CHAPTER 22 Basal ganglia
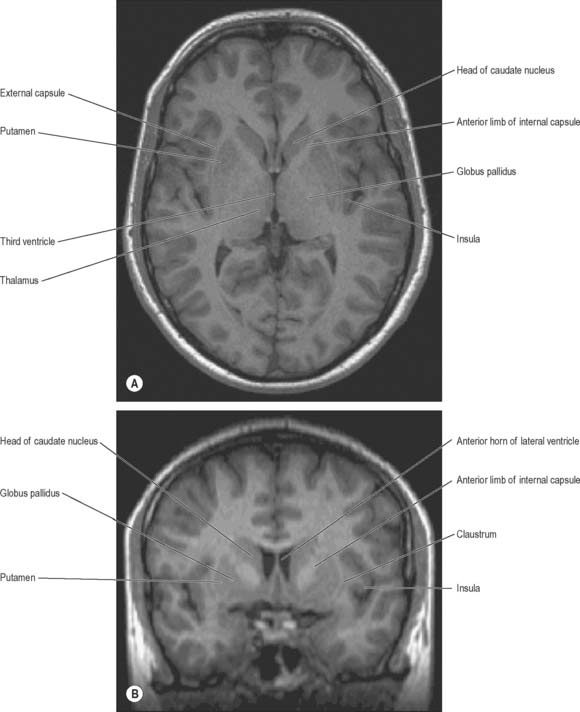
The term basal ganglia is used to denote a number of subcortical nuclear masses that lie in the inferior part of the cerebral hemisphere, in close relation with the internal capsule (Figs 22.1, 22.2; see Fig. 23.28). The traditional definition of the basal ganglia included the corpus striatum, claustrum, and amygdaloid complex. The term has now been restricted to the corpus striatum and its associated structures in the diencephalon and midbrain; collectively they form a functional complex involved in the control of movement and motivational aspects of behaviour. The function of the claustrum is unknown; the amygdala is more closely related to the limbic system and is therefore described in that context (see Ch. 23).
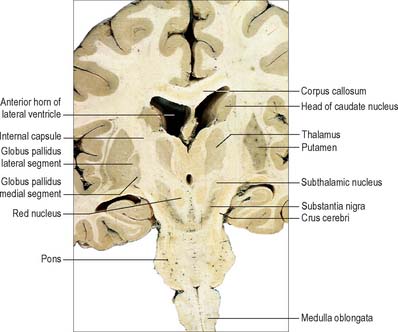
Fig. 22.2 Oblique coronal section of the brain.
(Dissection by EL Rees; photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
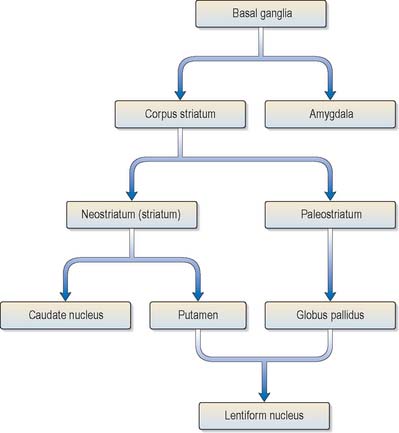
The corpus striatum consists of the caudate nucleus, putamen and globus pallidus (Fig. 22.3). Because of their close proximity, the putamen and globus pallidus were once considered as an entity, termed the lentiform (lenticular) complex or nucleus. However, although the name has been retained in gross anatomical terminology and in some compound names (e.g. sublenticular, retrolenticular), it is now known that the putamen and caudate nucleus share a common chemocytoarchitecture and connections, and they are referred to jointly as the neostriatum or simply the striatum.
Disorders of the basal ganglia are principally characterized by abnormalities of movement, muscle tone and posture. There is a wide spectrum of clinical presentations ranging from poverty of movement and hypertonia at one extreme (typified by Parkinson’s disease) to abnormal involuntary movements (dyskinesias) at the other. The underlying pathophysiological mechanisms that mediate these disorders have been much studied in recent years and are better understood than for any other type of complex neurological dysfunction. This has led to the introduction of new rational therapeutic strategies for both medical and neurosurgical treatment of movement disorders.
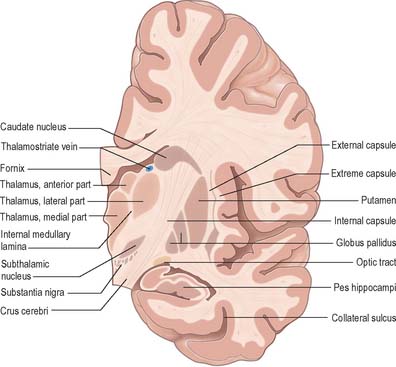
The caudate nucleus is a curved, tadpole-shaped mass. It has a large anterior head, which tapers to a body, and a down-curving tail (Fig. 22.4). The head is covered with ependyma and lies in the floor and lateral wall of the anterior horn of the lateral ventricle, in front of the interventricular foramen. The tapering body is in the floor of the body of the ventricle, and the narrow tail follows the curve of the inferior horn, and so lies in the ventricular roof, in the temporal lobe. Medially, the greater part of the caudate nucleus abuts the thalamus, along a junction that is marked by a groove, the sulcus terminalis. The sulcus contains the stria terminalis, lying deep to the ependyma (Fig. 22.5). The sulcus terminalis is especially prominent anterosuperiorly (because of the large size of the head and body of the caudate nucleus relative to the tail), and here the stria terminalis is accompanied by the thalamostriate vein.
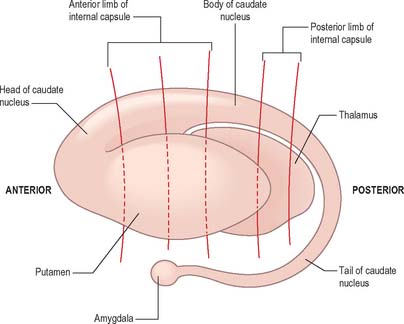
Fig. 22.4 The striatum within the left cerebral hemisphere.
(By permission from Crossman AR, Neary D 2000 Neuroanatomy, 3rd edn. Edinburgh: Churchill Livingstone.)
The corpus callosum lies above the head and body of the caudate nucleus. The two are separated laterally by the fronto-occipital fasciculus, and medially by the subcallosal fasciculus which caps the nucleus (Figs 22.5, 22.6). The caudate nucleus is largely separated from the lentiform complex by the anterior limb of the internal capsule (Figs 22.1, 22.6 and 22.7). However, the inferior part of the head of the caudate becomes continuous with the most inferior part of the putamen immediately above the anterior perforated substance; this junctional region is sometimes known as the fundus striati (Fig. 22.6). Variable bridges of cells connect the putamen to the caudate nucleus for most of its length. They are most prominent anteriorly, in the region of the fundus striati and the head and body of the caudate nucleus, where they break up the anterior limb of the internal capsule (Fig. 22.7). In the temporal lobe, the anterior part of the tail of the caudate nucleus becomes continuous with the posteroinferior part of the putamen. The vast bulk of the caudate nucleus and putamen are often referred to as the dorsal striatum. A smaller inferomedial part of the rostral striatum is referred to as the ventral striatum, and includes the nucleus accumbens.
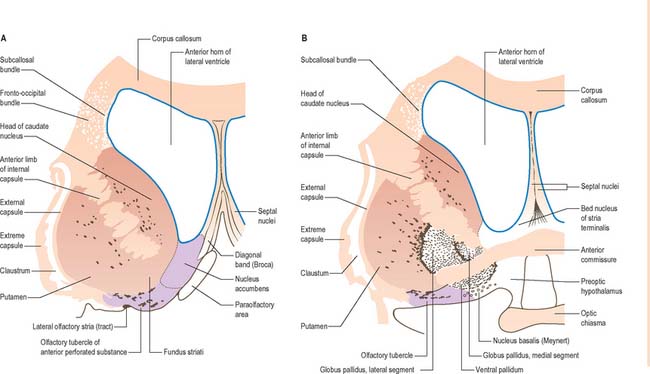
Fig. 22.6 Coronal sections through the corpus striatum and anterior perforated substance. A is anterior to B.
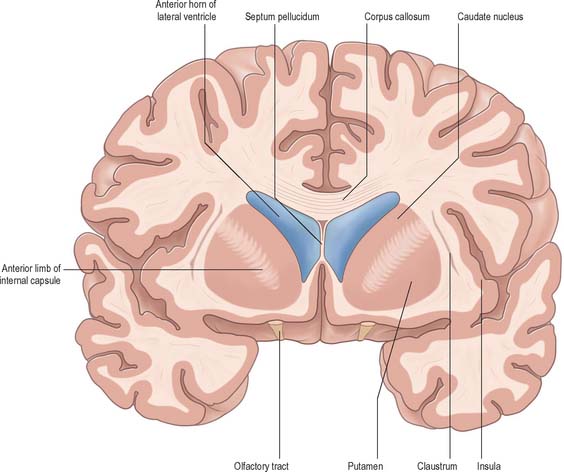
Fig. 22.7 Posterior aspect of a coronal section through the anterior horn of the lateral ventricles.
The lentiform complex (Figs 22.1, 22.2, 22.8) lies deep to the insular cortex, with which it is roughly coextensive, although they are separated by a thin layer of white matter and by the claustrum. The latter splits the insular subcortical white matter to create the extreme and external capsules; the external capsule separates the claustrum from the putamen. The internal capsule separates the lentiform complex from the caudate nucleus.
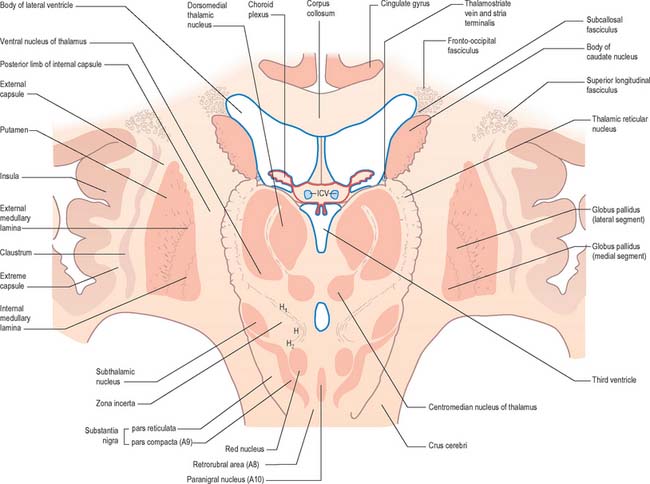
Inferiorly, a little behind the fundus striati, the lentiform complex is grooved by the anterior commissure, which connects inferior parts of the temporal lobes and the anterior olfactory cortex of the two sides (Fig. 22.6). The area above the commissure is referred to as the dorsal pallidum, and that below it as the ventral pallidum.
STRIATUM
The striatum consists of the caudate nucleus, putamen and ventral striatum, which are all highly cellular and well vascularized. The caudate and putamen are traversed by numerous small bundles of thinly-myelinated, or non-myelinated, small-diameter axons, which are mostly striatal afferents and efferents. They radiate through the striatal tissue as though converging on, or radiating from, the globus pallidus. The bundles are occasionally referred to by the archaic term ‘Wilson’s pencils’ and they account for the striated appearance of the corpus striatum.
All afferents to the striatum terminate in a mosaic manner. The size of a cluster of terminals is usually 100–200 μm across. Some afferent terminal clusters are not arranged in register with the clear striosome/matrix distributions seen in nigrostriatal and thalamostriatal axons. In general, afferents from neocortex end in striatal matrix and those from allocortex end in striosomes. However, the distinction is not absolute: although afferents from the neocortex arise in layers V and VI, those from the superficial part of layer V end predominantly in striatal matrix, whereas those from deeper neocortex project to striosomes. Striatal cell bodies, which are the sources of efferents, also form clusters, but again are not uniformly related to striosomes. For example, the cell bodies of some striatopallidal and striatonigral axons lie clustered within striosomes, and others lie outside them, but still in clusters. The neurones and neuropil of the ventral striatum are essentially similar to those of the dorsal striatum, but the striosomal/matrix organization is less well-defined, and seems to consist predominantly of striosomes.
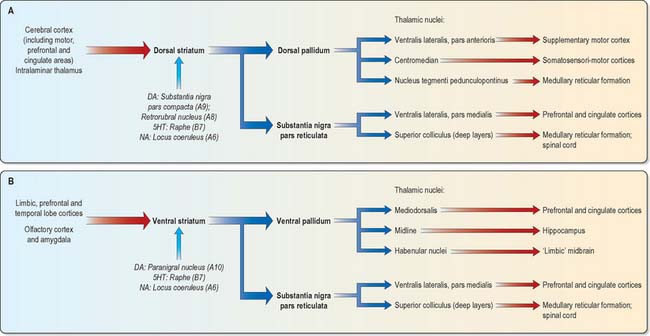
The major connections of the striatum are summarized in Fig. 22.9. Although the connections of the dorsal and ventral divisions overlap, the generalization can be made that the dorsal striatum is predominantly connected with motor and associative areas of the cerebral cortex, whilst the ventral striatum is connected with the limbic system and orbitofrontal and temporal cortices. For both dorsal and ventral striatum, the pallidum and substantia nigra pars reticulata are key efferent structures. The fundamental arrangement is the same for both divisions. The cerebral cortex projects to the striatum, which in turn projects to the pallidum and substantia nigra pars reticulata, and efferents from the pallidum and substantia nigra pars reticulata influence the cerebral cortex (either the supplementary motor area or prefrontal and cingulate cortices via the thalamus) and the superior colliculus (see below).
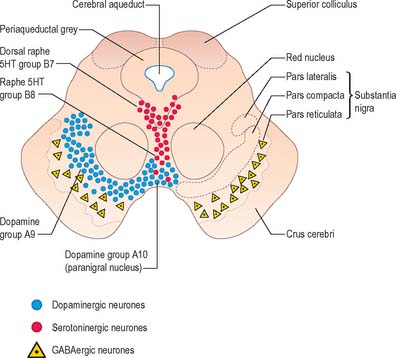
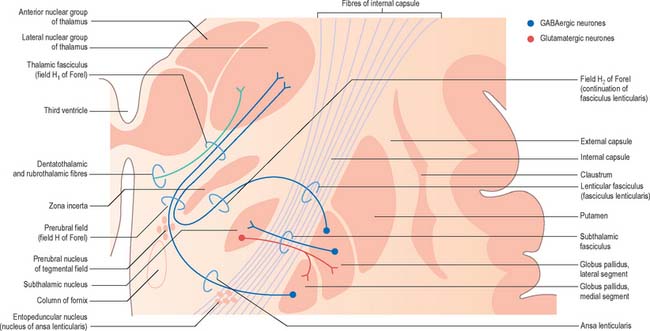
The aminergic inputs to the caudate and putamen are derived from the substantia nigra pars compacta (dopaminergic group A9; Fig. 22.10), the retrorubral nucleus (dopaminergic group A8), the dorsal raphe nucleus (serotoninergic group B7; Fig. 22.10) and the locus coeruleus (noradrenergic group A6). The nigrostriatal input is sometimes referred to as the ‘mesostriatal’ dopamine pathway. It reaches the striatum by traversing the H fields (of Forel) in the subthalamus and running in the median forebrain bundle. These aminergic inputs appear to modulate the responses of the striatum to cortical and thalamic afferent influences.
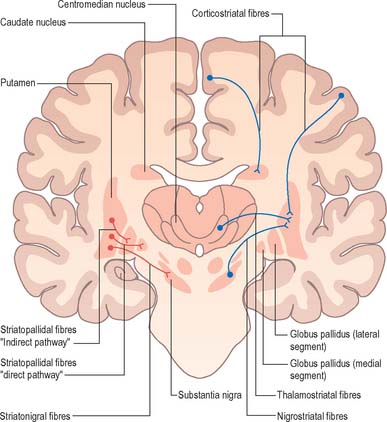
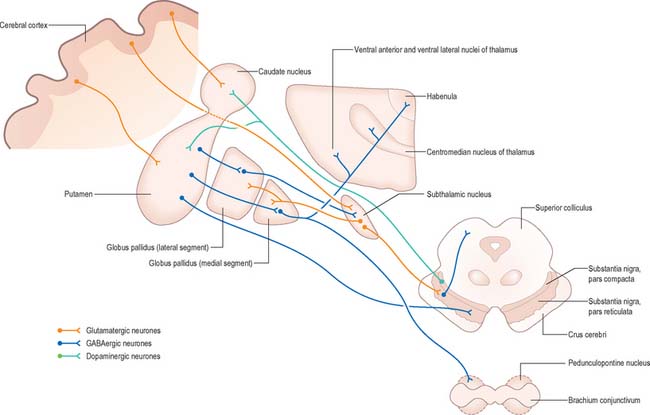
Efferents from the striatum pass to both segments of the globus pallidus and to the substantia nigra pars reticulata, where they end in a topically ordered fashion. Fibres ending in either the lateral or medial pallidal segment originate from different striatal cells (Fig. 22.9). Those to the lateral pallidum come from neurones which co-localize GABA and enkephalin and give rise to the so-called ‘indirect pathway’. This name refers to the fact that these striatal neurones influence the activity of basal ganglia output neurones in the medial pallidum via the intermediary of the subthalamic nucleus. Other striatal neurones, which co-localize GABA and substance P/dynorphin, project directly to the medial pallidum and are therefore described as the ‘direct pathway’.
A second outflow is established from the striatum to the pars reticulata of the substantia nigra. This also has both direct and indirect components, via the lateral pallidum and subthalamic nucleus (Figs 22.11, 22.12). The axons of the direct striatonigral projection constitute the laterally placed ‘comb’ system, which is spatially quite distinct from the ascending dopaminergic nigrostriatal pathway. Striatonigral fibres end in a spatially ordered way in the pars reticulata.
The ventral striatum is the primary target of cortical afferents from limbic cortices, including allocortex, and from limbic associated regions (Fig. 22.11). Thus, the hippocampus (through the fornix) and orbitofrontal cortex (through the internal capsule) project to the nucleus accumbens, and the olfactory, entorhinal, anterior cingulate and temporal visual cortices project to both the nucleus accumbens and olfactory tubercle in varying degrees. The tubercle also receives afferents from the amygdala. The contiguities of the cortical areas, which project to the ventral striatum and neighbouring dorsal striatum, emphasize the imprecise nature of the boundaries between the two divisions. All the cortical regions overlap and abut one another, and they project to neighbouring parts of the dorsal striatum as well as to the ventral striatum. The fundus striati and ventromedial caudate nucleus abut the olfactory tubercle and nucleus accumbens (Fig. 22.6), and receive connections from the orbitofrontal cortex and, to a lesser extent, from the lateral prefrontal and anterior cingulate cortices (which also project to the contiguous head of the caudate nucleus).
This continuity of the ventral and dorsal striata, as revealed by the arrangements of corticostriate projections, is reinforced by consideration of the aminergic inputs to the ventral striatum. They are derived from the dorsal raphe (serotoninergic group B7), the locus coeruleus (noradrenergic group A6) and from the paranigral nucleus (dopamine group A10), as well as the most medial part of the substantia nigra pars compacta (A9) (Fig. 22.10). The dopamine projections constitute the so-called mesolimbic dopamine pathway, which also projects to the septal nuclei, hippocampus and amygdala and prefrontal and cingulate cortices through the medial forebrain bundle. The lateromedial continuity of cell groups A9 and 10 is thus reflected in the relative positions of their ascending fibres in the subthalamus and hypothalamus (the H fields and median forebrain bundle, respectively), as well as in the lateromedial topography of the dorsal and ventral striata (Fig. 22.6), which in turn have contiguous and overlapping sources of cortical afferents.
As with the dorsal striatum, efferents from the ventral striatum project to the pallidum (in this case the ventral pallidum) and the substantia nigra pars reticulata (Figs 22.11, 22.12). In the latter case, the connection is both direct and indirect via the subthalamic nucleus. The projections from the pars reticulata are as described for the dorsal system, but axons from the ventral pallidum reach the thalamic mediodorsal nucleus (which projects to cingulate and prefrontal association cortex) and midline nuclei (which project to the hippocampus). Ventral pallidal axons also reach the habenular complex of the limbic system.
Nucleus accumbens
The ventral striatum consists of the nucleus accumbens and the olfactory tubercle. In front of the anterior commissure, much of the grey matter of the anterior perforated substance, and especially the olfactory tubercle, is indistinguishable from, and continuous with, the fundus striati, in terms of cellular composition, histochemistry and interconnections. The caudate nucleus is continuous medially with the nucleus accumbens (Fig. 22.6), which abuts the nuclei of the septum, close by the paraolfactory area, diagonal band of Broca and the fornix.
GLOBUS PALLIDUS
Striatopallidal fibres are of two main types. They project either to the lateral or the medial pallidal segment. Those projecting to the lateral segment constitute the beginning of the so-called ‘indirect pathway’. They utilize GABA as their primary transmitter and also contain enkephalin. Efferent axons from neurones in the lateral segment pass through the internal capsule in the subthalamic fasciculus, and travel to the subthalamic nucleus (Fig. 22.13).
Striatopallidal axons destined for the medial pallidum constitute the so-called ‘direct pathway’. Like the indirect projection, these also utilize GABA as their primary transmitter but they also contain substance P and dynorphin, rather than enkephalin. Efferent axons from the medial pallidal segment project through the ansa lenticularis and fasciculus lenticularis (Fig. 22.13). The former runs round the anterior border of the internal capsule and the latter penetrates the capsule directly. Having traversed the internal capsule, both pathways unite in the subthalamic region, where they follow a horizontal hairpin trajectory, and turn upwards to enter the thalamus as the thalamic fasciculus. The trajectory circumnavigates the zona incerta and creates the so-called ‘H’ fields of Forel (Figs 22.5, 22.13). Within the thalamus, pallidothalamic fibres end in the ventral anterior and ventral lateral nuclei and in the intralaminar centromedian nucleus. These in turn project excitatory (presumed glutamatergic) fibres primarily to the frontal cortex, including the primary and supplementary motor areas. The medial pallidum also projects fibres caudally to the pedunculopontine nucleus (Fig. 22.12). This lies at the junction of the midbrain and the pons, close to the superior cerebellar peduncle, and corresponds approximately to the physiologically identified ‘mesencephalic locomotor region’.
SUBSTANTIA NIGRA
The substantia nigra is a nuclear complex deep to the crus cerebri in each cerebral peduncle of the midbrain. It consists of a pars compacta, pars reticulata and a smaller pars lateralis (Fig. 22.10). The pars compacta and pars lateralis correspond to the dopaminergic cell group A9. Together with the retrorubral nucleus (A8) they comprise most of the dopaminergic neurone population of the midbrain and are the source of the mesostriatal dopamine system that projects to the striatum. The pars compacta of each side is continuous with its contralateral counterpart through the ventral tegmental dopamine group A10, which is sometimes known as the paranigral nucleus. This is the source of the mesolimbic dopamine system, which supplies the ventral striatum and neighbouring parts of the dorsal striatum, and the prefrontal and anterior cingulate cortices. The dopaminergic neurones of the pars compacta (A9) and paranigral nucleus (ventral tegmental group A10) also contain cholecystokinin (CCK) or somatostatin.
SUBTHALAMIC NUCLEUS
The subthalamic nucleus is a biconvex, lens-shaped nucleus in the subthalamus of the diencephalon. It lies medial to the internal capsule, immediately rostral to the level at which the latter becomes continuous with the crus cerebri of the midbrain (Figs 22.2, 22.5, 22.8). Within the nucleus, small interneurones intermingle with large multipolar cells with dendrites which extend for about one-tenth the diameter of the nucleus. The nucleus is encapsulated dorsally by axons, many of which are derived from the subthalamic fasciculus: they carry a major GABAergic projection from the lateral segment of the globus pallidus as part of the indirect pathway. It also receives afferents from the cerebral cortex. The subthalamic nucleus is unique in the intrinsic circuitry of the basal ganglia in that its cells are glutamatergic and project excitatory axons to both the globus pallidus and substantia nigra pars reticulata. Within the pallidum, subthalamic efferent fibres end predominantly in the medial segment but many also end in the lateral segment.
PATHOPHYSIOLOGY OF BASAL GANGLIA DISORDERS
Dopamine appears to have a dual action on medium spiny striatal neurones. It inhibits those of the indirect pathway and excites those of the direct pathway. Consequently, when dopamine is lost from the striatum, the indirect pathway becomes overactive and the direct pathway becomes underactive (Fig. 22.14). Overactivity of the striatal projection to the lateral pallidum results in inhibition of pallidosubthalamic neurones and, consequently, overactivity of the subthalamic nucleus. Subthalamic efferents mediate excessive excitatory drive to the medial globus pallidus and substantia nigra pars reticulata. This is exacerbated by underactivity of the GABAergic, inhibitory direct pathway. Overactivity of basal ganglia output then inhibits the motor thalamus and its excitatory thalamocortical connections. While this description is little more than a first approximation of the underlying pathophysiology, this model of the basis of parkinsonian symptoms has led to the introduction of new neurosurgical approaches to the treatment of Parkinson’s disease, based upon lesioning and deep brain stimulation of the medial globus pallidus and subthalamic nucleus (see below).
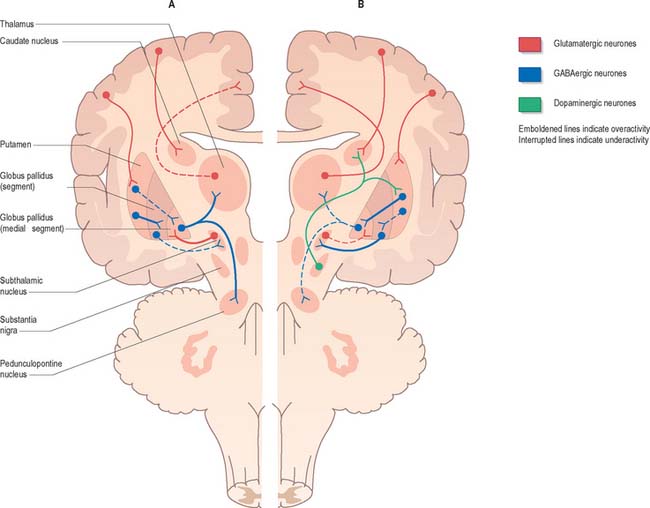
Fig. 22.14 Pathophysiology of Parkinson’s disease (A) and dyskinesias (B).
(Adapted from Crossman AR, Neary D 2000 Neuroanatomy, 2nd edition. Edinburgh: Churchill Livingstone.)
The current medical treatment for Parkinson’s disease relies upon levodopa (L-DOPA; L-dihydroxyphenylalanine), the immediate metabolic precursor of dopamine, or upon dopamine agonists. Whilst these usually provide good symptomatic relief for many years, eventually they lead to the development of side-effects, including dyskinesias. The involuntary movements that occur as a consequence of long-term treatment of Parkinson’s disease resemble those seen in Huntington’s disease, tardive dyskinesia and ballism. Experimental evidence suggests that these may share a common neural mechanism (Fig. 22.14). Thus, the indirect pathway becomes underactive, e.g. due to the effects of dopaminergic drugs in Parkinson’s disease, or the degeneration of the striatopallidal projection to the lateral pallidum in Huntington’s disease. This leads to physiological inhibition of the subthalamic nucleus by overactive pallidosubthalamic neurones. The involvement of the subthalamic nucleus explains why the dyskinetic movements of levodopa-induced dyskinesia and Huntington’s disease resemble those of ballism produced by lesion of the subthalamic nucleus. Underactivity of the subthalamic nucleus removes the excitatory drive from medial pallidal neurones, which are known to be underactive in dyskinesias. Once again this is an oversimplification. Whilst it is true that underactivity of the medial globus pallidus is associated with dyskinesias, it is also known that lesions of the globus pallidus alleviate them. This suggests that the dynamic aspects of pallidal and nigral efferent activity are important factors in the generation of dyskinesia.
There is evidence that dysfunction of the basal ganglia is involved in other complex, less well-understood, behavioural disorders. In animal experiments, lesions of the basal ganglia, especially of the caudate nucleus, induce uncontrollable hyperactivity (e.g. obstinate progression, incessant pacing and other constantly repeated behaviours). This and other evidence has led to the notion that the corpus striatum enables the individual to make motor choices and to avoid ‘stimulus-bound’ behaviour. PET scanning studies in man have shown that sufferers from obsessive-compulsive disorder (OCD), which is characterized by repeated ritualistic motor behaviour and intrusive thoughts, exhibit abnormal activity in the prefrontal cortex and caudate nuclei. There are similar suggestive observations in the hyperactive child syndrome. In this respect it may be significant that the basal ganglia, besides receiving connections from the frontal lobe and limbic cortices, also have an ascending influence on the prefrontal and cingulate cortices through the substantia nigra pars reticulata and dorsomedial and ventromedial thalamus (Fig. 22.11).
Before the advent of levodopa therapy, neurosurgery for Parkinson’s disease was commonplace. The globus pallidus and thalamus were favoured targets for chemical or thermal lesions. Pallidotomy and thalamotomy often improved rigidity and tremor, but they produced little consistent beneficial effect upon akinesia. With the arrival of levodopa therapy, which has a profound effect upon akinesia, the surgical treatment of Parkinson’s disease underwent progressive decline. However, it soon became clear that long-term use of levodopa was associated with a number of side-effects such as dyskinesias, ‘wearing-off’, and the ‘on–off’ phenomenon. More recent advances in understanding the pathophysiology of movement disorders, and in particular Parkinson’s disease, have stimulated a renaissance in the use of neurosurgery to treat movement disorders.
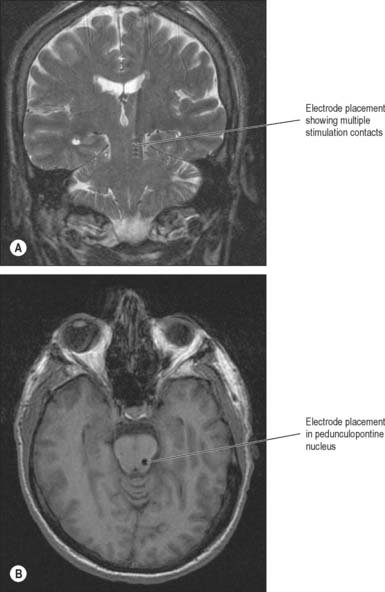
Implantation of deep-brain electrodes through which high-frequency pulses generated by a pacemaker could inhibit cells in the vicinity has been a concept since the early 1970s, but did not become a widespread reality until the late 1980s, as a result of technological advances. The introduction of the technique of deep brain stimulation (DBS), which avoids making permanent lesions, made bilateral surgery safer. There have been numerous reports of the effectiveness of both bilateral pallidal and subthalamic nucleus stimulation (Figs 22.15, 22.16). Subthalamic nucleus stimulation is favoured by most groups because, unlike pallidal stimulation, it allows patients to reduce their anti-parkinsonian medication.
Serendipity also has a role in such surgery. Clinically, parkinsonian patients can develop painful dystonic posturing of their limbs which responds dramatically to bilateral pallidal stimulation. This has led to preliminary studies of bilateral pallidal stimulation for dystonia with very promising results. Since it is held that in dystonia the pallidal neurones fire at rates below normal, this presents quite a puzzle as it is open to question how stimulation works. It also would appear that the neural mechanism that underlies this therapeutic effect on dystonia may differ from that in Parkinson’s disease and tremor, because in dystonia the improvement may take weeks to emerge, whereas it is immediate in the case of Parkinson’s disease.
The PPN has a caudal pars compacta that consists largely of cholinergic neurones and a rostral pars dissipata that consists largely of glutamatergic neurones. It is known to receive GABAergic efferent fibres from the globus pallidus and substantia nigra. In animal models of Parkinson’s disease these projections are overactive and the PPN is inhibited. Taken in conjunction with other experimental evidence, this suggests the possibility that the PPN is involved in the pathophysiology of disturbances of locomotion, gait and posture in Parkinson’s disease. Recently, DBS of PPN through implanted electrodes has been applied in drug-resistant akinetic parkinsonian patients (Fig. 22.17). Low frequency stimulation alleviates postural instability and on-medication gait freezing, symptoms which conventional medication and surgery fail to improve.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357-382.
Crossman AR. A hypothesis on the pathophysiological mechanisms that underlie levodopa- or dopamine agonist-induced dyskinesia in Parkinson’s disease: implications for future strategies in treatment. Mov Disord. 1990;5:100-108.
Hallett M. Pathophysiology of dystonia. Neural Transm Suppl. 2006;70:485-488.
Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925-1934.
Laitinen LV, Bergenheim AT, Hariz MI. Ventroposterolateral pallidotomy can abolish all Parkinsonian symptoms. Stereotact Funct Neurosurg. 1992;58:14-21.
A key paper which ignited widespread interest in functional neurosurgery for Parkinson’s disease..
Penney JBJr, Young AB. Striatal inhomogeneities and basal ganglia function. Mov Disord. 1986;1:3-15.