Chapter 4 Avoidance, Recognition, and Treatment of Complications in Cranial Neuromodulation for Pain
 Although not currently labeled for use in the United States for the treatment of pain, DBS and MCS are potentially effective therapies for medically intractable pain in carefully selected patients.
Although not currently labeled for use in the United States for the treatment of pain, DBS and MCS are potentially effective therapies for medically intractable pain in carefully selected patients. Complications of these therapies may arise during surgery from hardware complications, tissue injury, and from the undesired effects of stimulation.
Complications of these therapies may arise during surgery from hardware complications, tissue injury, and from the undesired effects of stimulation.Introduction
Cranial forms of neuromodulation for pain include deep brain stimulation (DBS), motor cortex stimulation (MCS), and non-invasive forms of electrical stimulation such as transcranial magnetic stimulation and transcranial direct-current stimulation.1 DBS and MCS involve the surgical implantation of electrodes within the brain or in the epidural space, respectively. The DBS and MCS electrodes are then connected subcutaneously to an implantable pulse generator (IPG), most often located in the chest. As with other forms of surgical neuromodulation, careful patient selection is critical to the success of DBS and MCS for pain, and a trial of externally driven stimulation is typically performed before placement of the IPG.
Deep Brain Stimulation for Pain
Deep brain stimulation for medically refractory pain was the first application of chronic intracranial DBS. In 1973 Hosobuchi et al2 implanted a stimulating electrode into the ventroposteromedial (VPM) thalamus to treat facial pain. Chronic stimulation was attempted after the observation by Hosobuchi and others that acute stimulation before lesion placement resulted in pain improvement. Today, DBS for medically intractable pain typically targets two regions, the sensory ventroposterolateral (VPL)/VPM thalamus and the periaqueductal/periventricular (PAG/PVG) grey matter. Common indications for DBS for pain include chronic poststroke pain syndromes and chronic facial pain syndromes. Rates of reported efficacy for these procedures vary widely, ranging from 12% to 60%. The U.S. Food and Drug Administration (FDA) initially approved and then rescinded the approval of DBS for pain.
The clinically significant complications of DBS take the form of surgical complications, hardware-related complications, and stimulation-dependent complications.3 Surgical complications of DBS surgery include intracranial hemorrhage and electrode misplacement. Hardware-related complications include electrode fracture, electrode erosion, and infection. Stimulation-dependent complications are the effect of undesired modulation of neural circuits adjacent to the targets of neuromodulation.
Surgical Complications and Avoidance
Motor Cortex Stimulation for Pain
Motor cortex stimulation for pain involves the surgical placement of stimulating electrodes in the epidural space overlying the primary motor cortex (precentral gyrus). Application of stimulation to the sensory cortex (postcentral gyrus) alone is ineffective in the treatment of pain. The paddle leads used are those designed for epidural spinal placement and may be oriented either along the precentral gyrus, parallel to the central sulcus, or bridging the precentral and postcentral gyri perpendicular to the central sulcus. MCS for the treatment of thalamic pain syndrome was reported first by Tsubokawa et al4 in 1991. Today the most common indications for MCS are trigeminal neuropathic pain and thalamic pain syndromes, although the therapy has been applied on a more limited basis to many other painful processes. For thalamic poststroke pain, approximately two-thirds of patients are reported to achieve adequate relief from the therapy. For trigeminal neuropathic pain, impressive response rates of up to 75% to 100% have been reported in some studies. As with DBS for pain, the FDA has not approved MCS as a therapy for pain, reflecting the difficulties of patient selection and treatment efficacy for this therapy.
Surgical Complications and Avoidance
Electrode Misplacement
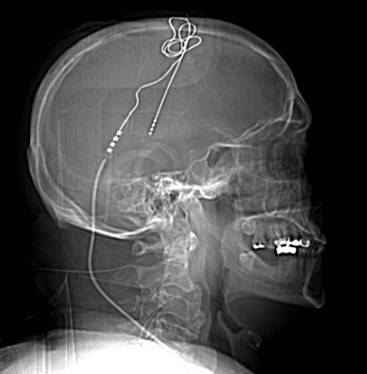
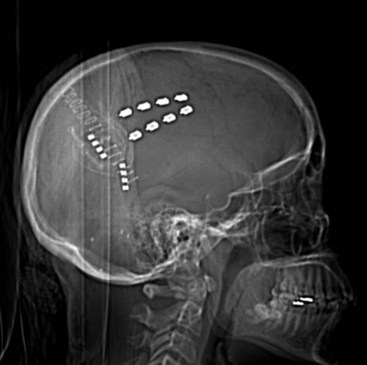
Several techniques allow identification of the sensory and motor cortex during surgery. In the author’s center, a stereotactic MRI and frameless stereotaxy are used to identify the location of the central sulcus. With a frameless imaging system, the location of the electrode with respect to the underlying brain may be directly evaluated. Ultrasound imaging of the cortical surface may also be used if an appropriately sized ultrasound probe is available. When a burr hole is used and therefore the location of the electrode cannot be directly visualized, fluoroscopy can be used to evaluate the precise location of the epidural lead (Fig. 4-2).
As in DBS surgery, electrophysiologic recording can also provide insight into physiologic lead location. Electrophysiologic pulsatile stimulation of the median nerve of the contralateral upper extremity evokes a negative postcentral N20 wave and a positive precentral P20 evoked potential.5 These sensory-evoked potentials can be used reliably to identify the location of the electrophysiologic border between sensory and motor cortical regions, allowing the lead to be placed over the motor cortex. A volumetric postoperative CT scan co-registered to a preoperative MRI can additionally allow simultaneous visualization of the brain and electrode contacts, verifying electrode locations with respect to the brain surface.
1 Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control. Pain Physician. 2010;13:157-165.
2 Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. 1973;29:158-161.
3 Lyons K, Koller W, Wilkinson S, et al. Surgical and hardware complications of subthalamic stimulation. Neurology. 2004;63:612-616.
4 Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir (Wein). 1991;52:137-139.
5 Brown JA, Pilitsis JG. Motor cortex stimulation. Pain Med. 2006;7(suppl):S140-S145.