Bacterial Infections
James Treat, Brian T. Fisher
Introduction
Bacterial skin infections in neonates and infants can present differently from those in older children, based on several factors: (1) the nature of the pathogen; (2) the developmental stage of the infant when infection is acquired: early (first or second trimester) versus late (third trimester) in gestation and early (first few days) versus late (2–8 weeks) postnatal life; and (3) the manner in which inoculation occurs (i.e. vertical transmission in utero, vertical transmission at the time of birth, or postnatally). Infection from hematogenous penetration of the placental barrier or through ruptured amniotic membranes can be more severe and widespread, involving multiple organ systems in addition to the skin, because of the vulnerable state of the developing neonate. Importantly, neonatal cutaneous infections, even those acquired postnatally, are potentially more serious compared with similar cutaneous infections in older children. The reason for these differences in severity is multifactorial, involving a complex interaction between host, pathogen, and environmental factors (Box 12.1).
Furthermore, the potential impact of a localized cutaneous infection is of greater concern in neonates, where alterations of the normal skin barrier are more common and can serve as a conduit for systemic infection. This is especially true for very low-birthweight infants where organisms that normally colonize the skin are also implicated as etiologic agents of sepsis. This suggests that sepsis may be a consequence of bacterial penetration at sites of skin injury or through the immature epidermal barrier.1 Edwards and colleagues highlighted this concern when they revealed the association of simple bland emollients in very low-birthweight infants with increased risk of neonatal bacterial sepsis caused by normal skin flora.2 Given the risk for severe consequences, neonatal cutaneous infections mandate a prompt and thorough evaluation and aggressive treatment to minimize morbidity and mortality.
The clinical presentation, pathogenesis, diagnostic modalities and suggested therapeutic interventions are reviewed for various common and less common neonatal cutaneous infections. The continued emergence of antibiotic resistance has complicated the empiric therapeutic options for some bacterial infections. This emergence of resistance has highlighted the importance of obtaining cultures when possible to identify the pathogen and its sensitivity profile. Recommendations regarding antibiotic choices and duration of therapy specific to the infection are provided for each clinical scenario noting the potential impact of resistance on antibiotic choices.
Superficial infections
Impetigo
Impetigo is the single most common primary skin infection in children,3 and impetiginization of atopic dermatitis is the most important secondary skin infection.4 Nonbullous impetigo is a superficial infection localized to the subcorneal portion of the epidermis typically caused Staphylococcus aureus and less commonly Streptococcus pyogenes (Group A beta-hemolytic streptococci). Bullous impetigo is almost exclusively caused by Staphylococcus aureus, most commonly phage group 2 (types 71 and 55) that elaborates toxins.5 These toxins cause epidermolysis and subsequent blister formation. Bullous impetigo is thought to be a localized form of staphylococcal scalded skin syndrome (SSSS).6
Cutaneous findings
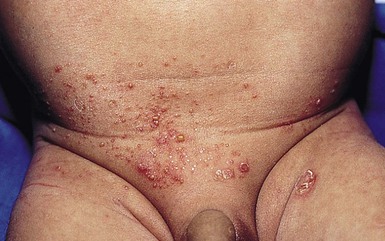
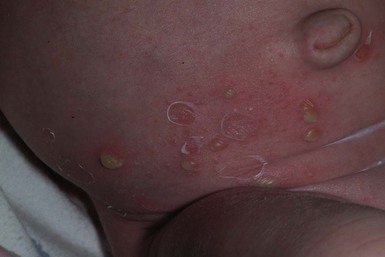
Nonbullous impetigo is characterized by erythematous, honey-colored crusted plaques (Fig. 12.1). Lesions tend to be localized in primary impetigo, but may become more widespread when superimposed on diseased skin (e.g., atopic dermatitis). Moist intertriginous, periorificial and periumbilical areas are commonly involved in both nonbullous and bullous impetigo. Bullous impetigo often presents during the first 2 weeks of life with flaccid, transparent, subcorneal bullae, which may be single or clustered, and often lack underlying cutaneous erythema. The bullae may contain pus that layers out in the dependent portion. The lesions rupture easily, leaving a shallow erosion surrounded by a narrow rim of peeling that heals without scarring (Fig. 12.2![]() ). However, postinflammatory pigmentary changes may persist for weeks to months. Multiple small pustules on the abdomen and diaper area are characteristic of staphylococcal pustulosis (Fig. 12.3).7 The healing sites from circumcision and the healing umbilical cord can be important niduses of infection in neonates.
). However, postinflammatory pigmentary changes may persist for weeks to months. Multiple small pustules on the abdomen and diaper area are characteristic of staphylococcal pustulosis (Fig. 12.3).7 The healing sites from circumcision and the healing umbilical cord can be important niduses of infection in neonates.
Extracutaneous findings
Most cases of impetigo, including neonatal bullous impetigo, are unaccompanied by constitutional signs of illness. Occasionally, hematogenous spread of bacteria can result in osteomyelitis, septic arthritis, pneumonia, or septicemia, particularly in neonates with bullous impetigo.3,7
Etiology and pathogenesis
S. aureus is isolated from approximately 85% of impetigo lesions and in 50–60% of cases is the sole pathogen. Streptococcus pyogenes causes the remainder of cases of nonbullous impetigo. The staphylococcal organisms that cause nonbullous impetigo are variable, but are generally not from phage group 2, whereas bullous impetigo is most commonly (80%) the result of S. aureus from phage group 2.8 Bullae production results from locally produced exfoliative or epidermolytic toxins A or B (ETA and ETB) which cleave desmoglein 1 (DSG1) within desmosomes, via serine proteases.9 If there is enough toxin produced, it can disseminate and cleave DSG1 in broader areas, leading to staphylococcal scalded skin syndrome (SSSS, see below). ETA is more commonly associated with bullous impetigo than is ETB. High titers of neutralizing antibodies against ETA and/or the inability of ETA to penetrate through the epidermis may explain why ETA does not commonly cause SSSS.10 Methicillin-resistant Staphylococcus aureus (MRSA) can cause impetigo, bullous impetigo, orbital cellulitis,11 abscesses, SSSS, necrotizing fasciitis, and toxic shock syndrome that are clinically indistinguishable from similar infections caused by methicillin-sensitive Staphylococcus aureus (MSSA).
Diagnosis
As the clinical presentation of MRSA and MSSA bullous impetigo and S. aureus and S. pyogenes nonbullous impetigo are indistinguishable, obtaining appropriate cultures is imperative to guide therapeutic choices. Culture of fluid from a vesicle, pustule or from beneath the lifted edges of a crusted plaque of impetigo, is usually sufficient to establish a diagnosis. Gram stains can be useful in establishing a rapid presumptive diagnosis but are less sensitive and less specific than a culture. When the diagnosis is in question, and Gram stain and culture are negative, a skin biopsy can be useful, although this is seldom necessary. When a biopsy is performed, the histopathology often reveals a vesicle or pustule in the subcorneal or granular region of the epidermis with marked dermal inflammation; the cavity is larger in the bullous form.
Differential diagnosis
Staphylococcal impetigo is clinically indistinguishable from streptococcal impetigo unless it is bullous, which is only caused by Staphylococcus aureus.3 Impetigo may be confused with several other infectious vesiculobullous or pustular disorders, such as herpes simplex virus (HSV), varicella, enterovirus, congenital cutaneous candidiasis, listeriosis, and scabies, as well as noninfectious disorders such as erythema toxicum neonatorum, transient neonatal pustular melanosis, eosinophilic pustulosis, chronic bullous disease of childhood, incontinentia pigmenti, epidermolysis bullosa, pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid.
Course, management, treatment, and prognosis
For superficial skin infections such as impetigo, the course is often self-limiting. Recently, there has been a trend towards using topical mupirocin, retapamulin (in children over 9 months of age) or fusidic acid (not available in the USA) for localized forms of impetigo and reserving oral antibiotics for those patients with more diffuse involvement or resistant organisms.12 When systemic antibiotic therapy is deemed necessary, antibiotic coverage should be inclusive of S. aureus. Oral antibiotics that are not cleaved by pencillinases present in most S. aureus isolates can be effective in this setting. This includes options such as cephalexin, cloxacillin, or dicloxacillin. Each of these options also has the benefit of covering S. pyogenes in the setting of nonbullous impetigo. Knowledge of the local epidemiology of MRSA infections is imperative, as a high rate of MRSA would prompt empiric coverage for MRSA in the setting of impetigo. Clindamycin is a reasonable empiric option; however, clindamycin resistance among MSSA and MRSA isolates is also on the rise.13
Strains of MRSA have been grouped primarily by the type of resistance genes that they carry in a genetic element referred to as the cassette chromosome element, called SCCMEC (SCCmec). Nosocomial MRSA contains types 1, 2, and 3 SCCmec, conferring multidrug resistance to non-β-lactam antibiotics. Community-acquired MRSA contains types 4 or 5 SCCmec, making this strain more susceptible to antimicrobial agents.14,15
There are a number of additional second-line oral agents that may be considered for the treatment of uncomplicated impetigo including cefadroxil, cefprozil, loracarbef, and amoxicillin/clavulanate. It should be noted that none of these second-line agents is effective against MRSA. The changing landscape of resistance necessitates that cultures be performed in order to guide appropriate therapeutic decisions.16 For isolates that are both MRSA and clindamycin resistant, trimethoprim-sulfamethoxazole may be an effective oral option but should not be used in children under 2 months of age, due to risk of hyperbilirubinemia and kernicterus. Additionally, the clinician should note that trimethoprim-sulfamethoxazole may not adequately cover Streptococcus pyogenes.
Various clinical scenarios of skin bacterial infection warrant the use of parenteral therapy. These include: infants presenting with deeper infections such as furuncles, abscesses, cellulitis, osteomyelitis, endocarditis, and pneumonia. Additionally, as bullous impetigo in neonates may advance rapidly, systemic enteral or parenteral therapy (instead of topical) with close clinical follow-up is encouraged.17 As with oral therapy, initial empiric parenteral therapy should be guided by local resistance patterns. Regions with low rates of MRSA can employ oxacillin or nafcillin as a first line agent. Those areas with higher rates of MRSA should use clindamycin or vancomycin empirically. Clindamycin, a protein synthesis inhibitor, may be advantageous for decreasing epidermolytic toxin production and is often used as an adjunctive agent in SSSS caused by MSSA or MRSA. Linezolid also shows excellent activity against MRSA and can be considered as an alternative parenteral option. The mortality rate in NICUs due to MRSA infection has been as high as 38% in some cohorts.18 Mortality rates are highest in infants developing bacteremia and septic shock.14,15,18 Late-onset neonatal sepsis, occurring 2–3 weeks after birth, has been seen with nosocomial MRSA infections. Thus, patients should be followed closely, even if they appear healthy.
Once signs of infection have begun to subside, and if constitutional signs are absent, therapy may be completed orally. Duration of therapy should be dictated by the clinical scenario as well as any associated complications. For uncomplicated impetigo, a 7-day course of therapy is typically sufficient. Secondary complications such as bacteremia, osteomyelitis and endocarditis warrant longer parenteral therapeutic courses, which should be managed with consultation from an infectious disease specialist.
Intertrigo
Intertrigo is an acute inflammation in a skin fold that can be irritant or infectious in etiology. Neonates and infants have abundant subcutaneous tissue and are often not mobile enough to allow the folds to air out. This occluded skin (neck, axillae and inguinal folds) is prone to overgrowth of yeast and bacteria. Clinicians often overlook staphylococcal and streptococcal infections as important and potentially dangerous causes of intertrigo.
Cutaneous findings
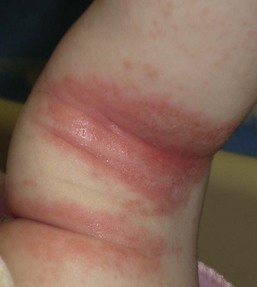
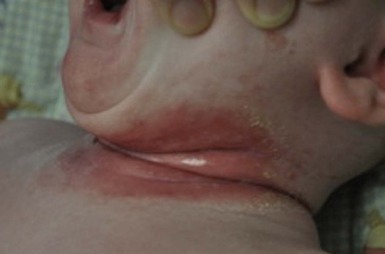
Bacterial intertrigo typically presents with red, slightly eroded, tender, shiny exudative patches centered on a skin fold (Figs 12.4, 12.5). Although the inguinal areas are most common, infants and neonates have many occluded skin folds due to their body habitus (including neck, axillae, antecubital and popliteal fossae) and any of these can become infected. The perianal area can be the initial source of infection and will also have a bright red, eroded appearance when infected with Strep. pyogenes or S. aureus. Intertrigo can also be present in several skin folds.19,20
Extracutaneous findings
Intertrigo is typically not associated with fever or extracutaneous spread. Untreated streptococcal disease can lead to post-streptococcal glomerulonephritis. Cutaneous streptococcal infections have also been associated with the subsequent development of psoriasis. Specifically, guttate psoriasis or napkin/perineal psoriasis should prompt an evaluation for perianal streptococcal infection in infants.21
Etiology and pathogenesis
Bacterial intertrigo and perianal infection is most commonly due to Strep. pyogenes but S. aureus (including MRSA) is becoming increasingly recognized as an etiologic pathogen for intertrigo.22,23 Pseudomonas species are less common causes of intertrigo and are more commonly indicative of a polymicrobial infection.
Differential diagnosis
Intertrigo can be due to irritation from over-washing or use of baby wipes. Seborrheic dermatitis, atopic dermatitis and contact dermatitis can also present with red patches in skin folds and each can become superinfected with bacteria or yeast. Candidal yeast infections often infect skin folds and present with peeling and satellite pustules.
Diagnosis
Although the clinical presentation is often enough to make the diagnosis, a Gram stain and culture from the most eroded or purulent area can be helpful in defining the causative pathogen. However, the results of these cultures need to be interpreted with the understanding that typical colonizing organisms, especially S. aureus, may not represent true infection. Although a rapid Strep test may be positive in streptococcal perianal dermatitis, this test has not been approved for use on non-posterior pharyngeal specimens. Potassium hydroxide scrapings and fungal cultures can help diagnose yeast infections.
Course, management, treatment, and prognosis
Intertrigo is worsened by skin occlusion, so allowing the area to aerate is important. Therapy with topical antibiotics such as mupirocin for S. aureus or Strep. pyogenes or bacitracin-polymyxin B for Pseudomonas spp. is often effective. For premature or immunosuppressed infants, or for multifocal and/or complicated infections, systemic therapy may be necessary. A similar approach, as described above for impetigo, should be employed when choosing an oral or parenteral therapeutic agent for intertrigo. A Gram stain and culture of the affected area should be taken before initiating empiric systemic antibiotics. If the culture results suggest a polymicrobial infection or a Pseudomonas spp. infection and the clinical scenario requires systemic therapy, then additional input from an infectious disease specialist should be considered.
Folliculitis/pustulosis/furunculosis
Pustules in the neonate or infant can be benign but must be differentiated from infectious pustulosis (bacterial, yeast, herpetic). Bacterial folliculitis is a superficial infection of the hair follicle ostium. Noninfectious pustulosis such as erythema toxicum neonatorum and transient neonatal pustular melanosis are addressed elsewhere in this book but typically are differentiated by their clinical presentation, including time course and superficial nature.
Cutaneous findings
Folliculitis presents as discrete, dome-shaped pustules with an erythematous base located at the ostia of the pilosebaceous canals. The lesions are usually asymptomatic, but may be pruritic or painful. Occasionally, a lesion may extend to involve deeper tissues and form an abscess (e.g., furuncle, carbuncle).
Extracutaneous findings
Extracutaneous findings are not typically present. Rarely, the local infection can progress to a systemic illness that is evident by fever onset and an ill-appearing child.
Etiology and pathogenesis
Folliculitis is caused predominantly by S. aureus, although coagulase-negative staphylococci are occasionally involved. A moist environment, maceration, poor hygiene, application of an occlusive emollient, and drainage from adjacent wounds and abscesses can be provoking factors. Pseudomonas aeruginosa is a rare cause of folliculitis in infants. It is found in circulating water, and the bacteria become trapped under the clothing, leading to infection. If pustulosis is due to P. aeruginosa it is often concentrated in areas that are covered by swimming suits or occluded such as axillae, inguinal folds and trunk.24
Diagnosis
The causative organism of folliculitis can be identified by Gram stain and culture of purulent material from the follicular orifice.
Differential diagnosis
Benign pustulosis of infancy is often on the differential of staphylococcal pustulosis in the neonate. Erythema toxicum neonatorum tends to be more transient and have a splotchy patch of erythema around fragile superficial pustules and the pustule contents contain eosinophils. Transient neonatal pustular melanosis is typically present at birth and the pustules rupture easily and are gone within hours to days; the pustules contain neutrophils similar to bacterial folliculitis but a Gram stain should be negative (although Gram stains have a significant false-negative rate in bacterial infection). Candida species, Pityrosporum ovale, and Malassezia furfur are all yeast pathogens capable of causing follicular papules and/or pustules. Identification of these fungal organisms can be made by potassium hydroxide examination of lesion scrapings or by skin biopsy. Several other conditions may mimic folliculitis, including miliaria, eosinophilic pustular folliculitis, acne neonatorum, tinea corporis, congenital cutaneous candidiasis, scabies, and erythema toxicum neonatorum.
Course, management, treatment, and prognosis
An attempt should be made to identify and eliminate predisposing factors. Localized uncomplicated folliculitis may be managed by removing causative factors, antiseptic cleansing (e.g., chlorhexidine in children over 2 months), and topical antibiotics. Severe or refractory folliculitis should be managed with oral therapy. Similar to impetigo and intertrigo, bacterial Gram stain and culture can be extremely useful to guide appropriate therapy. Initial empiric therapy should be directed at S. aureus. In areas with limited MRSA rates, cephalexin, cloxacillin or dicloxacillin are reasonable first-line agents. For regions where MRSA prevalence is higher, initial empiric therapy with clindamycin should be considered. Neonates and immunosuppressed infants may require intravenous therapy. Recurrent episodes should warrant investigation into asymptomatic nasal carriage of S. aureus by the patient, immediate caregivers, pets or other contaminated objects in the child’s environment.25 Decolonizing efforts including intranasal mupirocin and antibacterial washes should be considered for direct caretakers of infected infants, especially if the infection is recurrent.
Infection of pre-existing erosions and ulcerations
Open wounds that are not initially caused by a bacterial infection, such as those caused by epidermolysis bullosa (EB), autoimmune blistering diseases, ulcerated hemangiomas or vesicular infections (herpes simplex or varicella) often become colonized with bacteria but can also become secondarily infected. It is important for the clinician to be able to differentiate colonization from infection to determine if antibiotics are necessary.
Cutaneous findings
Erosions and ulcerations that are secondarily infected can present with a myriad of symptoms that include purulent drainage, crusting, erythema expanding from the wound, pain, and acute worsening that cannot be explained by the primary process. Green discharge or discoloration on dressings may be a marker of Pseudomonas infection.
Extracutaneous findings
Sepsis can occur secondary to bacterial infection of pre-existing erosions or ulcerations leading to fever, hypotension and potentially death. Sepsis complicating varicella-related skin ulcerations has been reported secondary to Gram-positive organisms including S. aureus and Strep. pyogenes, as well as Gram-negative pathogens such as Pseudomonas spp. and Escherichia coli.26
Etiology and pathogenesis
Cutaneous ulcerations are most typically colonized with normal skin flora such as diphtheroids, coagulase-negative staphylococci or anaerobes (especially in the perineum). S. aureus, streptococci (especially S. pyogenes) and Pseudomonas can also flourish in open wounds and are more pathogenic. The open wound lacks the physical protection of the epidermis and creates a warm, moist environment for bacterial overgrowth.
Diagnosis
Clinical interpretation of the likelihood of infection versus colonization is important but if infection is suspected, the wound should be cultured. The amount of bacteria, rapidity of bacterial growth in culture and the presence of just one pathogenic bacteria that has outcompeted the normal skin flora all suggest a true infection versus colonization.
Differential diagnosis
Ulcers can acutely worsen and drain due to the primary disease process, such as in pyoderma gangrenosum. Herpetic infections as well as yeast infections such as those caused by Candida spp. can also superinfect open wounds.
Course, management, treatment, and prognosis
Infected ulcers and erosions will often fail to heal at the same rate as the non-infected ulcers or erosions. Untreated, the areas may never heal, and may progress to cellulitis or systemic infection. Some erosions or ulceration in patients with EB are chronically colonized and so therapy should only be started if there is a clinical change or an area that is determined not to heal because of secondary infection. Topical therapy (such as mupirocin or bacitracin-polymyxin B) is often enough for localized areas. Metronidazole is often used for ulceration of perineal hemangiomas in order to cover anaerobic gut flora.27 Anecdotally, patients with chronic wounds such as those with EB will often rotate the type of topical antibiotic they use so as not to induce resistance. Systemic therapy should be reserved for patients with widespread infection, cellulitis or worsening infection, despite adequate topical therapy. As has been noted previously, therapy should be guided by the results of bacterial cultures. Initial empiric therapy should be directed at Gram-positive organisms with cephalexin, cloxacillin, dicloxacillin, or clindamycin. Infection secondary to S. pyogenes should always dictate systemic antibiotic therapy because of its potential for causing glomerulonephritis. Although less frequent, Gram-negative pathogens are possible and thus expanding antibiotic coverage for such pathogens should be considered if the clinical scenario is worsening, despite Gram-positive coverage, or if culture results suggest a Gram-negative pathogen.
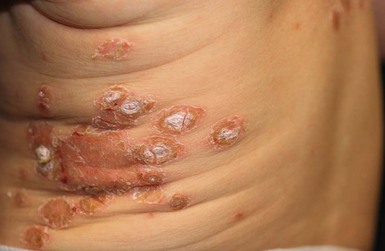
Paronychia
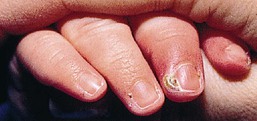
Paronychia is a localized inflammation of the nail fold, which is usually acquired, rather than congenital and can be either acute or chronic.28 Bacterial paronychia typically presents with the lateral nail fold becoming warm, erythematous, edematous, and painful (Fig. 12.6). A purulent exudate may develop. Dermatitis and loss of the cuticle in the affected area may contribute to initiation and/or perpetuation of the problem.
Although the primary disorder is separation of the eponychium from the nail plate, secondary infection is common. S. aureus and Strep. pyogenes are the most common aerobic organisms. Occasionally, Gram-negative organisms such as Pseudomonas spp., Proteus spp., and E. coli are involved.29 Paronychia is often associated with thumb-sucking.
The diagnosis is usually made clinically; in selected cases both aerobic and anaerobic cultures of purulent material may be useful in directing appropriate therapy.
The differential diagnosis includes Candida spp. infection, a frequent cause of acute and chronic paronychia in neonates, herpetic whitlow, and atopic dermatitis. Treatment includes measures directed at eliminating or reducing predisposing factors of nail-fold maceration and trauma (such as thumb-sucking). Warm compresses generally are curative for superficial lesions. Drainage of the abscess may be facilitated by gently pushing the nail fold away from the nail plate. In addition to incision and drainage, antibiotics are needed for treatment of deeper lesions. Dicloxacillin, cloxacillin, cephalexin or clindamycin (if MRSA is prevalent in the locality) are the antibiotics of choice for treatment of infections caused by S. aureus, whereas amoxicillin plus clavulanic acid is preferred for empiric treatment when additional anti-anaerobic therapy is desired. Concomitant Candida albicans can be present requiring topical or systemic anti-fungal therapy.
Funisitis/omphalitis
Funisitis is inflammation of the umbilical cord characterized by increased secretions and a foul odor. Funisitis may accompany chorioamnionitis. Omphalitis is an infection of the umbilical stump. Low-birthweight infants and those with complicated deliveries are at increased risk for omphalitis.30
Cutaneous findings
Excessive exudate from the umbilical stump, as seen in funisitis, may be a harbinger of subsequent infection. The exudate may be accompanied by bleeding from the umbilical vessels caused by a delay in closure. Omphalitis shows periumbilical erythema, edema, and tenderness, with or without discharge. Typically, signs of infection occur on the third day of life. The infection may extend subcutaneously to cause cellulitis, or along abdominal wall fascial planes leading to necrotizing fasciitis. Black discoloration or crepitus of the periumbilical tissues suggests more advanced infection.
Extracutaneous findings
The umbilical cord of the newborn infant is a particularly common portal of entry for invasive bacterial pathogens. Invasion may occur directly into the peritoneal cavity, with resultant peritonitis. Ascending infection along the umbilical vein is a particularly serious complication. Septic umbilical arteritis or suppurative thrombophlebitis of umbilical or portal veins, portal vein thrombosis, and liver abscesses may occur. Septic embolization from infected umbilical vessels (arteries or the vein) is uncommon, but may seed various organs, including the lungs, pancreas, kidneys, liver, brain, heart (i.e., endocarditis), or skin.
Etiology and pathogenesis
The umbilical cord stump may become highly colonized with pathogenic bacteria shortly after birth, including the mother’s vaginal and skin flora and bacteria from the hands of caregivers, including healthcare workers. Omphalitis is caused by a variety of these colonizing organisms, but S. aureus and Gram-negative organisms are most common, while anaerobes are an infrequent cause.31,32 Candidal funisitis has also been reported.33
Diagnosis
Gram stain and culture of moist umbilical cord or stump material may show organisms and be helpful in the early diagnosis and therapeutic decision process for funisitis or omphalitis. As with all cutaneous infections, the results of Gram stain and culture must be interpreted along with clinical signs to determine true infection as cultures will likely yield colonizing organisms, even in the absence of infection.
Differential diagnosis
The serous secretions of funisitis/omphalitis must be distinguished from those of a vitelline duct remnant, umbilical papilloma, or urachal remnant. Inflammatory disorders, such as seborrheic dermatitis and psoriasis, can affect the umbilical region.
Course, management, treatment, and prognosis
As progression to severe sequelae is a major concern, patients should be initiated on parenteral broad-spectrum antibiotics directed against Gram-positive and Gram-negative organisms.34 Definitive recommendations on empiric therapeutic choices are difficult owing to the variation in regional resistance rates. Empiric therapy should be dictated by each institution’s local resistance profile for S. aureus and Gram-negative organisms. Ampicillin-sulbactam is a reasonable empiric therapeutic option but if MRSA is a concern, the combination of clindamycin or vancomycin and gentamicin would be reasonable. In an infant who is ill-appearing, broadening coverage to include vancomycin, a higher generation cephalosporin and metronidazole may be warranted. Intravenous antibiotics should be continued until the erythema and drainage subside. Complications include evisceration, umbilical hernia, necrotizing fasciitis, cellulitis, peritonitis, superficial abscess, liver abscess and peritoneal adhesions.35 Predictors of poor outcome include early onset of the infection, unplanned home delivery, and temperature instability. Patients should be monitored closely for signs of necrotizing fasciitis, which has a high mortality rate.
Cellulitis
Cellulitis is characterized by infection of the dermis and subcutaneous tissues with relative sparing of the epidermis. A break in the skin resulting from trauma, surgery, or an underlying skin lesion predisposes to cellulitis. Cellulitis may be seen more commonly in patients predisposed to abnormal lymphatic drainage (e.g., Klippel–Trenaunay syndrome and lymphatic malformations) as well as in immunodeficiency disorders, but also can occur in otherwise healthy infants.
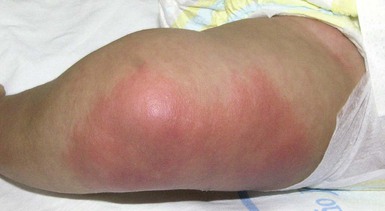
Cutaneous findings
Cellulitis presents as an area of edema, warmth, erythema, and tenderness (Fig. 12.7). The lateral margins tend to be indistinct because the process is deep in the skin. Application of pressure may produce pitting and pain. Cellulitis caused by S. aureus tends to be more localized and may be suppurative. Erysipelas is a superficial infection of the lymphatics typically caused by S. pyogenes that presents with bright red patches that tend to spread more rapidly and is often quite tender to palpation. There is often some break in the skin that is a portal of entry for the bacteria, leading to infection.
Extracutaneous findings
Regional adenopathy and constitutional signs and symptoms of fever, chills, and malaise are common. Complications of cellulitis include bacteremia with additional sequelae such as osteomyelitis, septic arthritis, and thrombophlebitis. Glomerulonephritis also can follow infection with S. pyogenes.
Etiology and pathogenesis
Besides S. aureus and Strep. pyogenes, group B streptococci are of major concern in the neonatal period. Additionally, Streptococcus pneumoniae, group G or C streptococci and Gram-negative agents, such as E. coli, have been implicated in cellulitis. In premature newborns, or newborns with immunologic defects, a number of other bacterial or fungal organisms need to be considered. Pasteurella multocida is implicated in cellulitis that follows dog or cat bites, whereas human bites may become infected with Eikenella corrodens. Immunization against Haemophilus influenzae type B has significantly reduced the incidence of cellulitis due to this organism.36
Diagnosis
Although not routinely necessary, aspirates from the leading edge of inflammation, skin biopsy, and blood cultures collectively allow for identification of the causal organism in approximately 25% of cellulitis cases. Importantly, an aspirate taken from the point of maximum inflammation yields the causal organism more often than does a leading-edge aspirate, and thus this approach may be favored.37 Lack of success in isolating an organism stems primarily from the low number of organisms present within the lesion.
Differential diagnosis
Wells syndrome, insect bites, drug reactions, contact dermatitis, subcutaneous fat necrosis, and cold panniculitis may resemble cellulitis.
Course, management, treatment, and prognosis
Empiric therapy for cellulitis should be directed by the history of the illness, the location and character of the cellulitis, and the age and immune status of the patient. Cellulitis in the neonate should prompt a sepsis workup, followed by initiation of empiric therapy intravenously with a penicillinase-stable antistaphylococcal antibiotic such as cefazolin, oxacillin, or nafcillin, and an aminoglycoside such as gentamicin or a cephalosporin such as cefotaxime. In areas with a high prevalence of MRSA, clindamycin or, if the child is severely ill, vancomycin should be considered. Once the regional erythema, warmth, edema, and fever have decreased significantly, transition to an oral regimen for completion in the outpatient setting is reasonable, provided that other sites of infection (e.g., the CSF) have been excluded.
Periorbital/orbital cellulitis
Infection of the soft tissues surrounding the eye is classified as periorbital (preseptal) cellulitis or orbital (postseptal) cellulitis.
Cutaneous findings
The orbital septum connects the orbital periosteum to the upper and lower eyelid structures. This tough fibrous band acts to prevent superficial infections from extending into the orbit and threatening vision. Periorbital cellulitis is a superficial infection that presents with redness, swelling, and tenderness of soft tissues anterior to the orbital septum. Orbital cellulitis involves deeper structures behind the orbital septum. Orbital cellulitis can be associated with sinusitis or direct penetrating trauma to the orbital septum. Orbital cellulitis presents with eyelid erythema, edema, and conjunctival hyperemia, and is distinguished clinically from periorbital cellulitis by ocular involvement.
Extracutaneous findings
Periorbital cellulitis can be associated with bacteremia, particularly when Streptococcus pneumoniae is involved. Orbital cellulitis is associated with proptosis, limited painful eye movement, decreased vision, and loss of corneal sensation. Constitutional signs such as fever can be seen with both periorbital and orbital cellulitis.
Etiology and pathogenesis
As noted above, periorbital cellulitis is a superficial infection and as such is more frequently caused by skin colonizers such as Staphylococcus spp. and Streptococcus spp.38 Orbital cellulitis is most commonly caused by an extension of ethmoiditis into the surrounding soft tissue. Although other sinus pathogens are linked to orbital cellulitis in children, S. aureus and, less commonly, Strep. pneumoniae are the more common pathogens described in neonatal orbital cellulitis.22,39 MRSA has less commonly been associated with orbital cellulitis in newborns23,24; however, with the recent increase in MRSA, it is likely that the frequency of neonatal orbital cellulitis caused by MRSA will also continue to increase.40
Diagnosis
As the landscape of antibiotic resistance changes, surgical intervention for drainage and attainment of cultures, if possible, can be extremely helpful in guiding antibiotic therapy choices. Positive blood cultures can be helpful in directing therapy but they are positive in a minority of cases. Ultrasound cannot reliably detect deep soft tissue infection; therefore, computed tomography (CT) is the most appropriate imaging study to define the extent of infection. Prompt imaging should be undertaken in any infant with proptosis and limited eye movement, as a delay in diagnosing orbital cellulitis can threaten vision and result in death.41
Differential diagnosis
Adenoviral conjunctivitis may mimic preseptal and septal cellulitis. Orbital pseudotumor, rhabdomyosarcoma, and Aspergillus fungal sinusitis must be excluded in the patient with periorbital swelling. A stye, or insect bite, can cause localized redness and edema in the eyelid, mimicking preseptal cellulitis but typically, there is a lack of fever, pain and constitutional symptoms.
Course, management, treatment, and prognosis
Deep soft tissue infection in the orbital region is a medical emergency. Making definitive recommendations on empiric antibiotic therapy for neonatal periorbital and orbital cellulitis is challenging. Clinicians need to be aware that the epidemiology of pathogens causing neonatal orbital cellulitis differs from that of older children.42 Generally speaking, antibiotic therapy should be directed at S. aureus and Strep. pneumoniae with less concern for anaerobes and other sinus commensals. In regions where MRSA rates are high, initial Gram-positive coverage should be broadened to include MRSA. Most pain can be managed adequately with decongestants and analgesics. Prompt consultation with ophthalmology and otorhinolaryngology should be obtained. Signs of optic nerve compression, such as decreased visual acuity, require urgent surgical decompression of the orbital space and infected sinuses.24 Transition to oral therapy can be considered once source control is established and clinical improvement has been shown to be robust. Although the most appropriate duration of therapy is not well studied, most experts continue therapy for 2–3 weeks.43
Subcutaneous and systemic infections
Abscesses
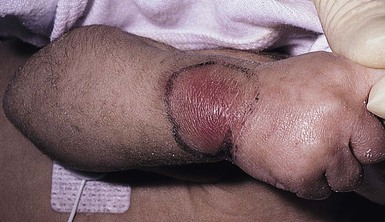
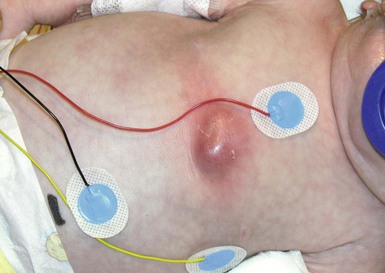
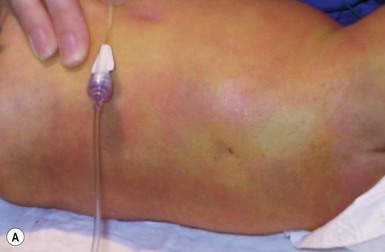
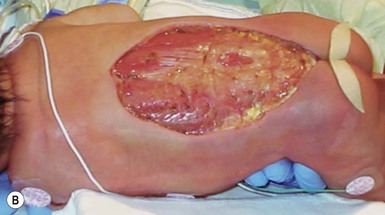
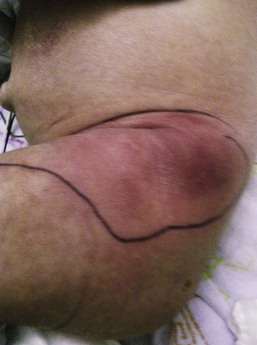
An abscess is a localized collection of pus in a cavity formed by the disintegration or necrosis of tissue, resulting in a firm, tender, erythematous nodule that becomes fluctuant (Figs 12.8, 12.9). In the neonatal period, an abscess is a potentially serious infection because of the higher risk for bacterial dissemination. Specific scenarios of breast and scalp abscesses are discussed below.
Cutaneous findings
Abscesses present with erythema, induration and pain to palpation. Depending on their depth and amount of time present, they may have fluctuance, or a pustule on top or drain to the surface.
Extracutaneous findings
Most localized furuncles are not associated with systemic findings. However, infants with an abscess should be closely monitored for signs and symptoms of bacterial sepsis, including temperature instability, irritability, poor oral intake, and leukocytosis (>15 000/mm3). Bacteremia can lead to hematogenous spread of the pathogen to other viscera and bones.
Etiology and pathogenesis
S. aureus is the single most common pathogen causing abscesses but Strep. pyogenes is found commonly as well. MRSA sometimes has virulence factors making infections caused by MRSA more likely to lead to abscess or furuncle formation. Abscesses can be seen in normal newborns, but multiple abscesses are characteristic of immunodeficiency syndromes, such as hyper-IgE syndrome and leukocyte adhesion deficiency.
Although S. aureus dominates as the causative pathogen of skin abscesses, other pathogens are possible. Prior to routine vaccination, Haemophilus influenzae type B was a well-documented cause of head and neck abscesses. In the setting of perianal and perineal abscesses, aerobic and anaerobic flora from the gastrointestinal and urinary tract should be considered. Neisseria gonorrhoeae has even been identified as the source of an abdominal abscess in a child.44 Identification of organisms not typically associated with abscess formation, especially organisms of low virulence, should raise concerns about underlying immunodeficiency.
Diagnosis
An abscess is typically a clinical diagnosis based on warmth, redness, induration, as well as pain and fluctuance on palpation. If the diagnosis is in question, an ultrasound can be useful to establish the presence of, size, and extent of any pocket of purulence. The therapy is to incise and drain amenable lesions; and the process of draining fluid also confirms the diagnosis of an abscess. Additional diagnostic testing such as blood cultures should be performed on a case-by-case basis. Recent data suggest that blood cultures in afebrile neonates with skin and soft tissue infections, including abscesses, are unnecessary.45 However, in febrile patients, blood cultures should be performed routinely. Additionally, blood cultures should be considered for premature and very low-birthweight infants as well as any child who is immunosuppressed.
Course, management, treatment, and prognosis
Incision and drainage is the treatment of choice for localized, uncomplicated abscesses in full-term neonates and infants who are otherwise well. Appropriate cultures should always be ordered for bacterial identification and sensitivities. The need for additional antibiotic coverage in the well-appearing neonate with adequate source control is not clear. Studies in children 6 months of age and older have shown that adequate drainage of an uncomplicated skin and soft tissue infection without subsequent administration of an antibiotic active against the identified pathogen is an effective approach.46 Although this study did not include those less than 6 months of age, it is reasonable to consider a similar approach assuming that close follow-up can be established. However, for premature, very low-birthweight or immunosuppressed neonates, intravenous antibiotics with MRSA coverage should be initiated (vancomycin or clindamycin typically); and an appropriate systemic workup should be done, including blood cultures.47
There are a few locations of abscesses in neonates and infants that have unique clinical diagnostic and therapeutic implications and are presented separately.
Breast abscess
Breast abscesses develop in full-term neonates during the first 1–6 weeks of life, most commonly during the 4th and 5th weeks.48 The incidence is approximately equal in males and females during the first 2 weeks of life, but thereafter, the incidence in girls is nearly twice that in boys.
Cutaneous findings
Breast abscesses present initially with breast enlargement, accompanied by varying degrees of erythema, induration, and tenderness. Fluctuance may or may not occur, depending in part on how early antibiotic therapy is initiated. Bilateral infection occurs in less than 5% of cases. Breast abscesses caused by S. aureus are accompanied by cutaneous pustules or bullae on the trunk, particularly in the perineal region, in 25–50% of patients. The symptoms, age at presentation, and clinical findings of infants with breast abscess caused by Gram-negative bacilli or anaerobes, are similar to those of infants infected with S. aureus. A noted exception is for infants infected with Salmonella species; they generally also have gastrointestinal illness.
Extracutaneous findings
A minority of patients develop fever (approx. one-third) and constitutional symptoms, such as irritability or toxicity, are uncommon. Leukocytosis (>15 000/mm3) is found in approximately half to two-thirds of patients. Concomitant bacteremia, pneumonia, osteomyelitis, or sepsis is unusual.
Etiology and pathogenesis
Breast abscess is usually due to S. aureus, but occasionally is caused by group B streptococci, E. coli, Salmonella spp., Proteus mirabilis, Pseudomonas aeruginosa, or Ureaplasma urealyticum.49 Although anaerobic organisms can be isolated from up to 40% of infections, their pathogenic role in neonates is questionable, and therapy directed specifically against them is generally unnecessary. The increased incidence in girls after 2 weeks of age (when breast gland development is more pronounced in girls than boys) and the lack of the disorder in the underdeveloped breast of premature infants, suggest that increased ductal tissue may be a factor in pathogenesis. Breast manipulation has also been suggested as a predisposing factor. Infants with S. aureus breast abscesses are typically colonized with the same organism in the nose or pharynx. It seems likely that S. aureus colonizing the skin of the nipple subsequently tracks up the ducts of the physiologically enlarged, predisposed breast, perhaps facilitated by breast manipulation, to infect deeper tissues.
Diagnosis
Gram stain of material expressed from the nipple or obtained by needle aspiration or incision and drainage can help identify the organism and guide initial antibiotic therapy. The presence of accompanying cutaneous vesicles or bullae may help in identifying S. aureus as the causal agent.
Course, management, treatment, and prognosis
Typically, breast abscesses remain localized and resolve with appropriate therapy. If fluctuance is absent, systemic antibiotic therapy may be curative and prevent abscess development. If fluctuance is present, the abscess must be drained by needle aspiration, by gently expressing pus from the nipple, or surgically, and Gram stain and culture obtained. Data in older children suggest that breast abscesses often result in the need for hospital admission.50 Although fewer data exist relative to neonatal breast abscesses, it is prudent to admit patients for parenteral antibiotic therapy. Antibiotics directed against S. aureus should be initiated as primary therapy. If local resistant patterns denote high rates of MRSA, then an agent such as vancomycin or clindamycin should be started. Blood cultures have been recommended in this clinical scenario, however the clinical impact of such testing has not been established.51 If the infant is displaying other concerning symptoms such as fever, or is ill-appearing, cultures of blood, urine and CSF should be considered. If Gram-negative bacilli are seen on the Gram stain, initial therapy should include Gram-negative coverage with an aminoglycoside or cefotaxime, while awaiting culture results. Once the infection has begun to subside, transition to oral therapy may be considered. In most instances, a total of 5–7 days of therapy is sufficient, although many experts continue treatment for 10–14 days. The most common complication of breast abscess is cellulitis, which develops in approximately 5–10% of affected infants. The cellulitis is generally localized, but can extend rapidly to involve the shoulder and/or abdomen. Scar formation leading to decreased breast size following puberty can occur as a late complication.
Scalp abscess
Scalp abscesses can develop in neonates at the insertion site of a fetal scalp monitoring electrode or because of local perinatal trauma to the scalp. The reported incidence of scalp abscess in neonates who undergo fetal scalp monitoring is between 0.1% and 5.2%.52
Cutaneous findings
Presentation occurs most commonly on day 3 or 4 of life, but may be as early as the first day and as late as 3 weeks. The lesion initially appears as a localized, erythematous area of induration, 0.5–2 cm in diameter. The site may become fluctuant or pustular.
Extracutaneous findings
Regional lymphadenopathy may be present, but other more serious complications, such as cranial osteomyelitis, subgaleal abscess, necrotizing fasciitis of the scalp, bacteremia, sepsis, and death, are rare.
Etiology and pathogenesis
Scalp abscesses are typically polymicrobial, including both aerobes and anaerobes. The anaerobic flora present reflect those found in the normal cervix during labor. The most common pathogenic organisms are Streptococcus spp. and E. coli.53 The most plausible hypothesis for the pathogenesis of scalp abscess is that the infection occurs via the ascent of normal cervical flora into the uterus following rupture of membranes, aided by procedures that access the uterine cavity. Placement of the electrode breaks the skin barrier, providing a foreign-body nidus for infection in the subcutaneous tissue. Risk factors include longer duration of ruptured membranes, longer duration of monitoring, monitoring for high-risk indications, amnionitis, and endometritis. In general, it appears that procedures that serve to provide increased access of vaginal flora to the infant, or more trauma to the scalp, may increase the risk of abscess development.
Diagnosis
Infants who are subjected to scalp electrode monitoring in utero should be followed closely during the first weeks of life for evidence of infection. If infection is suspected, culture for both aerobic and anaerobic organisms can be obtained ideally by needle aspiration or less ideally by swabbing the exudate expressed from the puncture site.
Differential diagnosis
The differential diagnosis includes cephalohematoma and HSV infection. A cephalohematoma is typically seen at birth and does not demonstrate erythema, warmth, or tenderness. It resolves spontaneously over 3 weeks and generally requires no treatment. Cutaneous HSV infection can mimic scalp abscess and occurs with a peak onset between 4 and 10 days of life. Prompt diagnosis and treatment of HSV is essential to prevent systemic dissemination. Aplasia cutis congenita can have clear drainage and resemble a scalp abscess.
Course, management, treatment, and prognosis
Scalp abscesses are often localized and self-limiting, leading some to suggest close monitoring with no antibiotics in the well-appearing child.52 However, owing to the risk of secondary complications, others have argued for the initiation of empiric antibiotics.54 Certainly, if fluctuance develops without spontaneous suppuration, incision and drainage with subsequent Gram stain and culture to guide antibiotic management are appropriate.
Necrotizing fasciitis
Necrotizing fasciitis is a potentially life-threatening infection that can arise as a complication in any number of cutaneous processes or can be without an identified incident event.55 Ultimately, the process results in rapid progression of infection along the superficial fascial layers causing significant morbidity and mortality. Although the incidence of necrotizing fasciitis is relatively rare in neonates and infants compared with older children and adults, the outcomes can be equally devastating.
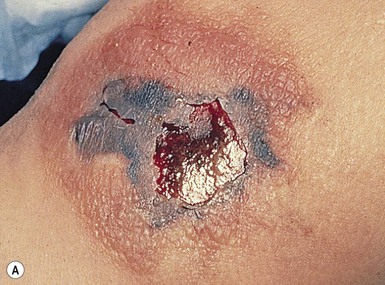
Cutaneous findings
At the site of the primary process, warmth, erythema swelling, and tenderness are typical (Fig. 12.10A). Often, the initial presentation can be difficult to distinguish from cellulitis.56 The process can start anywhere on the body, but the abdominal wall and thorax are the most frequent initial sites of disease in neonates. Involvement of the perineum and the external genitalia is specifically referred to as Fournier’s gangrene. As the infection progresses over the following 24–48 h, cutaneous findings are also likely to change. Later cutaneous signs can include a violaceous discoloration, formation of bullae initially filled with straw-colored and later bluish to hemorrhagic fluid, followed by darkening of affected tissues secondary to necrosis.57 Despite the initial significant pain, local anesthesia can ensue denoting compromise of local nerves. Infections resulting from one organism or a combination of organisms cannot be distinguished clinically. The presence of crepitus is often noted as a sign of an anaerobic pathogen such as Clostridium. However, the presence of crepitus is nonspecific and can be present with other pathogens such as E. coli.58
Extracutaneous findings
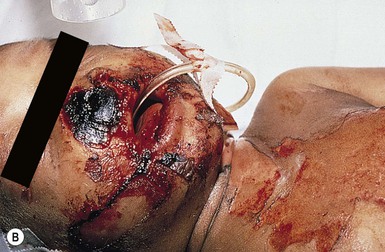
The clinical course of necrotizing fasciitis can vary considerably depending on the age of the patient. When considering pediatric patients of all ages, abdominal pain, vomiting, fever, chills and respiratory symptoms can be seen at presentation, while hypotension and other signs of shock are less common.59,60 However, rapid progression to more concerning events such as hypotension, coagulopathy and organ dysfunction is possible. For neonatal patients with necrotizing fasciitis, the initial presentation may be more ominous and include fever, tachycardia, lethargy, and poor cry.57,61 As the therapy for necrotizing fasciitis involves surgical resection of involved tissue (Fig. 12.10B), extensive postinfectious care of large cutaneous defects is often necessary.
Etiology and pathogenesis
The microbiologic etiology of necrotizing fasciitis is often grouped as type I, which is a polymicrobial infection including at least one anaerobic pathogen and type II, which is secondary to Strep. pyogenes and/or S. aureus.62 Variation in the epidemiology of causative pathogens does exist across pediatric age groups.63 Despite the limited number of neonatal cases, reasonable information on the microbiologic etiology and risk factors are available from multiple case reports and case series. Hsieh and colleagues reported on a review of 66 published case reports of neonatal necrotizing fasciitis.57 Almost three-quarters of the episodes with a microbiologically documented pathogen were polymicrobial in nature. More recently, a single center study retrospectively identified 15 patients from 2002–2006 who developed necrotizing fasciitis.61 Contrary to Hsieh and coworkers, monomicrobial infection secondary to staphylococci and streptococci was more common (66%). In both reports, over 60% of the cases had a predisposing condition (i.e., omphalitis, mastitis, bullous impetigo, balanitis, necrotizing enterocolitis, or an underlying immune deficiency) or exposure (i.e., fetal scalp monitor, surgical procedure or mother with mastitis). Case reports have also implicated fungal pathogens such as zygomycetes and Candida spp. as the cause of necrotizing fasciitis.61,64
As noted above, the initial presentation of necrotizing fasciitis may include a well-appearing child with cutaneous findings that can include warmth, tenderness, and erythema. Often upon palpation of the involved area, the tenderness elicited is said to be out of proportion to the appearance of the skin. In the neonatal population these exam findings may be less reliable. The infection can rapidly progress to include systemic signs such as sepsis and organ failure. As the infection spreads along the fascial planes it leaves in its wake areas of necrosis.65 Fatality rates in this patient population range from 20–59%.57,61
Diagnosis
As noted above, the initial presentation of necrotizing fasciitis can be nonspecific and the cutaneous findings not convincing. Tenderness to palpation out of proportion to what would be expected based on the actual appearance of the skin should alert the physician to the possibility of necrotizing fasciitis. Assessing tenderness in a neonatal patient can be challenging and thus clinicians should be concerned if the systemic disposition of the patient is worse than might be expected based on the skin appearance. Certainly, pre-existing conditions resulting in a breach of the skin barrier, such as omphalitis, impetigo, or mastitis, should also raise suspicion for necrotizing fasciitis. A definitive diagnosis can only be made by surgical exploration and visualization of the fascia, which must be undertaken as soon as the diagnosis is suspected. Although magnetic resonance imaging (MRI), CT scan or ultrasound may aid in delineating the extent and tissue planes of involvement, these imaging studies should not delay surgical intervention. Incisional biopsy with frozen section can be useful when gross appearance of the involved tissue cannot confirm the diagnosis.66 Histopathologically, necrotizing fasciitis shows necrosis and suppuration of the superficial fascia, edema, and an acute inflammatory infiltrate in the deep dermis, subcutaneous fat, and fascia. Tissue aerobic and anaerobic cultures should be taken at the time of the operative procedure. Additionally, blood cultures should also be drawn, as the causative pathogen is identified in the blood up to 50% of the time. Negative tissue and blood cultures do not completely exclude the possibility of necrotizing fasciitis, especially if the clinical scenario is consistent. Additional nonspecific laboratory evidence includes leukocytosis and thrombocytopenia.57 Attempts to establish predictive rules that leverage laboratory data to accurately identify patients with necrotizing fasciitis have been published. Unfortunately, the results of these studies have been contradictory and have not been specific to neonatal patients and thus are not applicable.63
Differential diagnosis
The initial cutaneous findings may be consistent with cellulitis in an otherwise stable patient. In a neonate, it is also plausible that a patient will present with systemic findings consistent with sepsis without an identified cutaneous lesion, as the lesion may not be apparent or may be covered by the diaper, clothing or blankets. It is imperative to do a full skin exam including the back, genital and perianal regions, especially if an etiology for sepsis has not been identified. Localizing the area of involvement for a patient with necrotizing fasciitis as early as possible is imperative to reducing the subsequent morbidity and mortality risk.
Course, management, treatment, and prognosis
Whenever possible, a multidisciplinary care team, including a neonatal intensive care specialist, a pediatric surgeon and a pediatric infectious disease specialist should immediately be established. Prompt surgical intervention is mandatory. The approach of the surgeon should include an initial incision to allow direct visualization of the fascia at the involved site. If grossly necrotic tissue is realized, then proceeding to removal of all devitalized tissue down to healthy appearing and bleeding tissue is warranted. If gross visualization cannot confirm the presence or absence of necrosis, then a biopsy with subsequent pathology interpretation of the frozen section can be helpful to dictate need for further debridement.66 Repeat exploration is generally indicated within 24–36 hours. In the interim, serial examinations should be performed and, if there is evidence of progression, then repeated surgical intervention is needed earlier. Surgery may need to be repeated on several occasions until devitalized tissue has ceased to form. Daily meticulous wound care is also paramount. Parenteral antibiotic therapy must be initiated immediately. Because monomicrobial and polymicrobial necrotizing fasciitis cannot be distinguished on clinical or surgical grounds, initial empiric therapy should include broad Gram-positive, Gram-negative, and anaerobic coverage. As with other infections, the local hospital antibiogram should help direct the need for coverage of potential resistant pathogens such as MRSA. Tissue culture data obtained at the onset of surgical intervention should inform directed antibiotic therapeutic decisions. For example if S. pyogenes is identified, transition to penicillin combined with clindamycin is optimal.67 Clindamycin is added to penicillin for necrotizing fasciitis caused by S. pyogenes because clindamycin is more effective against S. pyogenes bacteria that are slowly replicating and toxin-producing.67,68 If a polymicrobial infection is determined by culture results, then therapy should be employed to cover each pathogen. Importantly, with a polymicrobial infection, anti-anaerobic coverage should be continued even in the absence of growth of an anaerobic pathogen, as the yield of anaerobic cultures from tissue are highly dependent on culture collection techniques.
Ecthyma
Ecthyma refers to a red patch or nodule that necroses and ulcerates in the center due to bacterial overgrowth and disruption of blood vessels. The term ‘ecthyma gangrenosum’ is typically reserved for necrotic skin lesions specifically caused by Pseudomonas infection but there are other causes of ecthyma in neonates.69
Cutaneous findings
The lesion begins as a painful red or purpuric macule, that develops a pustular or vesicular center with a surrounding rim of pink or violaceous skin that rapidly ulcerates (Fig. 12.11A). The infection also may present with bullae. The ulcer develops raised edges with a necrotic, dense, black, depressed, and crusted center. Erythema multiforme-like lesions also have been described at onset. Lesions may be single or multiple, and sometimes cluster around areas of moisture such as the mouth or perineum (Fig. 12.11B).
Extracutaneous findings
The presence of ecthyma gangrenosum in an infant is often a complication of sepsis in which the skin lesions result from bacteremic spread of infection; however, only 1.3–13% of patients septic from P. aeruginosa ultimately develop cutaneous lesions.70 There have been nonsepticemic variants of ecthyma gangrenosum described in newborns. This localized form of ecthyma is likely from direct inoculation of the pathogen into the skin and therefore has a better prognosis.69
Etiology and pathogenesis
Ecthyma gangrenosum is most frequently due to P. aeruginosa, a Gram-negative bacillus. During septicemia, the organism tends to multiply in the walls of small blood vessels, which results in arterial and venous thrombosis, and ultimately dermal necrosis.71 Risk factors in neonates and young infants include prematurity, prolonged illness, necrotizing enterocolitis, previous bowel surgery, and an immunocompromised state, especially neutropenia. There are many other organisms that have been reported to cause ecthyma that include bacterial, fungal and viral pathogens such as S. aureus, Strep. pyogenes, Klebsiella pneumonia, Aeromonas hydrophila, E. coli, Proteus spp., Citrobacter freundii, Corynebacterium diphtheriae, Serratia marcescens, Stenotrophomonas maltophilia, Yersinia pestis, Aspergillus spp., mucormycosis, and Candida spp.69 The differential diagnosis for ecthyma in neonates who are premature or immunosuppressed for any reason should be appropriately broad.
Diagnosis/differential diagnosis
Lesions of ecthyma mandate a full sepsis work-up, tissue examination for Gram stain and culture, and histopathology. Gram stain is best performed on scrapings from the base of the ulceration. Histopathologic examination with proper staining for bacteria, fungi and, if indicated, virus, allows for the rapid differentiation of the source of infection. A pauci-inflammatory vasculitis, particularly of the veins, with surrounding edema, hemorrhage, and necrosis, is seen on histopathology of ecthyma gangrenosum. Bacteria may be seen in the perivascular tissue and occasionally in the vessel walls, particularly the adventitia and media of dermal veins, but not the arteries; the intima and lumina generally are spared.
Course, management, treatment, and prognosis
Ecthyma gangrenosum in association with bacteremia is a grave sign. Aggressive empiric parenteral antibiotic therapy should be initiated including a broad spectrum anti-pseudomonal agent such as cefepime, piperacillin-tazobactam, or an anti-pseudomonal carbapenem. Each of these agents also has reasonable Gram-positive coverage to include MSSA. If MRSA is suspected, vancomycin should be added. Some experts would suggest the inclusion of an aminoglycoside for broader Gram-negative coverage and additional anti-pseudomonal coverage. Finally, in an immunosuppressed, premature or critically ill child, there should be consideration for fungal coverage. Once the culture and susceptibility results are available, therapy may be narrowed.
Infections at the site of indwelling lines
When the skin is punctured and a foreign object (intravenous line or arterial line for instance) is placed, there is a risk of localized infection that can become systemic.
Cutaneous findings
Signs of catheter-related cutaneous infection include erythema, tenderness, and the presence of exudate at the exit site. Infection can occur at the catheter exit site, along the tunnel, or at its site of insertion into the vessel.72
Extracutaneous findings
Children can become bacteremic and possibly septic from the infected catheter site and can seed other organs with bacteria. Signs of systemic spread would include fever, lethargy, and temperature instability. S. aureus tends to lead to more fulminant infection. Coagulase-negative Staphylococci can cause indolent disease, although they are capable of producing fulminant infections. In developed countries, coagulase-negative staphylococci are the principal agents of sepsis in premature neonates.72,73 They also are an important cause of septicemia in neonates in many developing countries. Additional extracutaneous infections caused by coagulase-negative staphylococci include infective endocarditis, CSF shunt infections, necrotizing enterocolitis, pneumonia, urinary tract infection, and osteomyelitis.
Etiology and pathogenesis
Infection occurs because of the disruption of host defense mechanisms resulting from placement of the indwelling medical device. Any bacteria that colonize the skin can be pathogenic in this setting, especially in preterm or immunosuppressed infants. The normal human flora includes 13 species of coagulase-negative staphylococci. These colonize the skin of most neonates within 2–4 days of birth,74 are of relatively low virulence, and typically do not cause infection. However, they are an important cause of sepsis in preterm infants and may cause cutaneous infection. Staphylococcus epidermidis is the most prevalent member of this group, making up 60–90% of the coagulase-negative Staphylococci on the skin. The most important causes of clinical disease are S. epidermidis and S. haemolyticus. Colonization of the skin is a prerequisite for infection, and development of disease generally requires compromise of the epidermal barrier. Heavy skin colonization is a risk factor for the development of catheter-related infection. The increased incidence of infection in preterm infants is likely multifactorial but in part, may be due to the defective neutrophil oxidative burst compared with term infants.75
Diagnosis
Gram stain and cultures of skin and a culture of the blood via the line are important. Because this organism colonizes the skin, clinical correlation is necessary to determine whether it is the cause of the clinical disease. The line should be removed as early as possible.
Course, management, treatment, and prognosis
Strict handwashing is essential to limit the spread of such pathogens within the neonatal nursery. Recently, cleansing with chlorhexidine prior to line placement has been recommended in infants over 2 months of age to prevent line infections.76 When infection at the site of an indwelling catheter is suspected, broad Gram-positive coverage with an agent such as vancomycin should be initiated. In a child with signs of systemic infection, the addition of Gram-negative coverage is warranted. The treatment regimen can be modified appropriately once antibiotic susceptibility results are available.
Purpura fulminans
Purpura fulminans (PF) is a potentially disabling and life-threatening disorder characterized by an acute onset of progressive cutaneous hemorrhage and necrosis caused by dermal vascular thrombosis, and disseminated intravascular coagulation (DIC).77 Purpura fulminans usually arises in previously healthy neonates who become systemically ill, especially from meningococcal sepsis, varicella, pneumococcal sepsis, and meningitis.78
Cutaneous findings
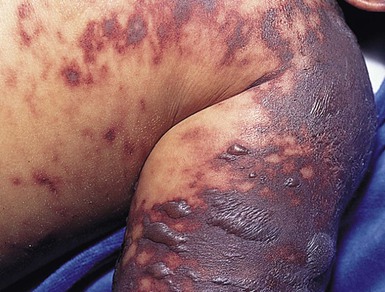
Cutaneous erythema with or without edema and petechiae develops first. Sites of involvement appear transiently to resemble ecchymoses, and up to this point, the pathologic process in the skin is reversible without progression to necrosis. Lesions evolve rapidly into painful, indurated, well-demarcated, irregularly bordered purpuric papules and plaques surrounded by a thin, advancing erythematous border. Late findings in necrotic areas are the formation of vesicles and bullae, which mark the development of hemorrhagic necrosis (Fig. 12.12), and finally firm eschar, which ultimately sloughs. The distal extremities are often the most severely involved, usually in a symmetric manner. This is probably a result of fewer collateral channels for tissue perfusion, and the relatively greater impact of circulatory collapse on perfusion of distal vascular beds. Acute infectious PF tends to progress proximally to form purpuric plaques of various sizes and shapes in a patchy distribution.
Extracutaneous findings
Shock is characteristic of acute infectious PF, and the development of systemic consumptive coagulopathy (i.e. DIC) is a defining feature. Thrombohemorrhagic manifestations may be found in multiple vascular beds and organ systems, and multiple organ dysfunction syndromes are common. Massive nontraumatic subdural hematoma can occur in extremely premature infants. Fibrinogen, coagulation factors (e.g., factors V and VIII), and platelets are consumed in ongoing thrombosis and fibrinolysis. Prothrombin time (PT) and partial thromboplastin time (PTT) are prolonged; fibrin degradation products (e.g., d-dimers) are elevated; and protein C, protein S, and antithrombin III levels are reduced.
Etiology/pathogenesis
Purpura fulminans develops in 15–25% of individuals with meningococcemia.79 In the neonate, acute infectious PF may be caused by early- or late-onset group B Streptococcus (GBS) disease, or occasionally a number of other Gram-positive and Gram-negative pathogens. PF results from an imbalance between anticoagulant and procoagulant activities of endothelial cells, shifting to a state of increased coagulation. In PF caused by Neisseria meningitidis this shift to a procoagulant state is triggered by endotoxin, producing an increase in interleukin (IL)-2, interferon-γ, tumor necrosis factor (TNF)-β, and IL-1, and leading to consumption of proteins C and S and antithrombin III.77
Diagnosis
Neisseria meningitidis can sometimes be identified as the cause of PF initially by finding the Gram-negative diplococci on Gram stain of material obtained by needle aspiration of a petechial skin lesion,80 or by scraping the lesion with a needle and making a smear of blood.81 The histopathologic hallmarks of PF are dermal vascular thrombosis and secondary hemorrhagic necrosis.82 Vasculitis, including a perivascular neutrophilic infiltrate, is a characteristic feature.
Differential diagnosis
Purpura fulminans in the neonatal period may be a manifestation of inherited, homozygous protein C, or rarely, protein S deficiency.83 Congenitally acquired infection with rubella, toxoplasmosis, and cytomegalovirus, and postnatally acquired infection with herpes simplex virus, viral hepatitis, certain enteroviruses, and respiratory syncytial virus, can produce purpura. Non-infectious causes of purpura in neonates include birth trauma, blood group incompatibility, neonatal isoimmune thrombocytopenia, maternal idiopathic thrombocytopenia purpura, maternal lupus erythematosus, drugs administered to the mother, Kasabach–Merritt syndrome, thrombocytopenia absent radius (TAR) syndrome, congenital histiocytosis, disseminated intravascular coagulation (DIC), congenital leukemia, and child abuse.84
Course, management, treatment, and prognosis
The size of the skin hemorrhage increases with disease severity, and the presence of purpura, particularly when generalized, is associated with high morbidity and mortality.85 Many who survive PF have cutaneous and/or skeletal deformities resulting from gangrene. Initial management of the patient with acute infectious PF must be focused on preserving life through respiratory and hemodynamic support and prompt broad-spectrum intravenous antibiotic coverage. Initial empiric therapy with a third-generation cephalosporin such as cefotaxime or ceftriaxone, is reasonable when targeting N. meningitidis.86 In immunocompromised patients, such as those with neutropenia, expanding coverage to include anti-pseudomonal activity is necessary. Additionally, if S. aureus is suspected and local rates of MRSA are increased, then the initiation of vancomycin may be necessary. Antibiotic therapy can be adjusted after the organism has been recovered and its susceptibility profile determined. Surgical consultation should be sought early in the course to monitor compartment pressures and intervene in compartment syndrome. Nutritional support is also important and should be continued during the rehabilitative phase. Therapeutic interventions that should be initiated for all patients with PF and DIC include vitamin K and fresh frozen plasma (FFP, 8–12 mg/kg every 12 hours) to correct possible deficiencies of vitamin K-dependent coagulation factors, antithrombin III, protein C, and protein S. A number of other newer, nonconventional treatment modalities, including concentrates of protein C or antithrombin III, recombinant tissue-type plasminogen activator, prostacyclin, plasmapheresis, hyperbaric oxygen, and a host of targeted immunotherapies (e.g., IL-1 receptor antagonist, monoclonal antibody to TNF-β, anti-endotoxin antibodies, platelet-activating factor receptor antagonist, and pentoxifylline) are available in some centers but the effectiveness of each of these interventions is unproven.77
Close contacts of patients with invasive meningococcal disease should receive prophylaxis with rifampin (or possibly ciprofloxacin for those aged 18 or over) as soon as possible. Options for prophylaxis include rifampin every 12 hours for 2 days or one dose of ceftriaxone intramuscularly. Patients at risk for meningococcal disease, such as those with complement or properdin deficiency, should be vaccinated. Current recommendations suggest that children down to age 9 months with a condition that predisposes them to meningococcal disease receive two doses of the quadrivalent meningococcal conjugate vaccine (MCV-4). Depending on the patient’s age, the second dose should be given either 2 (for age 2–18 years) or 3 (for age 9–23 months) months apart.87
Toxin-mediated disease
Exotoxin-mediated diseases are caused by the effects of extracellular toxins produced at a focus of infection or colonization. The site of bacterial replication is typically inconspicuous in relation to the clinical effects of the toxins. Toxins can act locally, as in bullous impetigo, or can cause widespread clinical signs resulting from hematogenous spread, as seen in staphylococcal scalded skin syndrome.
Staphylococcal scalded skin syndrome
Staphylococcal scalded skin syndrome (SSSS) is a staphylococcal epidermolytic toxin-mediated disease characterized by cutaneous tenderness and superficial, widespread blistering and/or desquamation.88 In the neonatal period, nursery outbreaks of the disease are described.89
Cutaneous findings
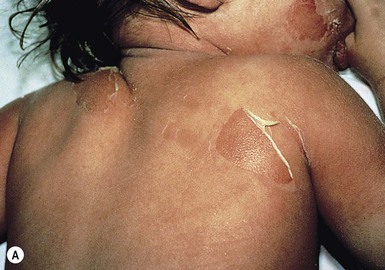
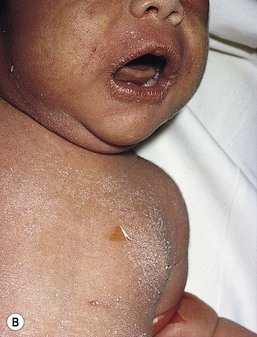
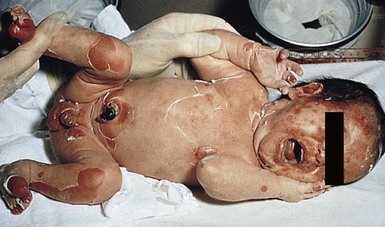
Exquisite tenderness of the skin may herald the onset of SSSS. Generalized macular erythema evolves rapidly into a scarlatiniform eruption that is accentuated in flexural and periorificial areas (Fig. 12.13). The brightly erythematous skin acquires a wrinkled appearance, leading to thick flaky desquamation, particularly in the flexures, over approximately 2–5 days. In severe cases, the erythrodermic phase is followed by the development of diffuse, sterile, flaccid blisters and erosions, and diffuse, bullous desquamation of large sheets of skin (Fig. 12.14). At this stage, areas of epidermis may separate in response to a gentle shear force (Nikolsky’s sign). As large sheets of epidermis peel away, moist, glistening, denuded areas become apparent, initially in the flexures and subsequently over much of the body surface. As the exposed denuded skin dries, it develops a crusted, flaky appearance. Distinctive radial crusting and fissuring around the eyes, mouth, and nose develop approximately 2–5 days after the onset of erythroderma. Secondary cutaneous infection, cellulitis, omphalitis, and severe surgical wound infections may occur.
Extracutaneous findings
Complications may include excessive fluid loss, electrolyte imbalance, faulty temperature regulation, pneumonia, endocarditis, and septicemia. Mortality, due predominantly to sepsis, is unusual, but is highest in the severe generalized form of the disease.
Etiology and pathogenesis
SSSS is caused predominantly by phage group II staphylococci, particularly strains 71 and 55; occasionally, a group I or III isolate is involved.90 Foci of infection include the nasopharynx, or less commonly the umbilicus, urinary tract, a cutaneous wound, conjunctivae, blood, and rarely through breastfeeding.91 These bacteria produce epidermolytic (i.e. exfoliative or exfoliating) toxins A (ETA), B (ETB), and/or D.92,93 ETB is more frequently associated with generalized SSSS than ETA. A reduced concentration of antibodies directed against ETB and an increased ability to penetrate into the bloodstream may explain why ETB is associated more frequently with SSSS.10 These epidermolytic toxins induce bulla formation by cleaving desmoglein I within desmosomes, via serine proteases. The severity of the disease is related to the toxin load, rather than the nature of the focal infection, and this load may be particularly high in neonates owing to reduced renal clearance.90
Diagnosis
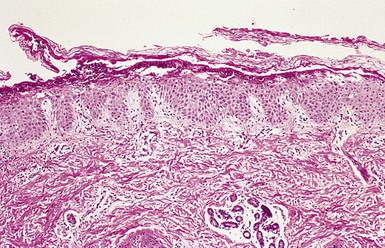
Recovery of a toxin-producing strain of S. aureus in a neonate or infant with widespread blistering establishes the diagnosis of SSSS. Although intact bullae on the skin are sterile, cultures should be obtained from multiple sites, including the blood, cerebrospinal fluid, nasopharynx, urine, umbilicus, and any suspected sites of localized infection, in an attempt to identify the source of the epidermolytic toxins. Histopathologically, subcorneal bulla formation through the granular layer without an inflammatory infiltrate is characteristic (Fig. 12.15![]() ). In cases that demand a rapid diagnosis, the exfoliated corneal layer can be seen on a frozen biopsy specimen of the desquamating epidermis. Scattered acantholytic cells that are evident histopathologically in the cleft-like bullae can also be seen in a Tzanck preparation.
). In cases that demand a rapid diagnosis, the exfoliated corneal layer can be seen on a frozen biopsy specimen of the desquamating epidermis. Scattered acantholytic cells that are evident histopathologically in the cleft-like bullae can also be seen in a Tzanck preparation.
Differential diagnosis
SSSS may be mistaken for a number of other blistering and exfoliating disorders, including scarlet fever, bullous impetigo, Kawasaki disease, epidermolysis bullosa, diffuse cutaneous mastocytosis, familial peeling skin syndrome with eosinophilia, epidermolytic hyperkeratosis, a viral exanthem, a drug eruption, erythema multiforme, and Stevens–Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN). SJS and TEN often can be distinguished by a history of drug ingestion, the presence of Nikolsky’s sign only at sites of erythema, and presence of mucosal blistering. The differentiation of SJS/TEN from SSSS may occasionally require a skin biopsy. SJS/TEN result in full-thickness epidermal necrosis, with a blister cleavage plane in the lowermost epidermis. Distinguishing between these conditions is particularly important because overall mortality rates as high as 30% have been reported with TEN, and avoidance of the offending drug is crucial to prevent recurrence.
Course, management, treatment, and prognosis
Recovery is usually rapid once appropriate antibiotic therapy has begun. Parenteral nafcillin or oxacillin should be given promptly. In regions where MRSA is prevalent, vancomycin should be considered. Strict isolation is imperative to avoid the spread of infection. (See Chapters 10 and 11 for skin care recommendations in blistering diseases.) General principles include minimizing the handling of the infant, and the use of emollients (e.g., petrolatum and petroleum jelly gauze) and semi-occlusive dressings to provide lubrication and minimize pain. Corticosteroids are detrimental and should be avoided. Healing occurs without scarring in 10–14 days.
Toxic shock syndrome
Toxic shock syndrome (TSS) is a severe acute infection characterized by fever, widespread macular erythema, shock, and multisystem organ failure. Toxins produced by certain strains of Strep. pyogenes and S. aureus act as superantigens leading to hypotension due to extreme activation of the immune system with cytokine release.
Etiology and pathogenesis
TSS is caused by a primary infection with either S. aureus or Strep. pyogenes that produce toxins that directly activate the immune system bypassing the usual antigen-mediated immune response system.94 Staphylococcal TSS occurs most frequently after primary cutaneous infection or infection secondary to surgery, foreign body or burns infections but sometimes the primary infection is not apparent.95,96 Staphylococcal TSS is caused primarily by an overproduction of toxic shock syndrome toxin 1 (TSST-1), but enterotoxins A, B and C have also been implicated.97 Streptococcal TSS can develop in various clinical settings including, but not limited to: cellulitis, pneumonia, septic arthritis, or pharyngitis.98 Most isolates of Strep. pyogenes that cause STSS are M protein types 1, 3, 12, and 28 that produce streptococcal pyrogenic exotoxins A and/or B.94,99,100 Lack of antibody against exotoxin appears to be a risk factor for severe, invasive disease and streptococcal TSS.94
Cutaneous findings
Staphylococcal TSS manifests as widespread erythema with accentuation in the skin folds. There is commonly a strawberry tongue and there is characteristic peeling of the hands, feet and sometimes perineum within 1–2 weeks.97
The primary Strep. pyogenes infection for some patients will include cellulitis, fasciitis, or myositis. In patients with these manifestations, development of severe pain at the site of infection is common. Primary varicella zoster virus infection (chickenpox) is also a risk factor for streptococcal TSS.94 Additionally, cutaneous findings are not always seen but can include erythroderma. The development of vesicles and bullae (5%) is a late, ominous sign of tissue devitalization.101,102 Other cutaneous signs in a minority of patients include a petechial, maculopapular, or diffuse scarlatiniform eruption.
Extracutaneous findings
In streptococcal TSS, patients without a soft tissue infection may have a variety of other focal infections that serve as the source of S. pyogenes, including endophthalmitis, osteomyelitis, myositis, pneumonia, perihepatitis, peritonitis, myocarditis, and sepsis.102 TSS can be acute or insidious in onset but most commonly presents with fever, generally accompanied by tachycardia and hypotension. There is then progression to shock and multiorgan compromise or failure that may include the kidneys, liver, hematologic systems and/or central nervous systems as noted below. Criteria for diagnosis of TSS are summarized in Box 12.2![]() .103,104
.103,104
Course, management, treatment, and prognosis
Patients suspected of having TSS should be managed in an intensive care setting because of the rapidly progressive, fulminant nature of the syndrome. Management consists of aggressive intravenous fluid resuscitation, culture of potential sites of infection, early surgical exploration of suspected deep-seated infections with debridement of devitalized tissue, removal of any potential foreign body nidus of infection and prompt administration of antibiotics. Inotropic agents may be necessary to manage shock due to toxic cardiomyopathy. The use of epinephrine (adrenaline) in patients with intractable hypotension may potentiate gangrene of digits. The use of intravenous immunoglobulin is still controversial but sometimes recommended in the setting of refractory disease. While ruling out septic shock from Gram-negative bacilli or polymicrobial necrotizing fasciitis, broad-spectrum antimicrobial therapy should be initiated. If a diagnosis of streptococcal TSS is made, therapy can be tailored to include an agent active against the cell wall of S. pyogenes such as penicillin. The addition of clindamycin has been recommended as an adjunctive therapy with the advantage of decreasing bacterial toxin production.105
Other infectious agents
There are a few bacterial pathogens that are either more specific to, or show unique cutaneous presentations in, neonates and infants and thus will be discussed separately.
Group B streptococcus
Group B Streptococcus, GBS, or Streptococcus agalactiae, a Gram-positive bacteria, is one of the most common causes of neonatal septicemia in developed countries. There are early and late forms of GBS disease. Early-onset disease presents within the first 6 days of life, although signs of infection are typically present at birth or within hours of delivery; preterm infants are particularly susceptible. Late-onset disease occurs from 7 days to 3 months of life, with a median age at onset of 27 days. The terminology of late, late onset is reserved for those infections presenting after 3 months of life.
Cutaneous findings
Cutaneous manifestations of GBS infection are rare.106 Skin lesions, however, may be an early sign of bacteremia or, alternatively, may provide a focus of infection from which bacteremia may occur. Cellulitis is the most common cutaneous manifestation of GBS infection and typically develops in late-onset disease. GBS cellulitis has a predilection for the face, submental, and submandibular regions in infants less than 12 weeks of age. Additional GBS soft tissue presentations have included facial or submandibular cellulitis with ipsilateral otitis media,107 and inguinal (Fig. 12.16), scrotal, prepatellar, or retropharyngeal. This organism can cause vesicles, bullae, and erosions that resemble impetigo.108 The lesions may be present at birth and appear anywhere on the body. Additional cutaneous manifestations of GBS infection include abscesses of the scalp, breast, submandibular gland, and subcutaneous tissue; erythema nodosum-like lesions; conjunctivitis; necrotizing fasciitis; acute necrotizing cellulitis of the scrotum; and purpura fulminans.
Extracutaneous findings
Extracutaneous findings vary depending on the timing of disease onset. Early-onset disease is characterized by septicemia, respiratory distress, apnea, shock, pneumonia, and less commonly, meningitis (5–15%). Bacteremia without a focus of infection (40–50%) is the most common presentation of late-onset disease, although other foci such as osteomyelitis, septic arthritis, and cellulitis/adenitis may be found. Cellulitis usually is associated with bacteremia (90%).
Etiology and pathogenesis
Cutaneous manifestations of GBS infection may be due to primary infection of the skin, often in association with surgery (e.g., circumcision) or cutaneous trauma (e.g., fetal scalp monitoring), or as a result of tertiary infection of the skin during bacteremia. Colonization of the neonate as a prelude to infection is thought to occur either in utero, via the ascent of organisms, or during delivery. Approximately 30% of women are carriers of GBS, yet only 1–2% of their infants develop early-onset disease, indicating that neonatal infection reflects a complex interaction between host defenses and bacterial virulence factors. In general, the likelihood of neonatal infection increases with greater bacterial inoculum, duration of exposure, and immaturity of the host. Infants who develop either early- or late-onset disease have low levels of antibody to type III polysaccharide, one of the organism’s major virulence factors.109
Diagnosis
The organisms may be visualized on Gram stain and recovered in cultures from skin lesions. Because skin findings may be an early indicator of occult bacteremia, evaluation should include urine, blood, and CSF cultures and a chest radiograph.
Differential diagnosis
Other bacterial organisms producing non-necrotizing soft tissue infections (e.g., impetigo, cellulitis, abscess), necrotizing soft tissue infections (e.g., necrotizing fasciitis) or purpura fulminans in the neonate can mimic GBS infection.
Course, management, treatment, and prognosis
Parenteral penicillin remains the drug of choice, but initial therapy for suspected GBS infection should consist of ampicillin and an aminoglycoside such as gentamicin, because this regimen provides broad coverage for the important pathogens in neonatal septicemia as well as synergic action against GBS. Once GBS has been identified as the pathogen and the bloodstream and CSF have been shown to be sterile for 24–48 hours, penicillin G alone can be given.110 The duration of therapy required for treatment of GBS skin infections depends on the nature and severity of the infection and the involvement of other sites (e.g., bloodstream, meninges). Interestingly, in a small case series of five patients with GBS cellulitis, all five had received ampicillin and gentamicin for at least 3 days for other indications prior to the onset of cellulitis. This suggests that neonatal GBS colonization persists, despite systemic antibiotic exposure.111 When there is GBS-induced meningitis, 30–50% of the neonates will have permanent neurologic defects.112 Overall mortality rates have improved greatly, from 50% in the 1970s to 5–6% in recent years.113 Vaccination against GBS is a potential effective method of prevention, but candidate vaccines are still under development.114
Listeria monocytogenes
Listeria monocytogenes is an uncommon cause of infection in newborn infants, with an incidence of 5.2 cases/100 000 live births. More recent data from Northern California suggest that the incidence of neonatal bacteremia secondary to Listeria monocytogenes is negligible.115 Worldwide, however, it is one of the three major causes of neonatal meningitis.116 Similar to infection with group B Streptococcus, affected infants present with disease shortly after birth, or with a late-onset form that generally develops between 2 and 5 weeks of life. Skin lesions, however, have only been described in early-onset disease.
Cutaneous findings
Lesions are usually evident at birth. Generalized petechiae and erythematous macules progress to erythematous pustules.117 Purulent conjunctivitis may also occur. Focal infection may occur from direct skin inoculation. Granulomatosis infanti septicum are widely disseminated granulomatous abscesses that are characteristic of severe listeriosis.116 These lesions are not only on the skin but can be seen in the liver, placenta, brain, spleen, kidneys, lungs, and GI tract.
Extracutaneous findings
Neonates with early-onset disease generally are delivered prematurely to mothers with a febrile illness and are septicemic (80%). Other symptoms include acute respiratory distress, pneumonia, meningitis, and myocarditis. Late-onset disease is usually seen days to weeks after an uncomplicated full-term delivery, with signs of sepsis or meningitis, including irritability, fever, lethargy, and diarrhea.
Etiology and pathogenesis
Listeria monocytogenes is usually acquired by the pregnant female via the consumption or aspiration of contaminated meat, raw dairy products, or raw vegetables.116 Affected pregnant women develop an influenza-like illness that may include vertical transmission to the fetus via hematogenous spread to the placenta, from an ascending vaginal infection, or during passage through the birth canal.118 Rarely, infection may result from cross-contamination in the delivery suite or nursery. The highest concentrations of organisms are found in the lungs and gut of the neonate, suggesting that infection may be acquired in utero via infected amniotic fluid. The organism has a predilection for the placenta and, once invasive disease has occurred, the central nervous system.
Diagnosis
A full sepsis evaluation should be performed in infants with suspected listeriosis, including bacterial cultures of the blood, urine, and CSF. The diagnosis may be aided by obtaining Gram stains and cultures from the mother’s vagina, the amniotic fluid, and the infant’s meconium, gastric washings, skin, posterior pharynx, and conjunctivae. Gram staining of meconium may be particularly revealing in granulomatosis infantisepticum. Cutaneous pustules show Gram-positive rods, but the organism may appear as cocci in pairs and simulate pneumococcus. L. monocytogenes also can produce white plaques on the surface of the cord. Serologic tests are not useful because of lack of sensitivity.
Differential diagnosis
Other pathogens that can cause a generalized vesiculopustular eruption must be excluded (see Chapter 10). The other pathogen that characteristically shows involvement of the umbilical cord is Candida.
Course, management, treatment, and prognosis
Infection acquired in utero may result in death of the fetus or congenital listeriosis. Early-onset Listeria infection in the first 2 days of life carries a 30% mortality rate, although mortality rates up to 38–60% have been reported.119,120 L. monocytogenes is sensitive in vitro to many antibiotics, including penicillin, ampicillin, erythromycin, trimethoprim-sulfamethoxazole, chloramphenicol, rifampin, tetracyclines, and aminoglycosides, but it is always resistant to cephalosporins.120 Although there have not been any controlled trials to determine optimal first line therapy, most experts recommend parenteral ampicillin and an aminoglycoside, such as gentamicin, which provides synergy against L. monocytogenes. After an adequate clinical response has been achieved, a course of therapy (10–14 days for invasive disease without meningitis, 2–3 weeks if meningitis is present) can be completed with ampicillin or penicillin alone.116
Treponema pallidum
Congenital syphilis (CS) occurs in infants born to mothers infected with the spirochete T. pallidum. In the 1980s and 1990s, the number of reported cases of CS and maternal syphilis in the USA increased, subsequently decreased to its lowest point in 2004 but unfortunately increased again nationwide from 2005–2008.121 Between 28% and 35% of neonates with CS had mothers who did not receive prenatal care.122 The lack of historical and laboratory data to make a diagnosis of CS in these cases emphasizes the importance of recognizing the dermatologic features of the disease.
Congenital syphilis is divided into early and late disease. Early disease usually presents before 3 months of age, although signs can appear anytime in the first 2 years of life. Late disease appears after age 2 years.
Cutaneous findings
Overall, approximately half of newborns with CS are asymptomatic at birth. Cutaneous findings, although highly variable, are present in only 38% of those affected.123 The palmar/plantar, perioral, and anogenital regions are classically involved. Mucous membrane involvement may present as snuffles (syphilitic rhinitis), which is often the first sign of CS. Syphilitic rhinitis begins as clear nasal discharge that may be mistaken clinically for a viral upper respiratory infection and may become profuse, chronic, and/or bloody. Associated inflammation and ulceration of the nasal mucosa can result in perforation of the nasal septum, with subsequent alteration of the nasal cartilage (saddle nose deformity). Condyloma lata refers to the highly infectious flat-topped papules and plaques that occur at the mucocutaneous junctions of the nares, angles of the mouth, and in the anogenital region; chronic induration in these areas leads to rhagades, or linear scars, which fan out from the corners of the mouth and the affected orifices. Mucous patches may also occur on the lips, tongue, and palate. Other early findings include petechiae (usually from thrombocytopenia), hemorrhagic vesicles and bullae (pemphigus syphiliticus), and erythematous macular, papulosquamous, annular, or polymorphous eruptions (Fig. 12.17). The papulosquamous eruption is most common on the posterior aspects, particularly the buttocks, back, and thighs, in addition to the soles. Although the papulosquamous rash resembles the coppery-red eruption of acquired secondary syphilis, pemphigus syphiliticus is unique to the newborn. Bullae form on an indurated, red base and rupture easily, leaving a macerated area that may form crusts. Changes on the palms and soles include erythema with a polished appearance; superficial peeling; indurated fissures; and oval ham-colored macules and papules that acquire a coppery-brown color as they age. Nail deformities, paronychia, and alopecia may occur. Untreated skin lesions resolve in 1–3 months with postinflammatory hyperpigmentation and/or hypopigmentation, but dyspigmentation is infrequent in the newborn period.
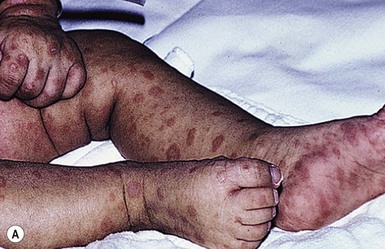
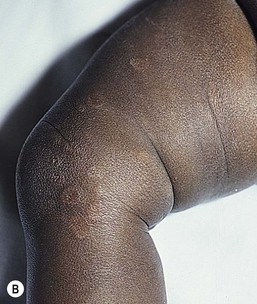
Extracutaneous findings
CS commonly presents with extracutaneous findings that include low birthweight, hepatomegaly with elevation of serum alkaline phosphatase, splenomegaly, anemia, thrombocytopenia, jaundice, osteochondritis, generalized lymphadenopathy, respiratory distress, hydrops fetalis, meningitis, meningoencephalitis, nephrotic syndrome, chorioretinitis, and pseudo-paralysis.123 Late manifestations (i.e., appearing after 2 years of age) involve the central nervous system (neurosyphilis, which may be asymptomatic), bones (frontal bossing, saddle nose, concave central face, saber shins, Clutton’s joints), teeth (Hutchinson peg-shaped notched central incisors, mulberry multicuspid first molars), skin (rhagades, nodular syphilids, gummata), eyes (interstitial keratitis, optic atrophy), and ears (eighth-nerve deafness). Hutchinson’s triad of defects includes interstitial keratitis, defects of the incisors, and sensorineural hearing loss.
Etiology and pathogenesis
Infection occurs via invasion of the placenta by the spirochete T. pallidum. The organism enters the bloodstream directly and invades the liver, with subsequent invasion of other organs, principally the skin, mucous membranes, bones, and central nervous system. Infection can occur at any time during pregnancy or at birth. Spirochetes preferentially adhere to endothelial cells and induce vasculitis. Nearly all neonates born to mothers with primary or secondary syphilis sustain congenital infection, but only about 50% are clinically symptomatic at birth.124 A high percentage will be preterm and at high risk for neurologic complications. As many as 25–40% of infected fetuses of mothers with untreated syphilis die in utero.125 Neonates infected during the third trimester typically are normal at birth, may become ill during the first weeks of life, but most commonly show signs at 2–6 weeks of age.
Diagnosis
The laboratory diagnosis of CS may be difficult because false-positive and -negative serologies confound interpretation and because T. pallidum cannot be cultured on artificial media.126 Maternal nontreponemal and treponemal IgG antibodies can be present in the fetus as a result of transplacental transfer even if the mother was adequately treated during pregnancy. Serum should be taken from the infant, rather than from cord blood, as the latter has yielded false-negative results especially when the mother was infected late in pregnancy.127 The diagnosis can be made by demonstration of spirochetes within a clinical specimen, obtained by scraping the base of a mucocutaneous lesion, using darkfield microscopy or direct fluorescent antibody testing. Specimens from mouth lesions require direct fluorescent antibody techniques to distinguish T. pallidum from commensal oral spirochetes. Histopathologic examination of the placenta and umbilical cord using specific fluorescent antitreponemal antibody staining also is recommended. Knowledge of the syphilis status for all new mothers should be documented in their newborn’s chart prior to discharge. A syphilis diagnosis should be strongly considered for a newborn if the mother had clinically documented syphilis during pregnancy or had reactive nontreponemal and treponemal tests before or during pregnancy. Additional intervention for the child depends on confirmation of appropriate treatment for the mother either before or during pregnancy. If treatment was before pregnancy and the child is well, no further intervention is required. If the mother’s treatment was not appropriate during pregnancy, then treatment of the child is necessary. If intrapartum treatment for the mother was appropriate then the decision to treat the child is based on the child’s serum nontreponemal titer (VDRL, RPR) results. If the infant’s nontreponemal test is four-fold the mother’s level, then the diagnosis of congenital syphilis is made and treatment administered; a titer less than four-fold that of the mother, however, does not exclude CS.128 Nontreponemal tests in both an infected mother and her congenitally infected infant may be falsely negative if the mother acquired the disease late in pregnancy, or in the case of a prozone phenomenon. False-positive reactivity of nontreponemal tests can be caused by certain infectious diseases (e.g., hepatitis, varicella, measles, infectious mononucleosis, tuberculosis, malaria, endocarditis), malignancies (e.g., lymphoma), and connective tissue disease (e.g., systemic lupus erythematosus). The nontreponemal tests are useful for screening, and, if reactive, should always be confirmed using a treponemal test. A CSF VDRL test should be performed on all neonates when there is a suspicion for CS, remembering that a negative result does not exclude neurosyphilis. A false-positive result, however, may occur in an uninfected newborn with a transplacentally acquired high serum VDRL titer. Newer techniques may improve our diagnostic capabilities, including immunoblotting to detect IgM against a 47 kDa membrane protein and PCR amplification of the genomic region encoding this same membrane protein.129 A skin biopsy is helpful in the evaluation of CS and shows swelling and proliferation of endothelial cells, and a predominantly perivascular infiltrate composed of lymphoid cells and plasma cells. Radiographic abnormalities are particularly important, as they are present in up to 95% of symptomatic and 20% of asymptomatic neonates with CS.130 Several long bones tend to be affected symmetrically, particularly the lower extremities. The metaphyseal lesions of osteochondritis vary from radiopaque bands to punctate lucencies to mottled regions. Diaphyseal lesions appear as periosteal new bone formation.
Differential diagnosis
As a result of the variable morphology of the lesions and the broad differential diagnosis it engenders, syphilis has been called ‘the great imitator.’ Blistering in CS must be differentiated from that in other vesiculobullous conditions that involve the palms and soles, including but not limited to congenital candidiasis, acropustulosis of infancy, scabies, and epidermolysis bullosa (see Chapters 10 and 11). Although unlikely, concern for possible congenital Lyme borreliosis has been described.129 However, although Lyme disease can result in a false-positive treponemal test, a nontreponemal VDRL test can be used to differentiate Lyme disease from syphilis because in the former the VDRL test is nonreactive.
Course, management, treatment, and prognosis
If CS is determined, or if there is inadequate maternal and newborn information to confirm appropriate maternal treatment and/or lack of evidence for neonatal infection, then penicillin therapy is recommended. The recommended dose, duration, and route of penicillin administration and follow-up vary according to a combination of specific historical, clinical, and laboratory factors for both the mother and child. Description of these recommendations is beyond the scope of this chapter but is well presented in guidelines published by both the Centers for Disease Control and Prevention as well as the American Academy of Pediatrics.131 Importantly, positive MHA-TP and FTA-ABS treponemal tests usually remain reactive for life, despite successful treatment. The prognosis in promptly treated early congenital syphilis is excellent.
Mycobacterium tuberculosis
Tuberculosis presenting in the neonatal timeframe can either be congenital (vertically acquired) or postnatally acquired. Although rare, the risk of congenital tuberculosis (CT) increased in the 1990s because of an increase in the number of tuberculosis cases in women of childbearing age. This reflects an increase in the incidence of tuberculosis overall, due in part to the human immunodeficiency virus (HIV) epidemic, as well as increased international adoption and lack of nonemergency medical care to illegal immigrants. The following definition for CT was suggested: presence of proven tuberculous lesions plus one of the following criteria: tuberculous lesions present in the first week of life; a primary hepatic complex or hepatic granulomas; tuberculous infection of the maternal genital tract or placenta; or exclusion of postnatal transmission of tuberculosis by a thorough investigation of contacts.132 In practice, distinguishing postnatally acquired tuberculosis from CT is not always possible. Even though the distinction is not important for therapeutic decisions, identification of the person infecting the infant postnatally has important public health implications in attempting to halt the spread of infection in the community.
Cutaneous findings
Cutaneous findings in CT are unusual and include scaly, erythematous, umbilicated papules and subcutaneous nodules.133 Infants with postnatal genital tuberculosis following circumcision may present with firm, nontender, enlarged, inguinal lymph nodes that progress to ulcerated, suppurative, bilateral, inguinal lymphadenopathy with sinus tract formation. Extensive ulceration of the penis and scrotum and varicella-like cutaneous lesions also have been described.134
Extracutaneous findings
Newborns with CT are frequently premature and have low birthweight. They may be symptomatic at birth. The symptoms of CT more commonly present during the 2nd to 4th weeks of life with poor feeding, listlessness, respiratory distress, fever, hepatosplenomegaly, abdominal distension, ear discharge, and lymphadenopathy.128 Most infected infants have abnormal chest radiographs (e.g., hilar lymphadenopathy, parenchymal infiltrates), and approximately 50% have a miliary pattern. Meningitis is present in about 20% of cases. Infants with postnatally acquired tuberculosis may present with fever, lethargy, respiratory distress, cough, vomiting, weight loss, lymphadenopathy, anemia, and hepatosplenomegaly at 4 weeks to several months after birth.135
Etiology and pathogenesis
Tuberculosis can be transmitted from mother to child in utero via hematogenous spread of Mycobacterium tuberculosis from the placenta or aspiration of amniotic fluid contaminated from the placenta or an ascending genital infection.132 The mother of a child with CT may be completely asymptomatic or may have a range of clinical manifestations for tuberculosis including but not limited to pleural effusions, meningitis or endometritis. Interestingly, while female genital tuberculosis is rare, acid-fast bacilli can often be identified in the placenta of women with children suffering from CT.131 Transplacental spread may produce a primary focus of infection in the liver, with involvement of the periportal lymph nodes and secondary hematogenous spread to the lungs, spleen, other viscera, and the central nervous system.132 Postnatal transmission is often via exposure of the child to a close contact with active respiratory tuberculosis. Additionally, postnatal exposure can also be from contaminated milk or local infection of compromised skin or mucous membranes. Women with isolated pulmonary tuberculosis are unlikely to infect the infant until after birth. Infection of the skin of the head and neck or the oral mucous membranes, associated with regional lymphadenopathy, may occur by kissing. In the past, tuberculosis of the skin and mucous membranes of the male genitalia was reported 1–4 weeks after ritual circumcision by an infected operator.
Diagnosis
CT should be suspected in any infant who presents with a sepsis-like syndrome, negative bacterial cultures, and lack of response to antibiotic therapy. A thorough history, including information regarding the risks for tuberculosis in the mother and other close contacts is invaluable to improving the time to diagnosis. Diagnosis is confirmed by identification of M. tuberculosis in tissue (e.g., liver, lymph node, bone marrow, skin) or fluid from the stomach, trachea, urinary tract, bone marrow, middle ear, or pleural spaces. Culture is the gold standard for identifying M. tuberculosis and confirming the diagnosis. However, because M. tuberculosis is slow growing, additional testing mechanisms can be helpful to guide initial therapy. Acid-fast stains are helpful if they are positive but a negative acid-fast stain does not rule out the presence of M. tuberculosis. False-positive smears from gastric aspirates in a newborn are rare. The use of a DNA probe may allow earlier detection but the accuracy of these probes used directly on specimens obtained from neonates is not well-defined. Other indirect testing modalities such as the Mantoux skin test, performed with 5 TU PPD (tuberculin purified protein derivative) can be done but are not typically clinically useful in this patient population. The PPD is usually initially negative and may take 3 weeks to 3 months to become positive. Interferon gamma release assays may be useful diagnostic tests in this patient population but further evaluation is necessary before they can be routinely endorsed.134 The mother of a neonate with suspected tuberculosis should also be evaluated for the presence of tuberculosis. A predelivery history can identify mothers with an increased risk for tuberculosis. If this is the case, the placenta should be examined and cultured at the time of birth.
Course, management, treatment, and prognosis
The course of CT may vary from acute and fulminant to subacute and insidious. Because CT is a relatively rare event, outcome data are limited. However, even with treatment, the case fatality rate of CT is substantial.132 Treatment for CT and postnatally acquired disease is the same and should be initiated promptly. A number of factors need to be considered prior to initiating therapy such as location of the infection (pulmonary, extrapulmonary, and central nervous system), regional resistance patterns, and knowledge of isolates previously obtained from the mother or other close contacts. Typically, initial therapy includes a four-drug regimen. Isoniazid, rifampin, and pyrazinamide make up the back-bone of the four-drug therapy. Options for the fourth agent include ethambutol, ethionamide, or an aminoglycoside (i.e., streptomycin, amikacin, kanamycin, or capreomycin).136 Decisions on the initial antibiotic regimen as well as decisions for adjustment in the regimen and total duration of therapy should be made in close concert with an infectious disease specialist with experience in caring for patients with tuberculosis.
Access the full reference list at ExpertConsult.com ![]()
Figures 2 and 15 are online at ExpertConsult.com ![]()
Box 2 is available online at ExpertConsult.com ![]()