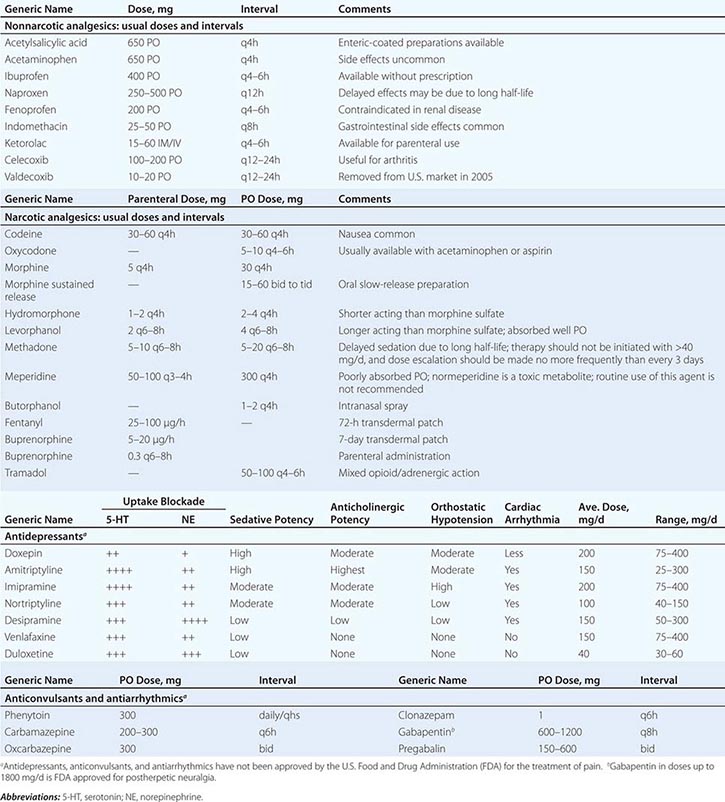
PART 2: Cardinal Manifestations and Presentation of Diseases
SECTION 1 PAIN
18 |
Pain: Pathophysiology and Management |
The province of medicine is to preserve and restore health and to relieve suffering. Understanding pain is essential to both of these goals. Because pain is universally understood as a signal of disease, it is the most common symptom that brings a patient to a physician’s attention. The function of the pain sensory system is to protect the body and maintain homeostasis. It does this by detecting, localizing, and identifying potential or actual tissue-damaging processes. Because different diseases produce characteristic patterns of tissue damage, the quality, time course, and location of a patient’s pain lend important diagnostic clues. It is the physician’s responsibility to provide rapid and effective pain relief.
THE PAIN SENSORY SYSTEM
Pain is an unpleasant sensation localized to a part of the body. It is often described in terms of a penetrating or tissue-destructive process (e.g., stabbing, burning, twisting, tearing, squeezing) and/or of a bodily or emotional reaction (e.g., terrifying, nauseating, sickening). Furthermore, any pain of moderate or higher intensity is accompanied by anxiety and the urge to escape or terminate the feeling. These properties illustrate the duality of pain: it is both sensation and emotion. When it is acute, pain is characteristically associated with behavioral arousal and a stress response consisting of increased blood pressure, heart rate, pupil diameter, and plasma cortisol levels. In addition, local muscle contraction (e.g., limb flexion, abdominal wall rigidity) is often present.
PERIPHERAL MECHANISMS
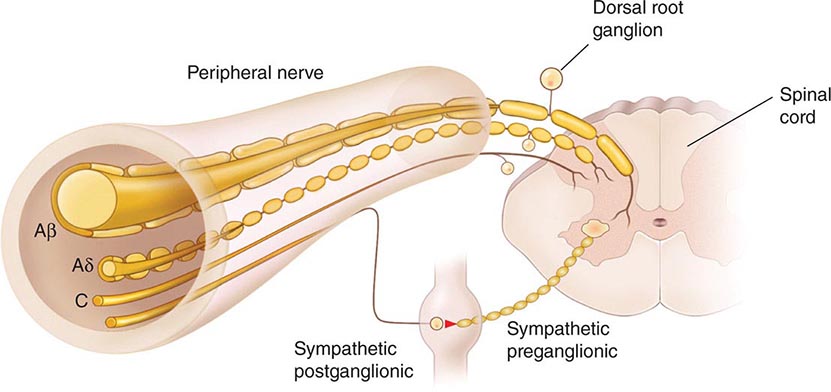
The Primary Afferent Nociceptor A peripheral nerve consists of the axons of three different types of neurons: primary sensory afferents, motor neurons, and sympathetic postganglionic neurons (Fig. 18-1). The cell bodies of primary sensory afferents are located in the dorsal root ganglia within the vertebral foramina. The primary afferent axon has two branches: one projects centrally into the spinal cord and the other projects peripherally to innervate tissues. Primary afferents are classified by their diameter, degree of myelination, and conduction velocity. The largest diameter afferent fibers, A-beta (Aβ), respond maximally to light touch and/or moving stimuli; they are present primarily in nerves that innervate the skin. In normal individuals, the activity of these fibers does not produce pain. There are two other classes of primary afferent nerve fibers: the small diameter myelinated A-delta (Aδ) and the unmyelinated (C) axons (Fig. 18-1). These fibers are present in nerves to the skin and to deep somatic and visceral structures. Some tissues, such as the cornea, are innervated only by Aδ and C fiber afferents. Most Aδ and C fiber afferents respond maximally only to intense (painful) stimuli and produce the subjective experience of pain when they are electrically stimulated; this defines them as primary afferent nociceptors (pain receptors). The ability to detect painful stimuli is completely abolished when conduction in Aδ and C fiber axons is blocked.
FIGURE 18-1 Components of a typical cutaneous nerve. There are two distinct functional categories of axons: primary afferents with cell bodies in the dorsal root ganglion, and sympathetic postganglionic fibers with cell bodies in the sympathetic ganglion. Primary afferents include those with large-diameter myelinated (Aβ), small-diameter myelinated (Aδ), and unmyelinated (C) axons. All sympathetic postganglionic fibers are unmyelinated.
Individual primary afferent nociceptors can respond to several different types of noxious stimuli. For example, most nociceptors respond to heat; intense cold; intense mechanical distortion, such as a pinch; changes in pH, particularly an acidic environment; and application of chemical irritants including adenosine triphosphate (ATP), serotonin, bradykinin, and histamine.
Sensitization When intense, repeated, or prolonged stimuli are applied to damaged or inflamed tissues, the threshold for activating primary afferent nociceptors is lowered, and the frequency of firing is higher for all stimulus intensities. Inflammatory mediators such as bradykinin, nerve-growth factor, some prostaglandins, and leukotrienes contribute to this process, which is called sensitization. Sensitization occurs at the level of the peripheral nerve terminal (peripheral sensitization) as well as at the level of the dorsal horn of the spinal cord (central sensitization). Peripheral sensitization occurs in damaged or inflamed tissues, when inflammatory mediators activate intracellular signal transduction in nociceptors, prompting an increase in the production, transport, and membrane insertion of chemically gated and voltage-gated ion channels. These changes increase the excitability of nociceptor terminals and lower their threshold for activation by mechanical, thermal, and chemical stimuli. Central sensitization occurs when activity, generated by nociceptors during inflammation, enhances the excitability of nerve cells in the dorsal horn of the spinal cord. Following injury and resultant sensitization, normally innocuous stimuli can produce pain (termed allodynia). Sensitization is a clinically important process that contributes to tenderness, soreness, and hyperalgesia (increased pain intensity in response to the same noxious stimulus; e.g., moderate pressure causes severe pain). A striking example of sensitization is sunburned skin, in which severe pain can be produced by a gentle slap on the back or a warm shower.
Sensitization is of particular importance for pain and tenderness in deep tissues. Viscera are normally relatively insensitive to noxious mechanical and thermal stimuli, although hollow viscera do generate significant discomfort when distended. In contrast, when affected by a disease process with an inflammatory component, deep structures such as joints or hollow viscera characteristically become exquisitely sensitive to mechanical stimulation.
A large proportion of Aδ and C fiber afferents innervating viscera are completely insensitive in normal noninjured, noninflamed tissue. That is, they cannot be activated by known mechanical or thermal stimuli and are not spontaneously active. However, in the presence of inflammatory mediators, these afferents become sensitive to mechanical stimuli. Such afferents have been termed silent nociceptors, and their characteristic properties may explain how, under pathologic conditions, the relatively insensitive deep structures can become the source of severe and debilitating pain and tenderness. Low pH, prostaglandins, leukotrienes, and other inflammatory mediators such as bradykinin play a significant role in sensitization.
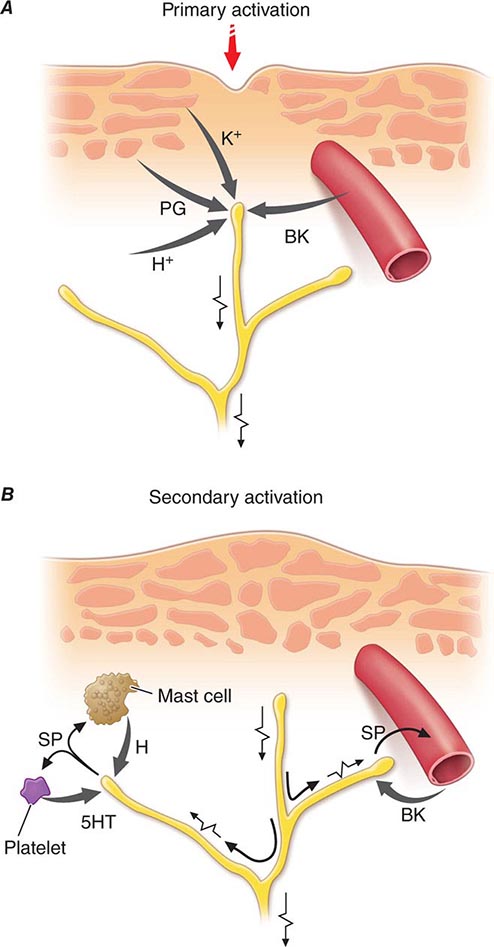
Nociceptor-Induced Inflammation Primary afferent nociceptors also have a neuroeffector function. Most nociceptors contain polypeptide mediators that are released from their peripheral terminals when they are activated (Fig. 18-2). An example is substance P, an 11-amino-acid peptide. Substance P is released from primary afferent nociceptors and has multiple biologic activities. It is a potent vasodilator, degranulates mast cells, is a chemoattractant for leukocytes, and increases the production and release of inflammatory mediators. Interestingly, depletion of substance P from joints reduces the severity of experimental arthritis. Primary afferent nociceptors are not simply passive messengers of threats to tissue injury but also play an active role in tissue protection through these neuroeffector functions.
FIGURE 18-2 Events leading to activation, sensitization, and spread of sensitization of primary afferent nociceptor terminals. A. Direct activation by intense pressure and consequent cell damage. Cell damage induces lower pH (H+) and leads to release of potassium (K+) and to synthesis of prostaglandins (PG) and bradykinin (BK). Prostaglandins increase the sensitivity of the terminal to bradykinin and other pain-producing substances. B. Secondary activation. Impulses generated in the stimulated terminal propagate not only to the spinal cord but also into other terminal branches where they induce the release of peptides, including substance P (SP). Substance P causes vasodilation and neurogenic edema with further accumulation of bradykinin (BK). Substance P also causes the release of histamine (H) from mast cells and serotonin (5HT) from platelets.
CENTRAL MECHANISMS
The Spinal Cord and Referred Pain The axons of primary afferent nociceptors enter the spinal cord via the dorsal root. They terminate in the dorsal horn of the spinal gray matter (Fig. 18-3). The terminals of primary afferent axons contact spinal neurons that transmit the pain signal to brain sites involved in pain perception. When primary afferents are activated by noxious stimuli, they release neurotransmitters from their terminals that excite the spinal cord neurons. The major neurotransmitter released is glutamate, which rapidly excites dorsal horn neurons. Primary afferent nociceptor terminals also release peptides, including substance P and calcitonin gene-related peptide, which produce a slower and longer-lasting excitation of the dorsal horn neurons. The axon of each primary afferent contacts many spinal neurons, and each spinal neuron receives convergent inputs from many primary afferents.
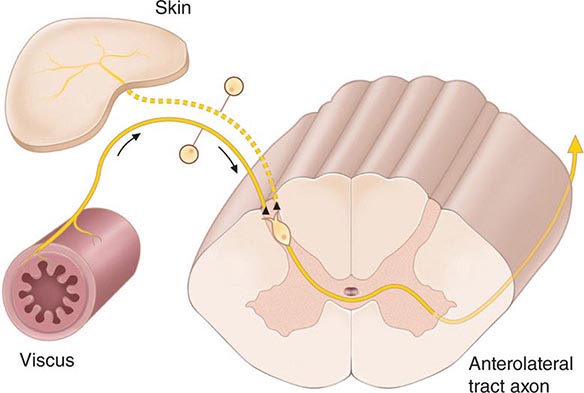
FIGURE 18-3 The convergence-projection hypothesis of referred pain. According to this hypothesis, visceral afferent nociceptors converge on the same pain-projection neurons as the afferents from the somatic structures in which the pain is perceived. The brain has no way of knowing the actual source of input and mistakenly “projects” the sensation to the somatic structure.
The convergence of sensory inputs to a single spinal pain-transmission neuron is of great importance because it underlies the phenomenon of referred pain. All spinal neurons that receive input from the viscera and deep musculoskeletal structures also receive input from the skin. The convergence patterns are determined by the spinal segment of the dorsal root ganglion that supplies the afferent innervation of a structure. For example, the afferents that supply the central diaphragm are derived from the third and fourth cervical dorsal root ganglia. Primary afferents with cell bodies in these same ganglia supply the skin of the shoulder and lower neck. Thus, sensory inputs from both the shoulder skin and the central diaphragm converge on pain-transmission neurons in the third and fourth cervical spinal segments. Because of this convergence and the fact that the spinal neurons are most often activated by inputs from the skin, activity evoked in spinal neurons by input from deep structures is mislocalized by the patient to a place that roughly corresponds with the region of skin innervated by the same spinal segment. Thus, inflammation near the central diaphragm is often reported as shoulder discomfort. This spatial displacement of pain sensation from the site of the injury that produces it is known as referred pain.
Ascending Pathways for Pain A majority of spinal neurons contacted by primary afferent nociceptors send their axons to the contralateral thalamus. These axons form the contralateral spinothalamic tract, which lies in the anterolateral white matter of the spinal cord, the lateral edge of the medulla, and the lateral pons and midbrain. The spinothalamic pathway is crucial for pain sensation in humans. Interruption of this pathway produces permanent deficits in pain and temperature discrimination.
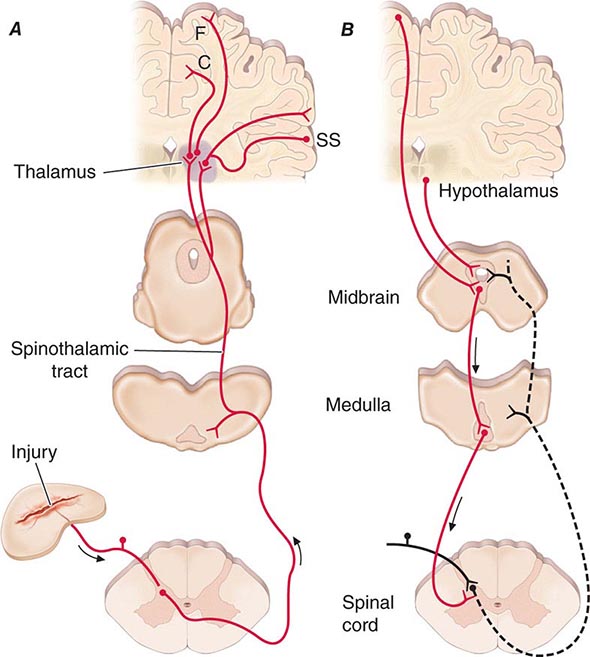
Spinothalamic tract axons ascend to several regions of the thalamus. There is tremendous divergence of the pain signal from these thalamic sites to several distinct areas of the cerebral cortex that subserve different aspects of the pain experience (Fig. 18-4). One of the thalamic projections is to the somatosensory cortex. This projection mediates the purely sensory aspects of pain, i.e., its location, intensity, and quality. Other thalamic neurons project to cortical regions that are linked to emotional responses, such as the cingulate gyrus and other areas of the frontal lobes, including the insular cortex. These pathways to the frontal cortex subserve the affective or unpleasant emotional dimension of pain. This affective dimension of pain produces suffering and exerts potent control of behavior. Because of this dimension, fear is a constant companion of pain. As a consequence, injury or surgical lesions to areas of the frontal cortex activated by painful stimuli can diminish the emotional impact of pain while largely preserving the individual’s ability to recognize noxious stimuli as painful.
FIGURE 18-4 Pain transmission and modulatory pathways. A. Transmission system for nociceptive messages. Noxious stimuli activate the sensitive peripheral ending of the primary afferent nociceptor by the process of transduction. The message is then transmitted over the peripheral nerve to the spinal cord, where it synapses with cells of origin of the major ascending pain pathway, the spinothalamic tract. The message is relayed in the thalamus to the anterior cingulate (C), frontal insular (F), and somatosensory cortex (SS). B. Pain-modulation network. Inputs from frontal cortex and hypothalamus activate cells in the midbrain that control spinal pain-transmission cells via cells in the medulla.
PAIN MODULATION
The pain produced by injuries of similar magnitude is remarkably variable in different situations and in different individuals. For example, athletes have been known to sustain serious fractures with only minor pain, and Beecher’s classic World War II survey revealed that many soldiers in battle were unbothered by injuries that would have produced agonizing pain in civilian patients. Furthermore, even the suggestion that a treatment will relieve pain can have a significant analgesic effect (the placebo effect). On the other hand, many patients find even minor injuries (such as venipuncture) frightening and unbearable, and the expectation of pain can induce pain even without a noxious stimulus. The suggestion that pain will worsen following administration of an inert substance can increase its perceived intensity (the nocebo effect).
The powerful effect of expectation and other psychological variables on the perceived intensity of pain is explained by brain circuits that modulate the activity of the pain-transmission pathways. One of these circuits has links to the hypothalamus, midbrain, and medulla, and it selectively controls spinal pain-transmission neurons through a descending pathway (Fig. 18-4).
Human brain–imaging studies have implicated this pain-modulating circuit in the pain-relieving effect of attention, suggestion, and opioid analgesic medications (Fig. 18-5). Furthermore, each of the component structures of the pathway contains opioid receptors and is sensitive to the direct application of opioid drugs. In animals, lesions of this descending modulatory system reduce the analgesic effect of systemically administered opioids such as morphine. Along with the opioid receptor, the component nuclei of this pain-modulating circuit contain endogenous opioid peptides such as the enkephalins and β-endorphin.
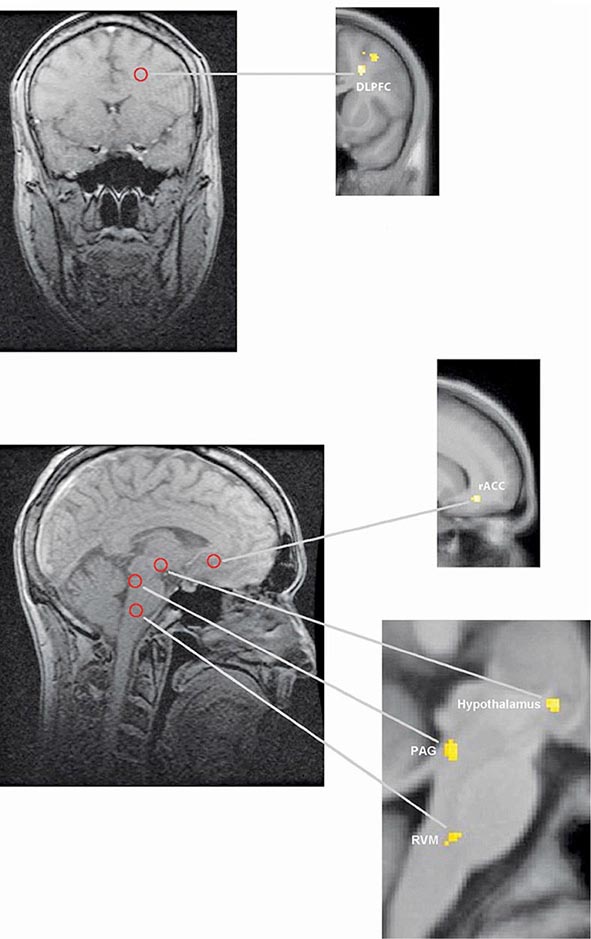
FIGURE 18-5 Functional magnetic resonance imaging (fMRI) demonstrates placebo-enhanced brain activity in anatomic regions correlating with the opioidergic descending pain control system. Top panel: Frontal fMRI image shows placebo-enhanced brain activity in the dorsal lateral prefrontal cortex (DLPFC). Bottom panel: Sagittal fMRI images show placebo-enhanced responses in the rostral anterior cingulate cortex (rACC), the rostral ventral medullae (RVM), the periaqueductal gray (PAG) area, and the hypothalamus. The placebo-enhanced activity in all areas was reduced by naloxone, demonstrating the link between the descending opioidergic system and the placebo analgesic response. (Adapted with permission from F Eippert et al: Neuron 63:533, 2009.)
The most reliable way to activate this endogenous opioid-mediated modulating system is by suggestion of pain relief or by intense emotion directed away from the pain-causing injury (e.g., during severe threat or an athletic competition). In fact, pain-relieving endogenous opioids are released following surgical procedures and in patients given a placebo for pain relief.
Pain-modulating circuits can enhance as well as suppress pain. Both pain-inhibiting and pain-facilitating neurons in the medulla project to and control spinal pain-transmission neurons. Because pain-transmission neurons can be activated by modulatory neurons, it is theoretically possible to generate a pain signal with no peripheral noxious stimulus. In fact, human functional imaging studies have demonstrated increased activity in this circuit during migraine headaches. A central circuit that facilitates pain could account for the finding that pain can be induced by suggestion or enhanced by expectation and provides a framework for understanding how psychological factors can contribute to chronic pain.
NEUROPATHIC PAIN
Lesions of the peripheral or central nociceptive pathways typically result in a loss or impairment of pain sensation. Paradoxically, damage to or dysfunction of these pathways can also produce pain. For example, damage to peripheral nerves, as occurs in diabetic neuropathy, or to primary afferents, as in herpes zoster infection, can result in pain that is referred to the body region innervated by the damaged nerves. Pain may also be produced by damage to the central nervous system (CNS), for example, in some patients following trauma or vascular injury to the spinal cord, brainstem, or thalamic areas that contain central nociceptive pathways. Such neuropathic pains are often severe and are typically resistant to standard treatments for pain.
Neuropathic pain typically has an unusual burning, tingling, or electric shock–like quality and may be triggered by very light touch. These features are rare in other types of pain. On examination, a sensory deficit is characteristically present in the area of the patient’s pain. Hyperpathia, a greatly exaggerated pain sensation to innocuous or mild nociceptive stimuli, is also characteristic of neuropathic pain; patients often complain that the very lightest moving stimulus evokes exquisite pain (allodynia). In this regard, it is of clinical interest that a topical preparation of 5% lidocaine in patch form is effective for patients with postherpetic neuralgia who have prominent allodynia.
A variety of mechanisms contribute to neuropathic pain. As with sensitized primary afferent nociceptors, damaged primary afferents, including nociceptors, become highly sensitive to mechanical stimulation and may generate impulses in the absence of stimulation. Increased sensitivity and spontaneous activity are due, in part, to an increased concentration of sodium channels in the damaged nerve fiber. Damaged primary afferents may also develop sensitivity to norepinephrine. Interestingly, spinal cord pain-transmission neurons cut off from their normal input may also become spontaneously active. Thus, both CNS and peripheral nervous system hyperactivity contribute to neuropathic pain.
Sympathetically Maintained Pain Patients with peripheral nerve injury occasionally develop spontaneous pain in the region innervated by the nerve. This pain is often described as having a burning quality. The pain typically begins after a delay of hours to days or even weeks and is accompanied by swelling of the extremity, periarticular bone loss, and arthritic changes in the distal joints. The pain may be relieved by a local anesthetic block of the sympathetic innervation to the affected extremity. Damaged primary afferent nociceptors acquire adrenergic sensitivity and can be activated by stimulation of the sympathetic outflow. This constellation of spontaneous pain and signs of sympathetic dysfunction following injury has been termed complex regional pain syndrome (CRPS). When this occurs after an identifiable nerve injury, it is termed CRPS type II (also known as posttraumatic neuralgia or, if severe, causalgia). When a similar clinical picture appears without obvious nerve injury, it is termed CRPS type I (also known as reflex sympathetic dystrophy). CRPS can be produced by a variety of injuries, including fractures of bone, soft tissue trauma, myocardial infarction, and stroke (Chap. 446). CRPS type I typically resolves with symptomatic treatment; however, when it persists, detailed examination often reveals evidence of peripheral nerve injury. Although the pathophysiology of CRPS is poorly understood, the pain and the signs of inflammation, when acute, can be rapidly relieved by blocking the sympathetic nervous system. This implies that sympathetic activity can activate undamaged nociceptors when inflammation is present. Signs of sympathetic hyperactivity should be sought in patients with posttraumatic pain and inflammation and no other obvious explanation.
CHRONIC PAIN
Managing patients with chronic pain is intellectually and emotionally challenging. The patient’s problem is often difficult or impossible to diagnose with certainty; such patients are demanding of the physician’s time and often appear emotionally distraught. The traditional medical approach of seeking an obscure organic pathology is usually unhelpful. On the other hand, psychological evaluation and behaviorally based treatment paradigms are frequently helpful, particularly in the setting of a multidisciplinary pain-management center. Unfortunately, this approach, while effective, remains largely underused in current medical practice.
There are several factors that can cause, perpetuate, or exacerbate chronic pain. First, of course, the patient may simply have a disease that is characteristically painful for which there is presently no cure. Arthritis, cancer, chronic daily headaches, fibromyalgia, and diabetic neuropathy are examples of this. Second, there may be secondary perpetuating factors that are initiated by disease and persist after that disease has resolved. Examples include damaged sensory nerves, sympathetic efferent activity, and painful reflex muscle contraction (spasm). Finally, a variety of psychological conditions can exacerbate or even cause pain.
There are certain areas to which special attention should be paid in a patient’s medical history. Because depression is the most common emotional disturbance in patients with chronic pain, patients should be questioned about their mood, appetite, sleep patterns, and daily activity. A simple standardized questionnaire, such as the Beck Depression Inventory, can be a useful screening device. It is important to remember that major depression is a common, treatable, and potentially fatal illness.
Other clues that a significant emotional disturbance is contributing to a patient’s chronic pain complaint include pain that occurs in multiple, unrelated sites; a pattern of recurrent, but separate, pain problems beginning in childhood or adolescence; pain beginning at a time of emotional trauma, such as the loss of a parent or spouse; a history of physical or sexual abuse; and past or present substance abuse.
On examination, special attention should be paid to whether the patient guards the painful area and whether certain movements or postures are avoided because of pain. Discovering a mechanical component to the pain can be useful both diagnostically and therapeutically. Painful areas should be examined for deep tenderness, noting whether this is localized to muscle, ligamentous structures, or joints. Chronic myofascial pain is very common, and, in these patients, deep palpation may reveal highly localized trigger points that are firm bands or knots in muscle. Relief of the pain following injection of local anesthetic into these trigger points supports the diagnosis. A neuropathic component to the pain is indicated by evidence of nerve damage, such as sensory impairment, exquisitely sensitive skin (allodynia), weakness, and muscle atrophy, or loss of deep tendon reflexes. Evidence suggesting sympathetic nervous system involvement includes the presence of diffuse swelling, changes in skin color and temperature, and hypersensitive skin and joint tenderness compared with the normal side. Relief of the pain with a sympathetic block supports the diagnosis, but once the condition becomes chronic, the response to sympathetic blockade is of variable magnitude and duration; the role for repeated sympathetic blocks in the overall management of CRPS is not established.
A guiding principle in evaluating patients with chronic pain is to assess both emotional and organic factors before initiating therapy. Addressing these issues together, rather than waiting to address emotional issues after organic causes of pain have been ruled out, improves compliance in part because it assures patients that a psychological evaluation does not mean that the physician is questioning the validity of their complaint. Even when an organic cause for a patient’s pain can be found, it is still wise to look for other factors. For example, a cancer patient with painful bony metastases may have additional pain due to nerve damage and may also be depressed. Optimal therapy requires that each of these factors be looked for and treated.
19 |
Chest Discomfort |
Chest discomfort is among the most common reasons for which patients present for medical attention at either an emergency department (ED) or an outpatient clinic. The evaluation of nontraumatic chest discomfort is inherently challenging owing to the broad variety of possible causes, a minority of which are life-threatening conditions that should not be missed. It is helpful to frame the initial diagnostic assessment and triage of patients with acute chest discomfort around three categories: (1) myocardial ischemia; (2) other cardiopulmonary causes (pericardial disease, aortic emergencies, and pulmonary conditions); and (3) non-cardiopulmonary causes. Although rapid identification of high-risk conditions is a priority of the initial assessment, strategies that incorporate routine liberal use of testing carry the potential for adverse effects of unnecessary investigations.
EPIDEMIOLOGY AND NATURAL HISTORY
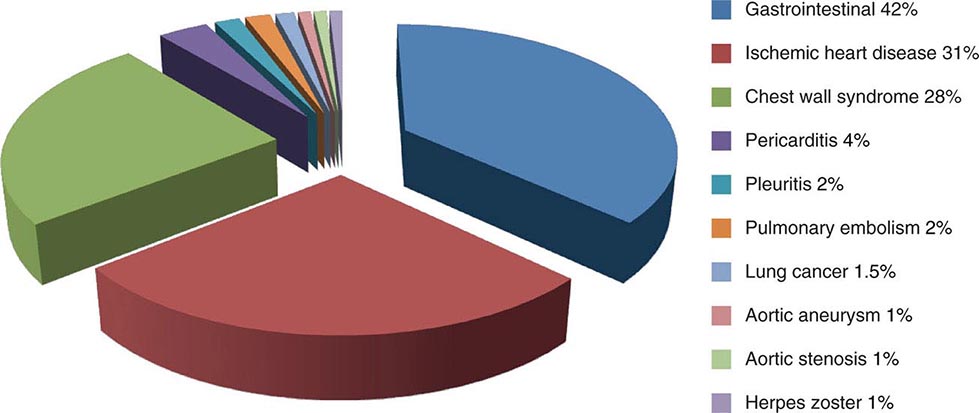
Chest discomfort is the third most common reason for visits to the ED in the United States, resulting in 6 to 7 million emergency visits each year. More than 60% of patients with this presentation are hospitalized for further testing, and the rest undergo additional investigation in the ED. Fewer than 25% of evaluated patients are eventually diagnosed with acute coronary syndrome (ACS), with rates of 5–15% in most series of unselected populations. In the remainder, the most common diagnoses are gastrointestinal causes (Fig. 19-1), and fewer than 10% are other life-threatening cardiopulmonary conditions. In a large proportion of patients with transient acute chest discomfort, ACS or another acute cardiopulmonary cause is excluded but the cause is not determined. Therefore, the resources and time devoted to the evaluation of chest discomfort in the absence of a severe cause are substantial. Nevertheless, a disconcerting 2–6% of patients with chest discomfort of presumed non-ischemic etiology who are discharged from the ED are later deemed to have had a missed myocardial infarction (MI). Patients with a missed diagnosis of MI have a 30-day risk of death that is double that of their counterparts who are hospitalized.
FIGURE 19-1 Distribution of final discharge diagnoses in patients with nontraumatic acute chest pain. (Figure prepared from data in P Fruergaard et al: Eur Heart J 17:1028, 1996.)
The natural histories of ACS, acute pericardial diseases, pulmonary embolism, and aortic emergencies are discussed in Chaps. 288, 294 and 295, 300, and 301, respectively. In a study of more than 350,000 patients with unspecified presumed non-cardiopulmonary chest discomfort, the mortality rate 1 year after discharge was <2% and did not differ significantly from age-adjusted mortality in the general population. The estimated rate of major cardiovascular events through 30 days in patients with acute chest pain who had been stratified as low risk was 2.5% in a large population-based study that excluded patients with ST-segment elevation or definite noncardiac chest pain.
CAUSES OF CHEST DISCOMFORT
The major etiologies of chest discomfort are discussed in this section and summarized in Table 19-1. Additional elements of the history, physical examination, and diagnostic testing that aid in distinguishing these causes are discussed in a later section (see “Approach to the Patient”).
|
TYPICAL CLINICAL FEATURES OF MAJOR CAUSES OF ACUTE CHEST DISCOMFORT |
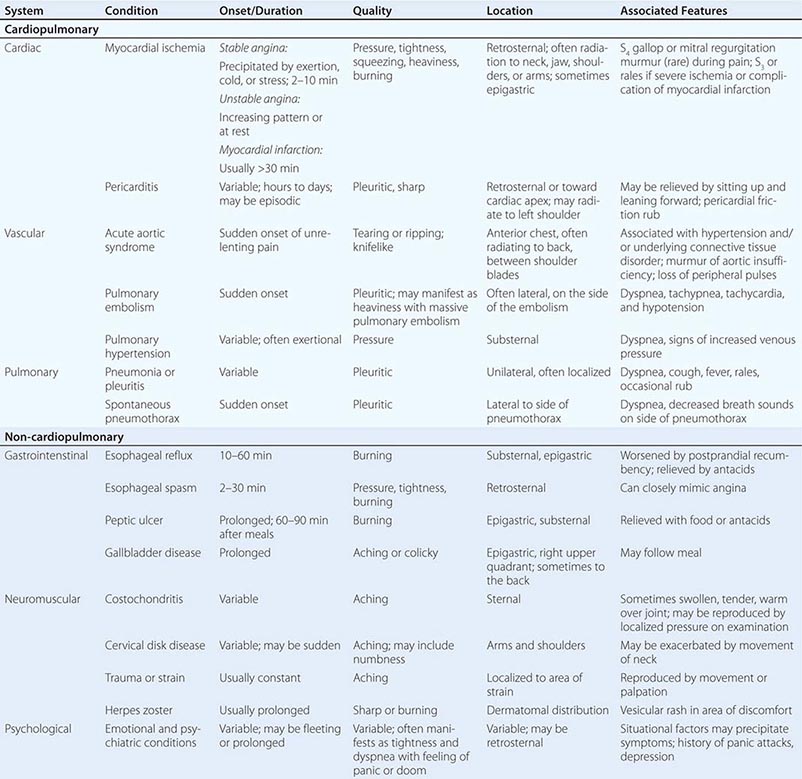
MYOCARDIAL ISCHEMIA/INJURY
Myocardial ischemia causing chest discomfort, termed angina pectoris, is a primary clinical concern in patients presenting with chest symptoms. Myocardial ischemia is precipitated by an imbalance between myocardial oxygen requirements and myocardial oxygen supply, resulting in insufficient delivery of oxygen to meet the heart’s metabolic demands. Myocardial oxygen consumption may be elevated by increases in heart rate, ventricular wall stress, and myocardial contractility, whereas myocardial oxygen supply is determined by coronary blood flow and coronary arterial oxygen content. When myocardial ischemia is sufficiently severe and prolonged in duration (as little as 20 min), irreversible cellular injury occurs, resulting in MI.
Ischemic heart disease is most commonly caused by atheromatous plaque that obstructs one or more of the epicardial coronary arteries. Stable ischemic heart disease (Chap. 293) usually results from the gradual atherosclerotic narrowing of the coronary arteries. Stable angina is characterized by ischemic episodes that are typically precipitated by a superimposed increase in oxygen demand during physical exertion and relieved upon resting. Ischemic heart disease becomes unstable most commonly when rupture or erosion of one or more atherosclerotic lesions triggers coronary thrombosis (Chap. 291e). Unstable ischemic heart disease is classified clinically by the presence or absence of detectable myocardial injury and the presence or absence of ST-segment elevation on the patient’s electrocardiogram (ECG). When acute coronary atherothrombosis occurs, the intracoronary thrombus may be partially obstructive, generally leading to myocardial ischemia in the absence of ST-segment elevation. Marked by ischemic symptoms at rest, with minimal activity, or in an accelerating pattern, unstable ischemic heart disease is classified as unstable angina when there is no detectable myocardial injury and as non–ST elevation MI (NSTEMI) when there is evidence of myocardial necrosis (Chap. 294). When the coronary thrombus is acutely and completely occlusive, transmural myocardial ischemia usually ensues, with ST-segment elevation on the ECG and myocardial necrosis leading to a diagnosis of ST elevation MI (STEMI, see Chap. 295).
Clinicians should be aware that unstable ischemic symptoms may also occur predominantly because of increased myocardial oxygen demand (e.g., during intense psychological stress or fever) or because of decreased oxygen delivery due to anemia, hypoxia, or hypotension. However, the term acute coronary syndrome, which encompasses unstable angina, NSTEMI, and STEMI, is in general reserved for ischemia precipitated by acute coronary atherothrombosis. In order to guide therapeutic strategies, a standardized system for classification of MI has been expanded to discriminate MI resulting from acute coronary thrombosis (type 1) from MI occurring secondary to other imbalances of myocardial oxygen supply and demand (type 2; see Chap. 294).
Other contributors to stable and unstable ischemic heart disease, such as endothelial dysfunction, microvascular disease, and vasospasm, may exist alone or in combination with coronary atherosclerosis and may be the dominant cause of myocardial ischemia in some patients. Moreover, non-atherosclerotic processes, including congenital abnormalities of the coronary vessels, myocardial bridging, coronary arteritis, and radiation-induced coronary disease, can lead to coronary obstruction. In addition, conditions associated with extreme myocardial oxygen demand and impaired endocardial blood flow, such as aortic valve disease (Chap. 301), hypertrophic cardiomyopathy, or idiopathic dilated cardiomyopathy (Chap. 287), can precipitate myocardial ischemia in patients with or without underlying obstructive atherosclerosis.
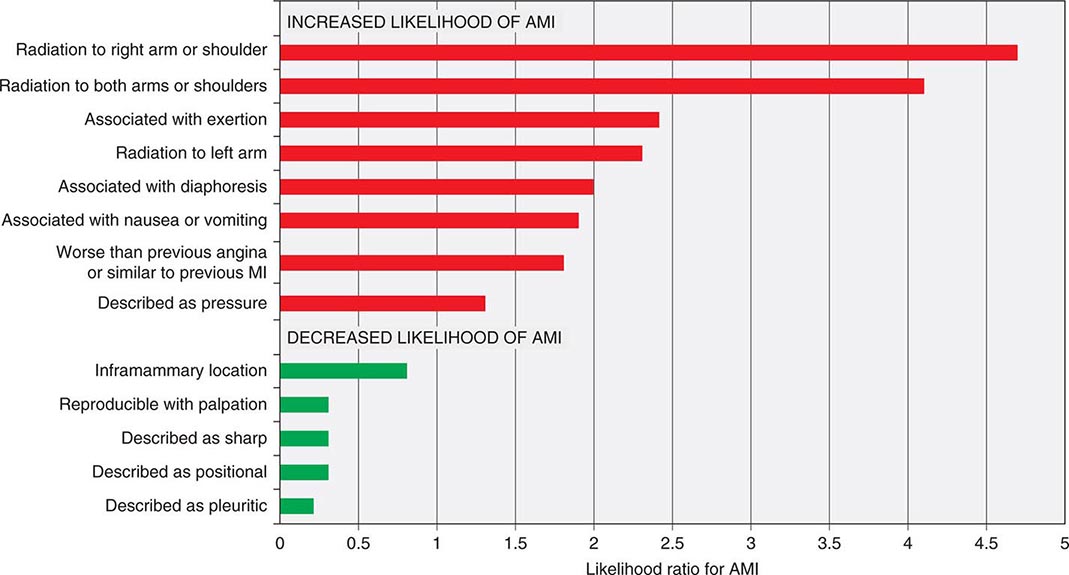
Characteristics of Ischemic Chest Discomfort The clinical characteristics of angina pectoris, often referred to simply as “angina,” are highly similar whether the ischemic discomfort is a manifestation of stable ischemic heart disease, unstable angina, or MI; the exceptions are differences in the pattern and duration of symptoms associated with these syndromes (Table 19-1). Heberden initially described angina as a sense of “strangling and anxiety.” Chest discomfort characteristic of myocardial ischemia is typically described as aching, heavy, squeezing, crushing, or constricting. However, in a substantial minority of patients, the quality of discomfort is extremely vague and may be described as a mild tightness, or merely an uncomfortable feeling, that sometimes is experienced as numbness or a burning sensation. The site of the discomfort is usually retrosternal, but radiation is common and generally occurs down the ulnar surface of the left arm; the right arm, both arms, neck, jaw, or shoulders may also be involved. These and other characteristics of ischemic chest discomfort pertinent to discrimination from other causes of chest pain are discussed later in this chapter (see “Approach to the Patient”).
Stable angina usually begins gradually and reaches its maximal intensity over a period of minutes before dissipating within several minutes with rest or with nitroglycerin. The discomfort typically occurs predictably at a characteristic level of exertion or psychological stress. By definition, unstable angina is manifest by self-limited anginal chest discomfort that is exertional but occurs at increased frequency with progressively lower intensity of physical activity or even at rest. Chest discomfort associated with MI is typically more severe, is prolonged (usually lasting ≥30 min), and is not relieved by rest.
Mechanisms of Cardiac Pain The neural pathways involved in ischemic cardiac pain are poorly understood. Ischemic episodes are thought to excite local chemosensitive and mechanoreceptive receptors that, in turn, stimulate release of adenosine, bradykinin, and other substances that activate the sensory ends of sympathetic and vagal afferent fibers. The afferent fibers traverse the nerves that connect to the upper five thoracic sympathetic ganglia and upper five distal thoracic roots of the spinal cord. From there, impulses are transmitted to the thalamus. Within the spinal cord, cardiac sympathetic afferent impulses may converge with impulses from somatic thoracic structures, and this convergence may be the basis for referred cardiac pain. In addition, cardiac vagal afferent fibers synapse in the nucleus tractus solitarius of the medulla and then descend to the upper cervical spinothalamic tract, and this route may contribute to anginal pain experienced in the neck and jaw.
OTHER CARDIOPULMONARY CAUSES
Pericardial and Other Myocardial Diseases (See also Chap. 288) Inflammation of the pericardium due to infectious or noninfectious causes can be responsible for acute or chronic chest discomfort. The visceral surface and most of the parietal surface of the pericardium are insensitive to pain. Therefore, the pain of pericarditis is thought to arise principally from associated pleural inflammation and is more common with infectious causes of pericarditis, which typically involve the pleura. Because of this pleural association, the discomfort of pericarditis is usually pleuritic pain that is exacerbated by breathing, coughing, or changes in position. Moreover, owing to the overlapping sensory supply of the central diaphragm via the phrenic nerve with somatic sensory fibers originating in the third to fifth cervical segments, the pain of pleural pericarditis is often referred to the shoulder and neck. Involvement of the pleural surface of the lateral diaphragm can lead to pain in the upper abdomen.
Acute inflammatory and other non-ischemic myocardial diseases can also produce chest discomfort. The symptoms of Takotsubo (stress-related) cardiomyopathy often start abruptly with chest pain and shortness of breath. This form of cardiomyopathy, in its most recognizable form, is triggered by an emotionally or physically stressful event and may mimic acute MI because of its commonly associated ECG abnormalities, including ST-segment elevation, and elevated biomarkers of myocardial injury. Observational studies support a predilection for women >50 years of age. The symptoms of acute myocarditis are highly varied. Chest discomfort may either originate with inflammatory injury of the myocardium or be due to severe increases in wall stress related to poor ventricular performance.
Diseases of the Aorta (See also Chap. 301) Acute aortic dissection (Fig. 19-1) is a less common cause of chest discomfort but is important because of the catastrophic natural history of certain subsets of cases when recognized late or left untreated. Acute aortic syndromes encompass a spectrum of acute aortic diseases related to disruption of the media of the aortic wall. Aortic dissection involves a tear in the aortic intima, resulting in separation of the media and creation of a separate “false” lumen. A penetrating ulcer has been described as ulceration of an aortic atheromatous plaque that extends through the intima and into the aortic media, with the potential to initiate an intramedial dissection or rupture into the adventitia. Intramural hematoma is an aortic wall hematoma with no demonstrable intimal flap, no radiologically apparent intimal tear, and no false lumen. Intramural hematoma can occur due to either rupture of the vasa vasorum or, less commonly, a penetrating ulcer.
Each of these subtypes of acute aortic syndrome typically presents with chest discomfort that is often severe, sudden in onset, and sometimes described as “tearing” in quality. Acute aortic syndromes involving the ascending aorta tend to cause pain in the midline of the anterior chest, whereas descending aortic syndromes most often present with pain in the back. Therefore, dissections that begin in the ascending aorta and extend to the descending aorta tend to cause pain in the front of the chest that extends toward the back, between the shoulder blades. Proximal aortic dissections that involve the ascending aorta (type A in the Stanford nomenclature) are at high risk for major complications that may influence the clinical presentation, including (1) compromise of the aortic ostia of the coronary arteries, resulting in MI; (2) disruption of the aortic valve, causing acute aortic insufficiency; and (3) rupture of the hematoma into the pericardial space, leading to pericardial tamponade.
Knowledge of the epidemiology of acute aortic syndromes can be helpful in maintaining awareness of this relatively uncommon group of disorders (estimated annual incidence, 3 cases per 100,000 population). Nontraumatic aortic dissections are very rare in the absence of hypertension or conditions associated with deterioration of the elastic or muscular components of the aortic media, including pregnancy, bicuspid aortic disease, or inherited connective tissue diseases, such as Marfan and Ehlers-Danlos syndromes.
Although aortic aneurysms are most often asymptomatic, thoracic aortic aneurysms can cause chest pain and other symptoms by compressing adjacent structures. This pain tends to be steady, deep, and occasionally severe. Aortitis, whether of noninfectious or infectious etiology, in the absence of aortic dissection is a rare cause of chest or back discomfort.
Pulmonary Conditions Pulmonary and pulmonary-vascular conditions that cause chest discomfort usually do so in conjunction with dyspnea and often produce symptoms that have a pleuritic nature.
PULMONARY EMBOLISM (See also Chap. 300) Pulmonary emboli (annual incidence, ~1 per 1000) can produce dyspnea and chest discomfort that is sudden in onset. Typically pleuritic in pattern, the chest discomfort associated with pulmonary embolism may result from (1) involvement of the pleural surface of the lung adjacent to a resultant pulmonary infarction; (2) distention of the pulmonary artery; or (3) possibly, right ventricular wall stress and/or subendocardial ischemia related to acute pulmonary hypertension. The pain associated with small pulmonary emboli is often lateral and pleuritic and is believed to be related to the first of these three possible mechanisms. In contrast, massive pulmonary emboli may cause severe substernal pain that may mimic an MI and that is plausibly attributed to the second and third of these potential mechanisms. Massive or submassive pulmonary embolism may also be associated with syncope, hypotension, and signs of right heart failure. Other typical characteristics that aid in the recognition of pulmonary embolism are discussed later in this chapter (see “Approach to the Patient”).
PNEUMOTHORAX (See also Chap. 317) Primary spontaneous pneumothorax is a rare cause of chest discomfort, with an estimated annual incidence in the United States of 7 per 100,000 among men and <2 per 100,000 among women. Risk factors include male sex, smoking, family history, and Marfan syndrome. The symptoms are usually sudden in onset, and dyspnea may be mild; thus, presentation to medical attention is sometimes delayed. Secondary spontaneous pneumothorax may occur in patients with underlying lung disorders, such as chronic obstructive pulmonary disease, asthma, or cystic fibrosis, and usually produces symptoms that are more severe. Tension pneumothorax is a medical emergency caused by trapped intrathoracic air that precipitates hemodynamic collapse.
Other Pulmonary Parenchymal, Pleural, or Vascular Disease (See also Chaps. 304, 305, and 316) Most pulmonary diseases that produce chest pain, including pneumonia and malignancy, do so because of involvement of the pleura or surrounding structures. Pleurisy is typically described as a knifelike pain that is worsened by inspiration or coughing. In contrast, chronic pulmonary hypertension can manifest as chest pain that may be very similar to angina in its characteristics, suggesting right ventricular myocardial ischemia in some cases. Reactive airways diseases similarly can cause chest tightness associated with breathlessness rather than pleurisy.
NON-CARDIOPULMONARY CAUSES
Gastrointenstinal Conditions (See also Chap. 344) Gastrointestinal disorders are the most common cause of nontraumatic chest discomfort and often produce symptoms that are difficult to discern from more serious causes of chest pain, including myocardial ischemia. Esophageal disorders, in particular, may simulate angina in the character and location of the pain. Gastroesophageal reflux and disorders of esophageal motility are common and should be considered in the differential diagnosis of chest pain (Fig. 19-1 and Table 19-1). Acid reflux often causes a burning discomfort. The pain of esophageal spasm, in contrast, is commonly an intense, squeezing discomfort that is retrosternal in location and, like angina, may be relieved by nitroglycerin or dihydropyridine calcium channel antagonists. Chest pain can also result from injury to the esophagus, such as a Mallory-Weiss tear or even an esophageal rupture (Boerhaave syndrome) caused by severe vomiting. Peptic ulcer disease is most commonly epigastric in location but can radiate into the chest (Table 19-1).
Hepatobiliary disorders, including cholecystitis and biliary colic, may mimic acute cardiopulmonary diseases. Although the pain arising from these disorders usually localizes to the right upper quadrant of the abdomen, it is variable and may be felt in the epigastrium and radiate to the back and lower chest. This discomfort is sometimes referred to the scapula or may in rare cases be felt in the shoulder, suggesting diaphragmatic irritation. The pain is steady, usually lasts several hours, and subsides spontaneously, without symptoms between attacks. Pain resulting from pancreatitis is typically aching epigastric pain that radiates to the back.
Musculoskeletal and Other Causes (See also Chap. 393) Chest discomfort can be produced by any musculoskeletal disorder involving the chest wall or the nerves of the chest wall, neck, or upper limbs. Costochondritis causing tenderness of the costochondral junctions (Tietze’s syndrome) is relatively common. Cervical radiculitis may manifest as a prolonged or constant aching discomfort in the upper chest and limbs. The pain may be exacerbated by motion of the neck. Occasionally, chest pain can be caused by compression of the brachial plexus by the cervical ribs, and tendinitis or bursitis involving the left shoulder may mimic the radiation of angina. Pain in a dermatomal distribution can also be caused by cramping of intercostal muscles or by herpes zoster (Chap. 217).
Emotional and Psychiatric Conditions As many as 10% of patients who present to emergency departments with acute chest discomfort have a panic disorder or related condition (Table 19-1). The symptoms may include chest tightness or aching that is associated with a sense of anxiety and difficulty breathing. The symptoms may be prolonged or fleeting.
|
CONSIDERATIONS IN THE ASSESSMENT OF THE PATIENT WITH CHEST DISCOMFORT |

CRITICAL PATHWAYS FOR ACUTE CHEST DISCOMFORT
Because of the challenges inherent in reliably identifying the small proportion of patients with serious causes of acute chest discomfort while not exposing the larger number of low-risk patients to unnecessary testing and extended ED or hospital evaluations, many medical centers have adopted critical pathways to expedite the assessment and management of patients with nontraumatic chest pain, often in dedicated chest pain units. Such pathways are generally aimed at (1) rapid identification, triage, and treatment of high-risk cardiopulmonary conditions (e.g., STEMI); (2) accurate identification of low-risk patients who can be safely observed in units with less intensive monitoring, undergo early exercise testing, or be discharged home; and (3) through more efficient and systematic accelerated diagnostic protocols, safe reduction in costs associated with overuse of testing and unnecessary hospitalizations. In some studies, provision of protocol-driven care in chest pain units has decreased costs and overall duration of hospital evaluation with no detectable excess of adverse clinical outcomes.
OUTPATIENT EVALUATION OF CHEST DISCOMFORT
Chest pain is common in outpatient practice, with a lifetime prevalence of 20–40% in the general population. More than 25% of patients with MI have had a related visit with a primary care physician in the previous month. The diagnostic principles are the same as in the ED. However, the pretest probability of an acute cardiopulmonary cause is significantly lower. Therefore, testing paradigms are less intense, with an emphasis on the history, physical examination, and ECG. Moreover, decision-aids developed for settings with a high prevalence of significant cardiopulmonary disease have lower positive predictive value when applied in the practitioner’s office. However, in general, if the level of clinical suspicion of ACS is sufficiently high to consider troponin testing, the patient should be referred to the ED for evaluation.
20 |
Abdominal Pain |
Correctly interpreting acute abdominal pain can be quite challenging. Few clinical situations require greater judgment, because the most catastrophic of events may be forecast by the subtlest of symptoms and signs. In every instance, the clinician must distinguish those conditions that require urgent intervention from those that do not and can best be managed nonoperatively. A meticulously executed, detailed history and physical examination are critically important for focusing the differential diagnosis, where necessary, and allowing the diagnostic evaluation to proceed expeditiously (Table 20-1).
|
SOME KEY COMPONENTS OF THE PATIENT’S HISTORY |
The etiologic classification in Table 20-2, although not complete, provides a useful framework for evaluating patients with abdominal pain.
|
SOME IMPORTANT CAUSES OF ABDOMINAL PAIN |
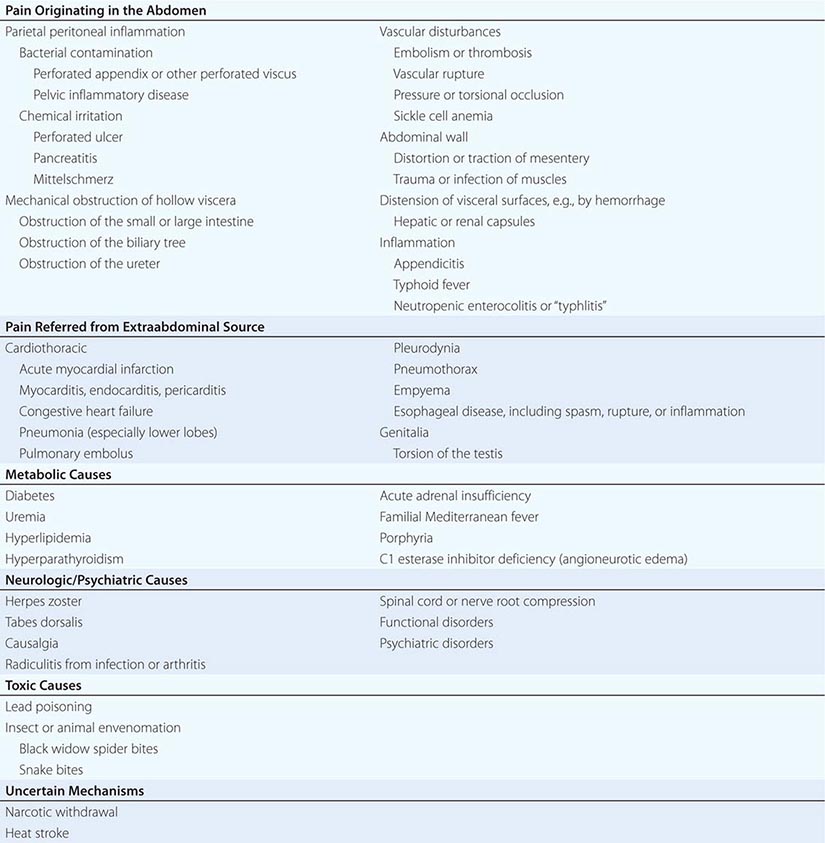
The most common causes of abdominal pain on admission are acute appendicitis, nonspecific abdominal pain, pain of urologic origin, and intestinal obstruction. A diagnosis of “acute or surgical abdomen” is not acceptable because of its often misleading and erroneous connotations. Most patients who present with acute abdominal pain will have self-limited disease processes. However, it is important to remember that pain severity does not necessarily correlate with the severity of the underlying condition. The most obvious of “acute abdomens” may not require operative intervention, and the mildest of abdominal pains may herald an urgently correctable lesion. Any patient with abdominal pain of recent onset requires early and thorough evaluation and accurate diagnosis.
SOME MECHANISMS OF PAIN ORIGINATING IN THE ABDOMEN
Inflammation of the Parietal Peritoneum The pain of parietal peritoneal inflammation is steady and aching in character and is located directly over the inflamed area, its exact reference being possible because it is transmitted by somatic nerves supplying the parietal peritoneum. The intensity of the pain is dependent on the type and amount of material to which the peritoneal surfaces are exposed in a given time period. For example, the sudden release into the peritoneal cavity of a small quantity of sterile acid gastric juice causes much more pain than the same amount of grossly contaminated neutral feces. Enzymatically active pancreatic juice incites more pain and inflammation than does the same amount of sterile bile containing no potent enzymes. Blood is normally only a mild irritant and the response to urine can be bland, so exposure of blood and urine to the peritoneal cavity may go unnoticed unless it is sudden and massive. Bacterial contamination, such as may occur with pelvic inflammatory disease or perforated distal intestine, causes low-intensity pain until multiplication causes a significant amount of inflammatory mediators to be released. Patients with perforated upper gastrointestinal ulcers may present entirely differently depending on how quickly gastric juices enter the peritoneal cavity. Thus, the rate at which any inflammatory material irritates the peritoneum is important.
The pain of peritoneal inflammation is invariably accentuated by pressure or changes in tension of the peritoneum, whether produced by palpation or by movement such as with coughing or sneezing. The patient with peritonitis characteristically lies quietly in bed, preferring to avoid motion, in contrast to the patient with colic, who may be thrashing in discomfort.
Another characteristic feature of peritoneal irritation is tonic reflex spasm of the abdominal musculature, localized to the involved body segment. Its intensity depends on the integrity of the nervous system, the location of the inflammatory process, and the rate at which it develops. Spasm over a perforated retrocecal appendix or perforation into the lesser peritoneal sac may be minimal or absent because of the protective effect of overlying viscera. Catastrophic abdominal emergencies may be associated with minimal or no detectable pain or muscle spasm in obtunded, seriously ill, debilitated, immunosuppressed, or psychotic patients. A slowly developing process also often greatly attenuates the degree of muscle spasm.
Obstruction of Hollow Viscera Intraluminal obstruction classically elicits intermittent or colicky abdominal pain that is not as well localized as the pain of parietal peritoneal irritation. However, the absence of cramping discomfort should not be misleading because distention of a hollow viscus may also produce steady pain with only rare paroxysms.
Small-bowel obstruction often presents as poorly localized, intermittent periumbilical or supraumbilical pain. As the intestine progressively dilates and loses muscular tone, the colicky nature of the pain may diminish. With superimposed strangulating obstruction, pain may spread to the lower lumbar region if there is traction on the root of the mesentery. The colicky pain of colonic obstruction is of lesser intensity, is commonly located in the infraumbilical area, and may often radiate to the lumbar region.
Sudden distention of the biliary tree produces a steady rather than colicky type of pain; hence, the term biliary colic is misleading. Acute distention of the gallbladder usually causes pain in the right upper quadrant with radiation to the right posterior region of the thorax or to the tip of the right scapula, but it is also not uncommonly found near the midline. Distention of the common bile duct often causes epigastric pain that may radiate to the upper lumbar region. Considerable variation is common, however, so that differentiation between these may be impossible. The typical subscapular pain or lumbar radiation is frequently absent. Gradual dilatation of the biliary tree, as can occur with carcinoma of the head of the pancreas, may cause no pain or only a mild aching sensation in the epigastrium or right upper quadrant. The pain of distention of the pancreatic ducts is similar to that described for distention of the common bile duct but, in addition, is very frequently accentuated by recumbency and relieved by the upright position.
Obstruction of the urinary bladder usually causes dull, low-intensity pain in the suprapubic region. Restlessness without specific complaint of pain may be the only sign of a distended bladder in an obtunded patient. In contrast, acute obstruction of the intravesicular portion of the ureter is characterized by severe suprapubic and flank pain that radiates to the penis, scrotum, or inner aspect of the upper thigh. Obstruction of the ureteropelvic junction manifests as pain near the costovertebral angle, whereas obstruction of the remainder of the ureter is associated with flank pain that often extends into the same side of the abdomen.
Vascular Disturbances A frequent misconception is that pain due to intraabdominal vascular disturbances is sudden and catastrophic in nature. Certain disease processes, such as embolism or thrombosis of the superior mesenteric artery or impending rupture of an abdominal aortic aneurysm, can certainly be associated with diffuse, severe pain. Yet, just as frequently, the patient with occlusion of the superior mesenteric artery only has mild continuous or cramping diffuse pain for 2 or 3 days before vascular collapse or findings of peritoneal inflammation appear. The early, seemingly insignificant discomfort is caused by hyperperistalsis rather than peritoneal inflammation. Indeed, absence of tenderness and rigidity in the presence of continuous, diffuse pain (e.g., “pain out of proportion to physical findings”) in a patient likely to have vascular disease is quite characteristic of occlusion of the superior mesenteric artery. Abdominal pain with radiation to the sacral region, flank, or genitalia should always signal the possible presence of a rupturing abdominal aortic aneurysm. This pain may persist over a period of several days before rupture and collapse occur.
Abdominal Wall Pain arising from the abdominal wall is usually constant and aching. Movement, prolonged standing, and pressure accentuate the discomfort and associated muscle spasm. In the case of hematoma of the rectus sheath, now most frequently encountered in association with anticoagulant therapy, a mass may be present in the lower quadrants of the abdomen. Simultaneous involvement of muscles in other parts of the body usually serves to differentiate myositis of the abdominal wall from other processes that might cause pain in the same region.
REFERRED PAIN IN ABDOMINAL DISEASE
Pain referred to the abdomen from the thorax, spine, or genitalia may present a vexing diagnostic challenge because diseases of the upper part of the abdominal cavity such as acute cholecystitis or perforated ulcer may be associated with intrathoracic complications. A most important, yet often forgotten, dictum is that the possibility of intrathoracic disease must be considered in every patient with abdominal pain, especially if the pain is in the upper abdomen.
Systematic questioning and examination directed toward detecting myocardial or pulmonary infarction, pneumonia, pericarditis, or esophageal disease (the intrathoracic diseases that most often masquerade as abdominal emergencies) will often provide sufficient clues to establish the proper diagnosis. Diaphragmatic pleuritis resulting from pneumonia or pulmonary infarction may cause pain in the right upper quadrant and pain in the supraclavicular area, the latter radiation to be distinguished from the referred subscapular pain caused by acute distention of the extrahepatic biliary tree. The ultimate decision as to the origin of abdominal pain may require deliberate and planned observation over a period of several hours, during which repeated questioning and examination will provide the diagnosis or suggest the appropriate studies.
Referred pain of thoracic origin is often accompanied by splinting of the involved hemithorax with respiratory lag and decrease in excursion more marked than that seen in the presence of intraabdominal disease. In addition, apparent abdominal muscle spasm caused by referred pain will diminish during the inspiratory phase of respiration, whereas it persists throughout both respiratory phases if it is of abdominal origin. Palpation over the area of referred pain in the abdomen also does not usually accentuate the pain and, in many instances, actually seems to relieve it.
Thoracic disease and abdominal disease frequently coexist and may be difficult or impossible to differentiate. For example, the patient with known biliary tract disease often has epigastric pain during myocardial infarction, or biliary colic may be referred to the precordium or left shoulder in a patient who has suffered previously from angina pectoris. For an explanation of the radiation of pain to a previously diseased area, see Chap. 18.
Referred pain from the spine, which usually involves compression or irritation of nerve roots, is characteristically intensified by certain motions such as cough, sneeze, or strain and is associated with hyperesthesia over the involved dermatomes. Pain referred to the abdomen from the testes or seminal vesicles is generally accentuated by the slightest pressure on either of these organs. The abdominal discomfort experienced is of dull, aching character and is poorly localized.
METABOLIC ABDOMINAL CRISES
Pain of metabolic origin may simulate almost any other type of intraabdominal disease. Several mechanisms may be at work. In certain instances, such as hyperlipidemia, the metabolic disease itself may be accompanied by an intraabdominal process such as pancreatitis, which can lead to unnecessary laparotomy unless recognized. C1 esterase deficiency associated with angioneurotic edema is often associated with episodes of severe abdominal pain. Whenever the cause of abdominal pain is obscure, a metabolic origin always must be considered. Abdominal pain is also the hallmark of familial Mediterranean fever (Chap. 392).
The problem of differential diagnosis is often not readily resolved. The pain of porphyria and of lead colic is usually difficult to distinguish from that of intestinal obstruction, because severe hyperperistalsis is a prominent feature of both. The pain of uremia or diabetes is nonspecific, and the pain and tenderness frequently shift in location and intensity. Diabetic acidosis may be precipitated by acute appendicitis or intestinal obstruction, so if prompt resolution of the abdominal pain does not result from correction of the metabolic abnormalities, an underlying organic problem should be suspected. Black widow spider bites produce intense pain and rigidity of the abdominal muscles and back, an area infrequently involved in intraabdominal disease.
IMMUNOCOMPROMISE
Evaluating and diagnosing causes of abdominal pain in immunosuppressed or otherwise immunocompromised patients is very difficult. This includes those who have undergone organ transplantation; who are receiving immunosuppressive treatments for autoimmune diseases, chemotherapy, or glucocorticoids; who have AIDS; and who are very old. In these circumstances, normal physiologic responses may be absent or masked. In addition, unusual infections may cause abdominal pain where the etiologic agents include cytomegalovirus, mycobacteria, protozoa, and fungi. These pathogens may affect all gastrointestinal organs, including the gallbladder, liver, and pancreas, as well as the gastrointestinal tract, causing occult or overtly symptomatic perforations of the latter. Splenic abscesses due to Candida or Salmonella infection should also be considered, especially when evaluating patients with left upper quadrant or left flank pain. Acalculous cholecystitis is a relative common complication in patients with AIDS, where it is often associated with cryptosporidiosis or cytomegalovirus infection.
Neutropenic enterocolitis is often identified as a cause of abdominal pain and fever in some patients with bone marrow suppression due to chemotherapy. Acute graft-versus-host disease should be considered. Optimal management of these patients may require meticulous follow-up including serial examinations to be certain that surgical intervention is not required to treat an underlying disease process.
NEUROGENIC CAUSES
Diseases that injure sensory nerves may cause causalgic pain. It has a burning character and is usually limited to the distribution of a given peripheral nerve. Normal nonpainful stimuli such as touch or a change in temperature may be causalgic and may frequently be present even at rest. The demonstration of irregularly spaced cutaneous pain spots may be the only indication that an old nerve injury exists. Even though the pain may be precipitated by gentle palpation, rigidity of the abdominal muscles is absent, and the respirations are not disturbed. Distention of the abdomen is uncommon, and the pain has no relationship to the intake of food.
Pain arising from spinal nerves or roots comes and goes suddenly and is of a lancinating type (Chap. 22). It may be caused by herpes zoster, impingement by arthritis, tumors, a herniated nucleus pulposus, diabetes, or syphilis. It is not associated with food intake, abdominal distention, or changes in respiration. Severe muscle spasm, as in the gastric crises of tabes dorsalis, is common but is either relieved or not accentuated by abdominal palpation. The pain is made worse by movement of the spine and is usually confined to a few dermatomes. Hyperesthesia is very common.
Pain due to functional causes conforms to none of the aforementioned patterns. Mechanisms of disease are not clearly established. Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by abdominal pain and altered bowel habits. The diagnosis is made on the basis of clinical criteria (Chap. 352) and after exclusion of demonstrable structural abnormalities. The episodes of abdominal pain are often brought on by stress, and the pain varies considerably in type and location. Nausea and vomiting are rare. Localized tenderness and muscle spasm are inconsistent or absent. The causes of IBS or related functional disorders are not known.
21 |
Headache |
Headache is among the most common reasons patients seek medical attention, on a global basis being responsible for more disability than any other neurologic problem. Diagnosis and management are based on a careful clinical approach augmented by an understanding of the anatomy, physiology, and pharmacology of the nervous system pathways mediating the various headache syndromes. This chapter will focus on the general approach to a patient with headache; migraine and other primary headache disorders are discussed in Chap. 447.
GENERAL PRINCIPLES
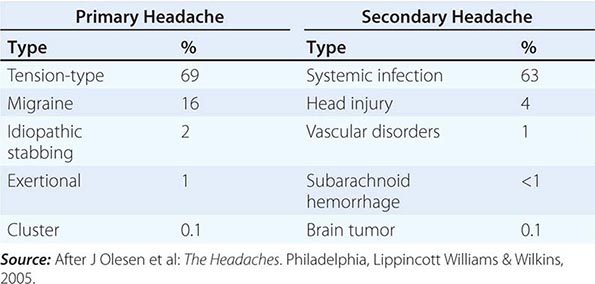
A classification system developed by the International Headache Society (www.ihs-headache.org/) characterizes headache as primary or secondary (Table 21-1). Primary headaches are those in which headache and its associated features are the disorder in itself, whereas secondary headaches are those caused by exogenous disorders (Headache Classification Committee of the International Headache Society, 2013). Primary headache often results in considerable disability and a decrease in the patient’s quality of life. Mild secondary headache, such as that seen in association with upper respiratory tract infections, is common but rarely worrisome. Life-threatening headache is relatively uncommon, but vigilance is required in order to recognize and appropriately treat such patients.
|
COMMON CAUSES OF HEADACHE |
ANATOMY AND PHYSIOLOGY OF HEADACHE
Pain usually occurs when peripheral nociceptors are stimulated in response to tissue injury, visceral distension, or other factors (Chap. 18). In such situations, pain perception is a normal physiologic response mediated by a healthy nervous system. Pain can also result when pain-producing pathways of the peripheral or central nervous system (CNS) are damaged or activated inappropriately. Headache may originate from either or both mechanisms. Relatively few cranial structures are pain-producing; these include the scalp, middle meningeal artery, dural sinuses, falx cerebri, and proximal segments of the large pial arteries. The ventricular ependyma, choroid plexus, pial veins, and much of the brain parenchyma are not pain-producing.
The key structures involved in primary headache appear to be the following:
• The large intracranial vessels and dura mater and the peripheral terminals of the trigeminal nerve that innervate these structures
• The caudal portion of the trigeminal nucleus, which extends into the dorsal horns of the upper cervical spinal cord and receives input from the first and second cervical nerve roots (the trigeminocervical complex)
• Rostral pain-processing regions, such as the ventroposteromedial thalamus and the cortex
• The pain-modulatory systems in the brain that modulate input from trigeminal nociceptors at all levels of the pain-processing pathways and influence vegetative functions, such as hypothalamus and brainstem structures
The innervation of the large intracranial vessels and dura mater by the trigeminal nerve is known as the trigeminovascular system. Cranial autonomic symptoms, such as lacrimation, conjunctival injection, nasal congestion, rhinorrhea, periorbital swelling, aural fullness, and ptosis, are prominent in the trigeminal autonomic cephalalgias, including cluster headache and paroxysmal hemicrania, and may also be seen in migraine, even in children. These autonomic symptoms reflect activation of cranial parasympathetic pathways, and functional imaging studies indicate that vascular changes in migraine and cluster headache, when present, are similarly driven by these cranial autonomic systems. Moreover, they can often be mistaken for symptoms or signs of cranial sinus inflammation, which is thus overdiagnosed and inappropriately managed. Migraine and other primary headache types are not “vascular headaches”; these disorders do not reliably manifest vascular changes, and treatment outcomes cannot be predicted by vascular effects. Migraine is a brain disorder and is best understood and managed as such.
CLINICAL EVALUATION OF ACUTE, NEW-ONSET HEADACHE
The patient who presents with a new, severe headache has a differential diagnosis that is quite different from the patient with recurrent headaches over many years. In new-onset and severe headache, the probability of finding a potentially serious cause is considerably greater than in recurrent headache. Patients with recent onset of pain require prompt evaluation and appropriate treatment. Serious causes to be considered include meningitis, subarachnoid hemorrhage, epidural or subdural hematoma, glaucoma, tumor, and purulent sinusitis. When worrisome symptoms and signs are present (Table 21-2), rapid diagnosis and management are critical.
|
HEADACHE SYMPTOMS THAT SUGGEST A SERIOUS UNDERLYING DISORDER |
A careful neurologic examination is an essential first step in the evaluation. In most cases, patients with an abnormal examination or a history of recent-onset headache should be evaluated by a computed tomography (CT) or magnetic resonance imaging (MRI) study. As an initial screening procedure for intracranial pathology in this setting, CT and MRI methods appear to be equally sensitive. In some circumstances, a lumbar puncture (LP) is also required, unless a benign etiology can be otherwise established. A general evaluation of acute headache might include cranial arteries by palpation; cervical spine by the effect of passive movement of the head and by imaging; the investigation of cardiovascular and renal status by blood pressure monitoring and urine examination; and eyes by funduscopy, intraocular pressure measurement, and refraction.
The psychological state of the patient should also be evaluated because a relationship exists between head pain and depression. This is intended to identify comorbidity rather than provide an explanation for the headache, because troublesome headache is seldom simply caused by mood change. Although it is notable that medicines with antidepressant actions are also effective in the prophylactic treatment of both tension-type headache and migraine, each symptom must be treated optimally.
Underlying recurrent headache disorders may be activated by pain that follows otologic or endodontic surgical procedures. Thus, pain about the head as the result of diseased tissue or trauma may reawaken an otherwise quiescent migraine syndrome. Treatment of the headache is largely ineffective until the cause of the primary problem is addressed.
Serious underlying conditions that are associated with headache are described below. Brain tumor is a rare cause of headache and even less commonly a cause of severe pain. The vast majority of patients presenting with severe headache have a benign cause.
SECONDARY HEADACHE
The management of secondary headache focuses on diagnosis and treatment of the underlying condition.
MENINGITIS
Acute, severe headache with stiff neck and fever suggests meningitis. LP is mandatory. Often there is striking accentuation of pain with eye movement. Meningitis can be easily mistaken for migraine in that the cardinal symptoms of pounding headache, photophobia, nausea, and vomiting are frequently present, perhaps reflecting the underlying biology of some of the patients.
Meningitis is discussed in Chaps. 164 and 165.
INTRACRANIAL HEMORRHAGE
Acute, severe headache with stiff neck but without fever suggests subarachnoid hemorrhage. A ruptured aneurysm, arteriovenous malformation, or intraparenchymal hemorrhage may also present with headache alone. Rarely, if the hemorrhage is small or below the foramen magnum, the head CT scan can be normal. Therefore, LP may be required to definitively diagnose subarachnoid hemorrhage.
Intracranial hemorrhage is discussed in Chap. 330.
BRAIN TUMOR
Approximately 30% of patients with brain tumors consider headache to be their chief complaint. The head pain is usually nondescript—an intermittent deep, dull aching of moderate intensity, which may worsen with exertion or change in position and may be associated with nausea and vomiting. This pattern of symptoms results from migraine far more often than from brain tumor. The headache of brain tumor disturbs sleep in about 10% of patients. Vomiting that precedes the appearance of headache by weeks is highly characteristic of posterior fossa brain tumors. A history of amenorrhea or galactorrhea should lead one to question whether a prolactin-secreting pituitary adenoma (or the polycystic ovary syndrome) is the source of headache. Headache arising de novo in a patient with known malignancy suggests either cerebral metastases or carcinomatous meningitis, or both. Head pain appearing abruptly after bending, lifting, or coughing can be due to a posterior fossa mass, a Chiari malformation, or low cerebrospinal fluid (CSF) volume.
Brain tumors are discussed in Chap. 118.
TEMPORAL ARTERITIS
(See also Chaps. 39 and 385) Temporal (giant cell) arteritis is an inflammatory disorder of arteries that frequently involves the extracranial carotid circulation. It is a common disorder of the elderly; its annual incidence is 77 per 100,000 individuals age 50 and older. The average age of onset is 70 years, and women account for 65% of cases. About half of patients with untreated temporal arteritis develop blindness due to involvement of the ophthalmic artery and its branches; indeed, the ischemic optic neuropathy induced by giant cell arteritis is the major cause of rapidly developing bilateral blindness in patients >60 years. Because treatment with glucocorticoids is effective in preventing this complication, prompt recognition of the disorder is important.
Typical presenting symptoms include headache, polymyalgia rheumatica (Chap. 385), jaw claudication, fever, and weight loss. Headache is the dominant symptom and often appears in association with malaise and muscle aches. Head pain may be unilateral or bilateral and is located temporally in 50% of patients but may involve any and all aspects of the cranium. Pain usually appears gradually over a few hours before peak intensity is reached; occasionally, it is explosive in onset. The quality of pain is only seldom throbbing; it is almost invariably described as dull and boring, with superimposed episodic stabbing pains similar to the sharp pains that appear in migraine. Most patients can recognize that the origin of their head pain is superficial, external to the skull, rather than originating deep within the cranium (the pain site for migraineurs). Scalp tenderness is present, often to a marked degree; brushing the hair or resting the head on a pillow may be impossible because of pain. Headache is usually worse at night and often aggravated by exposure to cold. Additional findings may include reddened, tender nodules or red streaking of the skin overlying the temporal arteries, and tenderness of the temporal or, less commonly, the occipital arteries.
The erythrocyte sedimentation rate (ESR) is often, although not always, elevated; a normal ESR does not exclude giant cell arteritis. A temporal artery biopsy followed by immediate treatment with prednisone 80 mg daily for the first 4–6 weeks should be initiated when clinical suspicion is high. The prevalence of migraine among the elderly is substantial, considerably higher than that of giant cell arteritis. Migraineurs often report amelioration of their headaches with prednisone; thus, caution must be used when interpreting the therapeutic response.
GLAUCOMA
Glaucoma may present with a prostrating headache associated with nausea and vomiting. The headache often starts with severe eye pain. On physical examination, the eye is often red with a fixed, moderately dilated pupil.
Glaucoma is discussed in Chap. 39.
PRIMARY HEADACHE DISORDERS
Primary headaches are disorders in which headache and associated features occur in the absence of any exogenous cause. The most common are migraine, tension-type headache, and the trigeminal autonomic cephalalgias, notably cluster headache. These entities are discussed in detail in Chap. 447.
CHRONIC DAILY HEADACHE
The broad diagnosis of chronic daily headache (CDH) can be applied when a patient experiences headache on 15 days or more per month. CDH is not a single entity; it encompasses a number of different headache syndromes, both primary and secondary (Table 21-3). In aggregate, this group presents considerable disability and is thus specially dealt with here. Population-based estimates suggest that about 4% of adults have daily or near-daily headache.
|
CLASSIFICATION OF CHRONIC DAILY HEADACHE |
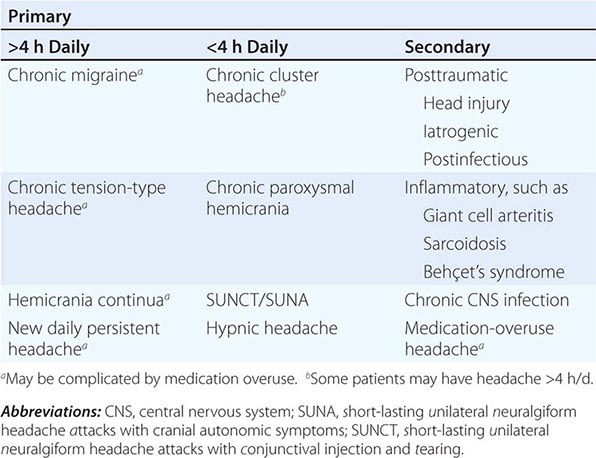
New daily persistent headache (NDPH) is a clinically distinct syndrome; its causes are listed in Table 21-4.
|
DIFFERENTIAL DIAGNOSIS OF NEW DAILY PERSISTENT HEADACHE |
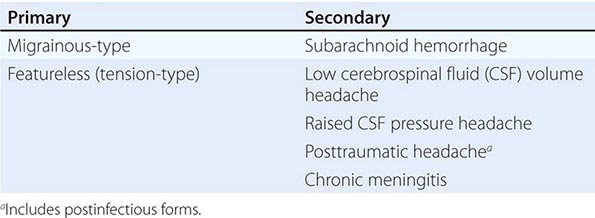
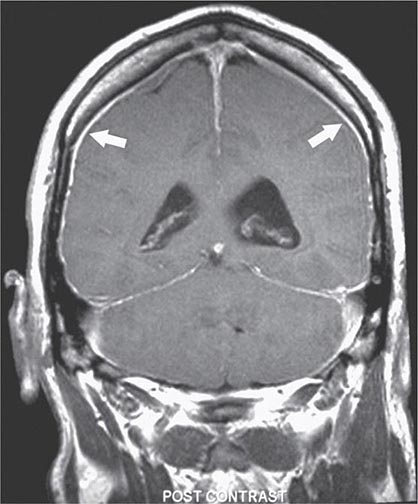
FIGURE 21-1 Magnetic resonance image showing diffuse meningeal enhancement after gadolinium administration in a patient with low cerebrospinal fluid (CSF) volume headache.
PRIMARY CARE AND HEADACHE MANAGEMENT
Most patients with headache will be seen first in a primary care setting. The task of the primary care physician is to identify the very few worrisome secondary headaches from the very great majority of primary and less troublesome secondary headaches (Table 21-2).
Absent any warning signs, a reasonable approach is to treat when a diagnosis is established. As a general rule, the investigation should focus on identifying worrisome causes of headache or on gaining confidence if no primary headache diagnosis can be made.
After treatment has been initiated, follow-up care is essential to identify whether progress has been made against the headache complaint. Not all headaches will respond to treatment, but, in general, worrisome headaches will progress and will be easier to identify.
When a primary care physician feels the diagnosis is a primary headache disorder, it is worth noting that more than 90% of patients who present to primary care with a complaint of headache will have migraine (Chap. 447).
In general, patients who do not have a clear diagnosis, have a primary headache disorder other than migraine or tension-type headache, or are unresponsive to two or more standard therapies for the considered headache type should be considered for referral to a specialist. In a practical sense, the threshold for referral is also determined by the experience of the primary care physician in headache medicine and the availability of secondary care options.
22 |
Back and Neck Pain |
The importance of back and neck pain in our society is underscored by the following: (1) the cost of back pain in the United States exceeds $100 billion annually; approximately one-third of these costs are direct health care expenses, and two-thirds are indirect costs resulting from loss of wages and productivity; (2) back symptoms are the most common cause of disability in those <45 years; (3) low back pain is the second most common reason for visiting a physician in the United States; and (4) 70% of persons will have back pain at some point in their lives.
ANATOMY OF THE SPINE
The anterior spine consists of cylindrical vertebral bodies separated by intervertebral disks and held together by the anterior and posterior longitudinal ligaments. The intervertebral disks are composed of a central gelatinous nucleus pulposus surrounded by a tough cartilaginous ring, the annulus fibrosis. Disks are responsible for 25% of spinal column length and allow the bony vertebrae to move easily upon each other (Figs. 22-1 and 22-2). Desiccation of the nucleus pulposus and degeneration of the annulus fibrosus increase with age and result in loss of disk height. The disks are largest in the cervical and lumbar regions where movements of the spine are greatest. The anterior spine absorbs the shock of bodily movements such as walking and running and, with the posterior spine, protects the spinal cord and nerve roots in the spinal canal.
FIGURE 22-1 Vertebral anatomy. (From A Gauthier Cornuelle, DH Gronefeld: Radiographic Anatomy Positioning. New York, McGraw-Hill, 1998; with permission.)
FIGURE 22-2 Spinal column. (From A Gauthier Cornuelle, DH Gronefeld: Radiographic Anatomy Positioning. New York, McGraw-Hill, 1998; with permission.)
The posterior spine consists of the vertebral arches and processes. Each arch consists of paired cylindrical pedicles anteriorly and paired lamina posteriorly. The vertebral arch also gives rise to two transverse processes laterally, one spinous process posteriorly, plus two superior and two inferior articular facets. The apposition of a superior and inferior facet constitutes a facet joint. The posterior spine provides an anchor for the attachment of muscles and ligaments. The contraction of muscles attached to the spinous and transverse processes and lamina works like a system of pulleys and levers that results in flexion, extension, and lateral bending movements of the spine.
Nerve root injury (radiculopathy) is a common cause of neck, arm, low back, buttock, and leg pain (see Figs. 31-2 and 31-3). The nerve roots exit at a level above their respective vertebral bodies in the cervical region (e.g., the C7 nerve root exits at the C6-C7 level) and below their respective vertebral bodies in the thoracic and lumbar regions (e.g., the T1 nerve root exits at the T1-T2 level). The cervical nerve roots follow a short intraspinal course before exiting. By contrast, because the spinal cord ends at the vertebral L1 or L2 level, the lumbar nerve roots follow a long intraspinal course and can be injured anywhere from the upper lumbar spine to their exit at the intervertebral foramen. For example, disk herniation at the L4-L5 level can produce not only L5 root compression, but also compression of the traversing S1 nerve root (Fig. 22-3). The lumbar nerve roots are mobile in the spinal canal, but eventually pass through the narrow lateral recess of the spinal canal and intervertebral foramen (Figs. 22-2 and 22-3). Neuroimaging of the spine must include both sagittal and axial views to assess possible compression in either the lateral recess or intervertebral foramen.
FIGURE 22-3 Compression of L5 and S1 roots by herniated disks. (From AH Ropper, MA Samuels: Adams and Victor’s Principles of Neurology, 9th ed. New York, McGraw-Hill, 2009; with permission.)
Pain-sensitive structures of the spine include the periosteum of the vertebrae, dura, facet joints, annulus fibrosus of the intervertebral disk, epidural veins and arteries, and the longitudinal ligaments. Disease of these diverse structures may explain many cases of back pain without nerve root compression. Under normal circumstances, the nucleus pulposus of the intervertebral disk is not pain sensitive.
CAUSES OF BACK PAIN
|
CAUSES OF BACK OR NECK PAIN |
LUMBAR DISK DISEASE
This is a common cause of acute, chronic, or recurrent low back and leg pain (Figs. 22-3 and 22-4). Disk disease is most likely to occur at the L4-L5 or L5-S1 levels, but upper lumbar levels are involved occasionally. The cause is often unknown, but the risk is increased in overweight individuals. Disk herniation is unusual prior to age 20 years and is rare in the fibrotic disks of the elderly. Complex genetic factors may play a role in predisposing some patients to disk disease. The pain may be located in the low back only or referred to a leg, buttock, or hip. A sneeze, cough, or trivial movement may cause the nucleus pulposus to prolapse, pushing the frayed and weakened annulus posteriorly. With severe disk disease, the nucleus may protrude through the annulus (herniation) or become extruded to lie as a free fragment in the spinal canal.
FIGURE 22-4 Left L5 radiculopathy. A. Sagittal T2-weighted image on the left reveals disk herniation at the L4-L5 level. B. Axial T1-weighted image shows paracentral disk herniation with displacement of the thecal sac medially and the left L5 nerve root posteriorly in the left lateral recess.
The mechanism by which intervertebral disk injury causes back pain is controversial. The inner annulus fibrosus and nucleus pulposus are normally devoid of innervation. Inflammation and production of proinflammatory cytokines within a ruptured nucleus pulposus may trigger or perpetuate back pain. Ingrowth of nociceptive (pain) nerve fibers into inner portions of a diseased disk may be responsible for some chronic “diskogenic” pain. Nerve root injury (radiculopathy) from disk herniation is usually due to inflammation, but lateral herniation may produce compression in the lateral recess or at the intervertebral foramen.
A ruptured disk may be asymptomatic or cause back pain, abnormal posture, limitation of spine motion (particularly flexion), a focal neurologic deficit, or radicular pain. A dermatomal pattern of sensory loss or a reduced or absent deep tendon reflex is more suggestive of a specific root lesion than is the pattern of pain. Motor findings (focal weakness, muscle atrophy, or fasciculations) occur less frequently than focal sensory or reflex changes. Symptoms and signs are usually unilateral, but bilateral involvement does occur with large central disk herniations that compress multiple roots or cause inflammation of nerve roots within the spinal canal. Clinical manifestations of specific nerve root lesions are summarized in Table 22-2.
The differential diagnosis covers a variety of serious and treatable conditions, including epidural abscess, hematoma, fracture, or tumor. Fever, constant pain uninfluenced by position, sphincter abnormalities, or signs of spinal cord disease suggest an etiology other than lumbar disk disease. Absence of ankle reflexes can be a normal finding in persons older than age 60 years or a sign of bilateral S1 radiculopathy. An absent deep tendon reflex or focal sensory loss may indicate injury to a nerve root, but other sites of injury along the nerve must also be considered. For example, an absent knee reflex may be due to a femoral neuropathy or an L4 nerve root injury. A loss of sensation over the foot and lateral lower calf may result from a peroneal or lateral sciatic neuropathy or an L5 nerve root injury. Focal muscle atrophy may reflect injury to the anterior horn cells of the spinal cord, a nerve root, peripheral nerve, or disuse.
A lumbar spine MRI scan or CT myelogram is necessary to establish the location and type of pathology. Spine MRIs yield exquisite views of intraspinal and adjacent soft tissue anatomy. Bony lesions of the lateral recess or intervertebral foramen are optimally visualized by CT myelography. The correlation of neuroradiologic findings to symptoms, particularly pain, is not simple. Contrast-enhancing tears in the annulus fibrosus or disk protrusions are widely accepted as common sources of back pain; however, studies have found that many asymptomatic adults have similar findings. Asymptomatic disk protrusions are also common and may enhance with contrast. Furthermore, in patients with known disk herniation treated either medically or surgically, persistence of the herniation 10 years later had no relationship to the clinical outcome. In summary, MRI findings of disk protrusion, tears in the annulus fibrosus, or hypertrophic facet joints are common incidental findings that, by themselves, should not dictate management decisions for patients with back pain.
The diagnosis of nerve root injury is most secure when the history, examination, results of imaging studies, and the EMG are concordant. The correlation between CT and EMG for localization of nerve root injury is between 65 and 73%. Up to one-third of asymptomatic adults have a lumbar disk protrusion detected by CT or MRI scans.
Management of lumbar disk disease is discussed below.
Cauda equina syndrome (CES) signifies an injury of multiple lumbosacral nerve roots within the spinal canal distal to the termination of the spinal cord at L1-L2. Low back pain, weakness and areflexia in the legs, saddle anesthesia, or loss of bladder function may occur. The problem must be distinguished from disorders of the lower spinal cord (conus medullaris syndrome), acute transverse myelitis (Chap. 456), and Guillain-Barré syndrome (Chap. 460). Combined involvement of the conus medullaris and cauda equina can occur. CES is commonly due to a ruptured lumbosacral intervertebral disk, lumbosacral spine fracture, hematoma within the spinal canal (e.g., following lumbar puncture in patients with coagulopathy), compressive tumor, or other mass lesion. Treatment options include surgical decompression, sometimes urgently in an attempt to restore or preserve motor or sphincter function, or radiotherapy for metastatic tumors (Chap. 118).
DEGENERATIVE CONDITIONS
Lumbar spinal stenosis (LSS) describes a narrowed lumbar spinal canal and is frequently asymptomatic. Typical is neurogenic claudication, consisting of back and buttock or leg pain induced by walking or standing and relieved by sitting. Symptoms in the legs are usually bilateral. Unlike vascular claudication, symptoms are often provoked by standing without walking. Unlike lumbar disk disease, symptoms are usually relieved by sitting. Patients with neurogenic claudication can often walk much farther when leaning over a shopping cart and can pedal a stationary bike with ease while sitting. These flexed positions increase the anteroposterior spinal canal diameter and reduce intraspinal venous hypertension, resulting in pain relief. Focal weakness, sensory loss, or reflex changes may occur when spinal stenosis is associated with neural foraminal narrowing and radiculopathy. Severe neurologic deficits, including paralysis and urinary incontinence, occur only rarely.
LSS by itself is frequently asymptomatic, and the correlation between the severity of symptoms and degree of stenosis of the spinal canal is variable. LSS can be acquired (75%), congenital, or both. Congenital forms (achondroplasia, idiopathic) are characterized by short, thick pedicles that produce both spinal canal and lateral recess stenosis. Acquired factors that contribute to spinal stenosis include degenerative diseases (spondylosis, spondylolisthesis, scoliosis), trauma, spine surgery, metabolic or endocrine disorders (epidural lipomatosis, osteoporosis, acromegaly, renal osteodystrophy, hypoparathyroidism), and Paget’s disease. MRI provides the best definition of the abnormal anatomy (Fig. 22-5).
FIGURE 22-5 Axial T2-weighted images of the lumbar spine. A. The image shows a normal thecal sac within the lumbar spinal canal. The thecal sac is bright. The lumbar roots are dark punctuate dots in the posterior thecal sac with the patient supine. B. The thecal sac is not well visualized due to severe lumbar spinal canal stenosis, partially the result of hypertrophic facet joints.
Conservative treatment of symptomatic LSS includes nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, exercise programs, and symptomatic treatment of acute pain episodes. There is insufficient evidence to support the routine use of epidural glucocorticoid injections. Surgical therapy is considered when medical therapy does not relieve symptoms sufficiently to allow for resumption of activities of daily living or when focal neurologic signs are present. Most patients with neurogenic claudication who are treated medically do not improve over time. Surgical management can produce significant relief of back and leg pain within 6 weeks, and pain relief persists for at least 2 years. However, up to one-quarter develop recurrent stenosis at the same spinal level or an adjacent level 7–10 years after the initial surgery; recurrent symptoms usually respond to a second surgical decompression.
Neural foraminal narrowing with radiculopathy is a common consequence of osteoarthritic processes that cause lumbar spinal stenosis (Figs. 22-1 and 22-6), including osteophytes, lateral disk protrusion, calcified disk-osteophytes, facet joint hypertrophy, uncovertebral joint hypertrophy (cervical spine), congenitally shortened pedicles, or, frequently, a combination of these processes. Neoplasms (primary or metastatic), fractures, infections (epidural abscess), or hematomas are other considerations. These conditions can produce unilateral nerve root symptoms or signs due to compression at the intervertebral foramen or in the lateral recess; symptoms are indistinguishable from disk-related radiculopathy, but treatment may differ depending on the specific etiology. The history and neurologic examination alone cannot distinguish between these possibilities. A spine neuroimaging (CT or MRI) procedure is required to identify the anatomic cause. Neurologic findings from the examination and EMG can help direct the attention of the radiologist to specific nerve roots, especially on axial images. For facet joint hypertrophy, surgical foraminotomy produces long-term relief of leg and back pain in 80–90% of patients. The usefulness of therapeutic facet joint blocks for pain is controversial. Medical causes of lumbar or cervical radiculopathy unrelated to anatomic spine disease include infections (e.g., herpes zoster, Lyme disease), carcinomatous meningitis, and root avulsion or traction (severe trauma).
FIGURE 22-6 Right L5 radiculopathy. A. Sagittal T2-weighted image. There is normal high signal around the exiting right L4 nerve root in the right neural foramen at L4-L5; effacement of the high signal in the right L5-S1 foramen is present one level caudal on the right at L5-S1. B. Axial T2-weighted image. The lateral recesses are normal bilaterally; the intervertebral foramen is normal on the left, but severely stenotic on the right. *Severe right L5-S1 foraminal stenosis.
SPONDYLOSIS AND SPONDYLOLISTHESIS
Spondylosis, or osteoarthritic spine disease, typically occurs in later life and primarily involves the cervical and lumbosacral spine. Patients often complain of back pain that increases with movement, is associated with stiffness, and is better when inactive. The relationship between clinical symptoms and radiologic findings is usually not straightforward. Pain may be prominent when x-ray, CT, or MRI findings are minimal, and prominent degenerative spine disease can be seen in asymptomatic patients. Osteophytes or combined disk-osteophytes may cause or contribute to central spinal canal stenosis, lateral recess stenosis, or neural foraminal narrowing.
Spondylolisthesis is the anterior slippage of the vertebral body, pedicles, and superior articular facets, leaving the posterior elements behind. Spondylolisthesis can be associated with spondylolysis, congenital anomalies, degenerative spine disease, or other causes of mechanical weakness of the pars (e.g., infection, osteoporosis, tumor, trauma, prior surgery). The slippage may be asymptomatic or may cause low back pain and hamstring tightness, nerve root injury (the L5 root most frequently), symptomatic spinal stenosis, or CES in severe cases. Tenderness may be elicited near the segment that has “slipped” forward (most often L4 on L5 or occasionally L5 on S1). Focal anterolisthesis or retrolisthesis can occur at any cervical or lumbar level and be the source of neck or low back pain. Plain x-rays with the neck or low back in flexion and extension will reveal the movement at the abnormal spinal segment. Surgery is considered for pain symptoms that do not respond to conservative measures (e.g., rest, physical therapy) and in cases with progressive neurologic deficit, postural deformity, slippage >50%, or scoliosis.
NEOPLASMS
Back pain is the most common neurologic symptom in patients with systemic cancer and is the presenting symptom in 20%. The cause is usually vertebral body metastasis but can also result from spread of cancer through the intervertebral foramen (especially with lymphoma), from carcinomatous meningitis, or from metastasis to the spinal cord. Cancer-related back pain tends to be constant, dull, unrelieved by rest, and worse at night. By contrast, mechanical low back pain usually improves with rest. MRI, CT, and CT myelography are the studies of choice when spinal metastasis is suspected. Once a metastasis is found, imaging of the entire spine reveals additional tumor deposits in one-third of patients. MRI is preferred for soft tissue definition, but the most rapidly available imaging modality is best because the patient’s condition may worsen quickly without intervention. Fewer than 5% of patients who are nonambulatory at the time of diagnosis ever regain the ability to walk; thus, early diagnosis is crucial. The management of spinal metastasis is discussed in detail in Chap. 118.
INFECTIONS/INFLAMMATION
Vertebral osteomyelitis is often caused by staphylococci, but other bacteria or tuberculosis (Pott’s disease) may be responsible. The primary source of infection is usually the urinary tract, skin, or lungs. Intravenous drug use is a well-recognized risk factor. Whenever pyogenic osteomyelitis is found, the possibility of bacterial endocarditis should be considered. Back pain unrelieved by rest, spine tenderness over the involved spine segment, and an elevated ESR are the most common findings in vertebral osteomyelitis. Fever or an elevated white blood cell count is found in a minority of patients. MRI and CT are sensitive and specific for early detection of osteomyelitis; CT may be more readily available in emergency settings and better tolerated by some patients with severe back pain. The intervertebral disk can also be affected by infection (diskitis) and, very rarely, by tumor.
Spinal epidural abscess (Chap. 456) presents with back pain (aggravated by movement or palpation), fever, radiculopathy, or signs of spinal cord compression. The subacute development of two or more of these findings should increase the index of suspicion for spinal epidural abscess. The abscess may track over multiple spinal levels and is best delineated by spine MRI.
Lumbar adhesive arachnoiditis with radiculopathy is due to fibrosis following inflammation within the subarachnoid space. The fibrosis results in nerve root adhesions and presents as back and leg pain associated with focal motor, sensory, or reflex changes. Causes of arachnoiditis include multiple lumbar operations, chronic spinal infections (especially tuberculosis in the developing world), spinal cord injury, intrathecal hemorrhage, myelography (rare), intrathecal injections (glucocorticoids, anesthetics, or other agents), and foreign bodies. The MRI shows clumped nerve roots or loculations of cerebrospinal fluid within the thecal sac. Clumped nerve roots may also occur with demyelinating polyneuropathy or neoplastic infiltration. Treatment is usually unsatisfactory. Microsurgical lysis of adhesions, dorsal rhizotomy, dorsal root ganglionectomy, and epidural glucocorticoids have been tried, but outcomes have been poor. Dorsal column stimulation for pain relief has produced varying results.
TRAUMA
A patient complaining of back pain and an inability to move the legs may have a spine fracture or dislocation; with fractures above L1 the spinal cord is at risk for compression. Care must be taken to avoid further damage to the spinal cord or nerve roots by immobilizing the back or neck pending the results of radiologic studies. Vertebral fractures frequently occur in the absence of trauma in association with osteoporosis, glucocorticoid use, osteomyelitis, or neoplastic infiltration.
Sprains and Strains The terms low back sprain, strain, and mechanically induced muscle spasm refer to minor, self-limited injuries associated with lifting a heavy object, a fall, or a sudden deceleration such as in an automobile accident. These terms are used loosely and do not clearly describe a specific anatomic lesion. The pain is usually confined to the lower back, and there is no radiation to the buttocks or legs. Patients with paraspinal muscle spasm often assume unusual postures.
Traumatic Vertebral Fractures Most traumatic fractures of the lumbar vertebral bodies result from injuries producing anterior wedging or compression. With severe trauma, the patient may sustain a fracture-dislocation or a “burst” fracture involving the vertebral body and posterior elements. Traumatic vertebral fractures are caused by falls from a height, sudden deceleration in an automobile accident, or direct injury. Neurologic impairment is common, and early surgical treatment is indicated. In victims of blunt trauma, CT scans of the chest, abdomen, or pelvis can be reformatted to detect associated vertebral fractures.
METABOLIC CAUSES
Osteoporosis and Osteosclerosis Immobilization, osteomalacia, the postmenopausal state, renal disease, multiple myeloma, hyperparathyroidism, hyperthyroidism, metastatic carcinoma, or glucocorticoid use may accelerate osteoporosis and weaken the vertebral body, leading to compression fractures and pain. Up to two-thirds of compression fractures seen on radiologic imaging are asymptomatic. The most common nontraumatic vertebral body fractures are due to postmenopausal or senile osteoporosis (Chap. 425). The risk of an additional vertebral fracture at 1 year following a first vertebral fracture is 20%. The presence of fever, weight loss, fracture at a level above T4, or the conditions described above should increase suspicion for a cause other than senile osteoporosis. The sole manifestation of a compression fracture may be localized back or radicular pain exacerbated by movement and often reproduced by palpation over the spinous process of the affected vertebra.
Relief of acute pain can often be achieved with acetaminophen or a combination of opioids and acetaminophen. The role of NSAIDs is controversial. Both pain and disability are improved with bracing. Antiresorptive drugs, especially bisphosphonates (e.g., alendronate), have been shown to reduce the risk of osteoporotic fractures and are the preferred treatment to prevent additional fractures. Less than one-third of patients with prior compression fractures are adequately treated for osteoporosis despite the increased risk for future fractures; even fewer at-risk patients without a history of fracture are adequately treated. Given the negative results of sham-controlled studies of percutaneous vertebroplasty (PVP) and of kyphoplasty for osteoporotic compression fractures associated with debilitating pain, these procedures are not routinely recommended.
Osteosclerosis, an abnormally increased bone density often due to Paget’s disease, is readily identifiable on routine x-ray studies and can sometimes be a source of back pain. It may be associated with an isolated increase in alkaline phosphatase in an otherwise healthy older person. Spinal cord or nerve root compression can result from bony encroachment. The diagnosis of Paget’s disease as the cause of a patient’s back pain is a diagnosis of exclusion.
For further discussion of these bone disorders, see Chaps. 424, 425, and 426e.
AUTOIMMUNE INFLAMMATORY ARTHRITIS
Autoimmune inflammatory disease of the spine can present with the insidious onset of low back, buttock, or neck pain. Examples include rheumatoid arthritis (Chap 380), ankylosing spondylitis, reactive arthritis, psoriatic arthritis, or inflammatory bowel disease (Chap. 384).
CONGENITAL ANOMALIES OF THE LUMBAR SPINE
Spondylolysis is a bony defect in the vertebral pars interarticularis (a segment near the junction of the pedicle with the lamina); the cause is usually a stress microfracture in a congenitally abnormal segment. It occurs in up to 6% of adolescents. The defect (usually bilateral) is best visualized on plain x-rays, CT scan, or bone scan and is frequently asymptomatic. Symptoms may occur in the setting of a single injury, repeated minor injuries, or during a growth spurt. Spondylolysis is the most common cause of persistent low back pain in adolescents and is often associated with sports-related activities.
Scoliosis refers to an abnormal curvature in the coronal (lateral) plane of the spine. With kyphoscoliosis, there is, in addition, a forward curvature of the spine. The abnormal curvature may be congenital due to abnormal spine development, acquired in adulthood due to degenerative spine disease, or occasionally progressive due to neuromuscular disease. The deformity can progress until ambulation or pulmonary function is compromised.
Spina bifida occulta is a failure of closure of one or several vertebral arches posteriorly; the meninges and spinal cord are normal. A dimple or small lipoma may overlie the defect. Most cases are asymptomatic and discovered incidentally during an evaluation for back pain.
Tethered cord syndrome usually presents as a progressive cauda equina disorder (see below), although myelopathy may also be the initial manifestation. The patient is often a young adult who complains of perineal or perianal pain, sometimes following minor trauma. MRI studies reveal a low-lying conus (below L1 and L2) and a short and thickened filum terminale.
REFERRED PAIN FROM VISCERAL DISEASE
Diseases of the thorax, abdomen, or pelvis may refer pain to the posterior portion of the spinal segment that innervates the diseased organ. Occasionally, back pain may be the first and only manifestation. Upper abdominal diseases generally refer pain to the lower thoracic or upper lumbar region (eighth thoracic to the first and second lumbar vertebrae), lower abdominal diseases to the midlumbar region (second to fourth lumbar vertebrae), and pelvic diseases to the sacral region. Local signs (pain with spine palpation, paraspinal muscle spasm) are absent, and little or no pain accompanies routine movements of the spine.
Low Thoracic or Lumbar Pain with Abdominal Disease Tumors of the posterior wall of the stomach or duodenum typically produce epigastric pain (Chaps. 109 and 348), but midline back or paraspinal pain may occur if retroperitoneal extension is present. Fatty foods occasionally induce back pain associated with biliary disease. Diseases of the pancreas can produce right or left paraspinal back pain. Pathology in retroperitoneal structures (hemorrhage, tumors, pyelonephritis) can produce paraspinal pain that radiates to the lower abdomen, groin, or anterior thighs. A mass in the iliopsoas region can produce unilateral lumbar pain with radiation toward the groin, labia, or testicle. The sudden appearance of lumbar pain in a patient receiving anticoagulants suggests retroperitoneal hemorrhage.
Isolated low back pain occurs in some patients with a contained rupture of an abdominal aortic aneurysm (AAA). The classic clinical triad of abdominal pain, shock, and back pain occurs in <20% of patients. The typical patient at risk is an elderly male smoker with back pain. The diagnosis may be missed because the symptoms and signs can be nonspecific. Misdiagnoses include nonspecific back pain, diverticulitis, renal colic, sepsis, and myocardial infarction. A careful abdominal examination revealing a pulsatile mass (present in 50–75% of patients) is an important physical finding. Patients with suspected AAA should be evaluated with abdominal ultrasound, CT, or MRI (Chap. 301).
Sacral Pain with Gynecologic and Urologic Disease Pelvic organs rarely cause low back pain, except for gynecologic disorders involving the uterosacral ligaments. The pain is referred to the sacral region. Endometriosis or uterine cancers may invade the uterosacral ligaments. Pain associated with endometriosis is typically premenstrual and often continues until it merges with menstrual pain. Uterine malposition may cause uterosacral ligament traction (retroversion, descensus, and prolapse) or produce sacral pain after prolonged standing.
Menstrual pain may be felt in the sacral region sometimes with poorly localized, cramping pain radiating down the legs. Pain due to neoplastic infiltration of nerves is typically continuous, progressive in severity, and unrelieved by rest at night. Less commonly, radiation therapy of pelvic tumors may produce sacral pain from late radiation necrosis of tissue. Low back pain that radiates into one or both thighs is common in the last weeks of pregnancy.
Urologic sources of lumbosacral back pain include chronic prostatitis, prostate cancer with spinal metastasis (Chap. 115), and diseases of the kidney or ureter. Lesions of the bladder and testes do not often produce back pain. Infectious, inflammatory, or neoplastic renal diseases may produce ipsilateral lumbosacral pain, as can renal artery or vein thrombosis. Paraspinal lumbar pain may be a symptom of ureteral obstruction due to nephrolithiasis.
OTHER CAUSES OF BACK PAIN
Postural Back Pain There is a group of patients with nonspecific chronic low back pain (CLBP) in whom no specific anatomic lesion can be found despite exhaustive investigation. These individuals complain of vague, diffuse back pain with prolonged sitting or standing that is relieved by rest. Exercises to strengthen the paraspinal and abdominal muscles are sometimes helpful.
Psychiatric Disease CLBP may be encountered in patients who seek financial compensation; in malingerers; or in those with concurrent substance abuse. Many patients with CLBP have a history of psychiatric illness (depression, anxiety states) or childhood trauma (physical or sexual abuse) that antedates the onset of back pain. Preoperative psychological assessment has been used to exclude patients with marked psychological impairments that predict a poor surgical outcome from spine surgery.
IDIOPATHIC
The cause of low back pain occasionally remains unclear. Some patients have had multiple operations for disk disease but have persistent pain and disability. The original indications for surgery may have been questionable, with back pain only, no definite neurologic signs, or a minor disk bulge noted on CT or MRI. Scoring systems based on neurologic signs, psychological factors, physiologic studies, and imaging studies have been devised to minimize the likelihood of unsuccessful surgery.
PAIN IN THE NECK AND SHOULDER
Neck pain, which usually arises from diseases of the cervical spine and soft tissues of the neck, is common. Neck pain arising from the cervical spine is typically precipitated by movement and may be accompanied by focal tenderness and limitation of motion. Many of the prior comments made regarding causes of low back pain also apply to disorders of the cervical spine. The text below will emphasize differences. Pain arising from the brachial plexus, shoulder, or peripheral nerves can be confused with cervical spine disease (Table 22-4), but the history and examination usually identify a more distal origin for the pain. Cervical spine trauma, disk disease, or spondylosis with intervertebral foraminal narrowing may be asymptomatic or painful and can produce a myelopathy, radiculopathy, or both. The same risk factors for serious causes of low back pain also apply to neck pain with the additional feature that neurologic signs of myelopathy (incontinence, sensory level, spastic legs) may also occur. Lhermitte’s sign, an electrical shock down the spine with neck flexion, suggests involvement of the cervical spinal cord.
|
CERVICAL RADICULOPATHY: NEUROLOGIC FEATURES |
TRAUMA TO THE CERVICAL SPINE
Trauma to the cervical spine (fractures, subluxation) places the spinal cord at risk for compression. Motor vehicle accidents, violent crimes, or falls account for 87% of cervical spinal cord injuries (Chap. 456). Immediate immobilization of the neck is essential to minimize further spinal cord injury from movement of unstable cervical spine segments. The decision to obtain imaging should be based on the nature of the injury. The NEXUS low-risk criteria established that normally alert patients without palpation tenderness in the midline; intoxication; neurologic deficits; or painful distracting injuries were very unlikely to have sustained a clinically significant traumatic injury to the cervical spine. The Canadian C-spine rule recommends that imaging should be obtained following neck region trauma if the patient is >65 years old or has limb paresthesias or if there was a dangerous mechanism for the injury (e.g., bicycle collision with tree or parked car, fall from height >3 feet or five stairs, diving accident). These guidelines are helpful but must be tailored to individual circumstances; for example, patients with advanced osteoporosis, glucocorticoid use, or cancer may warrant imaging after even mild trauma. A CT scan is the diagnostic procedure of choice for detection of acute fractures following severe trauma; plain x-rays can be used for lesser degrees of trauma. When traumatic injury to the vertebral arteries or cervical spinal cord is suspected, visualization by MRI with magnetic resonance angiography is preferred.
Whiplash injury is due to rapid flexion and extension of the neck, usually in automobile accidents. The exact mechanism of the injury is unclear. This diagnosis should not be applied to patients with fractures, disk herniation, head injury, focal neurologic findings, or altered consciousness. Up to 50% of persons reporting whiplash injury acutely have persistent neck pain 1 year later. Once personal compensation for pain and suffering was removed from the Australian health care system, the prognosis for recovery at 1 year from whiplash injury improved also. Imaging of the cervical spine is not cost-effective acutely but is useful to detect disk herniations when symptoms persist for >6 weeks following the injury. Severe initial symptoms have been associated with a poor long-term outcome.
CERVICAL DISK DISEASE
Herniation of a lower cervical disk is a common cause of pain or tingling in the neck, shoulder, arm, or hand. Neck pain, stiffness, and a range of motion limited by pain are the usual manifestations. Herniated cervical disks are responsible for ~25% of cervical radiculopathies. Extension and lateral rotation of the neck narrow the ipsilateral intervertebral foramen and may reproduce radicular symptoms (Spurling’s sign). In young adults, acute nerve root compression from a ruptured cervical disk is often due to trauma. Cervical disk herniations are usually posterolateral near the lateral recess. Typical patterns of reflex, sensory, and motor changes that accompany cervical nerve root lesions are summarized in Table 22-4. Although the classic patterns are clinically helpful, there are numerous exceptions because (1) there is overlap in sensory function between adjacent nerve roots, (2) symptoms and signs may be evident in only part of the injured nerve root territory, and (3) the location of pain is the most variable of the clinical features.
CERVICAL SPONDYLOSIS
Osteoarthritis of the cervical spine may produce neck pain that radiates into the back of the head, shoulders, or arms, or may be the source of headaches in the posterior occipital region (supplied by the C2-C4 nerve roots). Osteophytes, disk protrusions, or hypertrophic facet or uncovertebral joints may alone or in combination compress one or several nerve roots at the intervertebral foramina; these causes together account for 75% of cervical radiculopathies. The roots most commonly affected are C7 and C6. Narrowing of the spinal canal by osteophytes, ossification of the posterior longitudinal ligament (OPLL), or a large central disk may compress the cervical spinal cord and produce signs of radiculopathy and myelopathy in combination (myeloradiculopathy). When little or no neck pain accompanies cervical cord involvement, other diagnoses to be considered include amyotrophic lateral sclerosis (Chap. 452), multiple sclerosis (Chap. 458), spinal cord tumors, or syringomyelia (Chap. 456). The possibility of cervical spondylosis should be considered even when the patient presents with symptoms or signs in the legs only. MRI is the study of choice to define anatomic abnormalities of soft tissues in the cervical region including the spinal cord, but plain CT is adequate to assess bony spurs, foraminal narrowing, lateral recess stenosis, or OPLL. EMG and nerve conduction studies can localize and assess the severity of nerve root injury.
OTHER CAUSES OF NECK PAIN
Rheumatoid arthritis (RA) (Chap. 380) of the cervical facet joints produces neck pain, stiffness, and limitation of motion. Synovitis of the atlantoaxial joint (C1-C2; Fig. 22-2) may damage the transverse ligament of the atlas, producing forward displacement of the atlas on the axis (atlantoaxial subluxation). Radiologic evidence of atlantoaxial subluxation occurs in up to 30% of patients with RA. The degree of subluxation correlates with the severity of erosive disease. When subluxation is present, careful assessment is important to identify early signs of myelopathy. Occasional patients develop high spinal cord compression leading to quadriparesis, respiratory insufficiency, and death. Surgery should be considered when myelopathy or spinal instability is present. MRI is the imaging modality of choice. Ankylosing spondylitis can cause neck pain and less commonly atlantoaxial subluxation; surgery may be required to prevent spinal cord compression.
Acute herpes zoster can presents as acute posterior occipital or neck pain prior to the outbreak of vesicles. Neoplasms metastatic to the cervical spine, infections (osteomyelitis and epidural abscess), and metabolic bone diseases may be the cause of neck pain, as discussed above among causes of low back pain. Neck pain may also be referred from the heart with coronary artery ischemia (cervical angina syndrome).
THORACIC OUTLET SYNDROMES
The thoracic outlet contains the first rib, the subclavian artery and vein, the brachial plexus, the clavicle, and the lung apex. Injury to these structures may result in postural or movement-induced pain around the shoulder and supraclavicular region, classified as follows.
True neurogenic thoracic outlet syndrome (TOS) is an uncommon disorder resulting from compression of the lower trunk of the brachial plexus or ventral rami of the C8 or T1 nerve roots, caused most often by an anomalous band of tissue connecting an elongate transverse process at C7 with the first rib. Pain is mild or may be absent. Signs include weakness and wasting of intrinsic muscles of the hand and diminished sensation on the palmar aspect of the fifth digit. An anteroposterior cervical spine x-ray will show an elongate C7 transverse process (an anatomic marker for the anomalous cartilaginous band), and EMG and nerve conduction studies confirm the diagnosis. Treatment consists of surgical resection of the anomalous band. The weakness and wasting of intrinsic hand muscles typically does not improve, but surgery halts the insidious progression of weakness.
Arterial TOS results from compression of the subclavian artery by a cervical rib, resulting in poststenotic dilatation of the artery and in some cases secondary thrombus formation. Blood pressure is reduced in the affected limb, and signs of emboli may be present in the hand. Neurologic signs are absent. Ultrasound can confirm the diagnosis noninvasively. Treatment is with thrombolysis or anticoagulation (with or without embolectomy) and surgical excision of the cervical rib compressing the subclavian artery.
Venous TOS is due to subclavian vein thrombosis resulting in swelling of the arm and pain. The vein may be compressed by a cervical rib or anomalous scalene muscle. Venography is the diagnostic test of choice.
Disputed TOS accounts for 95% of patients diagnosed with TOS; chronic arm and shoulder pain are prominent and of unclear cause. The lack of sensitive and specific findings on physical examination or specific markers for this condition results in diagnostic uncertainty. The role of surgery in disputed TOS is controversial. Multidisciplinary pain management is a conservative approach, although treatment is often unsuccessful.
BRACHIAL PLEXUS AND NERVES
Pain from injury to the brachial plexus or peripheral nerves of the arm can occasionally mimic referred pain of cervical spine origin including cervical radiculopathy. Neoplastic infiltration of the lower trunk of the brachial plexus may produce shoulder or supraclavicular pain radiating down the arm, numbness of the fourth and fifth fingers or medial forearm, and weakness of intrinsic hand muscles innervated by the ulnar and median nerves. Delayed radiation injury may produce similar findings, although pain is less often present and almost always less severe. A Pancoast tumor of the lung (Chap. 107) is another cause and should be considered, especially when a concurrent Horner’s syndrome is present. Suprascapular neuropathy may produce severe shoulder pain, weakness, and wasting of the supraspinatus and infraspinatus muscles. Acute brachial neuritis is often confused with radiculopathy; the acute onset of severe shoulder or scapular pain is followed typically over days by weakness of the proximal arm and shoulder girdle muscles innervated by the upper brachial plexus. The onset may be preceded by an infection, vaccination, or minor surgical procedure. The long thoracic nerve may be affected resulting in a winged scapula. Brachial neuritis may also present as an isolated paralysis of the diaphragm with or without involvement of other nerves of the upper limb. Recovery may take up to 3 years.
Occasional cases of carpal tunnel syndrome produce pain and paresthesias extending into the forearm, arm, and shoulder resembling a C5 or C6 root lesion. Lesions of the radial or ulnar nerve can mimic a radiculopathy at C7 or C8, respectively. EMG and nerve conduction studies can accurately localize lesions to the nerve roots, brachial plexus, or peripheral nerves.
For further discussion of peripheral nerve disorders, see Chap. 459.
SHOULDER
Pain arising from the shoulder can on occasion mimic pain from the spine. If symptoms and signs of radiculopathy are absent, then the differential diagnosis includes mechanical shoulder pain (tendonitis, bursitis, rotator cuff tear, dislocation, adhesive capsulitis, or rotator cuff impingement under the acromion) and referred pain (subdiaphragmatic irritation, angina, Pancoast tumor). Mechanical pain is often worse at night, associated with local shoulder tenderness and aggravated by passive abduction, internal rotation, or extension of the arm. Pain from shoulder disease may radiate into the arm or hand, but focal neurologic signs (sensory, motor, or reflex changes) are absent.
SECTION 2 |
ALTERATIONS IN BODY TEMPERATURE |
23 |
Fever |
Body temperature is controlled by the hypothalamus. Neurons in both the preoptic anterior hypothalamus and the posterior hypothalamus receive two kinds of signals: one from peripheral nerves that transmit information from warmth/cold receptors in the skin and the other from the temperature of the blood bathing the region. These two types of signals are integrated by the thermoregulatory center of the hypothalamus to maintain normal temperature. In a neutral temperature environment, the human metabolic rate produces more heat than is necessary to maintain the core body temperature in the range of 36.5–37.5°C (97.7–99.5°F).
A normal body temperature is ordinarily maintained despite environmental variations because the hypothalamic thermoregulatory center balances the excess heat production derived from metabolic activity in muscle and the liver with heat dissipation from the skin and lungs. According to studies of healthy individuals 18–40 years of age, the mean oral temperature is 36.8° ± 0.4°C (98.2° ± 0.7°F), with low levels at 6 A.M. and higher levels at 4–6 P.M. The maximal normal oral temperature is 37.2°C (98.9°F) at 6 A.M. and 37.7°C (99.9°F) at 4 P.M.; these values define the 99th percentile for healthy individuals. In light of these studies, an A.M. temperature of >37.2°C (>98.9°F) or a P.M. temperature of >37.7°C (>99.9°F) would define a fever. The normal daily temperature variation is typically 0.5°C (0.9°F). However, in some individuals recovering from a febrile illness, this daily variation can be as great as 1.0°C. During a febrile illness, the diurnal variation is usually maintained, but at higher, febrile levels. The daily temperature variation appears to be fixed in early childhood; in contrast, elderly individuals can exhibit a reduced ability to develop fever, with only a modest fever even in severe infections.
Rectal temperatures are generally 0.4°C (0.7°F) higher than oral readings. The lower oral readings are probably attributable to mouth breathing, which is a factor in patients with respiratory infections and rapid breathing. Lower-esophageal temperatures closely reflect core temperature. Tympanic membrane thermometers measure radiant heat from the tympanic membrane and nearby ear canal and display that absolute value (unadjusted mode) or a value automatically calculated from the absolute reading on the basis of nomograms relating the radiant temperature measured to actual core temperatures obtained in clinical studies (adjusted mode). These measurements, although convenient, may be more variable than directly determined oral or rectal values. Studies in adults show that readings are lower with unadjusted-mode than with adjusted-mode tympanic membrane thermometers and that unadjusted-mode tympanic membrane values are 0.8°C (1.6°F) lower than rectal temperatures.
In women who menstruate, the A.M. temperature is generally lower in the 2 weeks before ovulation; it then rises by ~0.6°C (1°F) with ovulation and remains at that level until menses occur. Body temperature can be elevated in the postprandial state. Pregnancy and endocrinologic dysfunction also affect body temperature.
FEVER VERSUS HYPERTHERMIA
Fever is an elevation of body temperature that exceeds the normal daily variation and occurs in conjunction with an increase in the hypothalamic set point (e.g., from 37°C to 39°C). This shift of the set point from “normothermic” to febrile levels very much resembles the resetting of the home thermostat to a higher level in order to raise the ambient temperature in a room. Once the hypothalamic set point is raised, neurons in the vasomotor center are activated and vasoconstriction commences. The individual first notices vasoconstriction in the hands and feet. Shunting of blood away from the periphery to the internal organs essentially decreases heat loss from the skin, and the person feels cold. For most fevers, body temperature increases by 1–2°C. Shivering, which increases heat production from the muscles, may begin at this time; however, shivering is not required if heat conservation mechanisms raise blood temperature sufficiently. Nonshivering heat production from the liver also contributes to increasing core temperature. Behavioral adjustments (e.g., putting on more clothing or bedding) help raise body temperature by decreasing heat loss.
The processes of heat conservation (vasoconstriction) and heat production (shivering and increased nonshivering thermogenesis) continue until the temperature of the blood bathing the hypothalamic neurons matches the new thermostat setting. Once that point is reached, the hypothalamus maintains the temperature at the febrile level by the same mechanisms of heat balance that function in the afebrile state. When the hypothalamic set point is again reset downward (in response to either a reduction in the concentration of pyrogens or the use of antipyretics), the processes of heat loss through vasodilation and sweating are initiated. Loss of heat by sweating and vasodilation continues until the blood temperature at the hypothalamic level matches the lower setting. Behavioral changes (e.g., removal of clothing) facilitate heat loss.
A fever of >41.5°C (>106.7°F) is called hyperpyrexia. This extraordinarily high fever can develop in patients with severe infections but most commonly occurs in patients with central nervous system (CNS) hemorrhages. In the preantibiotic era, fever due to a variety of infectious diseases rarely exceeded 106°F, and there has been speculation that this natural “thermal ceiling” is mediated by neuropeptides functioning as central antipyretics.
In rare cases, the hypothalamic set point is elevated as a result of local trauma, hemorrhage, tumor, or intrinsic hypothalamic malfunction. The term hypothalamic fever is sometimes used to describe elevated temperature caused by abnormal hypothalamic function. However, most patients with hypothalamic damage have subnormal, not supranormal, body temperatures.
Although most patients with elevated body temperature have fever, there are circumstances in which elevated temperature represents not fever but hyperthermia (heat stroke). Hyperthermia is characterized by an uncontrolled increase in body temperature that exceeds the body’s ability to lose heat. The setting of the hypothalamic thermoregulatory center is unchanged. In contrast to fever in infections, hyperthermia does not involve pyrogenic molecules. Exogenous heat exposure and endogenous heat production are two mechanisms by which hyperthermia can result in dangerously high internal temperatures. Excessive heat production can easily cause hyperthermia despite physiologic and behavioral control of body temperature. For example, work or exercise in hot environments can produce heat faster than peripheral mechanisms can lose it. For a detailed discussion of hyperthermia, see Chap. 479e.
It is important to distinguish between fever and hyperthermia since hyperthermia can be rapidly fatal and characteristically does not respond to antipyretics. In an emergency situation, however, making this distinction can be difficult. For example, in systemic sepsis, fever (hyperpyrexia) can be rapid in onset, and temperatures can exceed 40.5°C (104.9°F). Hyperthermia is often diagnosed on the basis of the events immediately preceding the elevation of core temperature—e.g., heat exposure or treatment with drugs that interfere with thermoregulation. In patients with heat stroke syndromes and in those taking drugs that block sweating, the skin is hot but dry, whereas in fever the skin can be cold as a consequence of vasoconstriction. Antipyretics do not reduce the elevated temperature in hyperthermia, whereas in fever—and even in hyperpyrexia—adequate doses of either aspirin or acetaminophen usually result in some decrease in body temperature.