Basal ganglia
Basic anatomy
The functional nuclei of the basal ganglia (BG) include:
• Striatum (caudate nucleus and putamen), which receives information from all parts of the cerebral cortex except the primary visual and auditory cortices
• Globus pallidus internus (GPi)
• Globus pallidus externus (GPe)
• Substantia nigra (SN) – pars reticulata (pr) and pars compacta (pc) which contain dopamine-producing cells.
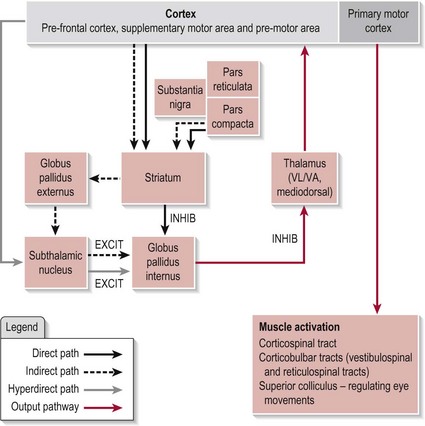
The circuitry between these functional nuclei forms several pathways, the direct, indirect and hyperdirect, the arrangement of which can be seen in Figure 11.1. The BG is also topographically arranged with each body part represented relative to its innervations.
Function of the basal ganglia
As well as the motor loop shown in Figure 11.1, there are three other loops within the BG which have their origins in different cortical areas and carry out specific functions via similar circuitry. Although previously described as discrete parallel loops, recent evidence indicates that there is a high level of interaction between these loops. However, the selection function described above is true for all of them, the motor loop (voluntary movement or learning), the limbic loop (emotions) and the associative loop (cognition and sensory integration). Based on its anatomical connections with the nociceptive system, the BG is also considered crucial in pain modulation. It appears that the interaction of several of these loops make it possible for the BG to influence several dimensions of pain (sensory discrimination, affective and cognitive) as well as the modulation of pain itself via its filtering role.
Function of the BG in motor control
The big picture
The pre-frontal cortex (S2.7) provides an idea for movement, a goal, based upon the internal and external environment. A relevant movement plan is selected from learned stored programmes in the supplementary motor area (SMA) and pre-motor area (PMA) (S2.7). At this stage, the BG assists in the selection of an appropriate plan, its initiation and termination. The selected plan is returned to the SMA and PMA for future reference and to the primary motor cortex from which the plan is executed via the descending tracts (S2.14).
The detail
The anatomical connections of the three pathways within the BG can be seen in Figure 11.1. Before exploring the detailed workings of how the BG achieves its function, a brief description of the role of each pathway is necessary. In essence, the direct pathway allows the selected motor programme through to the thalamus and is responsible for initiating the plan, while the indirect pathway halts any unwanted motor programmes and terminates the plan. These two pathways are also modulated by the SNpc circuit at the level of the striatum (Fig. 11.1). The neurotransmitter dopamine released by the SNpc cells has the opposite effect on the neurons of each pathway. It is excitatory to the direct pathway, reinforcing its action and is inhibitory to the indirect pathway so reducing its action. In both cases, this results in more chance of a motor programme being released to the thalamus when the SNpc neurons are active. Recent evidence has indicated the existence of a third pathway termed the hyperdirect (Fig. 11.1) which links the cortex directly to the STN and on to the GPi. This pathway is considered to be faster conducting than either the direct or indirect pathways. Its role is the same as the indirect pathway. Ultimately, it is the balance of activity between these three pathways at the GPi that acts as the filtering system to ensure the appropriate selection of a movement programme. The relative contribution of each of these pathways is at present unknown.
During movement
Selection of the appropriate motor programme. The decision as to the appropriate motor programme to select is based upon internal cues from the huge amount of sensory input received by the BG. Whether the programme is selected or not has recently been attributed to the competition/balance between the direct and indirect/hyperdirect pathways:
• In the direct pathway, the striatum stops the GPi from switching off the thalamus (to stop inhibition is termed ‘disinhibition’) and results in the release of the selected motor programme by the thalamus
• In the hyperdirect (via the cortex) and indirect (via the striatum) pathways, the GPi is excited or switched on so that again it stops the thalamus releasing any information and this results in unwanted motor programmes being halted.
Initiation and termination of the selected programme. This is achieved by adjusting the specific timing of activation of the three pathways:
• The hyperdirect pathway, which is fast conducting, excites or switches on the GPi so stopping the programme from being released by the thalamus
• Next, the direct pathway stops the GPi inhibiting the thalamus so allowing the selected programme to be initiated
• Finally, the indirect pathway switches on the GPi, to stop any further information leaving the thalamus and thus terminates the motor programme.
References and Further Reading
Boecker, H, Jankowski, J, Ditter, P, et al. A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. Neuroimage. 2008; 39:1356–1369.
Cunnington, R, Windischberger, C, Deecke, L, et al. The preparation and execution of self-initiated and externally-triggered movement: a study of event related fMRI. Neuroimage. 2002; 15:373–385.
Cunnington, R, Windischberger, C, Moser, E. Premovement activity of the presupplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Human Movement Science. 2005; 24:644–656.
Elsinger, CL, Harrington, DL, Rao, SM. From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage. 2006; 31:1177–1187.
Graybiel, AM. The basal ganglia: learning new tricks and loving it. Current Opinion in Neurobiology. 2005; 15:638–644.
Gerardin, E, Pochon, JB, Poline, JB, et al. Distinct striatal regions support movement selection preparation and execution. Neuroreport. 2004; 15:2327–2331.
Haber, SN, Calzavara, R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Research Bulletin. 2009; 78:69–74.
Jankowski, J, Scheef, L, Hüppe, C, et al. Distinct striatal regions for planning and executing novel and automated movement sequences. Neuroimage. 2009; 44:1369–1379.
Leblois, A, Boraud, T, Meissner, W, et al. Competition between feedback loops underlies normal and pathological demands in the basal ganglia. Journal of Neuroscience. 2008; 2613:3567–3583.
Nambu, A. A new dynamic model of the cortico-basal ganglia loop. Progress in Brain Research. 2004; 143:461–466.
Nambu, A. Seven problems on the basal ganglia. Current Opinion in Neurobiology. 2008; 18:595–604.
Nambu, A, Tokuno, H, Takada, M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neuroscience Research. 2008; 43:111–117.
Melrose, RJ, Poulin, RM, Stern, CE. An fMRI investigation of the role of the basal ganglia in reasoning. Brain Research. 2007; 1142:146–158.
Monchi, O, Petrides, M, Strafella, AP, et al. Functional role of the basal ganglia in the planning and execution of actions. Annals of Neurology. 2006; 59:257–264.
Pasupathy, A, Miller, EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005; 433:873–876.
Smith, Y, Raju, D, Nanda, B, et al. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Research Bulletin. 2009; 78:60–68.
The Parkinson’s Disease Society. The professional’s guide to Parkinson’s disease. www.parkinsons.org.uk/PDF/PubProfessionalGuideNov07pdf, 2007.
Weilke, F, Spiegel, S, Boecker, H, et al. Time-resolved fMRI of activation patterns in M1 and SMA during complex voluntary movement. Journal of Neurophysiology. 2001; 85:1858–1863.