. Their model seems to integrate many of the functions of the basal ganglia with the physiology and pharmacology of the entire system. These cognitive dimensions are important to remember when developing a plan of care for a patient with basal ganglia dysfunction.
Humans with basal ganglia disease also show problems in perceptual abilities, including deficits in tasks that involve perception of interpersonal and intrapersonal space.81 In pursuit-tracking tests individuals with Parkinson disease had particular difficulties in correcting errors77; if the motor system is inflexibly set, corrections can be made only by a complete reprogramming.
The ability to perform cognitive activities involves integrating sensory information and, on the basis of this information, making an appropriate response. The basal ganglia seem to have a sensory integrative function as evidenced by experiments that show a multisensory and heterotopic convergence of somatic, visual, auditory, and vestibular stimuli.26,71,72 Segundo and Machne82 hypothesized that the function of the basal ganglia was not subjective recognition of the stimuli but rather in the regulation of posture and movements of the body in space and in the production of complex motor acts. Nicola and colleagues had similar conclusions.54
For movements to be properly controlled and properly sequenced, the two sides of the body need to be well integrated. There is anatomical evidence that suggests some means of bilateral control for the basal ganglia. A lesion of one caudate nucleus or nigrostriatal pathway produces a change in the unit activity of the remaining caudate.78,83 Studies of the dopaminergic pathway also indicate interactions between the two sides of the body.83 For this reason one may find deficits in function even on the “uninvolved” side of an individual with disease of the basal ganglia. It is also possible that diseases of the basal ganglia may go unnoticed until damage is found bilaterally.
This summary of experimental results on the function of the basal ganglia illustrates several points. At least in some general way the basal ganglia are involved in the processes of movement related to preparing the organism for future motion and future reward. This may include preparing the cortex for approximate time activation, setting the postural reflexes or the gamma motor neuron system, organizing sensory input to produce a motor response in an appropriate environmental context, and inhibiting all unnecessary motor activity. Because of the multilevel involvement of the basal ganglia in movement, it is crucial that clinicians carefully observe all aspects of movement (simple and complex) with and without interference of sensory cues or performance of dual tasks as well as postural tone during examination and treatment and the responses to treatment (see Chapter 9).
Neurotransmitters
Before a detailed analysis of the diseases of the basal ganglia can be considered, a brief description of the neurotransmitters of this region is necessary. The most prevalent diseases discussed in this chapter indicate a deficit in specific neurotransmitters. The pharmacological treatment of Parkinson disease and, in the future, perhaps other “basal ganglia plus” diseases, is based on these neurochemical deficits. The basal ganglia possess high concentrations of many of the suspected neurotransmitters: dopamine (DA), acetylcholine (ACh), γ-aminobutyric acid (GABA), substance P, and the enkephalins and endorphins. This discussion, however, includes only the first three neurotransmitters. A diagram of the basal ganglia pathways, which includes the neurotransmitters, is shown in Figure 20-6.
DA is the major neurotransmitter of the nigrostriatal pathway. It is produced in the pars compacta of the substantia nigra. The axon terminals of these dopaminergic neurons are located in the caudate nucleus and putamen. DA appears to be excitatory to the neurons in the direct pathway (GABA and substance P neurons) and inhibitory to the neurons in the indirect pathway (GABA and enkephalin neurons).2 This dual effect means that a loss of DA will lead to a loss of excitation in the direct pathway and an excess of excitation of the indirect pathway, leading to a powerful decrease in activation of the thalamocortical pathway.
Several DA receptors exist; however, their chemical interactions permit the continued use of D1 and D2 receptor classes.7 The role of DA may modulate the effects of other neurotransmitters such as glutamate. Many new drugs (called the dopamine agonists) influence only one of these receptors. Recent experiments have been trying to determine which behaviors are mediated by which DA receptor in the hope that this research may lead to more effective drug treatment with fewer side effects.
Because various drugs and chemicals can act as agonists (similar to) and antagonists (blocking the action of) of DA, they are used in treating disease involving the basal ganglia. Agonists include amantadine, apomorphine, and a class of drugs called the ergot alkaloids (e.g., bromocriptine). Amphetamine, which prevents the reuptake of DA, can enhance the effect of any DA present in the system. Antagonists include haloperidol, clozapine, and antipsychotic drugs of the phenothiazine class. With time these drugs may deplete the basal ganglia of DA and thus cause Parkinson disease or tardive dyskinesia. Similar effects on the DA system are observed in a single dose of methamphetamine (see Chapter 36).84
ACh is believed to be the neurotransmitter of the small interneurons of the caudate and putamen. It is presumed to inhibit the action of DA in this region and classically must be “in balance” with DA (and GABA). Dopaminergic axon terminals are found on cholinergic neurons. Substances that increase dopaminergic activity decrease release of ACh and vice versa.85 The antagonists of ACh, such as belladonna alkaloids and atropine-like drugs, were one of the first class of drugs used in the treatment of Parkinson disease. ACh antagonists are still used as adjuncts to treatment for patients with Parkinson disease. As some of the drugs to treat dementia are ACh agonists, care must be used when these are prescribed for the person with basal ganglia dysfunction, especially Parkinson disease.
GABA is an inhibitory neurotransmitter that is found throughout the brain. In the basal ganglia it is synthesized in the caudate nucleus and putamen and transmitted to the globus pallidus and substantia nigra.86 GABA in the basal ganglia may permit movement to occur by allowing a distribution of neuronal firing. It also may provide a means of feedback inhibition in the efferent parts of the basal ganglia so that the program of activity is not repeated unless needed.86 Individuals with Huntington disease have a deficiency of this chemical. Although agonists of GABA exist (e.g., muscimol and imidazole acetic acid), a successful drug for the treatment of Huntington disease has not yet been found. This may be a result of either the ubiquitous nature of GABA or the very complex circuitry and interrelationships that exist among GABA, ACh, and DA.
In addition to the transmitters discussed, co-transmitters may be found in the basal ganglia. Two such co-transmitters are cholecystokinin and neurotensin. The interactions of these co-transmitters may alter the sensitivity of DA receptors. Fuxe and colleagues87 suggest that the interactions of co-transmitters may alter the “set point” of transmission in synapses. They may therefore be important in one of the side effects of DA therapy, supersensitivity.
Specific clinical problems arising from basal ganglia dysfunction
Parkinson disease
Parkinson disease, first described by Parkinson in 1807, is a disease characterized by rigidity, bradykinesia (slow movement), micrography, masked face, postural abnormalities, and a resting tremor. As might be suspected from the review of functional physiology of the basal ganglia, the postural abnormalities include an assumption of a flexed posture, a lack of equilibrium reactions, especially of the labyrinthine equilibrium reactions, and a decrease in trunk rotation. Parkinson disease is among the most prevalent of all CNS degenerative diseases. Presently there are an estimated 1 million people in the United States with this disease, with approximately 60,000 new cases each year; the incidence is 4.5 to 20.5 and the prevalence is 31 to 347 per 100,000. (Refer to the list of websites at the end of this chapter.) Incidence increases with advancing age, and it is estimated that one in three adults over the age of 85 will have this disease.2 The personal and societal burden of Parkinson disease is great and includes the costs of actual treatment, the burden of caregiving, and the costs of lost earnings in patients under the age of 65.88
The cause of Parkinson disease remains unknown, and the consensus is that it is multifactorial.89,90 A slow viral process or long-term effects of early infection were implicated in postencephalitic parkinsonism. Some evidence indicates involvement of environmental factors and that interaction of environment and aging lead to a critical decrease in DA. Several investigators have found a link between growing up in a rural area and Parkinson disease; the important factors include pesticide use, insecticide use, and elements in well water.91–97 Accumulation of free radicals, cell death to excitatory neurons from toxins, and dysfunction of nigral mitochondria have all been implicated in the pathological process. The genetics of Parkinson disease is still debated. Although twin studies indicate that there may not be a single gene involved in Parkinson disease, as in Huntington disease, a family history may be an important risk factor.93,98–101 Very recently a large-scale study found two genetic loci to be associated with Parkinson disease.102 So the debate continues, with most neurologists agreeing that the multifactorial approach will yield the best opportunity to develop a cure.
In view of possible treatment effects for Parkinson disease, it is interesting that a study by Sasco and others103 found an inverse relationship, albeit small, between participation in exercise or sports and later development of Parkinson disease. The loss of DA from the substantia nigra leads to alterations in both the direct and indirect pathways of the basal ganglia, resulting in a decrease in excitatory thalamic input to the cortex and perhaps a decrease in inhibitory surround that leads to the symptoms of Parkinson disease.
Symptoms
Bradykinesia and akinesia.
Bradykinesia (a decrease in motion) and akinesia (a lack of motion) are characterized by an inability to initiate and perform purposeful movements. They are also associated with a tendency to assume and maintain fixed postures. All aspects of movement are affected, including initiation, alteration in direction, and the ability to stop a movement once it is begun. Spontaneous or associated movements, such as swinging of the arms in gait or smiling at a funny story, are also affected. Bradykinesia is hypothesized to be the result of a decrease in activation of the supplementary motor cortex, premotor cortex, and motor cortex.104 The resting level of activity in these areas of the cortex may be decreased so that a greater amount of excitatory input from other areas of the brain would be necessary before movement patterns could be activated. In the individual with Parkinson disease, an increase in cortically initiated movement even for such “subcortical” activities as walking supports this hypothesis. Automatic activities are cortically controlled, and each individual aspect seems to be separately programmed. Associated movements in the trunk and other extremities are not automatic. This means that great energy must be expended whenever movement is begun.105
Bradykinesia and akinesia affect performance of all types of movements; however, complex movements are more involved than simple movements, such as dorsiflexing the foot at toe-off in walking as opposed to dorsiflexing the foot in a seated position.71,106–109 In addition, patients with parkinsonism have increased difficulty performing simultaneous or sequential tasks, over and above that seen with simple tasks. Parkinsonian patients must complete one movement before they can begin to perform the next, whereas control subjects are able to integrate two movements more smoothly in sequence. This deficit has been shown in a variety of tasks from performing an elbow movement and grip to tracing a moving line on a video screen. The patient with Parkinson disease behaves as if one motor program must be completely played out before the next one begins, and there is no advance planning for the next movement while the current movement is in progress.106–108,110,111 Morris and colleagues demonstrated a similar phenomenon in walking. Patients with parkinsonism were unable to perform walking while carrying a tray with a glass of water and had even more difficulty when walking and reciting a numerical sequence.112,113
Sequential movements become more impaired as more movements are strung together; for example, a square is disproportionately slower to draw than a triangle; a pentagon, more difficult than a square.5,106 These results indicate that patients with Parkinson disease have difficulty with transitions between movements. Transitional difficulties are more impaired in tasks requiring a series of different movements than tasks requiring a series of repetitive movements. For example, an individual will have less difficulty continually riding a stationary bike than movement requiring transitions such as coming from a chair to standing, walking, and turning a corner. Therefore treatment must include complex movements with directional changes to ensure that the patient is safe outside the treatment setting.
Bradykinesia is not caused by rigidity or an inability to relax. This was demonstrated in an electromyographic analysis of voluntary movements of persons with Parkinson disease.114 Although the pattern of electromyographic agonist-antagonists burst is correct, these bursts are not large enough, resulting in an inability to generate muscle force rapidly enough. Even in slow, smooth movements, however, these individuals demonstrated alternating bursts in the flexor and extensor muscle groups. This type of pattern, expected in rapid movements that require the immediate activation of the antagonist to halt the motion, interferes with slow, smooth, continuous motion. Other researchers have found an alteration in the recruitment order of single motor units.115,116 These alterations included a delay in recruitment, pauses in the motor unit once it was recruited, and an inability to increase firing rates. These persons therefore would have a delay in activation of muscles and an inability to properly sustain muscle contraction for movement, and a decreased ability to dissipate force rapidly.24,115,117 Such changes may account for perceived decreases in strength that are seen in persons with Parkinson disease. They are also important to remember in both treatment planning and the efficacy of treatment efficiency.
Rigidity.
The rigidity (increased resistance to passive movement) of Parkinson disease may be characterized as either “lead pipe” or “cogwheel.” The cogwheel type of rigidity is a combination of lead-pipe rigidity with tremor. In rigidity there is an increased resistance to movement throughout the entire range in both directions without the classic clasp-knife reflex so characteristic of spasticity. Procaine injections can decrease the rigidity without affecting the decrease of spontaneous movements, confirming that rigidity is not the same phenomenon as bradykinesia.118,119
Rigidity is not caused by an increase in gamma motor neuron activity, a decrease in recurrent inhibition, or a generalized excitability in the motor system.120 Long- and middle-latency reflexes are enhanced in parkinsonism, and the increase in long-latency reflexes approximates the observable increase in muscle tone. Short-latency reflexes (i.e., deep tendon reflexes), on the other hand, may be normal in persons with Parkinson disease.
Tatton and others121 found differences in certain cortical long-loop reflexes in normal and drug-induced parkinsonian monkeys, which led them to speculate that the “reflex gain” of the CNS may lose its ability to adjust to changing environmental situations. For example, in normal persons the background level of motor neuron excitability is different for the task of writing than for the task of lifting a heavy object; in individuals with Parkinson disease motor neuron excitability would be set at the same level. Similarly, in the normal individual there would be a difference in excitability if the environmental demands were for excitation or inhibition of a muscle; for the individual with Parkinson disease, there would be similar motor neuron excitability regardless of task demands. Furthermore, this lack of modulation may mean that the person with parkinsonism perceives himself or herself to be moving farther than he or she is actually moving. It is also consistent with a decrease in system flexibility and an inability to adjust to equilibrium perturbations.58,59,65
An important aspect of rigidity is that it might increase energy expenditure.122 This would increase the patient’s perception of effort on movement and may be related to feelings of fatigue, especially postexercise fatigue.123
Tremor.
The tremor observed in Parkinson disease is present at rest, usually disappears or decreases with movement, and has a regular rhythm of about 4 to 7 beats per second. Some people with Parkinson disease may have a postural tremor. The electromyographic tracing of a person with such a tremor shows rhythmical, alternating bursting of antagonistic muscles. Tremor can be produced as an isolated finding in experimental animals that have lesions in various parts of the brain stem or that have been treated with drugs, especially DA antagonists. DA depletion, however, is not the sole cause of tremor. It appears that efferent pathways, especially from the basal ganglia to the thalamus, must be intact because lesions of these fibers decrease or abolish the tremor.124 Poirier and colleagues124 proposed that tremor results from a combined lesion of the basal ganglia and cerebellar–red nucleus pathways. Because both the basal ganglia and the cerebellum project to the thalamus, a lesion of the thalamus can abolish the tremor regardless of the specific pathway(s). Although tremor may be cosmetically disabling, the tremor rarely interferes with activities of daily living (ADLs).
Postural instability.
Postural instability is a serious problem in parkinsonism that leads to increased episodes of falling and the sequelae of falls. More than two thirds of all patients with parkinsonism fall, and more than 10% fall more than once a week.125 People with Parkinson disease have a ninefold risk of recurrent falls compared with age-matched control subjects.60,126–130 Patients have an increased likelihood of falling as the duration of the disease increases. Drug treatment is not usually effective in reducing the incidence of falls. Deep brain stimulation and exercise, on the other hand, have been shown to be effective in increasing functional skills and/or motor performance; these improvements may decrease the number of falls.131–134 Large randomized clinical trials have been performed to determine the efficacy of exercise.135
Although the causes of balance difficulties are not known, several hypotheses exist. One explanation for postural instability is ineffective sensory processing. Several investigators have found deficits in proprioceptive and kinesthetic processing.55,74,117,136 For example, Martin55 found that labyrinthine equilibrium reactions were delayed in patients with Parkinson disease. Studies of the vestibular system itself, however, have shown that this system functions normally. Pastor and colleagues137 studied central vestibular processing in patients with Parkinson disease and found that the vestibular system responds normally and that patients can integrate vestibular input with the input from other sensory systems. This group hypothesized that the parkinsonian patients had an inability to adequately compensate for baseline instability. This theory is in partial agreement with studies by Beckley, Boehm, and others58,59,65 demonstrating that patients with Parkinson disease were unable to adjust the size of long- and middle-latency reflex responses to the degree of perturbation. These patients are therefore unable to activate muscle force proportional to displacement. Melnick and colleagues56 found that subjects with Parkinson disease were unable to maintain balance on a sway-referenced force plate. Glatt138 found that patients with Parkinson disease did not demonstrate anticipatory postural reactions and, in fact, behaved exactly as a rigid body with joints. Horak and colleagues,139,140 in a variety of studies, reported similar findings and found defects in strategy selection as well; patients with Parkinson disease chose neither a pure hip strategy nor a pure ankle strategy but mixed the two in an inappropriate and maladaptive response. Investigators have found that antiparkinsonian medications could improve background postural tone but did not improve automatic postural responses to external displacements.58,59,65,139–141 Other studies have demonstrated deficits in proprioceptive perception—what has been termed an “impaired proprioceptive body map.” Patients with Parkinson disease did not alter anticipatory postural adjustments in response to step width changes, unlike control subjects.142 Increased step width requires increased lateral reactive forces to unload the stance leg. The lack of ability to prepare for these extra forces may indicate that narrow stance width, start hesitation, and freezing of gait are compensatory mechanisms to proprioceptive loss.136 Likewise, when patients could not see their limbs, they had difficulty moving the foot to a predetermined location in response to perturbation. Control subjects had no difficulty.143,144 Taken together, it appears that postural instability results from inflexibility in response repertoire; an inability to inhibit unwanted programs; the interaction of akinesia, bradykinesia, and rigidity; and some disturbance in central sensory processing.
Gait.
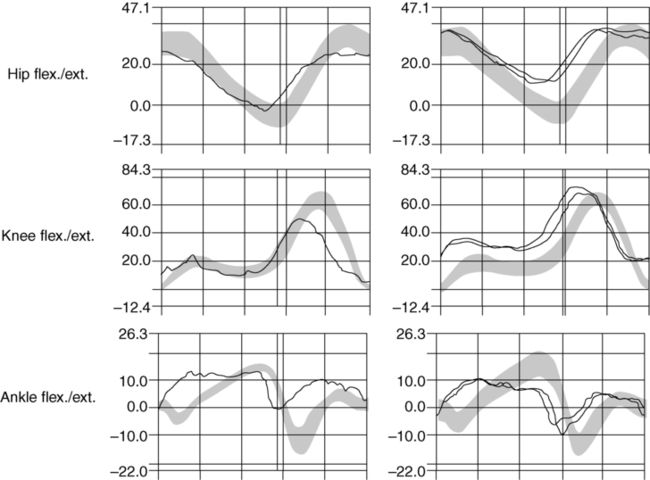
The typical parkinsonian gait is characterized by decreased velocity and stride length.145,146 As a consequence, foot clearance is decreased, which again places the individual at greater fall risk.147 In many patients, especially as the disease progresses, speed and shortening of stride progressively worsen as if the individual is trying to catch up with his or her center of gravity; this is termed festination. Forward festination is called propulsion; backward festination is known as retropulsion. One hypothesis is that festinating gait is caused by the decreased equilibrium responses. If walking is a series of controlled falls and if normal responses to falling are delayed or not strong enough, then the individual will either fall completely or continue to take short, running-like steps. The abnormal motor unit firing seen with bradykinesia may also be the cause of ever-shortening steps. If the motor unit cannot build up a high enough frequency or if it pauses in the middle of the movement, then the full range of the movement would decrease; in walking this would lead to shorter steps. Festination may also be the result of other changes in the kinematics of gait.
The changes in gait kinematics include changes in excursion of the hip and ankle joints (Figure 20-7). Instead of a heel-toe, the patient may have a flat-footed or, with disease progression, a toe-heel sequence. The patient with Parkinson disease appears to have lost the adult gait pattern and is using a more primitive pattern. The flat-footed gait decreases the ability to step over obstacles or to walk on carpeted surfaces. The use of three-dimensional gait analysis has shown that there is a decrease in plantarflexion at terminal stance. Changes are also seen in hip flexion, which may alter ankle excursion. However, qualitative aspects of the timing of joint excursion appear intact. Figure 20-7 illustrates the joint angles in a 55-year-old patient with Parkinson disease compared with adults without basal ganglia dysfunction.148
Perception, attention, and cognitive deficits.
Especially in recent years, researchers have tried to address the cognitive and perceptual impairments of people with Parkinson disease.136,149–152 Whereas the movement deficits are hypothesized to be caused by a decrease in putaminal excitation of the cortex, the learning and perceptual deficits are hypothesized to be caused by a decrease in cortical excitation from the caudate nucleus.111 The deficits are of frontal lobe function and include an inability to shift attention, an inability to quickly access “working memory,” and difficulty with visuospatial perception and discrimination. Research attention has focused on the specific deficits of parkinsonian patients compared with patients with Alzheimer disease, patients with frontal lobe damage, and those with temporal lobe damage.149,152,153 The perceptual deficits of all groups appear to increase with progression of the disease process. In general, patients have difficulty in shifting attention to a previously irrelevant stimulus,154 learning under conditions requiring selective attention,154 or selecting the correct motor response on the basis of sensory stimuli.155–157 There is also evidence that DA is involved in selection of responses that will be rewarding.54 These impairments will affect treatment strategies.
Learning deficits also have been found in patients with parkinsonism; procedural learning has been particularly implicated, as would be indicated based on the physiology of the system. Procedural learning is learning that occurs with practice or, as defined by Saint-Cyr and colleagues,158 “the ability gradually to acquire a motor skill or even a cognitive routine through repeated exposure to a specific activity constrained by invariant rules.” In their tests, patients with Parkinson disease did very poorly on tests of procedural learning, but their declarative learning was within normal limits. Pascual-Leone and colleagues111 studied procedural learning in more detail. They found that patients with Parkinson disease could acquire procedural learning but needed more practice than control subjects did. They also found that the ability to translate procedural knowledge to declarative knowledge was more efficient if it occurred with visual input alone rather than the combination of visual input with motor task. This may be a rationale for more therapy, not less.
Nonmotor symptoms.
Nonmotor symptoms are consistently seen in patients with Parkinson disease and may be attributable to dopaminergic pathways outside the basal ganglia. Braak159 hypothesized that Parkinson disease actually begins with DA deterioration in the medulla and progresses rostrally. Often the first signs are loss of sense of smell, constipation, vivid dreams (rapid-eye movement [REM] behavior disorder), and orthostatic hypotension.160,161 Orthostatic hypotension may cause some dizziness and requires coordination of medications for other medical problems. l-Dopa and DA agonists may lower blood pressure; blood pressure medication may need to be altered once antiparkinsonian drugs have been prescribed. Although not all people with these problems have Parkinson disease, when they are combined they may indicate risk for this disorder. Because physical therapy may be most effective when started early, researchers are trying to learn more about these early symptoms.
Other nonmotor symptoms that decrease quality of life include incontinence in men and women, sexual dysfunction, excess saliva, weight changes, and skin problems. Nonmotor symptoms that can interfere with and complicate physical and occupational treatment include fatigue, fear, anxiety, and depression. Urinary incontinence is important because it increases the risk of hospitalization and mortality.162
Sleep disorders are widespread in Parkinson disease and include more than just REM sleep disorder.163 The patient may experience daytime drowsiness and decreased sleep at night. There appears to be a lack of consolidation of sleep with decreased total sleep time as well as the presence of restless leg syndrome.164 Daytime drowsiness may be a side effect of medication; however, it can also be exacerbated after therapeutic exercise, so a cool-down period is necessary before the patient sits down and relaxes.
Nonmotor symptoms often predominate as the disease progresses.160 They contribute to severe disability, impaired quality of life, and shortened life expectancy. As the disease progresses, cognitive problems also become more frequent. Braak159 hypothesized that this was an indication of rostral progression of dopaminergic involvement. Cognitive involvement can include memory loss, confused thinking, and dementia. Parkinson disease medications may worsen these cognitive impairments. The nonmotor symptoms of Parkinson disease have been addressed in a practice parameter recommendation by the American Academy of Neurology.161
Stages of parkinson disease
Parkinson disease is a progressive disorder.165 The initial motor symptom is often a resting tremor or unilateral micrography (bradykinesia of the upper extremity). With time, rigidity and bradykinesia are seen bilaterally, and postural alterations and axial symptoms then begin to occur. This commonly starts with an increase in neck, trunk, and hip flexion that, accompanied by a decrease in righting and balance responses, leads to a decreased ability to maintain the center of gravity over the base of support.
Throughout this progressive deterioration of movement, there is also a decrease in higher-level sensory processing. In addition, the patient can perform only one task at a time. Reports of dementia range from 30% to 93% in patients with Parkinson disease.166 The presence of dementia in this population may indicate involvement of the ACh or noradrenergic mesolimbic system. In this case, treatment with anticholinergic drugs may increase a tendency toward dementia, especially in older patients. Sometimes cognitive deficits are inferred because of slowed responses, spatial problems, sensory processing problems, and a masked face (see Chapter 36).
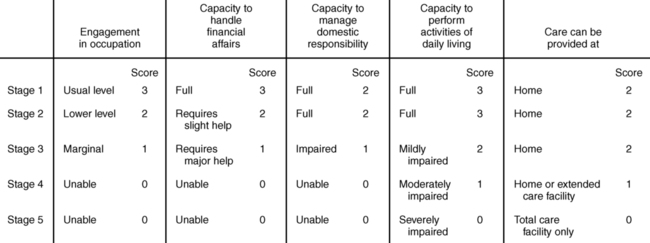
Staging of Parkinson disease uses the Hoehn and Yahr scale (Table 20-1).165 Originally developed as a 5-point scale, in recent years 0, 1.5, and 2.5 measurements have been added. The 1.5 and 2.5 ratings have not been validated, but because their use is so common, the latest recommendation is to continue using them while the validity is studied.167
TABLE 20-1 
HOEHN AND YAHR STAGING SCALE FOR PARKINSON DISEASE
| STAGE | PROGRESSION OF SYMPTOMS |
| 0 | No signs of disease. |
| 1 | Unilateral symptoms only. |
| 1.5 | Unilateral and axial involvement. |
| 2 | Bilateral symptoms. No impairment of balance. |
| 2.5 | Mild bilateral disease with recovery on pull test. |
| 3 | Balance impairment. Mild to moderate disease. Physically independent. |
| 4 | Severe disability, but still able to walk or stand unassisted. |
| 5 | Needing a wheelchair or bedridden unless assisted. |
| The Hoehn and Yahr scale is commonly used to describe how the symptoms of Parkinson disease progress. The original scale included stages 1 through 5.165 Stage 0 has since been added, and stages 1.5 and 2.5 have been proposed to best indicate the relative level of disability in this population.167 |

Pharmacological considerations and medical management
The knowledge that the symptoms of Parkinson disease are caused by a decrease in DA led to the pharmacological management of this disease. Because DA itself does not cross the blood-brain barrier, levo-dihydroxyphenylalanine (l-dopa), a precursor of DA that does, has been used to treat Parkinson disease since the late 1960s.168–170 An inhibitor of aromatic amino acid decarboxylation (carbidopa) is usually given with l-dopa to prevent the conversion to DA before entering the brain. The decarboxylase inhibitor allows a reduction in dosage of l-dopa itself, which helps decrease the cardiac and gastrointestinal side effects of DA.
Treatment of Parkinson disease with l-dopa in these various combinations is extremely helpful in reducing bradykinesia and rigidity. It is less effective in reducing tremor and the postural instability. Because Parkinson disease involves the nigral neurons, the receptors and the neurons in the striatum (which are postsynaptic to dopaminergic neurons) remain intact and initially are somewhat responsive to DA.171,172 With time, however, the receptors appear to lose their sensitivity, and the prolonged effectiveness (10 years or more) of l-dopa therapy is questionable.173–175 A further complication of l-dopa therapy is the development of involuntary movements (dyskinesias) and the “on-off” phenomenon—a short-duration response resulting in sudden improvement of symptoms followed by a rapid decline in symptomatic relief and perhaps the appearance of dyskinesias and/or dystonias.176,177 With time the “on” effect becomes of shorter and shorter duration.173,176,178,179 Controlled-release or slow-release l-dopa may decrease these side effects. The effectiveness of l-dopa does not appear to be closely correlated with the stage of the disease.
The use of l-dopa alone or in combination with carbidopa has not provided a cure or even prevented the degeneration of Parkinson disease.178,179 As more has become known about the DA receptor, specific agonists have been developed. Ropinirole, pramipexole, pergolide, and bromocriptine are examples of DA receptor D2 agonists that are used alone or with l-dopa. The agonists are thought to decrease the wearing-off effects as well as decrease the dyskinesias that occur with long-term l-dopa use, but l-dopa remains the most effective medication. It is quite likely that newer D2 and/or D2-D1 (DA receptor D1) agonists will be developed. Pharmacological interventions also include drugs that prevent the breakdown of DA (e.g., catechol-O-methyltransferase [COMT] inhibitors) and/or its reuptake. Entacapone is an example of a COMT inhibitor.180
Another approach to pharmacological treatment of individuals with Parkinson disease was developed from research on a designer drug that contained the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). It was found that the conversion of MPTP to the active neurotoxin MPP+ could be prevented by monoamine oxidase inhibitors such as deprenyl and pargyline.73,179 Deprenyl, rasagiline, and selegiline are now used before the initiation of, or in conjunction with, l-dopa and carbidopa.
Another treatment alternative is surgery performed in precise areas of the basal ganglia, known as stereotaxic surgery. Stereotaxic surgery is an old technique that has made a comeback based on the new knowledge of basal ganglia connectivity and improvements in the procedural instrumentation.* Initially, one of the structures of the basal ganglia was lesioned with freezing or high-frequency stimulation. Today the globus pallidus internal segment or the subthalamic nucleus is stimulated with implanted electrodes. This technique is known as deep-brain stimulation (DBS). DBS has now been approved by the U.S. Food and Drug Administration (FDA). An advantage of deep brain stimulation over permanent lesions is that DBS is reversible and is safer for bilateral surgeries. Stimulation of the globus pallidus internal segment or subthalamic nucleus has been shown to decrease all symptoms; subthalamic nucleus stimulation is also effective in reducing dyskinesias and may lessen the amount of medication taken.183–185 Effects of stimulation are greater for symptoms manifested in the “off” state. Deep brain stimulation has been demonstrated to improve rigidity, bradykinesia, and akinesia, as well as gait57,184,186–189 and balance.56,190 It has also been demonstrated to improve movement velocity and speed of muscle recruitment for activity.190,191 The proposed mechanism of action is interference with the abnormal neuronal firing.192,193 In a randomized, controlled, clinical trial, DBS was more effective in reducing symptoms and increasing quality of life than medication.194,195 This group also found that although some side effects were worse (e.g., brain hemorrhage), the total number of adverse reactions was greater in the medication group. Whether stimulation of the subthalamic nucleus is neuroprotective, that is, prevents further degeneration, is presently under investigation. Thalamic stimulation is used for decreasing tremor. Therapists may find that intense treatment immediately after these surgeries may be able to take advantage of neural plasticity.
Fetal transplantation of the substantia nigra to the caudate nucleus remains under investigation. A double-blind, placebo-controlled trial was completed with mixed results.192,196–198 Studies continue, including those of dose, cell type, and placement of cells. Recently, however, there was a report of Lewy-body inclusions in grafted cells 14 years after the transplant.199 The authors concluded that Parkinson disease was an ongoing process and that what caused the disease initially, also affected the grafted cells.
Examination of the client with parkinson disease
The Hoehn and Yahr Scale (see Table 20-1) is frequently used to describe the general severity of disease.165 The Unified Parkinson’s Disease Rating Scale (UPDRS) is the most widely used assessment tool to describe all facets of impairment: cognitive and emotional status, ADL ability, motor function, and side effects of medication.200–202 The UPDRS is also frequently used to measure the efficacy of treatments. Another clinical scale is the Core Assessment Program for Intracerebral Transplantation (CAPIT), which includes timed tests.203 This scale was designed to standardize assessments of patients with Parkinson disease who undergo surgical intervention. It is comprehensive and more time-consuming and therefore tends to be used more in research than in the clinic. Knowledge of these scales will help the physical therapist in communication and interactions with other health care professionals even though the scales may not be ideal for planning physical and occupational therapeutic interventions.
Assessment of functional activities will be most beneficial for treatment planning and reevaluation. In addition to assessing how the patient performs the activity, the time it takes to complete an activity must be measured. For example, gait is assessed by general pattern, speed, and distance, as well as the effects of interfering stimuli including walking while performing cognitive tasks. It is advantageous to evaluate forward and backward walking as well as braiding and the ability to alter gait speed in each of these conditions.145,146 Available objective tests of gait and functional mobility include the Timed Up-and-Go Test, 10-meter walk test, the 5 or 10 Times Sit-to-Stand Test, the Dynamic Gait Index, or any of the objective standardized tests presented in Chapter 8. Careful observation of how the person performs a task would be useful for treatment planning. For example, when rising from a chair, does the patient move forward in the chair, place the feet underneath the knees, and lean forward before rising?
A careful analysis of balance is imperative for the patient with Parkinson disease. This must include assessment with and without vision and the differences between the two conditions (see the section on balance in Chapter 22). Assessing challenges to balance such as tandem walking or standing on a compliant surface is important, especially in the early stages of the disease. This may be the first sign of balance impairment. Posturography is the most sensitive measure of postural instability, especially in the early stages of the disease (Hoehn and Yahr stages 1 and 2).58 A clinically useful tool to assess dynamic balance is the functional reach test, which has been shown to be an effective, predictive tool in people with Parkinson disease as it is in the elderly.204 The Balance Evaluation Systems Test (BESTest) is also an appropriate comprehensive measure for those with Parkinson disease. Obtaining a falls history continues to be a reliable predictor of future falls and is easy to measure. (Refer to Chapters 8 and 22 for specifics on these tests.)
General prognosis, treatment goals, and rationale
As with all treatment, the prognosis (functional goals and established time parameters) is based on the general goals related to the findings from the examination of each client and the client’s expectations and functional requirements. Parkinson disease must be understood as a degenerative disease when establishing the prognosis and treatment plan. Nonpharmacological and surgical interventions, especially physical therapy treatment, are especially important in the beginning of the disease.205 In general, goals include increasing movement and range of motion (ROM) in the entire trunk as well as the extremities, maintaining or improving chest expansion, improving balance reactions, and maintaining or restoring functional abilities. Increased movement may in fact modify the progression of the disease.206,207 It may further help to retard dementia. Although l-dopa decreases the bradykinesia, it alone will not be effective in increasing movement or improving balance; therefore, aggressive intervention in the early stages is necessary. Increasing trunk rotation goes hand in hand with increasing range of movement and motion in general. The longer clients are kept mobile, the less likely they are to develop pneumonia and the longer they can maintain independence in ADLs. Ideally, rehabilitation interventions should begin at the first sign of the disease, but this is not always possible. Treatment initiated while the disease is still unilateral (Hoehn and Yahr stage 1) is more advantageous.208,209
Treatment procedures
Overall, physical rehabilitation is effective in the treatment of people with Parkinson disease. The results are greater when treatment is started early in the disease process, but it has been shown to be effective in Hoehn and Yahr stages 1 to 3. The American Academy of Neurology recommends physical therapy in its practice parameters.210 The bottom line is that treatment by movement specialists that incorporates complex, sequential movements with multiple sensory inputs creates demands for responses that are environmentally appropriate, challenges balance, uses large-amplitude movements, and is fun and effective. Many treatment regimens have been used, and almost all have been successful. Animal research indicates that exercise and forced functional movements may protect the dopaminergic neurons.211 The following paragraphs will provide more precise information and more precise details.
Basic principles for treatment of the person with Parkinson disease will, of course, depend on the areas of impairment and handicap revealed in the evaluation. Certain principles, however, are true for all stages of the disease. First, the activities selected must engage the patient: the patient must find the activities interesting enough to do them regularly. Variety is important to facilitate shifts in movement as well as in thought. And movements must be big! (In fact, one treatment technique even uses that word in its name.) Activities that are designed to improve balance are valuable even in the early stages of the disease. To date, many rehabilitative techniques and exercises have demonstrated improvement in function for people with Parkinson disease, and there have now been a few randomized clinical trials with small numbers of patients to test efficacy of the varied techniques. Programs that emphasize sensory-motor integration, agility, and motor learning demonstrate decreased progression of disease and improved motor function.212–227 Programs that involve the coordination of dual motor-cognitive tasks and complex sequences of movements and that force the participant to quickly change movements dependent on environmental conditions have resulted in improved performance on the Timed Up-and-Go Test, the UPDRS, the 10-meter walk test, and a variety of balance tests. Some of these programs include the Lee Silverman Voice Treatment (LSVT BIG) program, sensory attention focused exercise (PD SAFEx), ballroom dance, Zumba, tai chi, karate, computer game playing, and alpine hiking.
Decreasing rigidity.
Movement throughout a full ROM is crucial, especially early in the disease process, to prevent changes in the properties of muscle itself. In Parkinson disease the contractile elements of flexors become shortened and those of the extensor surface become lengthened, enhancing the development of the flexed posture that is traditionally present.228 For most patients, treatment proceeds better if rigidity is decreased early in the treatment session. In fact, movement therapy interventions appear to have more lasting effects when the treatment is performed during the “on” phase of a medication cycle.
Many relaxation techniques appear to be effective in reducing rigidity, including gentle, slow rocking, rotation of the extremities and trunk, and the use of yoga (see Chapters 9 and 39). In the client with Parkinson disease, success in relaxation may be better achieved in the sitting or standing positions because rigidity may increase in the supine position.91 Furthermore, because the proximal muscles are often more involved than the distal muscles, relaxation may be easier to achieve by following a distal-to-proximal progression. The inverted position may be used with care. Initially this position facilitates some relaxation (increase in parasympathetic tone) and then increases trunk extension, which is important for the parkinsonian client. Relaxation may also be effective in reducing the tremor of Parkinson disease. Once a decrease in rigidity has been achieved, movement must be initiated in order to use the newfound range in a functional way.
Therapeutic programs.
Exercise itself is important for the person with Parkinson disease. There is a relationship between longevity and physical activity.229 Those who exercise have lower mortality rates.229 Some evidence also indicates that exercise may alter the magnitude of free radicals and other compounds linked to aging and parkinsonism. Immunological function may also be improved with exercise. Sasco and colleagues103 demonstrated a link between a lack of exercise and development of parkinsonism. Finally, the role of aerobic fitness itself may be a factor in reducing dysfunction.103 Animal data indicate that functional exercise decreased DA loss after a variety of lesion models.129,208,209,211,230 Some of the animal activities were similar to the complex, sensory-motor and agility programs now used in patient programs.213,218,221,222 Aerobic exercise may improve pulmonary function in patients with Parkinson disease because these functions appear to suffer from deficiencies in rapid force generation of the respiratory muscles, similar to limb musculature.231 Exercise is most beneficial when it is begun early in the disease process as is recommended in all books, pamphlets, and websites for the patient.232 (Refer to the list of websites at the end of this chapter.) All research on the effects of exercise programs in parkinsonism indicates this point. When the use of forced functional activities is delayed too long, few beneficial effects of exercise on the DA system have been shown in animal studies.208,209 Hurwitz233 found that patients who were still independently mobile at home and in the community benefited the most from a home program. Schenkman and Butler228 also indicate that patients in the earlier stages of the disease had the best potential for improvement. If patients practice regular physical exercise in conjunction with disease-specific exercises, the ill effects of inactivity will not potentiate the effects of the disease process itself. Although most patients with Parkinson disease can achieve an adequate exercise level, many clients have fitness levels that are poor or very poor before the medical diagnosis.122 Exercise, even once a week, can be effective in improving gait and balance in clients with Parkinson disease when practiced over several months.212,234
So far almost all studies have found that exercise under the guidance of a therapist is effective.213–227 Palmer and colleagues235 used precise, quantitative measures to assess motor signs, grip strength, coordination, and speed as well as measurements of the long-latency stretch reflex after two exercise programs in patients with Parkinson disease. These two programs were the United Parkinson Foundation program and karate training. Their results indicated improvement over 12 weeks in gait, grip strength, and coordination of fine motor control tasks and no change in a decline in movements requiring speed. The patients all felt an increase in general well-being. A study by Comella and colleagues236 as well as one by Patti and colleagues237 also found decreases in parkinsonian symptoms with physical and occupational therapy. However, these studies found no long-term carryover once therapy had been discontinued. The authors never explain the exercise program precisely nor the instructions provided for a home program.
Rhythmical exercise has been shown to decrease rigidity and bradykinesia and improve gait over time.212,238–254 Ballroom dancing is a form of rhythmical therapy for patients with Parkinson disease that incorporates rhythmical movement, rotation, balance, and coordination.212 A program of tango versus waltz and foxtrot indicated that although both groups improved on the UPDRS motor scale, Berg Balance Scale, 6-minute walk distance, and backward stride length, the tango group had greater improvements.214,217,225 The waltz and foxtrot, which are easier dances, may be beneficial for those at more advanced stages of the disease. The use of dance also facilitates changing direction. Our program using Latin dance (predominantly mambo and cha-cha) and other weight-bearing exercise demonstrated similar improvements in balance and especially initiation of gait.212,234,247,255 Similar effects were seen in tai chi, which demands attention to movement and increases challenges to balance and control of movement. Tai chi has been shown to be effective in improving gait and balance parameters.220
Studies using a program emphasizing sensory awareness of the size of movement have shown improvement in both speed of movement and gait parameters.224,226,227 PD SAFEx,226 a program that focuses attention on sensory awareness, was shown to improve gait and function on the UPDRS in a randomized controlled study. A group that engaged in aerobic exercise alone improved gait but not symptoms. A group that continued usual activities did not demonstrate improvements on any outcome measure. These authors concluded that programs emphasizing increased sensory feedback and awareness were superior in reducing the symptoms and improving the function of patients with Parkinson disease.
Treadmill training has been used in Parkinson disease exercise programs. Use of the treadmill with body-weight support increases safety and allows the therapist to control speed of movement as well as perturbations. Some studies have used cued treadmill training with good results and carryover to the home.256 Cognitive tasks and other dual tasks have also been added during treadmill training with good results.257 These studies found collectively improved measures of balance and gait, as well as reduced fear of falling and number of falls.213,221
Physical activity and movement appear to increase quality of life by decreasing depression and improving mood and initiative.248,258 Group classes can serve as an extra support system for patients with Parkinson disease and their spouses.* A carefully structured low-impact aerobics program appears to be beneficial to patients even with long-standing disease.234 One program designed for those at Hoehn and Yahr stage 2.5 or 3 begins with seated activities for upper extremities (Figure 20-8, A) and combination movements for warmup (Figure 20-8, B). The participants then progress to standing and marching activities that incorporate coordinated movements of arms and legs as well as balance and trunk rotation (Figure 20-9). All movements are performed to music similar to that used in aerobics classes in any gym or health club (Figure 20-10). (The rationale for the use of external cues and the role of rhythm in gait training are discussed in subsequent paragraphs.) A cool-down period allows participants to practice fine motor coordination activities of the hands (Figure 20-11). Many Parkinson disease associations also have audiotapes for exercises (e.g., United Parkinson Foundation).
The use of computer games to improve symptoms is currently under investigation. These games force the participant to move in precise ways or to shift weight to score points. Many “off-the-shelf” games exist and have been used with older adults (the predominant patient population of those with Parkinson disease) to increase activity levels. For some with Parkinson disease, even in the early stages, these games are too fast or too confusing. A feasibility study showed that disease-specific games could be used, and enjoyed by those with Parkinson disease.259
The most successful exercise programs appear to be those that incorporate context-dependent responses and a varied environment. All of the previously listed examples of these activities are presented in Box 20-1. Aerobic exercises that are not as effective in requiring context-dependent responses are presented in Box 20-2. Research has shown the importance of adjusting the response to the specific task and has also demonstrated the importance of practice for the parkinsonian patient.157,260 The principles of motor learning are of paramount importance in the treatment program of these patients. Random practice may enable the patient to learn the correct schema by which to regulate the extent, speed, and direction of the movement. Random practice also may be important in facilitating the ability of the patient to shift attention and to learn to access “working memory.” The parkinsonian patient may benefit from visual instruction and mental rehearsal before performing the movement.137,157 In addition, the instructions used need to be pertinent to the task at hand.
Strengthening.
Strengthening exercises have been promoted for the patient with Parkinson disease. With disuse comes decreased strength. Weakness occurs with initial contraction and also with prolonged contraction. Manual muscle testing may not reveal losses in strength; however, most of the successful exercise programs previously mentioned did include functional strength training as part of the program. High-resistance eccentric resistance can produce muscle hypertrophy and may effect improvements in mobility.261 Another study used “sports activities” in a twice-per-week program.158 The program included exercises on land designed to improve gait and balance and exercises in the water to increase strength. These investigators reported significant improvements in UPDRS scores, cognitive function, and mood in addition to ADL and motor scores during the 14-week program. Interestingly, they also found decreases in dyskinesia. The greatest changes in exercise appeared early and were maintained up to 6 weeks after cessation of the exercise program. According to the literature, functional strength training seems to be more effective than weightlifting if the goal is improvement in ADLs.158 An important part of any strengthening program is the trunk musculature. Spinal extensors need to be exercised, and spinal flexibility likewise encouraged.262
Use of cues for improving gait.
As the disease progresses, intensive exercise programs may need to be revised or altered. By stage 2.5, gait disorders are the most common diagnosis for which the person with Parkinson disease will see a therapist. Many aspects of gait are amenable to treatment. The problems that cause the biggest ambulation limitation are freezing and small steps. Both auditory and visual stimuli have been used in treatment of parkinsonian gait disorders. Thaut and colleagues263 demonstrated that use of a metronome or carefully synthesized music improves stride length and speed and that these improvements remain up to 5 weeks after the cessation of the auditory stimulus.234 Melnick and colleagues234 also demonstrated both immediate and longer-lasting improvements in gait with a rhythmical exercise program once a week in patients needing assistance to walk. A study by Nieuwboer and colleagues256 used auditory, visual, or somatosensory cues in the patient’s home. The patient chose the cue that was best for him or her. The cues were provided in a variety of tasks including walking with dual tasks and walking sideways and backward. Cues were effective in decreasing freezing, but there was only a small effect when the cues were stopped. There may be a difference in the use of cueing for those who freeze during gait and those who do not. The use of cues may be more effective for those who do not have frequent freezing episodes.264
People with Parkinson disease find climbing stairs easier than walking on a flat surface because of the visual stimulation provided by the stairs. Visual stimuli have been effective in freezing episodes. These include the use of lines on the floor and stair climbing. Martin55 found that parallel lines were more facilitating than other lines and that the space between lines was also important; the lines cannot be too close together. The use of visual stimuli has scant evidence of carryover. One client used visual stimuli in special glasses that provided constant lines for the client to step over. At present these glasses are not commercially available. Dunne and colleagues265 described a cane that could present a visual cue for the patient who has freezing episodes. Canes can be especially useful for patients who fall because of freezing. If a specialized cane is not available, the client can turn his or her own cane upside down. Other visual cues have been used to help initiate movement after freezing. For example, one patient tosses pennies ahead of him and steps over them. (He cautions that one should not bend to pick them up as this will again lead to freezing.) Another watches the movement of a person walking beside him; the movement of that person’s feet encourages his feet to move. The U walker has a laser line that can be added to provide lines on the ground. Morris and colleagues147 have tried to increase carryover of visual stimuli by incorporating them with a program of visualization. Their clients practiced walking with lines until the steps were near normal in size; the clients were then to visualize the lines on the floor as they walked. Their visualization program met with initial success. Increasing the magnitude of the step or the amplitude of the movement appears to be the most important component for improvement in gait and a decrease in freezing.147 Tactile cueing has also been demonstrated to improve gait ability.266
Gait rehabilitation must include walking in crowds, through doorways, and on different surfaces. Practicing walking slowly and quickly is important, as is walking with differing stride lengths, because in the real world step length and speed must change with environmental demands. The principles of motor learning presented in Chapter 4 appear to be very helpful for facilitating carryover of the therapeutic effects in our preliminary studies. One word of caution, however; as previously mentioned, the person with Parkinson disease has difficulty performing two tasks at once, such as walking and performing math problems or walking with a glass of water on a tray.113,130,155 The patient may have to concentrate only on walking as the disease progresses, to increase patient safety.
Balance.
Another problem for which therapy is indicated is impaired balance, especially because drug and surgical treatments are ineffective in remediating this problem. This problem will eventually affect all persons with Parkinson disease.267 If at all possible, the client should be instructed to practice balance exercises at the early stages of the disease. Equilibrium reactions in all planes of movement and under different controls should be encouraged. Techniques to increase dynamic balance control should be included, especially turning the body and turning the head. All three balance strategies need to be addressed and then practiced in a variety of environmental conditions. The newer computerized games that target balance have provided a fun and therapeutic method for keeping interest in balance exercises.130,135,136,142,143 (See Chapter 22 for other procedures to improve balance.)
Dual task performance.
Rarely will the client with Parkinson disease state that he or she has difficulty performing two tasks at once. Nonetheless, this is quite apparent in very simple activities, such as requiring the patient to count backward and walk at the same time.146,268 One solution is to instruct the client to attend to only one task at a time. Another is to have the client practice doing two things at the same time and constantly alter activities in a random practice mode during treatment. The efficacy of these two approaches has yet to be studied.
Activities of daily living.
Transitional movements pose great problems for the client, especially by Hoehn and Yahr stage 3. This is most likely because normal postural adjustments are no longer automatic and they become a sequential task. Practice with frequent review is helpful. Some researchers report improvement in moving from a seated to a standing position after practicing techniques designed to increase forward weight shift (e.g., leaning on a chair while standing up).269,270 Visualization of this task has demonstrated carryover. If getting up from a chair becomes too difficult, chairs with seats that lift up have been used effectively.
In addition to treatment in the therapy department, the parkinsonian client also should be given a home program. The home program should encourage moderate, consistent exercise as part of the normal day. Periodic checks may enhance compliance. Fatigue should be avoided and the exercise graded to the individual’s capability. The therapist should keep in mind that learned skills such as various sports are sometimes less affected than automatic movements, perhaps because these skills may rely on cortical involvement.16
Fatigue is a frequent complaint of people with Parkinson disease. Although it has been correlated with disease progression, depression, and sleep disturbances, it also exists in up to 44% of those without depression or sleep difficulty.271 This type of fatigue is over and above what is associated with the exertion of an exercise program and may be one reason people with Parkinson disease no longer exercise. The client with Parkinson disease frequently experiences postexercise fatigue. If a person is so tired after exercise that he or she cannot perform normal ADLs, exercise will not become a part of the client’s daily routine. Postexercise fatigue is easily alleviated by a gradual and extended cool-down period.
Patients frequently ask about the timing of medication and exercise. For any form of exercise in parkinsonism to be effective, movement must be possible, especially movement through the full arc of the joint. It seems plausible, therefore, that exercise should be performed during the “on” period of the medication cycle. On the other hand, perhaps a more long-lasting effect would result if the patient with Parkinson disease tried to exercise without medication. The question of the effects of exercise on DA agonist absorption was investigated by Carter and colleagues.272 These authors concluded that the effect was variable from patient to patient, but the response of each patient was consistent. However, none of the patients exercised vigorously, which may have skewed the results. Reuter and colleagues273 interpreted a decrease in dyskinesia seen after an exercise program as indicative of more efficient DA absorption. Nevertheless, this study supports the concept that the patient needs to be “in tune” with his or her own response and adjust medications and exercise to a schedule accordingly. The therapist is also involved in the prescription of assistive devices. The use of ambulatory aids for patients with Parkinson disease is an area with no clear-cut guidelines. Because coordination of upper and lower extremities is often difficult, the ability to use a cane or walker is often limited. The patient may drag the cane or carry the walker. Walkers with wheels sometimes increase the festinating gait, and the patient may simply fall over the walker. Nonetheless, four-wheel walkers with pushdown brakes appear to work best for many clients. A walker that is in the brake condition at the start and requires the patient to push on handles to walk may also be safer. For patients with a tendency to fall backward, an assistive device may simply be something to carry backward with them. Therefore the reason for using the assistive device must be carefully assessed. Walkers, walking sticks, or canes can be helpful for the person who is able to walk with a heel-toe gait pattern but lacks postural stability. The height of walker or cane should be adjusted carefully to promote trunk extension and avoid an increase in trunk flexion. A walking stick, or the use of two sticks as in hiking, is less likely to promote flexion than a cane. A survey by Mutch and colleagues250 in Ireland found that nearly half of the patients responding used some type of assistive device. These devices included devices for walking, reaching, rising from bed, and performing ADLs. Patients with Parkinson disease may also benefit from assistive devices for eating or writing.
As Parkinson disease progresses the patient may experience difficulty in swallowing and even chewing. Therapy for oral-motor control should be initiated, and a dietician consultation may be necessary to ensure adequate nutrition. A dietician may also be beneficial in guiding the patient’s protein intake. A diet high in protein may reduce the responsiveness of the patient to DA replacement therapy.146 Regulating the amount and timing of protein ingestion can improve the efficacy of drug treatment in some patients. Use of vitamins is a subject that appears on many websites for patients with Parkinson disease, and the patient should be reminded to consult with his or her physician when changing vitamins.
The resurgence of surgery as a treatment alternative in Parkinson disease, including stimulation of deep brain sites that may alter neuroplasticity, means that the therapist will face new and exciting challenges in treatment.274 Intense physical therapy, especially incorporating complex motor skills, has been demonstrated to be effective in improving function after a subthalamic nucleus lesion in animal studies.275 Therefore, intense physical therapy after surgery may be necessary to maximize benefits from all surgeries in Parkinson disease (as well as in Huntington disease and the dystonias).
Finally, therapeutic rehabilitation and exercise may modify but cannot halt or reverse the progression of this degenerative disease. The therapist can assist the client and family in coping with the constraints of this disease, enhancing the patient’s quality of life throughout its course. As stated in one study of Parkinson disease, the total cost of treatment must also include the cost to the spouse or other family members.2
Huntington disease
Huntington disease (formerly Huntington’s chorea) is another degenerative disease of the basal ganglia.276 It is the classic disorder representing hyperactivity in the basal ganglia circuitry.277 This disease gets its name from the family of physicians who described its patterns of inheritance. Huntington disease is inherited as an autosomal dominant trait and affects approximately 6.5 per 100,000 people.3 The defect is on the short arm of chromosome 4.278 The defect alters DNA so that there is an increase in the cytosine-adenine-guanine (CAG) sequence; in normal individuals there are 10 to 28 CAG triple repeats, but in the individual with Huntington disease there are 36 to 120 repeats.64 The longer the length of the CAG triple repeats, the earlier the onset of the disease. The CAG repeat is related to glutamine. The target protein affected by the polyglutamine expansion has been named huntingtin. Huntingtin combines with ubiquitin and induces intranuclear inclusions and interference with mitochondrial function. The defect is characterized by severe loss of the medium spiny neurons and preservation of the ACh aspiny neurons. There are decreases in choline acetyltransferase (CAT), ACh, the number of muscarinic ACh receptors, glutamic acid decarboxylase, and substance P. There is generally no decrease in DA, norepinephrine, or serotonin (5HT), although more recent studies with single-photon emission computed tomography (SPECT) indicate that DA does diminish significantly in the later stages of the disease.279
A marker for the Huntington gene has been detected.278 If the family pedigree is known and the chromosomes of the parents can be obtained, detection of which offspring have the faulty chromosome is possible presymptomatically. Of course, early detection of this disease involves ethical and practical issues. At present, although testing is available, it is not widely used. Furthermore, testing for Huntington disease is typically available only to those older than 18 years. Despite these problems, localization of the gene and the repeat is promising and offers hope for improved means of treatment.
Huntington disease affects neurons in the basal ganglia as well as the frontal cortex. The movement disorders are presumed to be related to degeneration of the striatal neurons, specifically the enkephalinergic neurons.16 The cognitive and emotional symptoms are associated with cortical destruction.
Symptoms
Some of the signs and symptoms of Huntington disease are similar to those of Parkinson disease: abnormalities in postural reactions, trunk rotation, distribution of tone, and extraneous movements. Individuals with Huntington disease, however, are at the other end of the spectrum; rather than a paucity of movement, they exhibit too much movement, which is evident in the trunk and face in addition to the extremities. The gait takes on an ataxic, dancing appearance (in fact, chorea means to dance in Greek), and fine movements become clumsy and slowed.280 As with the person with parkinsonism, there is a decrease in associated movements (e.g., arm swing). The extraneous movements are of the choreoathetoid type, that is, involuntary, irregular isolated movements that may be jerky and arrhythmical as in chorea, to rhythmical and wormlike as in athetosis. Usually, however, these occur in successive movements so that the entire picture is one of complex movement patterns. The “movement generator” aspects of the basal ganglia seem to be continuously active, as would fit the hypothesis of a disruption in the indirect pathway. As the disease progresses, the choreiform movements may give way to akinesia and rigidity.
Gait patterns of the person with Huntington disease are in some ways similar to those of Parkinson disease. Gait velocity and stride length are decreased. The decrease in velocity is correlated with disease progression. Unlike the person with Parkinson disease, however, the person with Huntington disease has a decreased cadence as well.281 The base of support is increased (again unlike the pattern seen in Parkinson disease). In addition, lateral sway is increased along with great variability in distal movements.
Disruptions in movement for the person with Huntington disease reflect the role of the basal ganglia in movement. For example, the person with Huntington disease, like the person with Parkinson disease, has difficulty responding to internal cues; he or she also has difficulty with internal rhythms. Kinematic analysis of upper-extremity complex tasks demonstrates that the person with Huntington disease must rely on visual guidance in the termination of a movement. This has been interpreted to indicate impairment in the development and fine-tuning of an internal representation of the task.282 These clients have increasing difficulty with more complex movements in the absence of advanced cues.283,284 The lack of internal cuing in the person with Huntington disease has been linked to the increased variability of response seen in these clients.285
The face is also affected in Huntington disease. Speech, breathing, and swallowing lack normal control and coordination. Speech lacks rhythm, as might be expected with decreased internal timing, and is often soft. Swallowing and therefore eating may also be difficult, are common problems, and often are accompanied by weight loss. In fact, some suggest that a person with decreased body weight and a parental history of the disease is at greater risk.286,287 Impaired voluntary eye movement is often the first sign of Huntington disease. The person with Huntington disease has difficulty with initiation and control of saccadic eye movements.
The exact mechanisms for the production of choreoathetoid movements are unknown. Because these extraneous movements are part of a person’s normal repertoire of movement patterns, they may be “released” at inappropriate times and without any modulation. A postmortem examination showed a decrease in GABA that was greater in the globus pallidus external segment than the internal segment. This agrees with the previously described current model.6 Recent use of positron emission tomography (PET) scans demonstrates loss of ACh and GABA neurons.288 A pattern therefore may be executed before it is necessary, and inappropriate portions of a movement pattern cannot be inhibited. Petajan289 found motor unit activity indicative of bradykinesia. Recordings of single motor units in the muscles indicates that persons with Huntington disease have a loss of control evidenced by an inability to recruit single motor units.289 As the efforts at control increased, these individuals demonstrated an overflow of motor unit activity that resulted in full choreiform movements. Those in the earlier stages of the disease demonstrated what the experimenters termed “microchorea,” or small ballistic activations of motor units.289 As in Parkinson disease, difficulty occurs in modulating motor neuron excitability. Another finding in this experiment revealed motor unit activity indicative of bradykinesia. Yanagasawa290 used surface EMG recordings to classify involuntary muscle contractions in Huntington disease patients with varying movement disorders from chorea to rigidity. He found brief, reciprocal, irregular contractions in those patients with classic chorea, and tonic nonreciprocal contractions in those patients with rigidity. Presence of athetosis or dystonia was associated with slow, reciprocal contractions. During sustained contractions, EMG activity demonstrated brief, irregular cessation of activity in the choreic patients. Thus patients with Huntington disease have interruption of normal motor function at rest and during sustained activity (e.g., stabilizing contractions).
In addition to the involvement of the motor systems, the individual with Huntington disease also shows signs of dementia and emotional disorders that become worse as the disease progresses. Neuropsychological tests are therefore part of the Unified Huntington’s Disease Rating Scale (UHDRS). The client may show lack of judgment and loss of memory, deterioration in speech and writing (i.e., severe decrease in ability to communicate), depression, hostility, and feelings of incompetence. IQ decreases, with performance measures decreasing more rapidly than verbal levels. Evidence of ideomotor apraxia is also present, especially as the disease progresses.291 Suicide is fairly common.
Stages of huntington disease
In many cases the dementia and psychological symptoms of Huntington disease occur after the onset of the neurological signs. In cases in which very subtle personality changes occur first, the diagnosis may be more difficult. Such persons may appear forgetful or unable to manage appointments and financial affairs. They may be thought to have early senility, or they may show signs of severe depression or schizophrenia. Early diagnosis may be important, and SPECT is showing promise for early detection of the disease.292
With time, the combination of the psychological and neurological problems causes the individual to lose all ability to work and perform ADLs. This person eventually can be cared for only in an extended care facility. By this time the choreiform movements have given way to rigidity, and the patient is bedridden. Death is usually caused by infection, but suicide is also common. Figure 20-12 shows the stages of Huntington disease according to Shoulson and Fahn.293
Pharmacological considerations and medical management
Advances in the pharmacological management of Parkinson disease have led to a great deal of research in an effort to find appropriate drugs for the management of Huntington disease.294–299 At present, however, no fully effective medication is available for this disease. Each symptom is treated with its own medication.
The symptoms of Huntington disease indicate an increase in dopaminergic effect. At autopsy a decreased number of intrinsic neurons of the striatum that contain the neurotransmitter GABA or ACh are found. Biochemical studies reveal a definite decrease in GABA concentration in addition to a decrease in ACh concentration in the basal ganglia. Therefore drug therapy depends on drugs that are cholinergic or GABA-containing agonists and those that act as DA antagonists. To date, the DA antagonists have been more effective in ameliorating neurological symptoms; however, these drugs have severe side effects including parkinsonism—for example, bradykinesia and rigidity—and tardive dyskinesia.294 There is no evidence that improvement of the choreiform movements leads to improved function.
In general, pharmacological treatment is not started until the choreiform movements interfere with function because these drugs have side effects that may be worse than the chorea.300 Perphenazine, haloperidol (Haldol), and reserpine are still the most commonly used medications. The first two block the DA receptors themselves; reserpine depletes DA stores in the brain. Side effects include depression, drowsiness, a parkinsonian type of syndrome, and sometimes dyskinesia. Drugs such as choline, which would increase ACh concentrations, have produced only transient improvement.188 Many efforts have been undertaken to find a GABA agonist that would reduce the symptoms of Huntington disease, but these have been unsuccessful so far.300,301 The problem with finding a medication to increase GABA is that such a drug will probably cause inhibition throughout the brain, not just in the basal ganglia. Thus the individual’s level of alertness and ability to function might be reduced—something the person with Huntington disease can ill afford.301 Riluzole, a drug that blocks glutaminergic neurotransmission, has been tried with initial success.302 A 2009 Cochrane review concluded that no pharmacological intervention demonstrated disease-modifying or disease-progression effects.303
The dementia and personality problems interfere more with life tasks than do the presence of movement disorders. Medications are usually prescribed as combinations of drugs to treat the specific emotional and psychological symptoms. Cortical degeneration is most certainly involved, but disruption of the heavy corticostriate projections also may be a factor in the progression of this disease. Although alterations in DA have been implicated in psychotic problems such as schizophrenia, the role of the basal ganglia in thought processes is, at best, little understood. In the words of Woody Guthrie, “There’s just not no hope. Nor not no treatment known to cure me of my dizzy called Chorea.”304
At present the best hope for the person with Huntington disease lies in a better understanding of the genetic mechanisms causing destruction of the GABA-containing cells in the striatum and cortical destruction. In the meantime, correct and early diagnosis is important in providing the proper early intervention, which must include counseling.305 In an effort to facilitate research into the causes as well as the treatment of the disease, the Commission for the Control of Huntington’s Disease has set up several research centers, including a brain and tissue bank. Research has also begun on the use of tissue transplantation. As with Parkinson disease, the tissue does survive, but the results are even more preliminary than for parkinsonism.
Examination of the client with huntington disease
The standard medical evaluation is the UHDRS.306 This comprehensive evaluation examines cognitive function as well as motor function. The physical or occupational therapy evaluation of a person with Huntington disease must include an assessment of the degree of functional ability and how the chorea interferes with function. Which extremities, including the face, are involved? Does the client have any cortical control of the chorea or any means (i.e., tricks) of allaying these extraneous movements? What exacerbates the symptoms? What lessens them? A simple rating scale is the capacity to perform ADLs (see Figure 20-12). A standardized ADL assessment with space to write in how the client performs these activities or why she or he cannot perform them would be helpful.
Treatment procedures
The Commission for the Control of Huntington’s Disease307 stated that these individuals are underserved by physical and occupational therapy. Peacock308 surveyed physical therapists in one state. Of the 585 therapists who responded, only 15.5% had worked with at least one patient with Huntington disease, and 6.2% had worked with more than one patient; this confirmed the underutilization of physical and occupational therapy today. Hayden304 and Peacock308 suggest that therapy can improve quality of life for this population. A 2008 article by Busse and colleagues309 demonstrated that there is still underutilization of therapy services. They also found that there no routine outcome measurements for the stages of the disease, and they suggested that management of falls and decreased mobility dysfunction could be a treatment goal of physical therapy interventions. Although animal models of Huntington disease exist, there have been few studies investigating possible movement interventions. In one animal study even a little environmental enrichment improved the ability of Huntington mice on a rotarod test and slowed the progression of the disease.310 The mice getting even more enrichment showed improvement on more behavioral tests as well as changes in the striatum. Recently a few articles have been published examining physical and occupational therapy treatment techniques for holistic therapy and for treating specific problems.311–313 See Chapter 9 for other specific therapeutic interventions.
A study by Zinzi and colleagues311 was a nonrandom pilot study that incorporated gait, balance, and transfer training. Strengthening of the extremities, trunk, and muscles of respiration as well as coordination and postural stability activities were included. The program was undertaken with occupational therapy to include cognitive, rehabilitation, and ADL training. Participants were engaged in the intensive inpatient program for 3 weeks for 8 hours per day, 5 days a week, 3 times a year. The data indicate that there was significant improvement in motor function and in ADL performance and that these subjects did not show deterioration over the 3 years of the study—a positive outcome for a degenerative disease.
Treatment of the person with Huntington disease has some parallels with the treatment of cerebral palsy athetosis. These techniques, however, must be adapted to the adult. Of critical importance are the techniques for improving coactivation and trunk stability. The use of the pivot-prone and withdrawal patterns of Rood are helpful, and their benefit may be increased with the use of Thera-Band. Neck co-contraction and trunk stability may improve, or at least oral functions may be maintained. In addition, the techniques of rhythmical stabilization in all positions as well as heavy work patterns of Rood should be helpful.314 Yet movements practiced out of context may not carry over into functional activities; thus practicing coactivation in functional patterns during treatment if at all possible is recommended. Whereas in Parkinson disease the emphasis is on large-amplitude movements, movements for the person with Huntington disease need to be of smaller amplitude and controlled.
The gait disorder of Huntington disease has been shown to respond to rhythmical auditory stimuli in one study.315 The ability to respond decreases in those most severely involved, indicating that treatment in the later stages of the disease may not be amenable to rhythmical stimuli. Another finding of this study was that cadence was a larger problem than stride length, especially at normal and fast speeds (compare this with the findings in Parkinson disease). Interestingly, people with Huntington disease were able to modulate gait to a metronome but had more difficulty with musical cues even when the tempos were identical. Subjects with Huntington disease demonstrated short-term carryover of metronome auditory stimuli to gait without auditory stimuli. Although the long-term carryover was not studied, using a metronome in gait training may be helpful in clients with Huntington disease. A more recent study using a metronome to cue gait during single and dual task gait activities found that participants with Huntington disease had difficulty synchronizing steps to a metronome in all conditions.313
The degree of dementia influences treatment options. Conscious efforts to control extraneous movements will be more difficult as cognitive function decreases. New memories and new patterns of movements are more difficult to establish. The therapist therefore must use techniques that require subcortical control and must keep in mind that the client can sometimes remember old, normal patterns of movement. An encouraging treatment method may be the use of imagery. Yágüez and colleagues312 found that patients with Huntington disease could use imagery to compensate for impairments in a graphomotor design task.
Peacock’s study308 suggests that group programs including strength, flexibility, balance, coordination, and breathing exercises may be very successful, especially in the early stages of the disease. This was confirmed in the study by Zinzi.311 No amount of physical or occupational therapy, however, can prevent neuronal cell loss. Because Huntington disease is a progressive, degenerative disease, the client’s condition will get worse, although the Zinzi study suggested that the progression might be slowed with integrated therapy.311 Eventually, goals must be aimed at preventing total immobility and assisting caretakers in transfer techniques and advising them in the use of adaptive equipment. One aspect of treatment that cannot be measured but is important in my view is the degree of hope offered just by the fact that a health professional is providing ongoing care. This may lessen the client’s degree of despair and depression and may help maintain quality of life.
Wilson disease
Wilson disease is characterized by an increase in the amount of copper absorbed from the intestinal tract, a subsequent elevation in the amount of copper in the blood serum, and an increase in the amount of copper deposited in tissue.316 Ceruloplasmin is concomitantly reduced. The increase in tissue copper may interfere with various enzyme systems of particular cells. The connection of copper with DA metabolism may account for the basal ganglia involvement.
Symptoms
If the onset of the disease occurs before age 20, the appearance of choreoathetoid movements of the face and upper extremities is usually present. The gait resembles that of the individual with Huntington disease. This early-onset form is accompanied by a rapid deterioration.317
The postures and movement patterns seen in people with Wilson disease include dystonic movements involving twisting and rotation of limbs, with sustained contraction at the end of the movement.16 As Wilson disease progresses, the classic abnormal posture of increased flexion occurs, along with rigidity that can progress to the inability to move if severe enough. Dystonia, like bradykinesia and choreoathetosis, belongs on a continuum of the extraneous movements present with basal ganglia involvement. Dysfunction of the cerebellum and intralaminar nuclei of the thalamus may also contribute to these impairments of posture and movement.318 A peculiar aspect of dystonia is that it can be decreased with proprioceptive or tactile inputs.16 As with other diseases of the basal ganglia, an imbalance or abnormal response in the neurotransmitters occurs in Wilson disease; however, the precise imbalance is not yet known.
Tardive dyskinesia
Tardive dyskinesia is usually a drug-induced disorder and thus will be used to indicate the problems that can arise from drug intoxication. In particular this section concentrates on the problems associated with drugs that affect DA metabolism and/or reuptake, including amphetamine, methamphetamine, haloperidol, and classes of drugs used in treatment of psychotic disorders: the phenothiazines, butyrophenones, and thioxanthenes. As the use and misuse of drugs becomes more common, these types of disorders may become more frequent. (Refer to Chapter 36 for additional information.)
Symptoms
Dyskinesia is defined as an inability to perform voluntary movement.305 In practical terms, however, dyskinesia is usually a series of rhythmical extraneous movements. In tardive dyskinesia this typically begins with, or may be confined to, the region of the face. These extraneous movements may include choreoathetoid or dystonic movements. Because of abnormality in basal ganglia function, abnormalities in postural tone and postural adjustments are also present. Instead of the typical flexed posture of Parkinson disease, clients with tardive dyskinesia show extension of the trunk with increased lordosis and neck flexion.319 This description of the disease is rather broad, but the problems of drug-induced movement disorders are varied. They may take the form of drug-induced Parkinson disease or dystonia. In tardive dyskinesia, akinesia and rigidity similar to that seen in parkinsonism may exist simultaneously with the choreoathetoid-like movements. The key factor in tardive dyskinesia is its slow onset after the ingestion of neuroleptic medications.
Etiology
Although many people take neuroleptic medication, only a small percentage acquires tardive dyskinesia. Many factors may predispose an individual to movement disorders. One of these is age.320 This might be expected because of the influence of aging processes on the concentration of DA. Gender may also be a factor. Women, and older women in particular, are more at risk for tardive dyskinesia, perhaps because of decreased estrogen.319 The absolute amount of neuroleptic ingested may also be a factor, but to date definitive studies have not been completed. So far the length of time the individual takes medication does not appear to be a strong predisposing factor. As the biological abnormalities of schizophrenia become better understood, further understanding of the causes of tardive dyskinesia also may be elucidated. The development of tardive dyskinesia is hypothesized to be caused by supersensitivity.305,321 With the use of drugs that deplete the brain of DA, the brain becomes more sensitive to it. And, in fact, in humans the withdrawal of neuroleptics tends to heighten the disease; essentially, withdrawal of the DA antagonist means that far more DA is able to act on these already sensitive terminals.305,321,322
Because of the effectiveness of long-term treatment for schizophrenia provided by neuroleptics, research into the underlying cause and therefore treatment of the major side effect, the motor disorders, has greatly increased.323 But as with Parkinson disease and Huntington disease, animal models are difficult to produce. However, experimental evidence indicates that the basal ganglia are involved in movements about the face, especially the mouth, and buccolingual dyskinesia is the most frequently encountered symptom in tardive dyskinesia.69,70 The response of basal ganglia neurons to sensory input shows increasing localization of response with age; the region about the mouth becomes increasingly sensitive.70 Further research along these lines, both in normal animals and in those with lesions, may answer the question of what is happening at a neuronal level. This would facilitate pharmacological and therapeutic interventions.
Pharmacological and medical management
The use of other drugs in conjunction with the neuroleptics has been tried in various animal models of the disease. As might be expected, anticholinergic drugs (which would worsen an imbalance between DA and ACh) worsen the dyskinesia. Lithium has been successful in one animal model of dyskinesia.305 Some neuroleptic drugs seem to have less effect on movement than others; however, the side effects of one such drug, chlorpromazine, are life-threatening. Reducing the buildup of phenylalanine is also indicated as a way to decrease occurrence of tardive dyskinesia. A medical food comprising branched-chain amino acids seems to reduce concentration of phenylalanine and was effective in reducing the movement disorder in one clinical trial. More research is needed into both the mechanisms of schizophrenia and the mechanisms for the production of the abnormal movements.
Evaluation and treatment interventions for dyskinesia
The effectiveness of rehabilitation therapy intervention in drug-induced dyskinesia is, as yet, not completely known. However, because the neuroleptics do provide an effective long-term treatment of schizophrenia, and because amphetamines and methamphetamine are being abused, therapists need to become aware of the problem and offer some assistance. Early drug holidays (time without use of drugs) may be of value in treatment of tardive dyskinesia, and therefore early awareness of incipient changes in motor function may be of value. Assessment of patients receiving drug therapy could perhaps begin before treatment and then at prescribed intervals. The knowledge that postural adjustments are abnormal in most basal ganglia diseases means that analysis of posture statically and in motion might provide early clues of development of movement disorders. The same would be true for balance reactions and changes in tone with changes in position. Once movement disorders appear, an assessment of when and where the extraneous movements occur is important. (See Chapter 8 for general examination tools and Chapter 22 specifically for tests of balance.)
Other considerations
Other drugs besides neuroleptics may also produce movement disorders. Amphetamine, for example, has been shown to cause long-term changes in brain function even with very small doses.324–326 Adults who were hyperactive as children sometimes show a decrease in the readiness potential.327 Further longitudinal research and research using PET scans and functional magnetic resonance imaging (fMRI) are underway to determine the role that medications used in treating hyperactive children, such as methylphenidate (Ritalin), might play in changing the architecture of the basal ganglia and causing movement disorders.84 The problem of drug-induced movement disorders may become an ever-increasing one for the therapist.
In 1982 several young people were treated for rigidity and “catatonia” after the use of what they thought was heroin. Careful examination of these patients revealed that they had parkinsonian-like symptoms.38,105 The chemical responsible for the symptomatology was MPTP, a meperidine analog that was an impurity in the designer heroin. This discovery has enabled research in animals and clinical studies in humans and may enable better understanding of the pathogenesis and, in turn, of the treatment of the disease. One hypothesized cause of Parkinson disease implicated environmental toxins (because some herbicides such as paraquat resemble the chemical structure of MPTP) and the involvement of superoxide free radicals.92,328,329 More complete epidemiological studies are now underway to investigate Parkinson disease to determine the relationship to specific herbicides and pesticides.
Methamphetamine use also induces movement disorders. The MRI of even infrequent users shows damage to the basal ganglia.330,331 A child born to a mother using methamphetamine may also have movement disorders and delayed achievement of developmental milestones. Another recreational drug, cocaine has long been known to produce parkinsonian movement disorders.332
Dystonia
General information
Generalized dystonia
Symptoms.
The person with generalized dystonia will begin a movement (such as walking) and then will experience a torsional contraction of the trunk; of the upper extremity, especially at the shoulder; and in the ankle, foot, and toes. These contractions may be so strong that further movement is impossible. Many patients experience pain as the muscles remain contracted for long periods of time.333
Evaluation and treatment intervention.
Movement therapy interventions are only now being developed for generalized dystonia. Overall, treatment similar to that in Huntington disease that emphasizes treating the symptoms may be beneficial. One successful program uses sensory integration and relearning techniques performed with attention.335 Practice is a crucial element of treatment, and the client must be willing to practice the sensory tasks many, many times throughout the day for benefit.
Focal dystonias
Spasmodic torticollis is the most common focal dystonia. The person with this disorder will have involuntary contractions of neck muscles that result in head turning and head extension and flexion movements that are often sustained for long periods of time. Other common sites of focal involvement are the vocal cords; the tongue and swallowing muscles; the facial muscles, especially about the eye; the hand; and the toes. Writer’s cramp is a task-specific dystonia, unlike other focal dystonias. An interesting phenomenon of dystonia is the fact that many patients will develop a sensory or motor “trick” that will decrease the severity of the muscle contraction(s) and may even stop these movements.333,336
Symptoms.
The signs and symptoms of focal hand dystonia are variable. The problem may initially manifest as an abnormality in the quality of sound produced by a musical instrument (e.g., a deterioration of vibration in a violinist),337 increasing errors in task performance, unusual fatigue or sense of weakness, or involuntary or excessive movement of a single digit or multiple digits. Initially the symptoms are subtle and virtually indistinguishable from the normal variations that may be seen in the execution experienced by all musicians studying technically demanding music or software engineers who spend excessive hours at the computer. Frequently, a person engaged in a profession with high repetition of tasks who has minimal pain but vague motor control problems or somatosensory dysfunction is manifesting early signs of focal dystonia.338 Although a co-contraction of flexors and extensors can be observed while an individual with hand dystonia performs the target task, at rest and during the performance of nontarget tasks the hand appears to function normally. Some patients demonstrate a variety of subtle abnormalities such as a reduced arm swing; loss of smooth, controlled grasping; a physiological tremor; hypermobility of the interphalangeal joints; decreased ROM in some upper limb joints (e.g., shoulder abduction, external rotation, finger abduction, forearm pronation); neurovascular entrapment; compression neuropathy; or poor posture.339–344
Etiology.
The cause of focal dystonias is unknown and multifactorial. Frucht344 observed that task-specific hand dystonia seemed to begin after motor skills had been acquired rather than during skill acquisition. Thus, focal hand dystonia in a musician is probably not a disorder of motor learning but a disruption of acquired, complex, motor programs. The data also suggested that peripheral environmental influences seem to play an important role in molding the dystonic phenotype. For example, the hand performing the more complex musical tasks (e.g., right hand in pianists and guitarists, left hand in violinists), seemed to be more predisposed to the development of dystonia. In addition, the dystonia usually began in one finger and spread to adjacent fingers, rarely skipping a finger. Furthermore, the ulnar side of the hand (fingers 4 and 5) was disproportionately affected, potentially because of the challenging ergonomics and technical stresses of the musical instrument required for this part of the hand in terms of gripping and activation of individual finger movements.345
Pharmacological and medical management.
The most common medical treatment for the focal dystonias is botulinum toxin. This toxin binds with the ACh receptors on the muscle and prevents the muscle from contracting. The injections are made under electromyographic guidance so that only those motor units involved in the production of the extraneous movements are paralyzed. However, the treatment does not cause permanent change, so the patient must repeat these injections every 3 to 4 months. Some people develop antibodies to the toxin, rendering it then ineffective.346,347 Therefore, medical management prevents the abnormal movement but is not a cure.
Evaluation and treatment.
Overall, treatment of focal dystonia will depend on the joint or joint involved. The duration of the dystonia, the trigger, and the person’s trick, if any, to relieve the dystonia must be noted. Tricks are sensory in nature and help relieve the pain often associated with the extreme movement. The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) is one evaluation for the person with spasmodic torticollis.348
Several ADLs should be examined. For example, in hand dystonia, the person should be evaluated using the instrument producing the dystonia (i.e., the pen in writer’s cramp) as well as other tools (e.g., a fork). In addition, there seems to be position dependence, so writing while prone may not evoke the dystonia despite severe inability to hold the pen at a desk.349
In addition to the full extent of the motoric abnormality, sensation, especially higher-level sensations such as precise localization of touch, graphesthesia, and kinesthesia must be assessed. Byl and colleagues found changes in the sensory cortex after development of focal hand dystonia.335,349–352 Recent evidence suggests that balance, particularly dynamic balance, should also be assessed in patients with torticollis.353 These balance difficulties have not been relieved with botulinum toxin.
Movement therapy interventions are now being developed. One successful program uses sensory integration and relearning techniques performed with attention.354 Practice is a crucial element of treatment and the client must be willing to practice the sensory tasks many times throughout the day for benefit. The client practices cognitively demanding sensory discrimination tasks throughout the day and tries to use only tension-free movements.349
Treatment for the person with torticollis must include a relearning of midline before the person can begin to practice normal movement away from midline. The client may find this relearning process easier after botulinum injection. See Chapter 9 for further treatment interventions appropriate for patients with focal dystonias.
Other considerations.
As with other extraneous movements associated with basal ganglia disorders, relaxation can reduce the muscle contraction. However, the time to incorporate relaxation is before the full-blown development of the muscle contraction—a difficult task. This task requires a shift in paradigm to a health and wellness model and prevention (see Chapter 2). Therefore clients should practice relaxation on a regular basis. A psychological aspect to the focal dystonias frequently necessitates intervention from a psychiatrist or psychologist.