Bariatric Surgery in Adolescents
Childhood obesity continues to be an increasingly prevalent and progressive disease with limited treatment options. Not only have increasing numbers of children and adolescents been affected, but the average weight of obese children continues to soar. In the USA alone, it is estimated that approximately 4% (over 2 million children and teens) are extremely obese (BMI > 99th percentile).1 Evidence from clinical trials show that behavioral weight management may have longer-lasting effects in younger children compared with adults, but good long-term outcomes are rare. These conventional treatment approaches are not effective for most who suffer with severe obesity,2–5 leading to the consideration of surgical weight loss options for select adolescents. A number of important factors must be considered when contemplating bariatric operations in adolescents. Surgical weight loss results in significant improvement, if not resolution, of most obesity-related comorbidity in adults.6 Short-term results suggest that this also is true for adolescents,7–11 but little is known about long-term efficacy and potential adverse consequences to an adolescent with the lifelong restriction of calories and certain micronutrients. The issue of recidivism and the potential multigenerational consequences necessitate that considerable care and deliberation be applied to decision-making concerning surgical weight management in this population.
Definitions
Obesity specifically refers to the condition of having excess body fat. Measurement of body mass index (BMI) is a reasonably accurate method for predicting adiposity, is reproducible in the clinical setting, and can be used as a screening tool.12–15 In children and adolescents, physiologic increases in adiposity, height, and weight during growth are expected. Given that childhood obesity is a global problem, it should be noted that there are ethnic and racial variations in onset, prevalence, and severity of the metabolic consequences of childhood obesity. Growth charts that are typically used to define obesity are age and gender specific.16–18
The terms overweight (BMI for age and gender >85th percentile), obese (BMI for age and gender >95th percentile), and extreme obesity (BMI for age and gender >99th percentile) have been used to refer to the increasing weight problem in children.18–20 The 85th and 95th percentiles of BMI for age were chosen mainly because these percentile boundaries approximate the BMI in young adults of 25 kg/m2 (overweight) and 30 kg/m2 (obese), respectively. Whereas more than 32% of adults in the USA are obese, about 18% of children and adolescents are obese, a prevalence that has more than tripled in the past two decades.1,21 Regarding extreme obesity, adolescent boys and most adolescent girls younger than age 18 with a BMI of 35 kg/m2 are above the 99th BMI percentile.1,18 Increasing metabolic risks associated with higher BMI for age, especially greater than or equal to the 99th BMI percentile, have been found when compared with lower levels of obesity.1 In addition, because all children with a BMI above the 99th percentile become obese adults (BMI ≥30 kg/m2) and because obese adults who were obese as children have more health complications and a higher mortality,1,22,23 bariatric surgical procedures in the mature adolescent may be a reasonable option for weight reduction that may well reduce the risk of obesity-related morbidity and early mortality.24
Consequences of Adolescent Obesity
Adolescent obesity is a multifaceted disease with serious immediate, intermediate, and long-term consequences.25,26 Critical periods exist between preconception and adolescence during which the risk of development of obesity is increased.27 Important risk factors for childhood and adolescent obesity are (1) low birth weight;28–31 (2) bottle feeding;32,33 (3) early adiposity rebound;34–38 (4) having a diabetic mother;39,40 (5) puberty;41–43 and (6) parental obesity.44–47 Knowledge of these risk factors for adolescent obesity gives insight into the genetic and environmental factors that result in obesity. With few exceptions, little understanding exists about which risk factors portend the development of extreme obesity.48–50
Associated with the remarkable increase in the prevalence of pediatric obesity is a parallel increase in the severity of obesity and in obesity-related chronic diseases. These diseases have an onset at a younger age and carry an increased risk for adult morbidity and mortality.51–55 Childhood obesity has adverse social and economic consequences.56–58 Numerous comorbid conditions exist in childhood obesity, affecting every major organ system.
Guidelines for Performing Bariatric Surgical Procedures in Adolescence
Patient Selection Criteria
National Institutes of Health (NIH) guidelines suggest that it is reasonable to consider weight loss surgery for adults with a BMI of 35 kg/m2 or greater in the presence of severe obesity-related comorbidities (e.g., sleep apnea, diabetes, joint disease severe enough to require joint replacement) or a BMI of 40 kg/m2 in the presence or absence of comorbidities.59 Although these criteria have remained unchanged since 1991, investigators at several institutions have used lower BMI guidelines for studying the effect of weight loss surgery on adults without adverse consequences. Data support the use of lowered BMI guidelines for patients with severe comorbidities, such as type 2 diabetes, which are responsive to weight loss.60
Recently published best practice guidelines consider a BMI greater than or equal to 35 kg/m2 with significant comorbidities or BMI of 40 kg/m2 with other comorbidities as factors to be used in the selection of adolescents who are most likely to benefit from bariatric surgical procedures.61 Physical maturity should be documented by either history and physical examination or radiographic study, thus generally limiting surgery to those individuals over the age of 12 years.
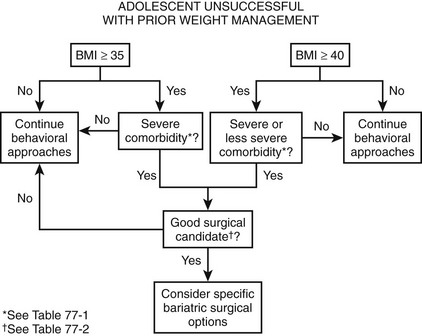
Specifically, when a teenager has reached physical maturity and has achieved a BMI greater than or equal to 35 kg/m2 with serious obesity-related comorbidity, and surgical therapy will be predictably successful in reversing the obesity and comorbidity, it is reasonable to consider a surgical treatment before adulthood. This is the case for adolescents with type 2 diabetes mellitus, pseudotumor cerebri, sleep apnea (apnea–hypopnea index >15), and severe steatohepatitis. For adolescents with less severe comorbidities or risk factors for long-term diseases, for which there is no disadvantage of waiting until adulthood, it is recommended that a BMI of 40 kg/m2 be used as a threshold for operative intervention. These comorbidities include, among others, mild obstructive sleep apnea syndrome (OSAS), hypertension, milder forms of obstructive sleep apnea (OSA), impaired quality of life, insulin resistance, glucose intolerance, or dyslipidemia.61 Figure 77-1 outlines a suggested algorithm for management.
Bariatric Programs for Adolescents
For highly motivated adolescents with comorbid conditions (Box 77-1) who have been unsuccessful with prior dedicated attempts at weight loss (generally at least 6 months), bariatric surgery should be considered as a therapeutic option. Young patients being considered for bariatric surgical procedures should be referred to a specialized center with a multidisciplinary bariatric team with pediatric expertise. Such a team is equipped for the sometimes difficult patient selection decisions and can provide long-term follow-up and management of the unique challenges posed by the severely obese adolescent. Guidelines have been established by the American Society for Bariatric Surgery (ASBS) (www.asbs.org), and the American College of Surgeons (ACS) that define such teams to include specialists with expertise in obesity evaluation and management, psychology, nutrition, physical activity, and bariatric surgical treatment.62 Depending on the individual needs of the adolescent patient, additional expertise in general pediatrics, adolescent medicine, endocrinology, pulmonology, gastroenterology, cardiology, orthopedics, and ethics should be readily available. At Cincinnati Children’s Hospital, the patient review process is similar to that used in our multidisciplinary oncology and transplant programs.63 This review by a panel of experts from various disciplines results in specific treatment recommendations for individual patients, including appropriateness and timing of possible operative intervention.
Factors Influencing Timing of Surgery
Physical Maturation
The timing for surgical treatment of extremely obese adolescents remains controversial and depends, in most cases, on the compelling health needs of the patient. However, certain physiologic factors must be considered in treatment planning. There is a theoretical concern about the impact of significant caloric restriction on the attainment of a genetically predetermined adult stature. Physiologic maturation is generally complete by sexual maturation (Tanner) stage 4.64 Skeletal maturation (adult stature) is normally attained by the age of 13 to 14 years in girls and 15 to 16 years in boys.65 Overweight children generally experience accelerated onset of puberty. As a result, they are likely to be taller and have advanced bone age compared with age-matched nonobese children.
If uncertainty exists about whether adult stature has been attained, skeletal maturation (bone age) can be objectively assessed with a radiograph of the hand and wrist.66 If an individual has attained more than 95% of adult stature, it is unlikely that a bariatric procedure would significantly impair completion of linear growth.67 For those who have not yet reached or nearly reached their predicted adult stature, one must balance the risks of growth delay due to caloric restriction against the potentially more significant risks of progression of obesity-related comorbid conditions if surgery is delayed.
Psychological Maturation
Adolescent psychological development also impacts the ability to participate in surgical decision-making and postoperative dietary compliance. Cognitive development refers to the development of the ability to think and reason. At any given age, adolescents are at varying stages of cognitive, psychosocial, and biologic maturity. The more mature adolescent who can reason and think abstractly is better able to consider the consequences of taking or not taking nutritional supplements or of following and adhering to the prescribed medical and nutritional regimens that are necessary for lifelong success (e.g., maintenance of weight loss and prevention of avoidable nutritional complications) after bariatric procedures.68
Before any decision for an operation is made, all candidates should undergo a comprehensive psychological evaluation.69 Goals of this evaluation include:
• To determine the level of cognitive and psychosocial development, primarily to judge the extent to which the adolescent is capable of participating in the decision to proceed with the intervention
• To identify past and present psychiatric, emotional, behavioral, or eating disorders
• To define potential support for, or barriers to, regimen compliance, the family readiness for surgical treatment, and the required lifestyle changes (particularly if one or both parents are obese)
• To assess reasoning and problem-solving ability
• To assess whether reasonable outcome expectations exist
• To assess family unit stability and identify psychological stressors or conflicts within the family
• To determine whether the adolescent is autonomously motivated to consider bariatric surgical treatment or whether any element of coercion is present
Unfortunately, no ‘relative value scale’ exists that would enable one to assign appropriate significance to the wealth of information (subjective and objective) obtained in the psychological evaluation. However, during a comprehensive assessment, we have found that a complete psychological assessment is very helpful for team decision-making and that generally good team agreement is reached about whether a particular patient has a majority (or conversely a minority) of the attributes of a good candidate for bariatric surgery (Box 77-2).
Surgical Options
In 1991, the NIH Bariatric Consensus Development Conference established parameters that led to a more uniform application of bariatric operations for adults. After that conference, an increase in the use of these procedures occurred. This conference concluded that, at that time, insufficient data existed to make recommendations about bariatric surgical treatment for patients younger than 18 years. Fortunately, outcome data are emerging for the adolescent age group, and adolescent bariatric research is now receiving some attention at the NIH. Indeed, multiple studies with an adolescent bariatric focus have now been funded by the National Institute of Diabetes and Digestive and Kidney Diseases to examine various outcomes of adolescent weight loss surgery. The largest and most comprehensive study to date, a multicenter research consortium called Teen-Longitudinal Assessment of Bariatric Surgery (TEEN-Labs), has been formed that includes investigators at Cincinnati Children’s Hospital, Texas Children’s Hospital, Children’s Hospital of Alabama, Nationwide Children’s Hospital, and the University of Pittsburgh. Recruitment for this study is now complete with the anticipation of numerous outcome papers to be published from the data set in the very near future. More information about this research consortium can be obtained on the website www.cchmc.org/teen-LABS.
Of the many procedures that have been advocated for weight loss, the operations that have been used primarily can be classified as either purely restrictive or a combination of restriction and malabsorption (Fig. 77-2). The laparoscopic adjustable gastric band (AGB) and laparoscopic vertical sleeve gastrectomy (LSG) are purely restrictive procedures, and the degree of weight loss with these operations in adults has generally been satisfactory. The LSG is a relatively new operation that produces significant initial weight loss with low operative risk in both adult70,71 and pediatric studies.72 Because it likely does not affect micronutrient absorption, it may be a safe alternative with fewer nutritional risks than Roux-en-Y gastric bypass (RYGB), and also may avoid device-related long-term risks inherent in the AGB procedure.
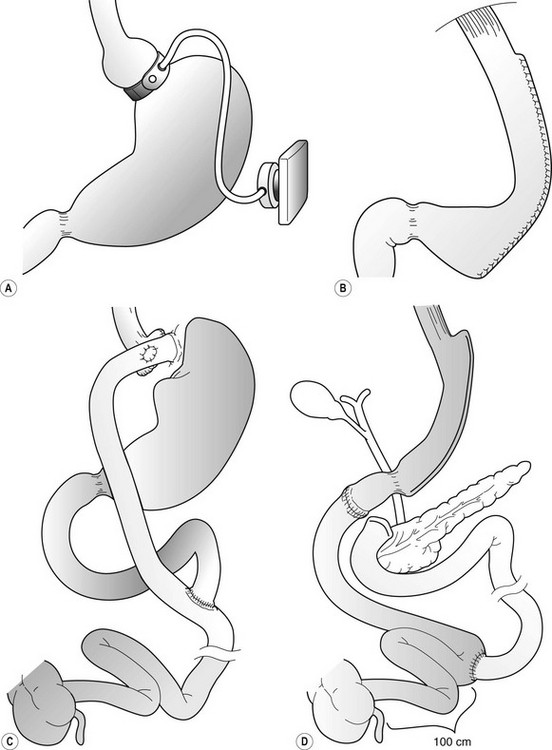
FIGURE 77-2 Operative procedures for weight loss that are performed laparoscopically. (A) Adjustable gastric band (AGB). (B) Vertical sleeve gastrectomy (VSG). (C) Roux-en-Y gastric bypass (RYGB). (D) Duodenal switch operation.
There are good reasons to think that adolescents may be better served by purely restrictive options such as the AGB73 (Figs 77-3 and 77-4) or LSG72 (Figs 77-5 and 77-6). Although nutritional deficiencies are not as likely to develop from the purely restrictive operations as compared with diversionary operations (gastric bypass, duodenal switch), these operations still impair overall energy intake significantly. This may lead to impaired intake of important vitamins and minerals if not adequately supplemented. With operations that do not transect the gastrointestinal tract, there is less risk and a reportedly lower mortality risk compared with gastric bypass.74 A specific patient group for which one might suspect that a purely restrictive operation would be well suited is the younger adolescent, or even preadolescent, with a significant, progressive comorbid condition (e.g., type 2 diabetes, OSAS, or pseudotumor cerebri). However, these patients have the greatest potential for noncompliance with postoperative nutritional recommendations.
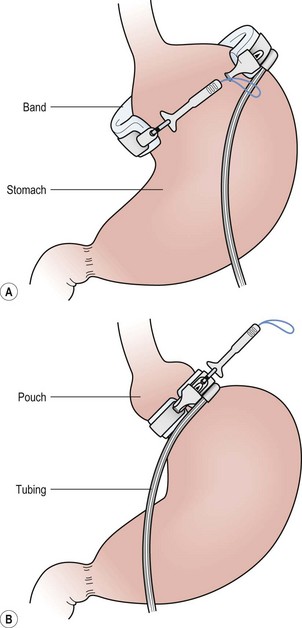
FIGURE 77-3 (A) After careful dissection in the retrogastric space, the adjustable gastric band is pulled behind the stomach with a grasping instrument. (B) The instrument is then placed through the buckle, and the locking portion is grasped and pulled through the buckle and locked in place.
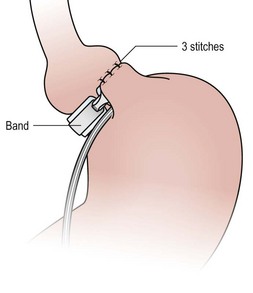
FIGURE 77-4 Plication of the anterior wall of the stomach over the gastric band is accomplished with three or four sutures using 2-0 permanent suture. These sutures should be deep and full thickness. It is important to remove the orogastric tube before placement of these sutures so that the tube is not incorporated in these plication sutures.
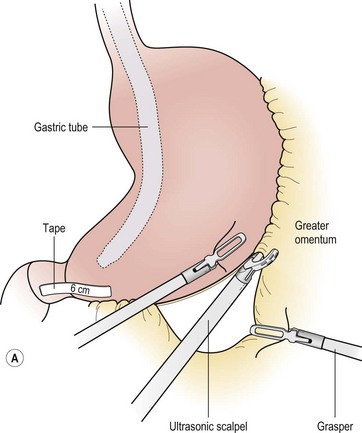
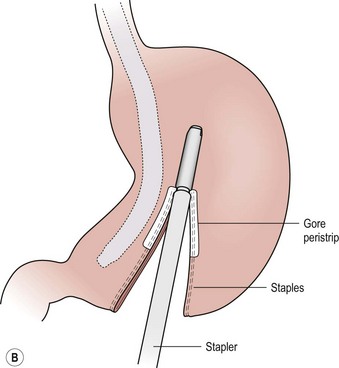
FIGURE 77-5 (A) For the LSG the greater omental attachments and the short gastric vessels are taken down along the entire extent of the greater curvature of the stomach using the ultrasonic scalpel. This dissection is started 6 cm from the end of the pylorus. (B) The endoscopic stapler, first using a green load and then a blue load, is used to ligate and divide the stomach. A 34 French bougie is inserted along the lesser curve of the stomach to act as a guide for resection.
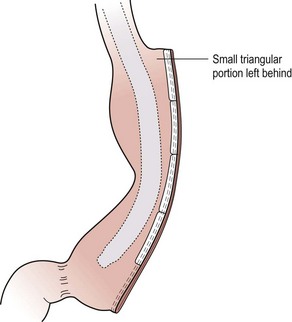
FIGURE 77-6 The completed sleeve gastrectomy is seen. Note that a small triangle of stomach is left near the gastroesophageal junction to help prevent any inadvertent resection or encroachment on the esophagus.
The AGB is not currently approved by the USA Food and Drug Administration (FDA) for patients younger than 18 years of age. A randomized control trial from Australia demonstrated a modest amount of weight loss at two years when compared to lifestyle intervention (BMI reduction of 28% vs 3%) but noted a high (33%) rate of reoperation.75 The theoretical benefits of nontransectional operations are lost when considering LSG owing to the long staple line. Both the AGB and the LSG must be viewed as procedures with less than adequate long-term outcome data in the adolescent population. Any patient undergoing these operations must be carefully counseled about the unknown long-term efficacy. Clinical outcome data is currently being collected prospectively to enable objective assessment of efficacy and safety.
The RYGB (Figs 77-7 to 77-9) consists of both a restrictive pouch and a mildly malabsorptive component (the bypass of the stomach and duodenum). Moreover, it also offers an additional negative reinforcement of ‘dumping syndrome’ in some patients, providing excellent weight loss in adolescents and adults.76 The partial biliopancreatic bypass with duodenal switch is primarily a malabsorptive procedure that results in good weight loss for adults with the highest classes of obesity (generally >60 kg/m2 BMI), but with higher risks of operative complications and postoperative nutritional problems. Thus, the duodenal switch is not widely recommended for adolescents.
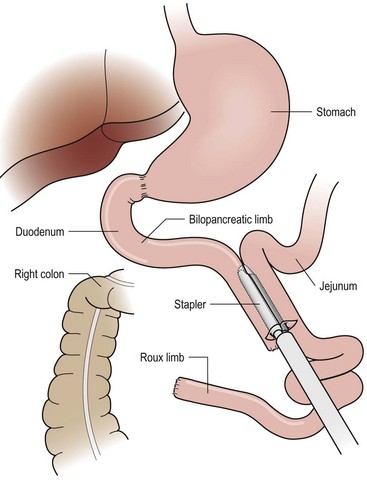
FIGURE 77-7 For the RYGB, a jejunostomy is created with a linear stapler connecting a 50 cm bilopancreatic limb and 100–150 cm roux limb as shown. The mesenteric defect is then closed to prevent herniation of bowel through this space.
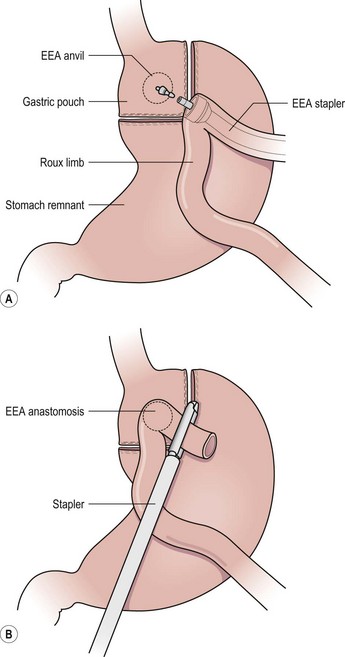
FIGURE 77-8 (A) A 30 mL gastric pouch is created along the lesser curve of the stomach just beyond the gastroesophageal junction using a linear cutting stapler with the anvil of the 25 mm EEA stapler in place. The Roux limb is brought anterior to the colon and gastric remnant. The end of the Roux limb is then opened and the EEA stapler is introduced, extended, and then mated with the anvil as shown to perform the gastrojejunostomy. (B) The open end of the roux limb is then stapled closed, thus completing the gastrojejunostomy.
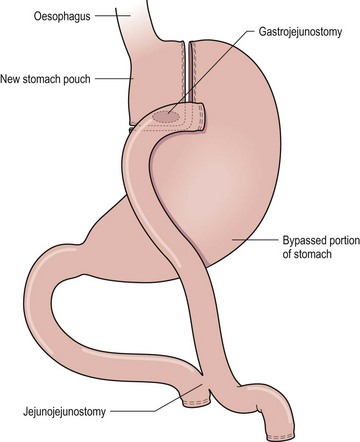
FIGURE 77-9 The Roux-en-Y gastric bypass consists of both a restrictive pouch size and a mildly malabsorptive component (the bypass of the stomach and duodenum). The gastric pouch is based on the lesser curve and is created using a 34 French orogastric tube as a guide. A 75–150 cm Roux limb is used in most patients.
Regardless of the procedure used, surgeons and allied health personnel new to the field should, at a minimum, undergo a basic training course offered by one of the professional surgical organizations (ASBS, ACS, or the Society of American Gastrointestinal Endoscopic Surgeons). Before performing laparoscopic bariatric operations, surgeons must meet all local credentialing requirements for the performance of bariatric procedures and advanced laparoscopic operations. Credentialing guidelines for both open and laparoscopic bariatric procedures have been outlined previously.77–81
Perioperative and Surgical Management
Preoperative Education and Management
The multidisciplinary preoperative evaluation that leads to the decision to offer surgical treatment is followed by considerable patient and family preoperative education. It is important that this process is organized and not rushed because patients must comprehend a great deal of information about the postoperative anatomic and physiologic changes that impact success as well as the risks for the short- and long-term complications. This process generally lasts a minimum of 6 months. Patients may also benefit from discussion with others who have undergone surgical treatment. At our institution, we find that support groups involving the patient’s own peers at various stages of the process provides the most benefit. In the weeks before the operation, a final outpatient visit for anesthesiology consultation, final informed permission (consent) discussion, and final review of the postoperative regimen is scheduled.
General Aspects of Surgical Techniques for the Morbidly Obese
In general, an open laparotomy should be avoided in morbidly obese individuals due to difficulty with adequate visualization and the higher risk of wound complications. For initial abdominal access, we have found that a laparoscopically guided technique using a transparent, bladeless, 12 mm cannula is safe and effective. Alternatively, some surgeons prefer a blind puncture using the Veress needle in the left upper quadrant. To access the gastroesophageal junction for bariatric procedures, a wide variety of surgical instruments have been developed with the morbidly obese patient in mind. However, it should be noted that the majority of laparoscopic procedures in morbidly obese individuals can be efficiently and safely accomplished using standard adult 5 mm instrumentation. The details and nuances of the procedures used for adolescents can be obtained from a variety of excellent bariatric texts.82,83
Postoperative Management
Bariatric operations reduce the intake and decrease the absorption of food rich in essential fatty acids, vitamins, and other specific nutrients, the long-term results of which are not well understood and are of legitimate concern. Poor nutrition during fetal development can result in a variety of adverse health outcomes, including future obesity, as suggested by the Dutch famine cohort.28 Therefore, success in adolescent bariatric surgical treatment requires an expanded definition—not only sustained weight loss but also subsequent normal progression through the remainder of adolescence, adulthood, and eventually uncomplicated reproduction with normal offspring.
Nutritional and metabolic consequences of bariatric operations have been well delineated in adults84–95 and adolescents.96 To avoid nutritional complications, patients must adhere to procedure-specific guidelines for diet and vitamin/mineral supplementation. Restrictive procedures (including gastric bypass) essentially require a very low-calorie intake, thus requiring attention to an adequate (0.5 g/kg) daily protein intake to minimize lean mass loss during the rapid weight loss phase (generally the first postoperative year). Impaired absorption of iron, folate, calcium, and vitamin B12 may occur after gastric bypass.87,96,97 Often obese adolescents will have vitamin deficiency even before operative intervention.96,98 Even with postoperative supplementation, severe deficiencies can occur. Adolescence also may be a particular risk for thiamine deficiency.99 In addition, poor postoperative compliance among adolescents who have undergone bariatric operations has been reported.100 As certain micronutrient deficiencies, such as folate and calcium, have established ramifications for the patient and potential offspring, both warrant special consideration.
Folate is a water-soluble B vitamin that is essential for growth, cell differentiation and embryonic morphogenesis, gene regulation, repair, and host defense.101 Adequate maternal periconceptional folic acid consumption during critical periods of organ formation early in the first trimester may reduce the likelihood of fetal malformations, including neural tube defects and perinatal complications such as low birth weight, prematurity, and placental abruption and infarction. This information is particularly relevant because the majority of adolescents seeking bariatric surgical treatment are girls, many of whom will want to be mothers. Thus, physicians caring for adolescents who undergo bariatric surgical procedures must stress the importance of daily folate and other B-complex vitamin intake. Moreover, patients should be monitored annually (and before planned conception) for serum vitamin levels, particularly when uncertainty about compliance exists.101
When compared to their obese counterparts, women who become pregnant after bariatric surgery appear to have improved fertility and lower maternal and neonatal complications.102,103 There are no studies examining outcomes of pregnancy after bariatric operations in the adolescent population. However, unplanned adolescent pregnancy is a legitimate concern after massive weight loss.104 We strongly recommend that all girls and caregivers are informed about increased fertility (a physiologic change due to increased insulin sensitivity) and the likelihood for increased risk-taking after surgical weight loss. These patients should be counseled to avoid pregnancy during the two-year period after operation and should be offered reliable contraception. The modern intrauterine devices are effective and safe, may be placed at the time of the bariatric operation, and are commonly used by our program.
Adolescence is a period of rapid skeletal mineral accretion and is, therefore, a window of opportunity to influence lifelong bone health, both positively and negatively. While obese adolescents have higher than average bone mineral density/content, they may well have less than normal bone mineral density and content for their weight. This may translate into greater risk for fractures.105 Furthermore, impaired accretion of bone mineral content in adolescence increases the risk for osteoporosis and results in a two-fold greater risk of fracture in later life.106 Given the impaired absorption of both vitamin D and calcium after bariatric surgical procedures and the large individual variation in bone accretion, it is essential to monitor closely the bone mineral density of adolescents undergoing bariatric procedures. Behavioral strategies can and should be used to encourage compliance with postoperative vitamin and mineral intake, which should positively influence nutritional outcomes after adolescent bariatric operations.106
Adolescent compliance may be enhanced by: (1) visual aids; (2) focus on immediate benefit from treatment; (3) participation in self-management; (4) self-monitoring; and (5) reinforcement (Box 77-3).107 With the alterations in eating patterns required after bariatric surgical procedures, repetitive reinforcement is needed to facilitate the formation of lifelong health-promoting habits. The adolescent bariatric surgical program should build on the best practices of other adolescent disease-management programs. Success will be based on the premise that sustained weight control for the adolescent requires ongoing behavioral intervention, structured family involvement, and continued support.106, 108–110
Long-Term Management
Postoperative follow-up visits after adolescent bariatric operations are intensive but depend in part on the type of operation performed. For example, we see gastric bypass and sleeve gastrectomy patients at 2 weeks, 6 weeks, 3 months, 6 months, 12 months, 18 months, 24 months, and then annually following operation. Dietary advancement after the first month is a methodical process of introducing new items of gradually increasing complexity toward the goal of a well-balanced, small portion (approximately one cup per meal) diet, which includes the daily intake of 0.5–1 g of protein per kilogram of ideal weight. Nonsteroidal anti-inflammatory medications should be avoided to reduce the risk of intestinal ulceration and bleeding after gastric bypass. Ranitidine is often prescribed postoperatively and switched to a proton pump inhibitor if reflux symptoms persist. This is often seen following sleeve gastrectomy, but these patients can often be weaned off by 6 months. Postoperative vitamin and mineral supplementation typically consists of two pediatric chewable multivitamins, a calcium/vitamin D supplement, and an iron supplement for menstruating females (Table 77-1). Due to the severity of thiamine deficiency, additional B-complex vitamins beyond what is contained in multivitamin preparations should be given.99 We routinely re-emphasize five basic ‘rules’ with each patient encounter: (1) eat protein first; (2) drink 64 to 96 ounces of water or sugar-free liquids daily; (3) do not snack between meals; (4) exercise 30 to 60 minutes per day; and (5) always remember vitamins and minerals.
TABLE 77-1
Recommended Nutritional Supplementation Following Weight Loss Surgery
| Supplement | Recommendation |
| Multivitamin (with folic acid) | One to two daily |
| Calcium citrate with vitamin D | 1500–1800 mg/day |
| Vitamin D | 1000 IU/day (if deficient preoperatively) |
| Vitamin B12 | 500 µg/day oral or 1000 µg/month intramuscular |
| Elemental iron | 65 mg elemental iron for menstruating females |
| Vitamin B1 | Consider 50 mg daily in first 6 months |
| Vitamin A, K | Treat if symptomatic |
Laboratories are checked at 6 months postoperatively and then yearly unless symptoms arise.
Adapted from Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am 2009;56:1105–21.
Outcomes in Adolescents
Results of RYGB have been retrospectively reviewed in small series of adolescents with generally satisfactory results.76,111–121 However, a 14-year follow-up of a small cohort of nine patients demonstrating considerable late weight re-gain suggests that adolescents may require different selection criteria or postoperative management than adults to achieve optimal long-term weight loss outcomes.7 A recent study from our institution demonstrated significant weight loss (decrease in BMI by 37% at one year) in patients following gastric bypass. It was noted that the timing of operation is an important consideration given that those patients with the highest BMI values, even with significant weight loss, still do not reach a nonobese nadir weight.76
Although the AGB is currently not approved by the FDA for adolescents younger than 18 years of age, there have been a few short-term studies. These studies show a modest amount of weight loss at 1 year, but long-term follow-up is lacking.122–124 A randomized trial from Australia, discussed earlier, demonstrated a decrease in BMI by 28% at two years compared to 3% in those treated medically, but with a 33% re-operative rate.75
Results for adolescents undergoing sleeve gastrectomy are also scarce with one reasonable size study published to date.72 This demonstrated comparable weight loss at 2 years to gastric bypass and an excellent safety profile. Although encouraging, more long-term studies are needed. The current TEEN-Labs trial hopes to answer many of the questions regarding all three operations while providing significant data in regards to the long-term weight loss and comorbidity reduction seen in these obese adolescents.
References
1. Freedman, DS, Mei, Z, Srinivasan, SR, et al. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr. 2007; 150:12–17.
2. Epstein, LH, Valoski, A, Wing, RR, et al. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990; 264:2519–2523.
3. Yanovski, JA. Intensive therapies for pediatric obesity. Pediatr Clin North Am. 2001; 48:1041–1053.
4. Spear, BA, Barlow, SE, Ervin, C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007; 120(Suppl 4):S254–S288.
5. Hearnshaw, C, Matyka, K. Managing childhood obesity: When lifestyle change is not enough. Diabetes Obes Metab. 2010; 12(11):947–957.
6. Wittgrove, AC, Clark, GW. Laparoscopic gastric bypass, Roux-en-Y in 500 patients: Technique and results, with 3–60 month follow-up. Obes Surg. 2000; 10:233–239.
7. Sugerman, HJ, Sugerman, EL, DeMaria, EJ, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003; 7:102–108.
8. Strauss, RS, Bradley, LJ, Brolin, RE. Gastric bypass surgery in adolescents with morbid obesity. J Pediatr. 2001; 138:499–504.
9. Ippisch, HM, Inge, TH, Daniels, SR, et al. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008; 51:1342–1348.
10. Zeller, MH, Noll, JG, Long, JD, Inge, TH. Psychosocial functioning improves following adolescent bariatric surgery. Obesity. 2009; 17:985–990.
11. Inge, TH, Miyano, G, Bean, J, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009; 123:214–222.
12. Himes, JH, Dietz, WH. Guidelines for overweight in adolescent preventive services: Recommendations from an expert committee. The Expert Committee on Clinical Guidelines for Overweight in Adolescent Preventive Services. Am J Clin Nutr. 1994; 59:307–316.
13. Daniels, SR, Khoury, PR, Morrison, JA. The utility of body mass index as a measure of body fatness in children and adolescents: Differences by race and gender. Pediatrics. 1997; 99:804–807.
14. Pietrobelli, A, Faith, MS, Allison, DB, et al. Body mass index as a measure of adiposity among children and adolescents: A validation study. J Pediatr. 1998; 132:204–210.
15. Dietz, WH, Robinson, TN. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr. 1998; 132:191–193.
16. Cole, TJ, Bellizzi, MC, Flegal, KM, et al. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ. 2000; 320:1240–1243.
17. Rolland-Cachera, MF, Sempe, M, Guilloud-Bataille, M, et al. Adiposity indices in children. Am J Clin Nutr. 1982; 36:178–184.
18. Flega, KM, Wei, R, Ogden, CL, et al. Characterizing extreme values of body mass index-for-age by using the 2000 centers for disease control and prevention growth charts. Am J Clin Nutr. 2009; 90:1314–1320.
19. Strauss, RS, Pollack, HA. Epidemic increase in childhood overweight, 1986–1998. JAMA. 2001; 286:2845–2848.
20. Ogden, CL, Flegal, KM, Carroll, MD, et al. Prevalence and trends in overweight among ultrasound children and adolescents, 1999–2000. JAMA. 2002; 288:1728–1732.
21. Ogden, CL, Carroll, MD, Curtin, LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006; 295:1549–1555.
22. Whitaker, RC, Wright, JA, Pepe, MS, et al. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997; 337:869–873.
23. Vanhala, M, Vanhala, P, Kumpusalo, E, et al. Relation between obesity from childhood to adulthood and the metabolic syndrome: Population-based study. BMJ. 1998; 317:319–320.
24. Inge, TH. Bariatric surgery for morbidly obese adolescents: Is there a rationale for early intervention? Growth Horm IGF Res. 2006; 16:S15–S19.
25. Must, A, Strauss, RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999; 23:S2–11.
26. Nadler, EP, Brotman, LM, Miyoshi, T, Fryer, GE, Weitzman, M. Morbidity in obese adolescents who meet the adult national institutes of health criteria for bariatric surgery. J Pediatr Surg. 2009; 44:1869–1876.
27. Wahlqvist, ML. Chronic disease prevention: A life-cycle approach which takes account of the environmental impact and opportunities of food, nutrition and public health policies—the rationale for an eco-nutritional disease nomenclature. Asia Pac J Clin Nutr. 2002; 11:S759–S762.
28. Ravelli, AC, van Der Meulen, JH, Osmond, C, et al. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999; 70:811–816.
29. Parsons, TJ, Power, C, Logan, S, et al. Childhood predictors of adult obesity: A systematic review. Int J Obes Relat Metab Disord. 1999; 23:S1–107.
30. Sorensen, HT, Sabroe, S, Rothman, KJ, et al. Relation between weight and length at birth and body mass index in young adulthood: Cohort study. BMJ. 1997; 315:1137.
31. Wei, JN, Li, HY, Sung, FC, et al. Birth weight correlates differently with cardiovascular risk factors in youth. Obesity (Silver Spring). 2007; 15:1609–1616.
32. Bergmann, KE, Bergmann, RL, Von Kries, R, et al. Early determinants of childhood overweight and adiposity in a birth cohort study: Role of breast-feeding. Int J Obes Relat Metab Disord. 2003; 27:162–172.
33. Michels, KB, Willett, WC, Graubard, BI, et al. A longitudinal study of infant feeding and obesity throughout life course. Int J Obes (Lond). 2007; 31:1078–1085.
34. He, Q, Karlberg, J. Probability of adult overweight and risk change during the BMI rebound period. Obes Res. 2002; 10:135–140.
35. Cameron, N, Demerath, EW. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol. 2002; 35:159–184.
36. Whitaker, RC, Pepe, MS, Wright, JA, et al. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998; 101:E5.
37. Eriksson, JG, Forsen, T, Tuomilehto, J, et al. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia. 2003; 46:190–194.
38. Rolland-Cachera, MF, Deheeger, M, Bellisle, F, et al. Adiposity rebound in children: A simple indicator for predicting obesity. Am J Clin Nutr. 1984; 39:129–135.
39. Silverman, BL, Rizzo, TA, Cho, NH, et al. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998; 21:B142–B149.
40. Jouret, B, Ahluwalia, N, Cristini, C, et al. Factors associated with overweight in preschool-age children in southwestern France. Am J Clin Nutr. 2007; 85:1643–1649.
41. Caprio, S. Insulin resistance in childhood obesity. J Pediatr Endocrinol Metab. 2002; 15:487–492.
42. Heald, FP, Khan, MA. Teenage obesity. Pediatr Clin North Am. 1973; 20:807–817.
43. Ong, KK, Northstone, K, Wells, JC, et al. Earlier mother’s age at menarche predicts rapid infancy growth and childhood obesity. PLoS Med. 2007; 4:e132.
44. Whitaker, RC, Wright, JA, Pepe, MS, et al. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997; 337:869–873.
45. Whitaker, RC. Understanding the complex journey to obesity in early adulthood. Ann Intern Med. 2002; 136:923–925.
46. Durand, EF, Logan, C, Carruth, A. Association of maternal obesity and childhood obesity: Implications for healthcare providers. J Community Health Nurs. 2007; 24:167–176.
47. Santiago, S, Zazpe, I, Cuervo, M, Martinez, JA. Perinatal and parental determinants of childhood overweight in 6–12 years old children. Nutr Hosp. 2012; 27:599–605.
48. Farooqi, IS, O’Rahilly, S. Recent advances in the genetics of severe childhood obesity. Arch Dis Child. 2000; 83:31–34.
49. Clement, K, Boutin, P, Froguel, P. Genetics of obesity. Am J Pharmacogenomics. 2002; 2:177–187.
50. Haqq, AM, Farooqi, IS, O’Rahilly, S, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003; 88:174–178.
51. Fontaine, KR, Redden, DT, Wang, C, et al. Years of life lost due to obesity. JAMA. 2003; 289:187–193.
52. Must, A, Jacques, PF, Dallal, GE, et al. Long-term morbidity and mortality of overweight adolescents: A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992; 327:1350–1355.
53. Biro, FM, Wien, M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010; 91(Suppl.):S1499–S1505.
54. The, N, Suchindran, C, North, KE, Popkin, BM, Gordon-Larsen, P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010; 304:2042–2047.
55. Reilly, JJ, Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int J Obes (Lond). 2011; 35(7):891–898.
56. Strauss, RS. Childhood obesity. Pediatr Clin North Am. 2002; 49:175–201.
57. Gortmaker, SL, Must, A, Perrin, JM, et al. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993; 329:1008–1012.
58. Wang, G, Dietz, WH. Economic burden of obesity in youths aged 6 to 17 years: 1979–1999. Pediatrics. 2002; 109:E81.
59. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991; 115:956–961.
60. O’Brien, PE, Dixon, JB, Laurie, C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: A randomized trial. Ann Intern Med. 2006; 144:625–633.
61. Pratt, JS, Lenders, CM, Dionne, EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring). 2009; 17(5):901–910.
62. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992; 55:S615–S619.
63. Inge, TH, Garcia, VF, Daniels, SR, et al. A multidisciplinary approach to the adolescent bariatric surgical patient. J Pediatr Surg. 2004; 39:442–447.
64. Tanner, JM. Growth at Adolescence, 2nd ed. Oxford: Blackwell Scientific Publications; 1962.
65. Marshall, WA, Tanner, JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970; 45:13–23.
66. Greulich, WW, Pyle, SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. Palo Alto, CA: Stanford University Press; 1959.
67. Tanner, JM. Assessment of Skeletal Maturity and Prediction of Adult Height (TW2 method). San Diego, CA: Academic Press; 1983.
68. Piaget, J. The stages of the intellectual development of the child. Bull Menninger Clin. 1962; 26:120–128.
69. Greenberg, I, Sogg, S, Perna, FM. Behavioral and psychological care in weight loss surgery: Best practice update. Obesity. 2009; 17:880–884.
70. Aggarwal, S, Kini, SU, Herron, DM. Laparoscopic sleeve gastrectomy for morbid obesity: A review. Surg Obes Relat Dis. 2007; 3:189–194.
71. Cottam, D, Qureshi, FG, Mattar, SG, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006; 20:859–863.
72. Alqahtani, AR, Antonisamy, B, Alamri, H, et al. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg. 2013; 256:266–273.
73. Dolan, K, Creighton, L, Hopkins, G, et al. Laparoscopic gastric banding in morbidly obese adolescents. Obes Surg. 2003; 13:101–104.
74. Chapman, A, Game, P, O’Brien, P, et al. Laparoscopic adjustable gastric banding for the treatment of obesity: A systematic literature review. Surgery. 2004; 135:326–351.
75. O’Brien, PE, Sawyer, SM, Laurie, C, et al. Laparoscopic adjustable gastric banding in severely obese adolescents. JAMA. 2010; 303:519–526.
76. Inge, TH, Jenkins, TM, Zeller, M, et al. Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. J Pediatr. 2010; 156:103–108.
77. Martin, LF, O’Leary, JP. Standards of excellence for bariatric surgery: Great concept, but how? Obes Surg. 1998; 8:229–231.
78. Buchwald, H. Mainstreaming bariatric surgery. Obes Surg. 1999; 9:462–470.
79. Al-Saif, O, Gallagher, SF, Banasiak, M, et al. Who should be doing laparoscopic bariatric surgery? Obes Surg. 2003; 13:82–87.
80. Guidelines for laparoscopic and open surgical treatment of morbid obesity: American Society for Bariatric Surgery and Society of American Gastrointestinal Endoscopic Surgeons. Obes Surg. 2000; 10:378–379.
81. Oria, HE, Brolin, RE. Performance standards in bariatric surgery. Eur J Gastroenterol Hepatol. 1999; 11:77–84.
82. Inge, TH. Laparoscopic Roux-en-Y gastric bypass. In: Holcomb GW, III., Georgeson KE, Rothenberg SS, eds. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008:181–187.
83. Barnett, SJ, Inge, TH, Bariatric surgery: PrinciplesSpitz L, Coran A, eds. Operative Pediatric Surgery. 7th ed, 2013.
84. Gollobin, C, Marcus, WY. Bariatric beriberi. Obes Surg. 2002; 12:309–311.
85. Chaves, LC, Faintuch, J, Kahwage, S, et al. A cluster of polyneuropathy and Wernicke-Korsakoff syndrome in a bariatric unit. Obes Surg. 2002; 12:328–334.
86. Amaral, JF, Thompson, WR, Caldwell, MD, et al. Prospective hematologic evaluation of gastric exclusion surgery for morbid obesity. Ann Surg. 1985; 201:186–193.
87. Halverson, JD. Vitamin and mineral deficiencies following obesity surgery. Gastroenterol Clin North Am. 1987; 16:307–315.
88. Halverson, JD. Micronutrient deficiencies after gastric bypass for morbid obesity. Am Surg. 1986; 52:594–598.
89. MacLean, LD, Rhode, BM, Shizgal, HM. Nutrition following gastric operations for morbid obesity. Ann Surg. 1983; 198:347–355.
90. Mason, EE. Starvation injury after gastric reduction for obesity. World J Surg. 1998; 22:1002–1007.
91. Schilling, RF, Gohdes, PN, Hardie, GH. Vitamin B12 deficiency after gastric bypass surgery for obesity. Ann Intern Med. 1984; 101:501–502.
92. Boylan, LM, Sugerman, HJ, Driskell, JA. Vitamin E, vitamin B-6, vitamin B-12, and folate status of gastric bypass surgery patients. J Am Diet Assoc. 1988; 88:579–585.
93. Lynch, RJ, Eisenberg, D, Bell, RL. Metabolic consequences of bariatric surgery. J Clin Gastroenterol. 2006; 40:659–668.
94. Shikora, SA, Kim, JJ, Tarnoff, ME. Nutrition and gastrointestinal complications of bariatric surgery. Nutr Clin Pract. 2007; 22:29–40.
95. Tucker, ON, Szomstein, S, Rosenthal, RJ. Nutritional consequences of weight-loss surgery. Med Clin North Am. 2007; 91:499–514.
96. Xanthakos, SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am. 2009; 56:1105–1121.
97. Alvarez-Leite, JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004; 7:569–575.
98. Carrodeguas, L, Kaidar-Person, O, Szomstein, S, et al. Preoperative thiamine deficiency in obese population undergoing laparoscopic bariatric surgery. Surg Obes Relat Dis. 2005; 1:517–522.
99. Towbin, A, Inge, TH, Garcia, VF, et al. Beriberi after gastric bypass surgery in adolescence. J Pediatr. 2004; 145:263–267.
100. Rand, CS, Macgregor, AM. Adolescents having obesity surgery: A 6-year follow-up. South Med J. 1994; 87:1208–1213.
101. Hall, JG, Solehdin, F. Folate and its various ramifications. Adv Pediatr. 1998; 45:1–35.
102. Guelinckx, I, Devlieger, R, Vansant, G. Reproductive outcome after bariatric surgery: A critical review. Hum Reprod Update. 2009; 15:189–201.
103. Maggard, MA, Yermilov, I. Pregnancy and fertility following bariatric surgery: A systematic review. JAMA. 2008; 300:2286–2296.
104. Roehrig, HR, Xanthakos, SA, Sweeney, J, et al. Pregnancy after gastric bypass surgery in adolescents. Obes Surg. 2007; 17:873–877.
105. Goulding, A, Taylor, RW, Jones, IE, et al. Spinal overload: A concern for obese children and adolescents? Osteoporos Int. 2002; 13:835–840.
106. Wysocki, T, Greco, P, Harris, MA, et al. Behavior therapy for families of adolescents with diabetes: Maintenance of treatment effects. Diabetes Care. 2001; 24:441–446.
107. Rapoff MA, et al, eds. Compliance with Pediatric Medical Regimens. New York: Raven Press, 1991.
108. Zeller, MH, Guilfoyle, SM, Reiter-Purtill, J, et al. Adolescent bariatric surgery: Caregiver and family functioning across the first postoperative year. Surg Obes Relat Dis. 2011; 7:145–150.
109. Fielding, D, Duff, A. Compliance with treatment protocols: Interventions for children with chronic illness. Arch Dis Child. 1999; 80:196–200.
110. Rapoff, MA. Assessing and enhancing adherence to medical regimens for juvenile rheumatoid arthritis. Pediatr Ann. 2002; 31:373–379.
111. Jen, HC, Rickard, DG, Shew, SB, et al. Trends and outcomes of adolescent bariatric surgery in California, 2005–2007. Pediatrics. 2010; 126:e746–e753.
112. Breaux, CW. Obesity surgery in children. Obes Surg. 1995; 5:279–284.
113. Strauss, RS, Bradley, LJ, Brolin, RE. Gastric bypass surgery in adolescents with morbid obesity. J Pediatr. 2001; 138:499–504.
114. Sugerman, HJ, Sugerman, EL, DeMaria, EJ, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003; 7:102–107.
115. Greenstein, RJ, Rabner, JG. Is adolescent gastric-restrictive antiobesity surgery warranted? Obes Surg. 1995; 5:138–144.
116. Anderson, AE, Soper, RT, Scott, DH. Gastric bypass for morbid obesity in children and adolescents. J Pediatr Surg. 1980; 15:876–881.
117. Soper, RT, Mason, EE, Printen, KJ, et al. Gastric bypass for morbid obesity in children and adolescents. J Pediatr Surg. 1975; 10:51–58.
118. Rand, CS, Macgregor, AM. Adolescents having obesity surgery: A 6-year follow-up. South Med J. 1994; 87:1208–1213.
119. Inge, TH, Garcia, V, Daniels, S, et al. A multidisciplinary approach to the adolescent bariatric surgical patient. J Pediatr Surg. 2004; 39:442–447.
120. Lawson, ML, Kirk, S, Mitchell, T, et al. One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: A multicenter study from the Pediatric Bariatric Study Group. J Pediatr Surg. 2006; 41:137–143.
121. Barnett, SJ, Stanley, C, Hanlon, M, et al. Long-term follow-up and the role of surgery in adolescents with morbid obesity. Surg Obes Relat Dis. 2005; 1:394–398.
122. Ananthapavan, J, Moodie, M, Haby, M, et al. Assessing cost-effectiveness in obesity: Laparoscopic adjustable gastric banding for severely obese adolescents. Surg Obes Relat Dis. 2010; 6:377–385.
123. Holterman, AX, Browne, A, Tussing, L, et al. A prospective trial for laparoscopic adjustable gastric banding in morbidly obese adolescents: An interim report of weight loss, metabolic and quality of life outcomes. J Pediatr Surg. 2010; 45:74–78.
124. de la Cruz-Munoz, N, Messiah, SE, Cabrera, JC, et al. Four-year weight outcomes of laparoscopic gastric bypass surgery and adjustable gastric banding among multiethnic adolescents. Surg Obes Relat Dis. 2010; 6:542–547.