33 Basal ganglia
Introduction
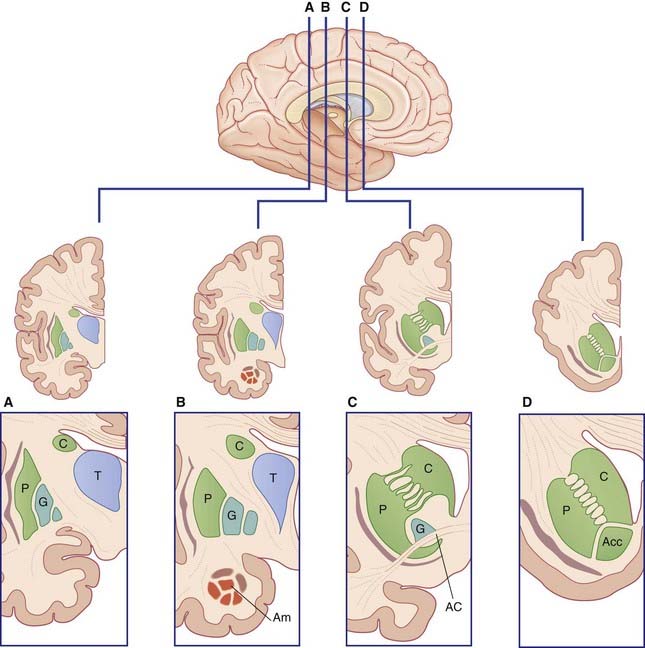
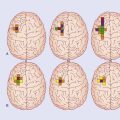
The term basal ganglia is used to designate the areas of basal forebrain and midbrain known to be involved in the control of movement (Figure 33.1). It includes:
Basic Circuits
Motor loop
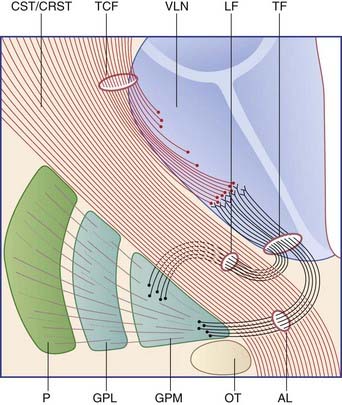
Figure 33.2 is derived from Figure 33.1A. It is a schematic coronal section including the posterior part of the striatum, depicting component parts of the motor loop. Two pathways are known. The ‘direct’ pathway traverses the corpus striatum and thalamus and involves five consecutive sets of neurons (Figure 33.2A). The ‘indirect’ pathway engages the subthalamic nucleus in addition and involves seven sets of neurons (Figure 33.2B). Separate from these pathways are two thalamic projections from the medial pallidum (ansa lenticularis and lenticular fasciculus) shown in Figure 33.3.
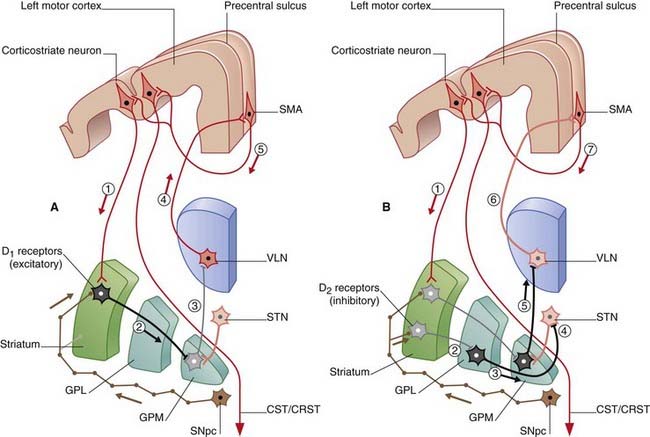
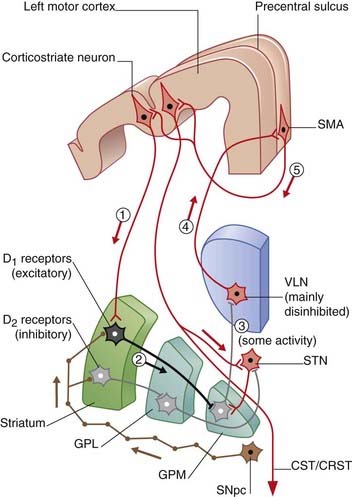
Figure 33.2 Coronal section through the motor loop, based on Figure 33.1A. (A) The sequence of five sets of neurons involved in the ‘direct’ pathway from sensorimotor cortex to thalamus with final return to sensorimotor cortex via SMA. (B) The sequence of seven sets of neurons involved in the ‘indirect’ pathway.
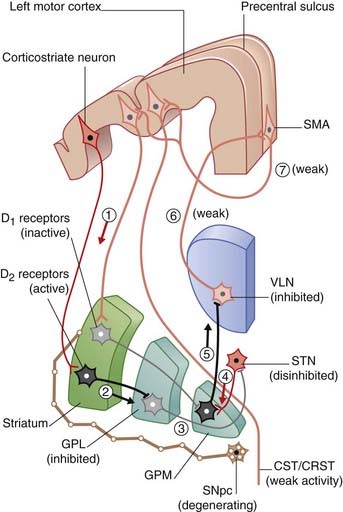
The nigrostrial pathway projects from the compact part of the substantia nigra to the striatum, where it makes two kinds of synapses upon the projection neurons there (Figure 33.4). Those upon ‘direct’ pathway neurons are facilitatory, by way of dopaminergic type 1 (D1) receptors on the dendritic spines; those upon ‘indirect’ pathway neurons are inhibitory, by way of type 2 (D2) receptors. Cholinergic internuncial neurons within the striatum are excitatory to projection neurons, and they are inhibited by dopamine.
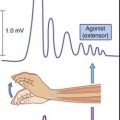
A healthy substantia nigra is tonically active, favoring activity in the ‘direct’ pathway. Facilitation of this pathway is necessary for the SMA to become active before and during movement. SMA activity immediately prior to movement can be detected by means of recording electrodes attached to the scalp. This activity is known as the (electrical) readiness potential, and its manner of production is described in the caption to Figure 33.4. Impulses pass from SMA to the motor cortex, where a cerebello-thalamocortical projection selectively enhances pyramidal and corticoreticular neurons within milliseconds prior to discharge.
Progressive failure of dopamine production by the compact part of substantia nigra is the precipitating cause of Parkinson’s disease (PD) (Clinical Panel 33.1).
Clinical Panel 33.1 Hypokinesia
Parkinson’s disease
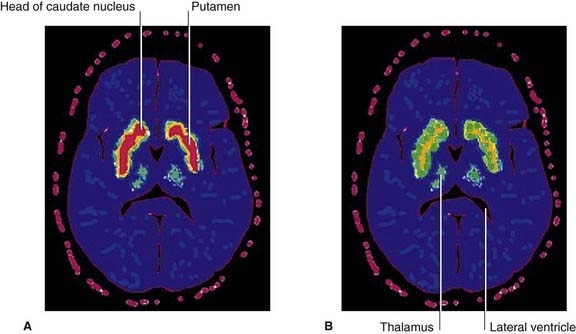
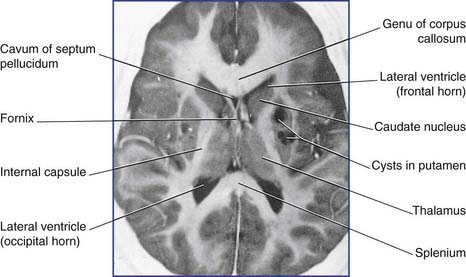
Parkinson’s disease (PD) affects about 1% of people over 65 years of age in all countries. The primary underlying pathology is degeneration of nigrostriatal neurons, resulting in diminished dopamine content within the striatum. [18F]fluorodopa is a mildly radioactive compound which, when injected intravenously, binds with dopamine receptors in the striatum. In symptomatic PD, a significant reduction of [18F]fluorodopa binding (and therefore of receptors) is revealed by means of PET scanning (Figure CP 33.1.1). One consequence is increased striatal activity, with a shift from the direct to the indirect motor pathway (Figure CP 33.1.2).
Tremor
Tremor is associated with rhythmic bursting activity within all five cell groups of the direct motor loop (Figure 28.2A) and in anterior horn cells of the spinal cord. The contribution of disordered autogenic inhibition to both resting tremor and rigidity is described below.
Rigidity
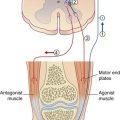
Historically, rigidity has been abolished by section of dorsal nerve roots, thus proving its peripheral sensory origin. It can also be alleviated by a surgical lesion of the pallidum or of the VL nucleus of the thalamus. Because muscle spindle stretch reflexes are not exaggerated in PD, attention has focused on the Golgi tendon organ afferents responsible for autogenetic inhibition. As illustrated in Chapter 10, these afferents synapse upon inhibitory, 1b internuncials which, when activated by muscle contraction, dampen activity of motor neurons supplying the same muscle and any homonymous contributors to the same movement (e.g. impulses generated in biceps brachii tendon organs will depress both brachialis and biceps motor neurons). In PD patients, autogenetic inhibition is reduced, and it is also delayed to the extent that it becomes entrained with the pulses descending from the brain, with the effect of contributing to the tremor. It may also contribute to the rigidity, because in PD there is some degree of co-contraction of prime movers and antagonists.
In addition to its massive projections into the pallidum, the putamen projects to another group of GABAergic neurons, namely the reticular part of the substantia nigra. The compact part of the substantia nigra also projects to the reticular part. The reticular part projects in turn to the brainstem locomotor center (Ch. 24). In PD, the overactive putamen would be expected to have the knock-on effect of inhibiting impulse traffic in the projections from the locomotor area to the pontine and medullary reticular reticulospinal tracts.
Impairment of postural reflexes
Two other symptoms, oculomotor hypokinesia and dementia, are mentioned in the main text.
Misdiagnosis
Treatment of Parkinson’s disease
Drugs
The first line of treatment of PD is administration of L-dopa which can cross the blood–brain barrier and is metabolized to dopamine by surviving nigral neurons. Some 75% of patients benefit, with a reduction of symptoms by 50% or more. After several years of L-dopa therapy, many patients develop spontaneous choreiform movements (described in Clinical Panel 33.2) owing to excessive striatal response. After a year or more, the effectiveness of L-dopa declines with the progressive loss of nigral neurons, and dopamine agonist drugs are often used instead to stimulate striatal postsynaptic dopamine receptors.
Anticholinergic drugs reduce activity of the cholinergic internuncials in the striatum. They ameliorate tremor (of both kinds) in particular, but the required dosage is liable to produce one or more of the autonomic side effects listed in Clinical Panel 12.3.
Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje number in essential tremor. Arch Neurol. 2008;65:101-107.
Benabid AI. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67-81.
Benarroch EE. Subthalamic nucleus and its connections. Neurology. 2008;70:1991-1995.
Braak H, Tredici KT. Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916-1927.
Defazio G, et al. Pain as a nonmotor symptom of Parkinson disease. Arch Neurol. 2008;65:1191-1194.
Manto M. Tremorgenesis: a new conceptual scheme using reciprocally innervated circuits of neurons. J Transl Med. 2008;6:71-76.
Romanelli P, Esposito V, Schaal DW, Heit G. Somatotopy in the basal ganglia: experimental and clinical evidence for segregated sensorimotor channels. Brain Res Rev. 2005;48:112-128.
Schapira AHV. Etiology and pathogenesis of Parkinson Disease. Neurol Clin. 2009;27:583-603.
Timmermann L, Gross J, Dirks M, et al. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199-212.
Limbic loop
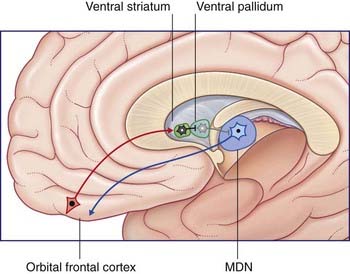
Figure 33.5 depicts the limbic basal ganglia loop. This loop passes from inferior prefrontal cortex through nucleus accumbens (anterior end of the striatum, Figure 33.1D) and ventral pallidum, with return via the mediodorsal nucleus of thalamus to the inferior prefrontal cortex. (The nucleus accumbens, along with the nearby olfactory tubercle (not shown) are known as the ventral striatum.)
Oculomotor loop
The oculomotor loop commences in the frontal eye field and posterior parietal cortex (area 7). It passes through the caudate nucleus and through the reticular part of the substantia nigra (SNpr). It returns via the ventral anterior nucleus of the thalamus to the frontal eye field and prefrontal cortex. SNpr sends an inhibitory GABAergic projection to the superior colliculus, where it synapses upon cells controlling automatic saccades (Ch. 23). These cells are also supplied directly from the frontal eye field.
Other disorders involving the basic ganglia include several hyperkinetic states briefly described in Clinical Panel 33.2.
Clinical Panel 33.2 Other extrapyramidal disorders
Cerebral palsy
The ventricular system in spastic diplegia is dilated owing to maldevelopment of periventricular oligodendrocytes in the 6th to 8th month of gestation, notably those myelinating corticospinal fibers destined for lumbosacral segments of the spinal cord are affected. Intrauterine infection (Figure CP 33.2.1), ischemia, and metabolic disorders are etiological suspects.
Benarroch EE. Subthalamic nucleus and its connections. Neurology. 2008;70:1991-1995.
Brown P, Steiger MJ. Basal ganglia disorders. In: Bronstein AM, Brandt T, Woollacott M, editors. Clinical disorders of balance, posture and gait. London: Arnold; 2004:156-166.
Crossman AR. Functional anatomy of movement disorders. J Anat. 2000;196:519-525.
Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2008;78:69-74.
Kuban KCK, Leviton A. Cerebral palsy. N Engl J Med. 1994;333:88-195.
Meunier S, Pol S, Houeto JL, Vidailhet M. Abnormal reciprocal inhibition between antagonist muscles in Parkinson’s disease. Brain. 2000;123:1017-1026.