19 Basal Ganglia
The Basal Ganglia Include Five Major Nuclei
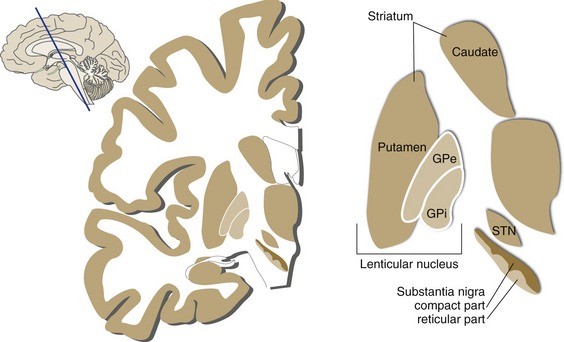
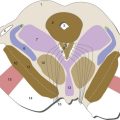
The meaning of the term “basal ganglia” has changed over the years, but most folks would now agree that there are five major structures on the list: the caudate nucleus, putamen, globus pallidus, substantia nigra, and subthalamic nucleus (Fig. 19-1). The caudate nucleus and putamen have similar but parallel connections and are referred to in combination as the striatum. The putamen and globus pallidus have very different connections but are physically stuck together; in combination, they are referred to as the lenticular nucleus (from the Latin word for “lentil”).
Basal Ganglia Circuitry Involves Multiple Parallel Loops That Modulate Cortical Output
How does damage to the basal ganglia cause movement (and other) disorders? For the most part, we know only the broad outlines of an answer, but there is one basic fact to keep in mind: The basal ganglia have no major outputs to lower motor neurons. Instead, they work primarily by influencing what comes out of the cerebral cortex.
The striatum is, in a sense, the major input part of the basal ganglia, collecting excitatory (glutamate) inputs from large cortical areas (different areas for different parts of the striatum). GPi and SNr are the principal output structures, sending inhibitory (GABA) projections to the thalamus, which in turn projects back to a restricted portion of this large cortical area (Fig. 19-2). Because thalamocortical projections are excitatory, the globus pallidus is in a position to suppress or facilitate cortical activity by way of varying patterns of inhibition in the thalamus; the balance of excitatory and inhibitory connections interposed between the striatum and GPi/SNr helps determine the pattern. For example, inhibiting an inhibitory GPi neuron could have the same net effect on the thalamus as increasing excitatory inputs to the same part of the thalamus (Fig. 19-3).
Although the cortex-striatum-globus pallidus-thalamus-cortex pathway is commonly drawn as a single loop, it is actually a system of parallel, overlapping loops, each wired up according to the same principles (Fig. 19-4). So the caudate nucleus, for example, receives its inputs from a different widespread area of the cortex than does the putamen and influences its own part of the globus pallidus, which in turn projects to its own part of the thalamus and its own restricted cortical area.
The putamen subsystem is the part most directly involved in movement disorders. Its inputs come from the motor and somatosensory areas flanking the central sulcus. Its outputs reach motor areas of cortex (particularly the supplementary motor area) by way of the ventral lateral and ventral anterior nuclei of the thalamus (VL and VA). This set of connections is consistent with the notion that the putamen and supplementary motor area are somehow involved in planning or initiating voluntary movements.
Finally, nucleus accumbens and adjacent striatal areas (collectively called the ventral striatum) receive inputs from limbic structures and project, by way of part of the globus pallidus and thalamus, back to limbic cortex (described in Chapter 23).
Interconnections of the Basal Ganglia Determine the Pattern of Their Outputs
The Cerebral Cortex, Substantia Nigra, and Thalamus Project to the Striatum
The Internal Segment of the Globus Pallidus and the Reticular Part of the Substantia Nigra Provide the Output from the Basal Ganglia
The cortex-striatum-GPi/SNr-thalamus-cortex loop is often considered to be a direct pathway through the basal ganglia that facilitates movement (Fig. 19-5), a circuit whose malfunction partially explains the slow, diminished movements of Parkinson’s disease.
The Subthalamic Nucleus Is Part of an Indirect Pathway through the Basal Ganglia
The output of GPi and SNr is determined by the balance of inhibitory and excitatory inputs they receive. The striatum is a major source of inhibitory inputs. The subthalamic nucleus, on the other hand, provides a powerful excitatory input. The subthalamic nucleus is often considered to be part of an indirect pathway through the basal ganglia (Fig. 19-6), one that has just the opposite effect on thalamic activity as the direct pathway. According to one model of basal ganglia function, the balance of activity in the direct and indirect pathways helps to determine which cortical activities are facilitated and which are suppressed. The fibers that travel back and forth between the subthalamic nucleus and the globus pallidus penetrate the internal capsule as small bundles that are collectively called the subthalamic fasciculus (THB6 Figure 19-16, p. 486).
Many Basal Ganglia Disorders Result in Abnormalities of Movement
The best-known basal ganglia disorders are characterized by a combination of positive and negative signs—positive signs being involuntary muscle contractions in various patterns and negative signs being difficulty producing muscle contraction. Parkinson’s disease is the classic example. Positive signs include a resting tremor, especially pronounced in the hands, and a general increase in tone in all muscles, referred to as rigidity. Negative signs include slow movements (bradykinesia) and reduced numbers of movements (hypokinesia or akinesia). There’s no particular change in strength or reflexes. Other basal ganglia disorders can be accompanied by different kinds of involuntary movements that fall into three general categories: rapid movements called chorea; slow, writhing movements called athetosis; and flailing movements of entire limbs, called ballism. In some disorders, tone is increased even more than in Parkinson’s; in others, it is decreased.