Bariatric surgery
Introduction
Only a few operations have stood the test of time. The original jejuno-ileal bypass was abandoned due to the blind loop syndrome and resulting liver and bone metabolism problems. The vertical banded gastroplasty (VBG) has been superseded by gastric banding due to the advantage of being able to adjust the size of the gastric stoma. The gastric bypass has evolved from a continuously stapled horizontal gastroplasty and loop gastroenterostomy into a smaller pouch with a Roux-en-Y reconstruction.1,2 The Magenstrasse and Mill procedure has evolved into the sleeve gastrectomy, an operation that is rapidly being taken up worldwide due to its perceived ease and lesser risk compared to gastric bypass.3 Other procedures specifically for type 2 diabetes, such as duodeno-jejunal bypass and a temporary implanted endoscopic sheath that prevents absorption in the duodenum and proximal jejunum (‘Endobarrier’), are being evaluated. The revolution of laparoscopic surgery has allowed most bariatric surgery to be performed laparoscopically with low mortality and morbidity. As a result, bariatric surgery is now established as a cost-effective intervention that should be increasingly provided.
Obesity as a public health problem
Obesity is a chronic, relapsing, debilitating, lifelong disease, recognised by the World Health Organisation as a global pandemic.4 The influential Foresight report estimated that by 2010 28% of women and 33% of men in the UK would be obese, rising to 50% of women and 60% of men and, even more worrying, 25% of children as well by 2050.5 The definitions of obesity are shown in Table 20.1. The term ‘obesogenic environment’ was used to describe the ‘results of people responding normally to the obesogenic environments they find themselves in’, given the current trends of reduced physical activity and easily available, highly advertised, relatively cheap, energy-dense foods.6 An example of an obesogenic food environment is the observation that the price per calorie of healthy foods (whole grains, lean meats, low fat dairy, fruit and vegetables) increased to eight times the equivalent price of unhealthy foods (sweets, calorific drinks and fatty foods) between 2004 and 2008 in Seattle, USA.7 Even payment methods have been implicated in unhealthy food choices: in a study in Buffalo, USA, buying of unhealthy compared to healthy foods significantly increased when the payment method was by card compared to cash (P < 0.01).8
Table 20.1
Definition of obesity according to body mass index
| BMI (kg/m2) | WHO classification | Common clinical description |
| 18.5–24.9 | Normal range | Desirable |
| 25–29.9 | Pre-obese | Overweight |
| 30–34.9 | Obese class I | Obese |
| 35–39.9 | Obese class II | Clinically severe and complex obesity |
| 40–49.9 | Obese class III | |
| 50 and over | Super-obesity |
Adapted from Dixon JB, Zimmet P, Alberti KG et al. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med 2011; 28: 628-642.
The association between obesity and the metabolic syndrome of type 2 diabetes, hypertension, sleep apnoea and polycystic ovarian syndrome is well recognised.11 These, together with other harmful obesity-related comorbid disease such as non- alcoholic steatohepatosis, asthma, back and lower limb degenerative problems, cancer and depression, present a massive financial burden on health services.
In addition, obese people are known to have higher unemployment and higher rates of claiming state benefit, and consume a disproportionate amount of the healthcare budget.12,13
The traditional advice ‘eat less and exercise more’ given to patients, based on understanding of the basic energy equation, does not work in the majority: a large study in the USA of 107 000 individuals using the Behavioural Risk Factor Surveillance System found that 63% of men and 78% of women were attempting to lose weight or maintain weight at any one time. However, 34% of men and 40% of women maintained the same energy intake despite reducing fats, and only 1 in 5 of those trying to control their weight were able to eat fewer calories and do 150 minutes exercise per week.14
In chronic obesity it is recognised that patients have usually missed the boat of prevention.15 Most patients are able to diet to some extent but nearly always reach a plateau; thus a typical pattern in someone who has already become obese is a lifetime of repeated dieting and weight regain.
It is thus not surprising that the worldwide rate of bariatric surgery has increased dramatically. In the USA, the most obese nation, the volume of surgery was 136 000 operations in 2004, while the American Society for Bariatric and Metabolic Surgery (ASMBS) estimated that 220 000 procedures were carried out in 2008.18 This figure is similar to cholecystectomy, and at this rate of surgery the UK would be doing more than 50 000 operations per year.
Worldwide the commonest bariatric procedure is the Roux-en-Y gastric bypass (RYGB). Data from the UK and Ireland National Bariatric Surgery Registry (NBSR) indicated that in 6483 operations in 2009–10, 67% of the NHS patients had a gastric bypass, 21% gastric banding and 10.5% a sleeve gastrectomy.19 In Europe the rate of gastric banding fell during the 2000s, having far exceeded gastric bypass during the 1990s.20 By contrast, the popularity for banding increased in the USA after the FDA granted approval in 2001, and currently is estimated to exceed bypass (46% vs. 44%), with sleeve gastrectomy at 7.8%.21
Diabetes risk with obesity
Type 2 diabetes is the most important comorbid disease of obesity, affecting around 280 million people, a figure that is likely to rise to 438 million by 2030.22 About 85% of type 2 diabetics are obese and the increased risk of becoming diabetic is estimated to be 93-fold for women and 42-fold for men who are severely obese.23 Medical treatment cannot stop diabetes from progressing and thus the epidemic is one of the largest threats to global health in the 21st century.
Cancer risk with obesity
Obesity is the second most common cause of preventable cancer after smoking. In data from the Cancer Prevention Study II in the USA from 1982 to 1998, the relative risk (RR) of death from cancer increased above a BMI of 30 for both men and women.24 In 900 000 people the risk of most, if not all, cancers was increased in non-smokers.25 For women with BMI > 40, there was an RR of 6.25 for uterine cancer, and the overall RR was 2.51 for other cancers, in particular kidney, cervix and pancreas. For men with BMI > 35, there was an RR of 4.52 for liver cancer, and for BMI > 30 the RR was 1.68 for all other cancers. Thus the impact of obesity appears to be important for most, if not all, organs.
Psychosocial morbidity and prejudice
Up to 60% of bariatric patients have psychiatric disorders and substantial psychiatric comorbidity.26 They also have impaired self-esteem, lower quality of life, and more depression and anxiety than the general population. About one-third are victims of sexual child abuse. Anti-obesity stereotypes are common in society and also in healthcare professionals: the obese are regarded as ‘lazy, unmotivated and non-compliant’, they ‘lack self-discipline’, and they face discrimination and prejudice.26 Obesity is regarded as being ‘self-inflicted’ and is associated with ‘moral failure and guilt’, underlining the need for sensitivity and non-judgmental approaches to management.
Baseline obesity-related disease
Data from the NBSR
In common with the international literature, a very high proportion (80.1%) of patients undergoing bariatric surgery were female. It is not known why so few men have surgery, but men were older, heavier and had more comorbidity. For example, 43% of the men were diabetic, 50% had hypertension, 28% had dyslipidaemia and 37% had sleep apnoea (P < 0.001). The average BMI was 50.6 compared to 47.7 in females. The differences are not simply explained by the older age of males, since on age group comparison males age 40–49 had a median of three comorbidities compared to a median of two in females of the same age group (P < 0.001).19
Multidisciplinary work-up
The team should include bariatric physicians, dieticians, nurse specialists, psychologists, anaesthetists and surgeons, and support groups.29–31 Current National Institute for Clinical Excellence (NICE) guidelines, which were based on National Institutes of Health guidelines (1991), are shown in Box 20.1. It is not known what proportion of patients who fulfil the weight threshold for surgery would ever be suitable as patients must show that they are engaged and committed to the process and this is difficult to measure. Some believe that demonstration of weight loss before surgery is necessary to gauge this, but the evidence base for this is poor or lacking.28 Rare endocrine causes should be excluded. Current drug or alcohol misuse and uncontrolled psychiatric disease are regarded as contraindications, as are a ‘lack of comprehension of risks and lifestyle changes required’, which might exclude patients with learning difficulties.20,28 Patients should not be operated on when pregnant.28 Crohn’s disease is a relative contraindication and portal hypertension is an absolute contraindication.20
Optimisation of patients
Multidisciplinary teams should ensure that medical comorbidities can be identified and treated, so that high-risk patients with multiple medical comorbidities can be made as fit as possible before surgery.28,30 This includes indentifying and treating obstructive sleep apnoea before surgery, and careful perioperative control of diabetes.23 It is recommended that patients stop smoking at least 8 weeks before surgery.28 Medications for epilepsy should be considered carefully as the absorption may be insufficient after gastric bypass or duodenal switch.
Obesity Surgery–Mortality Risk score (OS-MRS)
Thus a male patient with high BMI and hypertension, both of which are associated with central obesity, is likely to be more difficult to operate on due to the extra torque on laparoscopic instruments. Usual practice, in addition to medical work-up, is to put patients on a ‘liver shrinkage diet’ for at least 2 weeks before surgery, as this has been shown to reduce liver size, and probably reduces torque in central obesity (Grade B recommendation).28,33 Weight reduction before surgery may also downgrade the risk group.
Current bariatric operations and surgical techniques
Gastric bypass
There are several laparoscopic techniques described and there is as yet no standardisation. However, the weight loss results appear very much the same. Most agree that a short vertical lesser curvature-based gastric pouch of no more than 5–6 cm, that is separated from fundus, should be constructed, as this has been shown by MacLean and colleagues to produce weight loss over 15 years.2 Starting just below the oblique fat pad on the lesser curve and usually taking the second branch of the left gastric artery, the pouch is made with linear staplers directed transversely over 2–3 cm then vertically up to just to the left of the angle of His, staying to the right of the posterior gastric artery if identified. Routing of the Roux limb can be retro- or antecolic and particular attention must be paid to the correct identification of the bowel limb to prevent ‘Roux-en-O’34,35 (Fig. 20.1).
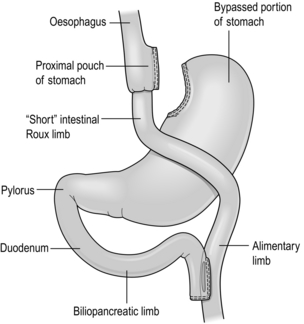
Figure 20.1 Gastric bypass showing short vertical lesser curve-based gastric pouch with Roux-en-Y jejuno-jejunostomy.
In open surgery exact calibration of the diameter of the anastomosis of the Roux limb to the gastric pouch was thought to be critical to the rate of emptying and thus weight loss after the operation. However, as for banded gastric bypass (Fobi/Capella), there were no good data to support this.36,37 In fact, an early study by Naslund in Sweden of 29 patients found that between-patient variation in the size of the gastric outlet did not correlate with weight loss 1 year after bypass.38 In the UK NBSR, the techniques of pouch-enterostomy are reported as 39% linear stapler with suture closure of the defect (after Lonroth), 23% circular stapler (after Wittgrove) and 38% hand sewn(after Higa).19,34,35,39
The lengths of the biliary and Roux limbs are not standardised and are impossible to measure accurately but in the US literature the biliary limb length is not usually mentioned since the assumption is that it is ‘kept short’ in order to reduce malabsorption of vitamins and minerals. The commonest description of the Roux limb is around 100–150 cm, after Brolin et al. in New Jersey and MacLean and colleagues in Montreal.2,40 Limited evidence suggests that a longer Roux limb of 150 cm gives more weight loss for BMI > 50, but this is not maintained in the long term. In standard bypass protein/calorie malabsorption is not the mechanism of weight loss. Since the common channel length is the determinant of available gut for absorption, and this is never measured in standard bypass, it is unlikely that longer lengths will ever be studied because of the worse nutritional consequences that would ensue.41
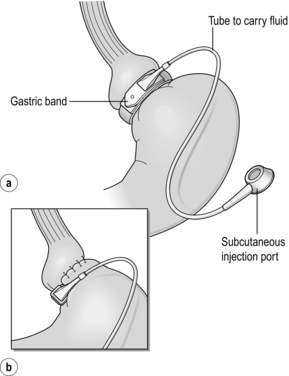
Gastric banding
The early perigastric approach for tunnelling the band posteriorly has now been abandoned as it is associated with high rates of band erosion into the stomach. The pars flaccida technique (through the window of the lesser omentum) is now preferred, keeping above the lesser omental bursa posteriorly. A band placed around the gastro-oesophageal junction would produce weight loss by dysphagia, and correct placement is just below, producing a small ‘virtual pouch’ of gastric mucosa above the band20 (Fig. 20.2).
The access port is usually sutured to the rectus sheath in the upper abdomen for ease of access by a non-coring, Huber needle for band adjustments. Placement of the band is just the start of the process of weight loss. Adjustments of the band are made by injecting saline progressively in order to reach the so-called ‘sweet spot’ of optimal restriction. A patient should probably be offered monthly clinic visits, at least for the first year. One study found that patients who attended seven or more clinics achieved 50% EWL in the first year compared to only 42% EWL in those who attended six times or fewer (P < 0.01).42 The ability to provide good follow-up should be part and parcel of the process, but is often lacking.20
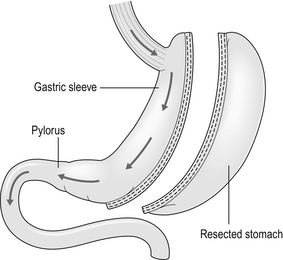
Sleeve gastrectomy
Technically simpler than gastric bypass, the operation evolved from the Magenstrasse and Mill operation described by Johnston in Leeds, where the divided fundus (the ‘mill’) was left in continuity with the lesser curve-based tube (the ‘main street’).3 In the sleeve the lesser curve-based tube is constructed over a size 32 or 34Fr bougie. The dissection uses linear stapling devices and starts 3–6 cm proximal to the pylorus upwards to just lateral to the angle of His.43 Some descriptions of the sleeve now remove more of the antrum, leaving a true sleeve upwards from the pylorus (Fig. 20.3).
Sleeve gastrectomy is seen as a less risky alternative to gastric bypass, and a number of trials have now been reported (see below). The ASMBS issued an update statement on sleeve gastrectomy in October 2011, summarising the current data.44 Himpens et al. from Belgium have the largest published series, with 5-year follow-up in which they found that 30 sleeve patients had 77.5% EWL at 3 years and 53.3% EWL at 6 years.45 However, the starting BMI was only 39.9 and an additional 11 patients had a further bariatric procedure during follow-up, including ‘re-sleeve’ because of weight regain. Although there is much enthusiasm for sleeve gastrectomy, the lack of long-term studies and demonstrable superiority compared to both gastric bypass and banding, together with the concern about weight regain due to expansion of the sleeve, mean this operation is unlikely to replace either in the foreseeable future. The longest follow-up so far reported is 8–9 years, when 13 patients with an initial BMI of 45.8 had 68% EWL.46
Biliopancreatic diversion/duodenal switch
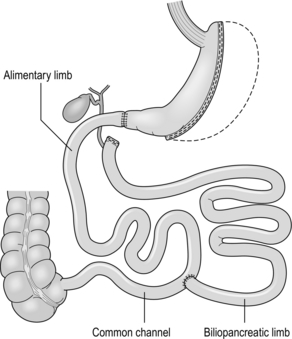
Although the biliopancreatic diversion (BPD) described by Scopinaro in Naples produces greater weight loss than gastric bypass or banding, it requires very careful nutritional follow-up due to malabsorption, which is the mechanism of action of the operation.31 BPD appears to be done rarely; however, its duodenal switch (DS) variant, combined with sleeve gastrectomy, is performed in a few centres.47 DS is increasingly seen as a rescue operation for weight regain after sleeve gastrectomy.45 All patients after DS need a high-protein diet and regular vitamin and mineral supplements with monitoring for life.
The DS variant of the BPD involves a sleeve gastrectomy followed by division of the duodenum just distal to the pylorus. The ileum is divided with a linear stapler and a duodeno-ileostomy and ileo-ileostomy are made such that the common channel for food absorption measures 75–125 cm and the alimentary channel measures 100–250 cm. The long remaining biliary limb is not measured47 (Fig. 20.4).
Mechanisms of bypass and banding
Both bypass and banding appear to control appetite and satiety by physiological mechanisms. In a blinded crossover trial of filling/de-filling of gastric bands, Dixon et al. demonstrated that satiety before and after eating was greater when the band was filled than when not.48 It is proposed that stimulation of vagal afferent fibres adjacent to the band mediates the feeling of satiety, as there is no marked change, if any, in gut hormone profiles.20
In contrast to banding, there has been an explosion of publications demonstrating rises in gut hormone levels after gastric bypass that favour reduced appetite and early satiety. Hormones such as peptide YY (PYY), glucagon-like peptide-1 (GLP-1) and oxyntomodulin have attracted particular attention and there are currently publications exploring changes in energy expenditure and bile acids as mediators of improved insulin resistance.49,50 In the foregut theory, diabetes has been proposed to be a disease of the duodenum and proximal jejunum, referring to the exclusion of this part of the anatomy to the passage of food in gastric bypass. In the original research, diabetic rats were made non-diabetic after duodeno-jejunal bypass, in which only the duodenum and proximal jejunum were excluded from the passage of food, leaving the stomach intact. This is the basis for the development of the duodeno-jejunal bypass in humans and the endoscopic duodenal sleeve technique (Endobarrier).51 If current trials show success, the endoscopic sleeve could become a useful adjunct in making patients fitter for surgery.
In the distal gut theory, rapid delivery of food from the gastric pouch via the Roux limb to the terminal ileum is proposed as the mechanism by which gut hormones rise and produce early reduction in appetite and early satiety.49 In this study, patients with good weight loss produced high levels of PYY and GLP-1 compared to patients who did not lose as much weight (P < 0.05). In addition, in a randomised controlled trial (RCT) those receiving somatostatin after gastric bypass had attenuated gut hormone responses and experienced return of appetite compared to those receiving placebo (P < 0.05).49 The distal gut theory contradicts the thinking behind the banded bypass.
Weight loss outcomes
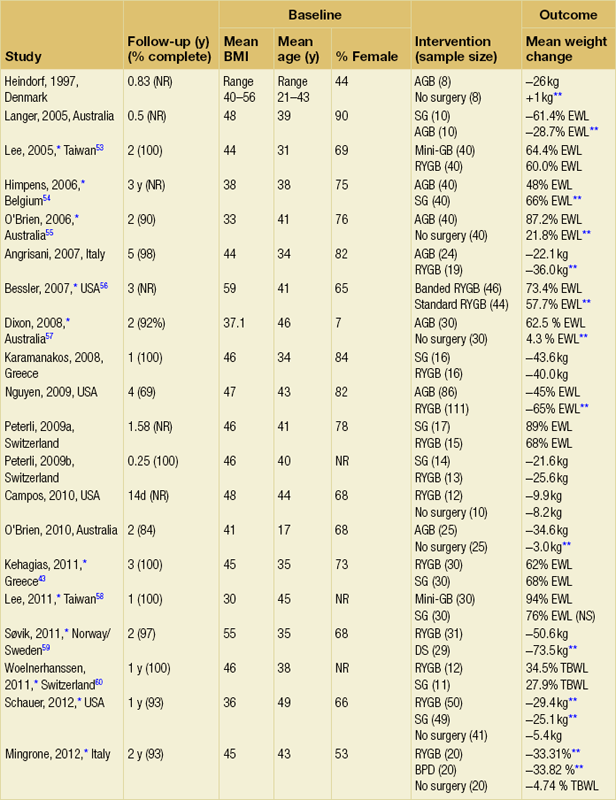
A measurement of weight loss is the most consistent variable used in reporting results of bariatric surgery, being regarded by surgeons as the paramount clinical outcome. There is, however, almost complete lack of consistency about how this is measured or presented, as illustrated in Table 20.2, in which the known RCTs of weight loss outcomes from currently performed procedures are summarised.52 This lack of consistency leads to difficulties in combining data from studies in systematic reviews and meta-analyses, and it makes comparisons between studies and centres difficult. Most of the available evidence consists of case series (level III evidence).
Table 20.2
Summary of RCTs of currently performed procedures52
Band versus bypass RCTs
Two RCTs have compared gastric bypass and adjustable gastric banding. Angrisani et al. in Naples randomised 24 patients to gastric bypass and 27 patients to banding.62 There was no mortality and one band patient was lost to follow-up. Four band patients required revision to other bariatric operations during follow-up, two for gastric pouch dilatation and two for unsatisfactory weight loss. Three bypass patients required early re-operations because of life-threatening complications (12.5%). Fifteen bypass patients (62.5%) achieved BMI < 30 compared to three (11.5%) band patients (P < 0.001). The authors concluded that gastric bypass produces better weight loss and fewer failures, acknowledging that the high complication rate was accounted for by their learning curve in the bypass operation.
Nguyen et al. in California randomised 111 patients to gastric bypass and 86 to banding.63 There was no mortality in either group. At 4 years patients were grouped into quintiles of percentage excess weight loss (% EWL). The highest quintiles representing 60% EWL or more had 64.1% of bypass patients and 15.3% of band patients. The lowest quintiles representing < 40% EWL had 5.1% of bypass patients and 50.0% of band patients (P < 0.05). Although the authors concluded that weight loss at 4 years was superior after bypass, the follow-up rates achieved were only 83.1% for bypass and 93.3% for band, and no explanation was given for the different numbers randomised to each arm.
Band versus sleeve gastrectomy RCT
In the largest RCT, Himpens et al. in Belgium randomised 80 patients to either operation.54 Weight loss was better for sleeve at each time point measured: 57.7% EWL vs. 41.4% EWL at 1 year and 66% EWL vs. 48% at 3 years (both P < 0.01). Although there were several operations for complications in each group, an additional two patients in each was converted to another bariatric operation due to lack of weight loss.
Banded versus non-banded gastric bypass RCT
There has been one RCT of banded versus non-banded (standard) gastric bypass. The banded gastric bypass involves placing a silicone ring around the gastric pouch above the pouch-enterostomy anastomosis, with the intention of causing a fixed amount of restriction and thus greater weight loss. In an RCT, Bessler et al. randomised 46 patients to banded bypass and 44 to standard bypass.56 There were no significant differences in EWL at 6, 12 and 24 months (43.1% vs. 24.7%, 64.0% vs. 57.4%, and 64.2% vs. 57.2%). However, there was a small advantage at 36 months for the banded group (73.4% vs. 57.7%, P < 0.05), although the number available for follow-up was not specified. Other than in a few centres, this operation has not received widespread uptake and the proposed mechanism of action (hold-up of food in the pouch) is at variance with the distal gut theory.49
There has been one RCT of standard gastric bypass versus the so-called mini-gastric bypass. In this variant a long gastric pouch/tube (midway in length between the pouch in standard gastric bypass and that in a sleeve gastrectomy) is created and an antecolic loop gastroenterostomy is made without a Roux-en-Y. Although the weight loss outcomes are reported to be similar to standard gastric bypass, it is an uncommon operation and there has been reluctance to recognise it for credentialing purposes by the ASMBS and the American College of Surgeons (ACS).31,53
Sleeve versus bypass RCTs
There are at least three RCTs of sleeve gastrectomy versus gastric bypass and one of sleeve versus mini-gastric bypass. Kehagias et al. in Greece have published the longest follow-up in an RCT of bypass versus sleeve and they found no significant difference in weight loss between the two at any time point up to 3 years (3-year data: 68% EWL for sleeve and 63% EWL for bypass).43 An earlier report of the same trial found that weight loss at 1 year was similar for the two operations and this is the general consensus for medium-term comparison of bypass with sleeve.52
Bypass versus duodenal switch RCT
There is one published trial, from Norway/Sweden.59 In this study, Søvik et al. found much greater weight loss after sleeve gastrectomy with DS compared to bypass at 2 years. Cholesterol and other measures of lipid metabolism were improved more by duodenal switch but measures of vitamin D metabolism were worse. Although quality of life was broadly similar in follow-up between the two, adverse events were more frequent after DS (62% vs. 32%, P < 0.02). These were mainly due to the unavoidable nutritional consequences of DS and this illustrates the main challenge in follow-up after this operation.
Complications of surgery
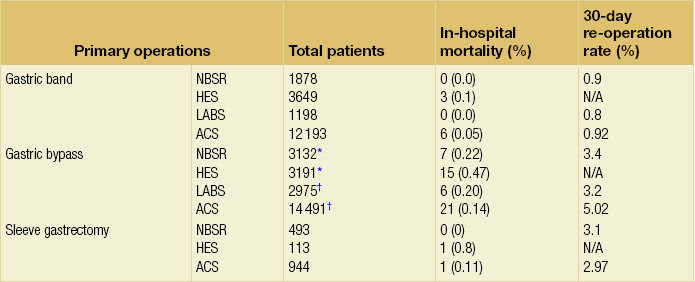
Open bypass has higher mortality (data not shown), and the Hospital Episode Statistics (HES) data from the UK were from 2000 to 2008, when much of the surgery was not yet laparoscopic. By far the largest report of outcomes is from the Bariatric Outcomes Longitudinal Database of the ASMBS Centers of Excellence programme, in which 57 918 patients had gastric bypass (54.7%), banding (39.6%) or sleeve gastrectomy (2.3%).66 The 90-day mortality rate for those with follow-up was 0.22%, but neither mortality per procedure nor 30-day re-operation rates were presented. The ACS Bariatric Surgery Center Network reported higher morbidity, re-operation and re-intervention rates for gastric bypass than for banding, with sleeve between the two.21 Similarly, the Michigan Bariatric Surgery Collaborative reported 30-day complication rates for bypass of 3.6% compared to 0.9% for gastric banding and 2.2% for sleeve.67
Gastric bypass
Anastomotic leak, particularly at the pouch-enterostomy, is a feared and potentially deadly complication and may be present in up to 3%.68 It typically occurs within 24 hours, i.e. the anastomosis is unlikely to have been water-tight initially. It is essential that a patient who has abdominal pain and a sense of ‘impending doom’ be considered for same-day re-laparoscopy or contrast-enhanced computed tomography (CT) scan.68 Tachycardia is an unreliable sign, particularly when the patient is taking beta-blockers for hypertension. The pouch-enterostomy is more likely to leak than the jejuno-jejunostomy and, because of its accessibility, it is usual to do a leak test during surgery. Same-day re-operation and direct suturing or T-tube placement and drainage are essential due to the limited reserve in patients with severe comorbidity. Poor healing is characteristic and patients are severely catabolic, with very high energy requirements.
Bleeding from anastomoses and staple lines may present postoperatively as melaena in up to 4% and almost always settles, sometimes needing transfusion.69 Closed loop obstruction, particularly if there is a ‘Roux-en-O’ or acute internal hernia, is life threatening and must be excluded or rectified quickly.
DVT/PE is still the commonest cause of mortality after bariatric surgery, probably accounting for half the deaths. It should be routine to give appropriate prophylaxis with calf compression stockings and inflatable leggings during surgery and at least one postoperative week of appropriate anticoagulant for any operation other than banding.70 All patients should be encouraged to mobilise on the same day of surgery and enhanced recovery regimens are common. Data from the UK NBSR showed that > 90% of banding patients are discharged home by 24 hours and > 80% of bypass and sleeve patients are discharged home by postoperative day 3.19
Late
Stricture at the pouch-enterostomy occurs in up to 5%, probably depending on the technique of anastomosis and any tension.35 It usually responds to endoscopic dilatation. Marginal ulcer is reported to occur and it may be less common if Helicobacter pylori infection is sought and treated preoperatively. Internal herniation with bowel ischaemia is a known serious risk as the hernia spaces made by the anatomical rearrangement enlarge as weight is lost. It is mandatory for every patient to be on lifelong vitamin and trace mineral supplementation due to the unavailability of the duodeno-proximal jejunal segment for absorption (see below).
The risk of symptomatic internal hernia is at least 2% over 1–2 years after surgery.71
Gastric Band
Late
Every patient must be carefully counselled about the long-term risk of re-operation. Suter et al. in Switzerland found there was a 21% cumulative re-operation rate over 7 years.73 The real-life re-operation rate is not known since so many patients are lost to follow-up, but a realistic estimation may be 10–20% over 5–10 years. Re-operations may be minor, e.g. a needle stick injury to the tubing adjacent to the port or failure of the port membrane causing leakage and requiring local attention to replace the port or shorten the tubing. Much more serious complications are infection, requiring removal of the whole band, slippage of the position of the band around the upper part of the stomach, and erosion, where the band migrates into the lumen of the stomach.
Slippage: This typically presents at 2–3 years, and produces vomiting and loss of restriction. This is usually a non-urgent situation and treatment consists of de-filling the band, a contrast X-ray to make the diagnosis and laparoscopic repositioning of the band. Concentric pouch dilatation, sometimes with a dilated oesophagus and lack of weight loss, can be confused with slippage and may indicate lack of a satiety response from the band.
Erosion: This occurs in about 1% of patients long term and may result from, or be the cause of, port-site infection. It does not present as an emergency. Sudden loss of restriction is the norm, and a red port scar de novo is worrying for erosion. Endoscopy or contrast X-ray provides the diagnosis and band removal is usually done laparoscopically, although it can be done by endoscopy. Revision surgery, usually to convert to a gastric bypass, should be delayed for at least 6 months to allow healing.
Sleeve gastrectomy
The main early complication of sleeve is leakage along the staple line, usually at the angle of His. This is thought to be due to the relative back pressure produced by an intact pylorus against oesophageal peristalsis. With an incidence reported consistently at up to 3%, leaks can take months to heal, and many surgeons routinely use reinforcement materials or continuous suturing along the staple line to try to prevent a leak.74 Treatment options include percutaneous drainage, endoscopic stenting, parenteral nutrition/jejunal feeding, proton-pump inhibitors and fibrin glue.
High-volume specialisation
Data from the Michigan Bariatric Surgery Collaborative assessed hospital complication rates in 15 275 patients having bypass, banding and sleeve gastrectomy in 2006–2009. The overall risk for all procedures (after adjusting for comorbidity status and OS-MRS risk; see above) of serious complication was 4.0% (CI 2.8–5.3) for annual volumes of < 150 cases per hospital and < 100 cases per surgeon combined, compared to 1.9% (CI 1.4–2.3) for ≥ 300 cases per hospital and ≥ 250 per surgeon combined (P < 0.001).67 In addition to specific surgical complications, the team approach characteristic of high-volume hospitals appears to facilitate better outcomes (Grade D recommendation).30,31 Most experts recommend round-table discussion of patients on a regular basis. Since the success of banding is completely reliant on good follow-up, the multidisciplinary team should be structured to enable this.31 In addition, the International Diabetes Federation statement (see below) made the following comment:
There are a number of other case series supporting high-volume specialisation in gastric bypass surgery that also recognise the high risk of the learning curve and the need for mentoring for this operation.75
Comorbidity outcomes
Data from a meta-analysis of 21 studies showed improvements in diabetes, hypertension, dyslipidaemia and sleep apnoea in nearly all patients.76 Numerous other studies show improvements in individual organ failures. For example, liver biopsies on 36 patients were assessed blindly before and 2 years after gastric banding.77 Histology showed marked improvements in indices of grade and stage of non-alcoholic steatohepatosis (P < 0.01). At follow-up only three patients had a fibrosis score of 2 or more compared with 18 patients at baseline (P < 001). Another example is cardiac function; in one study 423 gastric bypass patients were compared to 733 non-operated controls with an echocardiogram at baseline and at 2 years. Left ventricular mass and right ventricular cavity area had both decreased at follow-up (P < 0.01), and left atrial volume stayed the same in bypasses but had increased in controls (P < 0.05), all indicating improved cardiac function.78
Swedish Obese Subjects study
The largest series of detailed long-term comorbidity data is the Swedish Obese Subjects (SOS) study (Table 20.4).79 This study is also probably the best known in bariatric surgery. It was initiated in 1987, recruiting to 2001, and is a non-randomised cohort study of the effect of bariatric surgery versus medical therapy. At the time, before laparoscopic techniques, bariatric surgery was considered too dangerous to allow ethical randomisation between surgery and best medical therapy. Thus, 2037 patients choosing not to have surgery were matched and compared to 2010 surgical patients. Over a mean follow-up of 10.9 years (range 4.9–18.2) there was no significant weight loss in the medical group, who received all appropriate therapy for their comorbid disease. In contrast, weight loss in the surgical groups, consisting of non-adjustable gastric banding, gastric bypass and vertical banded gastroplasty, was between 13.2% and 25%. There were improvements in every variable studied, but it was noted that hypertension tended to recur, associated with advancing age of the study population.
Table 20.4
Incidence of comorbidity: 10-year data from the Swedish Obese Subjects study79
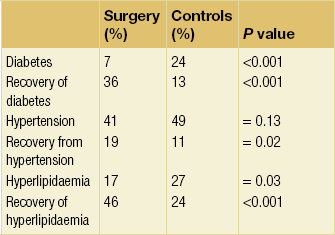
Reproduced from Sjöström L, Lindroos A-K, Peltonen M et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 35:2683–93. With permission from Massachusetts Medical Society.
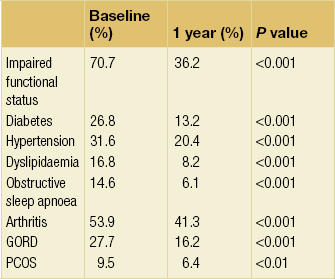
Data from the NBSR
The proportion of patients recorded as showing no indication of comorbidities at 1 year in the NBSR is shown in Table 20.5.19 The functional improvement was dramatic and at 2 years the rate of diabetes had fallen by 85.5%. Most of the effect of surgery in the NBSR results was from gastric bypass; in fact, for banding only dyslipidaemia and functional impairment showed significant improvement at 1 year, although all other variables showed non-significant improvement. The data are also consistent with the slower rate of weight loss universally found with gastric banding compared to RYGB.20 Because of a lack of well-controlled randomised studies comparing the two operations, it is not clear whether the apparently slower rate of improvement in comorbidity with banding translates to different outcomes in the medium to long term.
Quality of life (QoL) after bariatric surgery
There are many studies indicating that quality of life improves after surgery compared to no surgery. Again, the largest study is the SOS, which reported that all QoL measures improved in 655 surgery patients compared to 621 controls when assessed at 2–10 years.17 Variables included General Health Rating Index, Sickness Impact Profile, Mood Adjective Check List, and Hospital Anxiety and Depression score. Improved QoL was also found in the RCT by O’Brien et al. where band patients had improvement at 2 years in five of eight domains in the SF-36 tool – physical function, physical role, general health, vitality and emotional role.61 Improvement in psychological functioning is not universal, however, as it also appears that there is an increased risk of suicide after surgery.80
Diabetes outcomes
In the systematic review of outcomes in 4070 diabetic patients by Buchwald et al., it was estimated that remission at 2 years was achieved in 57% of patients after gastric banding, 80% after gastric bypass and 95% after biliopancreatic diversion.81 Also, HbA1c and fasting glucose levels fell significantly, indicating better diabetes control. However, the main drawback of such a meta-analysis is lack of consistent reporting of the terms used to define remission.
Given that bariatric surgery is presently only being offered to a very small proportion of those who could benefit, even in countries with highly developed healthcare, the International Diabetes Federation perceived the need in 2011 to offer international guidance.23 The recommendations included: ‘bariatric surgery is an appropriate treatment for type 2 diabetes and BMI ≥ 35 not achieving recommended treatment targets with medical therapy, especially where there is other obesity-related comorbidity’ (author’s italics).
Various terms for improvement of diabetes control after surgery have been used, including ‘resolution’ and ‘cure’, but ‘remission’ is now the preferred term. In 2009 the American Diabetes Association introduced a more strict definition of complete remission as being a return to normal measures of glucose metabolism (HbA1c < 6%, fasting glucose < 5.6 mmol/L) in the absence of hypoglycaemic medication over at least 1 year after bariatric surgery.82 One study has assessed remission rates with the new definition. At 2-year follow-up in 209 diabetics, HbA1c was reduced in all surgical groups (P < 0.001), and complete remission rates were 40.6% after gastric bypass, 7% after gastric banding and 26% after sleeve gastrectomy.83 Although the study was not randomised, the differences between the procedures were significant (P < 0.001).
The study by Dixon et al. in 2008 is the only randomised trial comparing diabetes outcomes between banding and intensive medical therapy. Recently, two further studies have provided randomised evidence for gastric bypass, sleeve gastrectomy and BPD as well compared to medical therapy. Both RCTs highlight the main therapeutic aim of controlling HbA1c rather than purely focusing on the ability of surgery to reduce medication usage. In the RCT by Schauer et al., HbA1c fell from 9.3 to 6.4 with gastric bypass, and 9.5 to 6.6 with sleeve gastrectomy (2.9 percentage points for both).84 In comparison, HbA1c only fell from 8.9 to 7.5 (1.4 percentage points, both P < 0.001) with intensive medical therapy. The primary end-point (proportion with HbA1c < 6.0 at 1 year) was achieved by 42% with gastric bypass, 37% with sleeve gastrectomy and 12% with medical therapy (both P < 0.001 compared to medical therapy).In the RCT by Mingrone et al., the starting HbA1c was 8.65 ± 1.45, and this fell to 6.35 ± 1.42 with gastric bypass, 4.95 ± 0.49 with BPD and 7.69 ± 0.57 with intensive medical therapy (P < 0.01 between each group).85 The primary end-point (fasting glucose < 5.6 mmol/L and HbA1c < 6.5% on no medication) was achieved by 75% with gastric bypass, 95% with BPD and none with medical therapy (P < 0.001 between groups). These are the only randomised data between bypass and BPD, and suggest that the latter gives better glycaemic control. There are no randomised data studying the effect of DS on HbA1c.
The different rates of improvement/remission between operations supports the consensus that bypass (and BPD) has effects other than pure weight loss on improving glycaemic control. It is widely presumed that this is due to the postoperative rise in gut hormones such as the incretin GLP-1, which is associated with rapid improvement in diabetic control in the immediate postoperative period before significant weight loss.86
While all of the currently popular operations dramatically improve glycaemic control, many patients can still expect to be on diabetic medications postoperatively. Previously, surgeons tended to champion reduction in medication usage and to gauge the success of surgery in these terms. Focusing on glycaemic control (HbA1c) as well should not detract from the very substantial financial benefit of patients coming off treatment (see below). Thus, surgery is ‘a component of the ongoing treatment of chronic disease management of type 2 diabetes and obesity’ and patients still need long-term follow-up by interested physicians.23
Nutritional support in follow-up
The frequency of assessment should be at least 3- to 6-monthly in the first year if there are known deficiencies, at least 6- to 12-monthly in the second year and at least annually thereafter.28
Recommended supplements are: multi-vitamin, calcium with vitamin D, folic acid, iron and vitamin B12 (Grade B recommendation). In addition, folic acid should be supplemented in all women of child-bearing age who are sexually active because of the risk of neural tube defects and the likelihood that fertility will improve after surgery (Grade A recommendation).28
Long-term survival benefit after surgery
Several population-based studies now indicate that bariatric surgery confers survival benefit. The SOS study was the first prospective study to show that bariatric surgery confers survival benefit; even after the 90-day mortality rate from surgery of 0.25%, 129 control patients died compared to 101 in the surgical group, hazard ratio (HR) 0.76 (95% CI 0.59–0.99), with a time to reach significance (P = 0.04) of 13 years.87
Another study that also reported in 2007 was a retrospective analysis of prospectively collected data.80 Using self-reported BMI data collected from driving licences in Utah, Adams et al. were able to match, for age, sex and BMI, 7925 patients who had undergone gastric bypass with 7925 controls. The average age was 39.5 and BMI 45.3, but it was not possible to collect data on pre- or postoperative comorbidity or BMI at follow-up. The study period was 1984–2002 and the mean follow-up was 7.1 years. Expressing mortality as deaths/10 000 patient years, 37.6 patients died in the years after surgery compared to 57.1 controls (40% reduction, P < 0.001). Disease-specific reductions in mortality were also seen for coronary artery disease (56% reduction, 2.6 vs. 5.9, P = 0.006), diabetes (92% reduction, 0.4 vs. 3.4, P = 0.005) and cancer (60% reduction, 5.5 vs. 13.3, P = 0.001). The post-surgical mortality at 1 year was 0.53%, which compared to 0.52% of controls dying in the same period.
The reports described confirmed survival benefit after mainly gastric bypass surgery. O’Brien and Dixon’s group in Melbourne has also reported survival benefit after gastric banding.88 In a retrospective series of 966 operated patients followed up for 4 years, the HR for death was 0.28 (95% CI 0.10–0.85) compared to a matched cohort of 2119 community controls followed up for 12 years.
To date there are no data on survival benefit from other bariatric operations. However, a more recent study of male gastric bypass patients in Veterans Administration hospitals in the USA, who were predominantly older than in the studies above, also reported in 2011.90 No survival benefit at 6.7 years was found in the 850 operated patients compared to 847 controls. This is the first study not to show survival benefit after bariatric surgery, possibly because of relatively high mortality in the operated patients and the relatively short follow-up.
After surgery, patients may not return to the level of risk of the general population. In Sweden 13 270 bariatric surgery patients still had an HR for mortality of 1.24 (CI 1.15–1.34) when compared to 132 700 individuals from the general population who did not have surgery.91 One of the many unknowns regarding the mechanism by which surgery reduces mortality is whether any of the operations halt or reverse the characteristic microvascular changes of diabetes.
Cancer incidence after bariatric surgery
A further finding from the SOS study is the reduction in incidence of all cancers in follow-up. Using the Swedish National Cancer Registry to observe cancer incidence from 1987 to 2005, Sjöström et al. found that 117 cancers developed in 2010 operated patients compared to 169 cancers in 2037 controls (HR 0.67, P < 0.0009).92 Although the absolute numbers are small, Sjöström et al. found it useful to compare this effect of surgery to statin treatment, where the HR for development of fatal or non-fatal myocardial infarction was 0.80 in > 90 000 patients.93
Cost-effectiveness of bariatric surgery
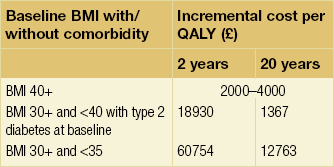
Large studies from the UK, USA and Canada have recently evaluated the economics of bariatric surgery. In 2009 the HTA programme published a systematic review in which Picot et al. analysed 5386 journal articles.95 Of these, 26 were used in the clinical effectiveness review, including 23 RCTs. Bariatric surgery was found to be more clinically effective for weight loss than non-surgical methods, with some measures of quality of life improving but not others. In addition, cost-effectiveness was estimated using incremental cost-effectiveness ratios (ICERs) per quality-adjusted life year (Table 20.6). Particularly for longer time horizons up to 20 years, these ratios were deemed cost-effective, although not for the BMI group 30 + and < 35.
Another, updated systematic review has recently reported and expressed health economic outcomes as incremental cost-utility ratios (ICURs) in 2009 US dollars.52 ICURs ranged from $1000 to $40 000 per quality-adjusted life year (QALY), and it was not possible to distinguish between different procedures. O’Brien’s group has analysed the lifetime projected cost savings for recently diagnosed diabetics treated with gastric banding compared to medical therapy from their RCT, and found surgery cost less and led to more QALYs.96 Using a different model in the USA that is based on a meta-analysis, other health economists found the cost-effectiveness over a 10-year timescale for gastric banding to be between $US11 000 and 13 000/QALY for patients with diabetes.97 Gastric bypass was found to be more cost-effective at between $US7000 and 12 000/QALY for diabetes, and all of these estimates were well below the threshold of cost/QALY usually used to justify expenditure.
In another study the 3651 patients in a US employer claims database of 5 million who were operated between 1999 and 2005 were matched to similarly obese non-operated controls with similar baseline comorbidity using the ICD9 code 278.01.98 The operations were mainly gastric bypass. Total healthcare costs including hospital visits and medications were recorded during the 6 months before surgery and continuously up to 5 years. Using a model adjusted for inflation, Cremieux et al. estimated that all costs were recouped for laparoscopic bariatric surgery within 2 years and for open bariatric surgery within 4 years.
In a study using a US insurance database of 8.5 million people, Klein et al. analysed the pre- and postoperative drug costs in diabetic patients (n = 808) matched to non-operated controls (n = 808).99 They found that at 3 months after surgery insulin usage had fallen to 43% in operated patients and stayed at 84% in controls. Medication and supply costs of insulin at this time were $US33 and $US123, respectively (both P < 0.001). At 6 months only 28% of operated patients still had a diagnosis of diabetes and it was found that the break-even point for the cost of surgery due to the cessation of diabetes medications alone was 26 months.
Despite the assumed benefits, the rate of bariatric surgery for the NHS in England in 2009–10 was approximately 0.30% of those who might benefit (3642 of 1.2 million). The availability of surgery appears to be no greater in other countries with a similar scale of obesity, with healthcare providers seemingly caught by the imperative to balance budgets within a 1-year timescale given that the cost of surgery may not be recouped until at least 1–2 years.100 The case for diabetes is an illustration of this.99 It is not known how much the willingness of healthcare systems to provide this surgery might be influenced by societal prejudice.
Wider economic benefit of bariatric surgery
The above cost-effectiveness models do not include the benefits to society as a whole, such as gainful employment after surgery. A UK Office of Health Economics (OHE) report used a novel approach in which they estimated the expected gains arising from unemployed patients going back to work.101 The estimate was based on a study of patients who at a median 14 months follow-up after surgery increased their paid hours worked by 57% and reduced their state benefit claims by 75%.102 The model found that if 25% of eligible patients (140 000 in their estimate) received surgery the boost to the GDP would total £1.295 billion at 3 years due to increases in paid employment, with an additional £151 million being returned to the economy by reducing benefits costs. Although this was a single study, reports from at least three other European countries, including the SOS study, have also shown increases in paid work after surgery.103–105 If the additional economic factors alone are considered, without including any cost savings from medication reduction or less hospitalisation, surgery pays for itself within 1 year.101
Who should have surgery?
The greater challenge is to estimate the proportion of the morbidly obese population who should be offered bariatric surgery, as there is no medical argument to limit the number treated to 1% or fewer of those who can benefit. The upper limit for how many should be treated is also unknown, but the recent Edmonton Obesity Surgery Score attempts to estimate life expectancy (akin to TMN staging) for a given level of disease.106 After analysing data from 7967 individuals in the US NHANES studies, it was found that dying within a 20-year timescale was more likely for those scoring 2 (HR 1.57, 95% CI 1.16–2.13) and 3 (HR 2.69, 95% CI 1.98–3.67) than for those scoring 0 or 1, irrespective of BMI. Score 2 included established chronic disease (e.g. diabetes, hypertension, sleep apnoea, arthritis) and score 3 also included end-organ damage and significant functional limitation. Thus, if there are limited resources those likely to die earlier might be preferred for surgery, and not those with a high BMI but no comorbidity, in contrast to the NICE guidance.
References
1. Mason, E.E., Ito, C., Gastric bypass in obesity. Surg Clin North Am 1967; 47:1345–1351. 6073761
2. Christou, N.V., Look, D., MacLean, L.D., Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg 2006; 244:734–740. 17060766
3. Johnston, D., Dachtler, J., Sue-Ling, H.M., et al, The Magenstrasse and Mill operation for morbid obesity. Obes Surg 2003; 13:10–17. 12630607
4. Friedrich, M.J., Epidemic of obesity expands its spread to developing countries. JAMA 2002; 287:1382–1386. 11903011
5. Foresight – Tackling obesity, future choices. Government Office for Science, October 2007. The UK government report that used sophisticated computer modelling techniques to predict the likely increase in obesity and its financial consequences.
6. Swinburn, B.A., Sacks, G., Hall, K.D., et al, Obesity 1. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011; 378:804–814. 21872749
7. Monsivais, P., Mclain, J., Drewnowski, A., The rising disparity in the price of healthful foods: 2004–2008. Food Policy 2010; 35:514–520. 22290786
8. Thomas, M., Desai, K.K., Seenivasan, S. How credit card payments increase unhealthy food purchases: visceral regulation of vices. J Consumer Res. 2011; 38:126–139.
9. Finucane, M.M., Stevens, G.A., Cowan, M.J., et al, National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011; 377:557–567. 21295846 Data from 960 country-years and 9.1million participants were analysed to produce these estimates.
10. National Obesity Observatory. www.noo.org.uk; [accessed July 10]. The public health observatory website that publishes rates of surgery in the different regions of the UK and also updates on the current prevalence of obesity.
11. Dixon, J.B., Pories, W.J., O’Brien, P.E., et al, Surgery as an effective early intervention for diabesity: why the reluctance? Diabetes Care 2005; 28:472–474. 15677819
12. Suhrcke, M., McKee, M., Arce, R.S., et al, Investment in health could be good for Europe’s economies. Br Med J 2006; 333:1017–1019. 17095786
13. Lenzer, J. Obesity related illness consumes a sixth of the US healthcare budget. Br Med J. 2010; 341:c6014.
14. Sedula, M.K., Mokdad, A.H., Williamson, D.F., et al, Prevalence of attempting weight loss and strategies for controlling weight. JAMA 1999; 282:1353–1358. 10527182
15. Leff, D.R., Heath, D., Surgery for obesity in adulthood. Br Med J 2009; 339:b3402. 19773325
16. Leibel, R.L., Rosenbaum, M., Hirsch, J., Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995; 332:621–628. 7632212 This paper demonstrated clearly the fall in basal metabolic rate after volitional weight loss in volunteer subjects, showing that energy expenditure reduces so as to mitigate against weight loss in a period of reduced intake. It is regarded as a classic paper demonstrating the physiological difficulties in losing weight by dieting.
17. Colquitt, J.L., Picot, J., Loveman, E., et al, Surgery for obesity. Cochrane Database Syst Rev. 2009;(2) CD003641. 19370590 This is the current update of the Cochrane review process enumerating extracted data from all available clinical trials by professional researchers and is a standard reference for clinical outcomes data.
18. www.win.niddk.nih.gov/publications/labs.htm; [accessed December 2011].
19. Welbourn, R., Fiennes, A., Kinsman, R., et al. The National Bariatric Surgery Registry: First registry report to March 2010. Henley-on-Thames: Dendrite Clinical Systems; 2011.
20. O’Brien, P.E., Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol 2010; 25:1358–1365. 20659224
21. Hutter, M.M., Schirmer, B.D., Jones, D.B., et al, First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011; 254:410–422. 21865942
22. Shaw, J.E., Sicree, R.A., Zimmet, P.Z., Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87:4–14. 19896746
23. Dixon, J.B., Zimmet, P., Alberti, K.G., et al, Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med 2011; 28:628–642. 21480973 The International Diabetes Federation is the umbrella organisation for 200 national diabetes associations in 160 countries. The working group for this statement reviewed the literature from 1991 to 2010 on diabetes and bariatric surgery before a consensus conference in Brussels in December 2010. The paper details recommendations on clinical practice and future research.
24. Adami, H.O., Trichopoulos, D., Obesity and mortality from cancer. N Engl J Med 2003; 348:1623–1624. 12711736
25. Calle, E.E., Rodriguez, C., Walker-Thurmond, K., et al, Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348:1625–1638. 12711737
26. Hofman, B., Stuck in the middle: the many moral challenges with bariatric surgery. Am J Bioethics 2010; 10:3–11. 21161829
27. Belle, S.H., Chapman, W., Courcoulas, A.P., et al, The relationship of BMI with demographic and clinical characteristics in the Longitudinal Assessment of Bariatric Surgery (LABS). Surg Obes Relat Dis 2008; 4:474–480. 18514583 While the increasing level of comorbidity as the BMI rises is well established in the general population, this was the first major report showing the incidence of comorbidity for different BMI groups in patients undergoing bariatric surgery.
28. Mechanick, J.I., Kushner, R.F., Sugerman, H.J., et al. AACE/TOS/ASMBS guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008; 14:S1–83. The major evidence-based reference for bariatric surgery for clinical practice.
29. NICE. Obesity, the prevention, identification, assessment and management of overweight and obesity in adults and children, Clinical Guideline 43. National Institute for Clinical Excellence; 2006. NICE guidance is provided in the UK for commissioning bodies but at present the NHS is not legally obliged to follow the guidance.
30. McMahon, M.M., Sarr, M.G., Clark, M.M., et al, Clinical management after bariatric surgery: value of a multidisciplinary approach. Mayo Clin Proc 2006; 81:S34–S45. 17036577
31. Kelly, J.J., Shikora, S., Jones, D.B., et al, Best practice updates for surgical care in weight loss surgery. Obesity 2009; 17:863–870. 19396064
32. DeMaria, E.J., Murr, M., Byrne, T.K., et al, Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg 2007; 246:578–582. 17893494 This is the paper that shows the validation of the OS-MRS. Mortality was 0.2% in class A patients compared to 2.4% in class C patients.
33. Fris, R.J., Preoperative low energy diet diminishes liver size. Obes Surg. 2004;14(9):1165–1170. 15527628
34. Olbers, T., Lonroth, H., Fagevik-Olsen, M., et al, Laparoscopic gastric bypass: development of technique, respiratory function, and long-term outcome. Obes Surg 2003; 13:364–370. 12841895
35. Higa, K., Ho, T., Tercero, F., et al, Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;7(4):516–525. 21333610
36. Fobi, M.A.L., Lee, H., Holness, R., et al, Gastric bypass operation for obesity. World J Surg 1998; 22:925–935. 9717418
37. Capella, R.F., Capella, J.F., Vertical banded gastroplasty–gastric bypass: preliminary report. Obes Surg 1991; 1:389–395. 10775940
38. Naslund, I., The size of the gastric outlet and the outcome of surgery for obesity. Acta Chir Scand 1986; 152:205–210. 3716740
39. Wittgrove, A.C., Clark, G.W., Tremblay, L.Y., Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg 1994; 4:353–357. 10742801
40. Brolin, R.E., Kenler, H.A., Gorman, J.H., et al, Long-limb gastric bypass in the superobese. Ann Surg 1992; 215:387–395. 1558421
41. Stefanidis, D., Kuwada, T.S., Gersin, K.S., The importance of the length of the limbs for gastric bypass patients – an evidence-based review. Obes Surg 2011; 21:119–124. 20680504
42. Shen, R., Dugay, G., Rajaram, K., et al, Impact of patient follow-up on weight loss after bariatric surgery. Obes Surg 2004; 14:514–519. 15130229
43. Kehagias, I., Karamanakos, S.N., Argentou, M., et al, Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg 2011; 21:1650–1656. 21818647
44. American Society for Metabolic and Bariatric Surgery. Updated position statement on sleeve gastrectomy as a bariatric procedure. Available from http://s3.amazonaws.com/publicASMBS/GuidelinesStatements/PositionStatement/ASMBS-SLEEVE-STATEMENT-2011_10_28.pdf, 2011. [[accessed December 2011]].
45. Himpens, J., Dobbeleir, J., Peeters, G., Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg 2010; 252:319–324. 20622654
46. Sarela, A.I., Dexter, S.P.L., O’Kane, M., et al, Long-term follow-up after laparoscopic sleeve gastrectomy; 8–9 year results. Surg Obes Relat Dis. 2012;8(6):679–684. 21890430
47. Hess, D.S., Hess, D.W., Oakley, R.W., The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg 2005; 15:408–416. 15826478
48. Dixon, A.F., Dixon, J.B., O’Brien, P.E., Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J Clin Endocrinol Metab 2005; 90:813–819. 15585553
49. le Roux, C.W., Welbourn, R., Werling, M., et al, Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007; 246:780–785. 17968169
50. Wynne, K., Park, A.J., Small, C.J., et al, Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes 2006; 30:1729–1736. 16619056
51. Rubino, F., Forgione, A., Cummings, D.E., et al, The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006; 244:741–749. 17060767
52. Padwal, R., Klarenbach, S., Wiebe, N., et al, Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med. 2011;26(10):1183–1194. 21538168
53. Lee, W-J., Yu, P-J., Wang, W., et al, Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity. Ann Surg 2005; 242:20–28. 15973097
54. Himpens, J., Dapri, G., Cadiere, G.B., A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy. Obes Surg 2006; 16:1450–1456. 17132410
55. O’Brien, P.E., Dixon, J.B., Laurie, C., et al, Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program. A randomized trial. Ann Intern Med 2006; 144:625–633. 16670131
56. Bessler, M., Daud, A., Kim, T., et al, Prospective randomized trial of banded versus nonbanded gastric bypass for the super obese: early results. Surg Obes Relat Dis 2007; 3:480–484. 17544335
57. Dixon, J.B., O’Brien, P.E., Playfair, J., et al, Adjustable gastric banding and conventional therapy for type 2 diabetes. A randomized controlled trial. JAMA 2008; 299:316–332. 18212316 This is the only RCT that compares banding to medical therapy for new-onset diabetics in BMI range 30–40.
58. Lee, W.J., Chong, K., Ser, K.H., et al, Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg 2011; 146:143–148. 21339423
59. Søvik, T.T., Aasheim, E.T., Taha, O., et al, Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch. A randomized trial. Ann Intern Med 2011; 155:281–291. 21893621
60. Woelnerhanssen, B., Peterli, R., Steinert, R.E., et al, Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy – a prospective randomized trial. Surg Obes Relat Dis 2011; 7:561–568. 21429816
61. O’Brien, P.E., McPhail, T., Chaston, T.B., et al, Systematic review of medium-term weight loss after bariatric operations. Obes Surg 2006; 16:1032–1040. 16901357 This systematic review compared weight loss outcomes for bypass, banding, banded bypass, and BPD with or without DS from 43 papers up to 10 years after surgery.
62. Angrisani, L., Lorenzo, M., Borrelli, V., Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis 2007; 3:127–132. 17331805
63. Nguyen, N.T., Slone, J.A., Nguyen, X-MT., et al, A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg 2009; 250:631–641. 19730234
64. Burns, E.M., Naseem, H., Bottle, A., et al, Introduction of laparoscopic bariatric surgery in England: observational population cohort study. Br Med J 2010, Aug 26; 341:c4296. 20798224
65. Flum, D., Perioperative safety in the Longitudinal Assessment of Bariatric Surgery. The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. N Engl J Med 2009; 361:445–454. 19641201
66. DeMaria, E.J., Pate, V., Warthen, M., et al, Baseline data from American Society for Metabolic and Bariatric Surgery-designated bariatric surgery centers of excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis 2010; 6:347–355. 20176512
67. Birkmeyer, N.J., Dimick, J.B., Share, D., et al, Hospital complication rates with bariatric surgery in Michigan. JAMA 2010; 304:435–442. 20664044
68. Lee, S., Effect of location and speed of diagnosis on anastomotic leak outcomes in 3828 gastric bypass cases. J Gastrointest Surg 2007; 11:708–713. 17562118
69. Nguyen, N.T., Longoria, M., Chalifoux, S., et al, Gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg 2004; 14:1308–1312. 15603643
70. Podnos, Y.D., Jimenez, J.C., Wilson, S.E., et al, Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg 2003; 138:957–961. 12963651
71. Ahmed, A.R., Rickards, G., Husain, S., et al, Trends in internal hernia incidence after laparoscopic Roux-en-Y gastric bypass. Obes Surg 2007; 17:1563–1566. 18004631
72. , ASMBS position statement on emergency care of patients with complications related to bariatric surgery. Surg Obes Relat Dis 2010; 6:115–117. 20189469
73. Suter, M., Calmes, J.M., Paros, A., et al, A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg 2006; 16:829–835. 16839478
74. Casella, G., Soricelli, E., Rizzello, M., Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg 2009; 19:821–826. 19381737
75. Flum, D.R., Salem, L., Elrod, J.A., et al, Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA 2005; 294:1903–1908. 16234496
76. Maggard, M.A., Shugarman, L.R., Suttorp, M., Meta-analysis: surgical treatment of obesity. Ann Intern Med 2005; 142:547–559. 15809466
77. Dixon, J.B., Bhathal, P.S., Hughes, N.R., et al, Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology 2004; 39:1647–1654. 15185306
78. Owan, T., Avelar, E., Morley, K., et al, Favorable changes in cardiac geometry and function following gastric bypass surgery. J Am Coll Cardiol 2011; 57:732–739. 21292133
79. Sjöström, L., Lindroos, A-K., Peltonen, M., et al, Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. 15616203
80. Adams, T.D., Gress, R.E., Smith, S.C., et al, Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357:753–761. 17715409
81. Buchwald, H., Estok, R., Fahrbach, K., et al, Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009; 122:248–256 e5. 19272486
82. Buse, J.B., Caprio, S., Cefalu, W.T., How do we define cure of diabetes? Diabetes Care 2009; 32:2133–2135. 19875608
83. Pournaras, D.J., Aasheim, E.T., Søvik, T.T., et al, Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg 2012; 99:100–103. 22021090
84. Schauer, P.R., Kashyap, S.R., Wolski, K., et al, Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–1576. 22449319
85. Mingrone, G., Panunzi, S., De Gaetano, A., et al, Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366:1577–1585. 22449317
86. Pournaras, D.J., Osborne, A., Hawkins, S.C., et al, Rapid remission of type 2 diabetes after Roux en Y gastric bypass. Ann Surg 2010; 252:966–971. 21107106
87. Sjöström, L., Narbro, C., Sjöström, C.D., et al, Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357:741–752. 17715408
88. Peeters, A., O’Brien, P.E., Laurie, C., et al, Substantial intentional weight loss and mortality in the severely obese. Ann Surg 2007; 246:1028–1033. 18043106
89. Pontiroli, A.E., Morabito, A., Long-term prevention of mortality in morbid obesity through bariatric surgery: a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg 2011; 253:484–487. 21245741 This is the only systematic review so far published on mortality after bariatric surgery.
90. Maciejewski, M.L., Livingston, E.H., Smith, V.A., et al, Survival among high-risk patients after bariatric surgery. JAMA 2011; 305:2419–2426. 21666276
91. Ostlund, M.P., Marsk, R., Rasmussen, F., et al, Morbidity and mortality before and after bariatric surgery for morbid obesity compared with the general population. Br J Surg 2011; 98:811–816. 21351078
92. Sjöström, L., Gummesson, A., Sjöström, C.D., et al, Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects study): a prospective, controlled intervention trial. Lancet Oncol 2009; 10:653–662. 19556163
93. Preiss, D., Sattar, N., Lipids, lipid modifying agents and cardiovascular risk: a review of the evidence. Clin Endocrinol (Oxf) 2009; 70:815–828. 19067719
94. Adams, T.D., Stroup, A.M., Gress, R.E., et al, Cancer incidence and mortality after gastric bypass surgery. Obesity 2009; 17:796–802. 19148123 This and the SOS paper by Sjöström et al.92 were among the first publications illustrating the powerful effect of bariatric surgery on reducing cancer incidence, which it is commented is much greater than that of statins on reducing cardiovascular events. Statins are universally accepted while bariatric surgery is not.
95. Picot, J., Jones, J., Colquitt, J.L., et al, The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009; 13:1–214. 19954682
96. Keating, C.L., Dixon, J.B., Moodie, M.L., et al, Cost-effectiveness of surgically induced weight loss for the management of Type 2 diabetes: modeled lifetime analysis. Diabetes Care 2009; 32:567–574. 19171720
97. Hoerger, T.J., Zhang, P., Segel, J.E., et al, Cost effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care 2010; 33:1933–1939. 20805271
98. Cremieux, J., Buchwald, H., Shikora, S.A., et al, A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14(9):589–596. 18778174
99. Klein, S., Ghosh, A., Cremieux, J., et al, Economic impact of the clinical benefits of bariatric surgery in diabetes patients with BMI ≥ 35 kg/m2. Obesity 2011; 19:581–587. 20829800
100. McCartney, M., Slimmed down surgery. Br Med J 2010; 341:c5499. 20978064
101. Office of Health Economics. Shedding the pounds. www.rcseng.ac.uk/news/docs/BariatricReport.pdf, 2010. [[accessed 23.11.12]].
102. Hawkins, S.C., Osborne, A., Finlay, I.G., et al, Paid work increases and state benefit claims decrease after bariatric surgery. Obes Surg 2007; 17:434–437. 17608252
103. van Gemert, W.G., Adang, E.M., Greve, J.W., et al, Quality of life assessment of morbidly obese patients: effect of weight-reducing surgery. Am J Clin Nutr 1998; 67:197–201. 9459366
104. Narbro, K., Agren, G., Jonsson, E., et al, Sick leave and disability pension before and after treatment for obesity: a report from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord 1999; 23:619–624. 10411235
105. Andersen, J.R., Aasprang, A., Bergsholm, P., et al, Health-related quality of life and paid work participation after duodenal switch. Obes Surg 2010; 20:340–345. 19352783
106. Padwal, R.J., Pajewski, N.M., Allison, D.B., et al, Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people. CMAJ. 2011;183(14):E1059–E1066. 21844111