13 Autonomic nervous system and visceral afferents
Sympathetic Nervous System
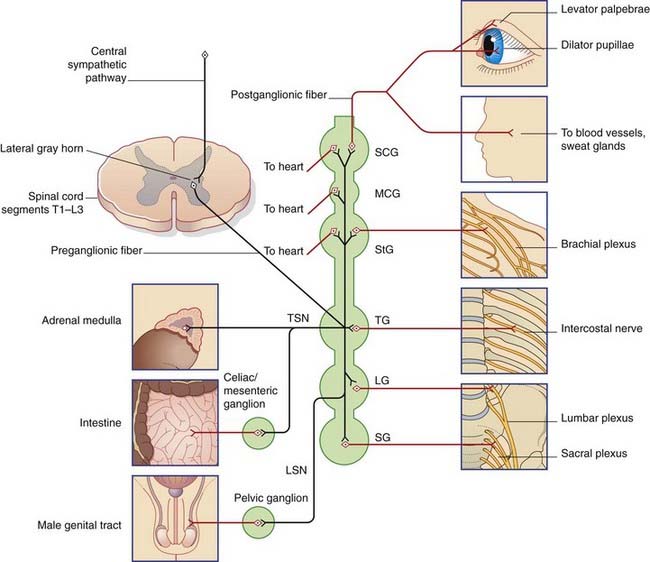
The sympathetic outflow from the nervous system is thoracolumbar, the preganglionic neurons being located in the lateral gray horn of the spinal cord at thoracic and upper two (or three) lumbar segmental levels. From these neurons, preganglionic fibers emerge in the corresponding anterior nerve roots and enter the paravertebral sympathetic chain. The fibers do one of four things (Figure 13.1):
Clinical Panel 13.1 Sympathetic interruption
Stellate block
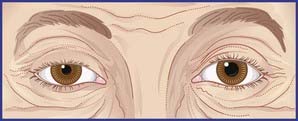
Injection of local anesthetic around the stellate ganglion – stellate block – is a procedure used in order to test the effects of sympathetic interruption on blood flow to the hand. Both pre- and postganglionic fibers are inactivated, producing sympathetic paralysis in the head and neck on that side, as well as in the upper limb. A successful stellate block is demonstrated by (a) a warm, dry hand, (b) Horner’s syndrome, which consists of a constricted pupil owing to unopposed action of the pupillary constrictor, and (c) ptosis (drooping) of the upper eyelid owing to paralysis of smooth muscle fibers contained in the levator muscle of the upper eyelid (Figure CP 13.1.1).
Dominance of the right stellate ganglion in control of the heart rate is shown by the marked slowing of the pulse following a right, but not a left, stellate block. (See also Box 13.1.)
The sympathetic supply to the eye is considered further in Chapter 23.
The sympathetic system exerts tonic (continuous) constrictor activity on blood vessels in the limbs. In order to improve the blood flow to the hands or feet, impulse traffic along the sympathetic system can be interrupted surgically (Clinical Panel 13.1).
Parasympathetic Nervous System
The parasympathetic system generally has the effect of counterbalancing the sympathetic system. It adapts the eyes for close-up viewing, slows the heart, promotes secretion of salivary and intestinal juices, and accelerates intestinal peristalsis. A notable instance of concerted sympathetic and parasympathetic activity occurs during sexual intercourse (Box 13.4).
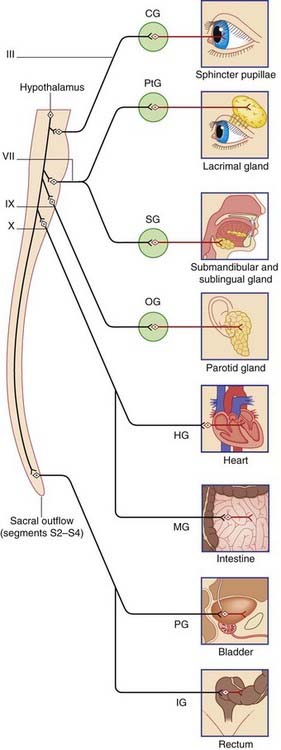
The parasympathetic outflow from the CNS is craniosacral (Figure 13.2). Preganglionic fibers emerge from the brainstem in four cranial nerves – the oculomotor, facial, glossopharyngeal, and vagus – and from sacral segments of the spinal cord.
Cranial parasympathetic system
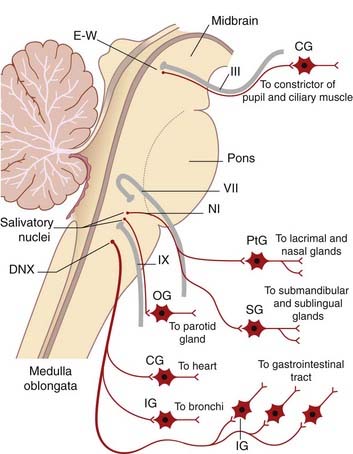
Preganglionic parasympathetic fibers emerge in four cranial nerves (Figure 13.3):
Neurotransmission in the Autonomic System
Ganglionic transmission
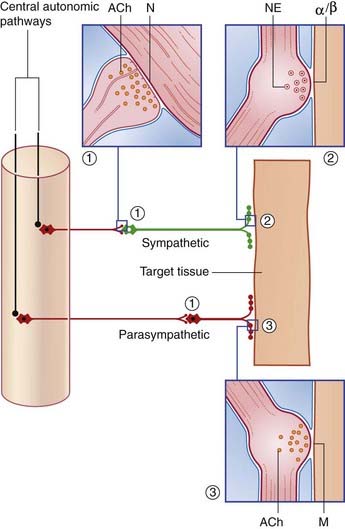
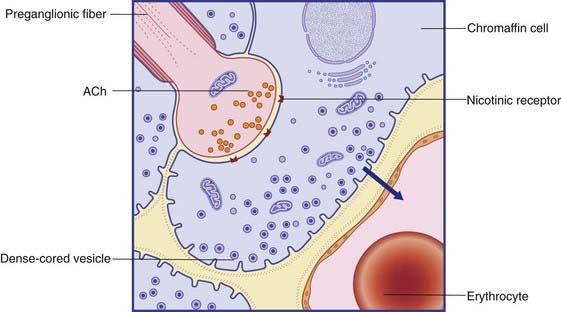
The preganglionic neurons of the sympathetic and parasympathetic systems are cholinergic: the neurons liberate acetylcholine (ACh) on to the ganglion cells at axodendritic synapses (Figure 13.4). The receptors on the ganglion cells are nicotinic, so named because the excitatory effect can be imitated by locally applied nicotine.
Junctional transmission
Postganglionic fibers of the sympathetic and parasympathetic systems form neuroeffector junctions with target tissues (Figure 13.4). Transmitter substances are liberated from innumerable varicosities strung along the course of the nerve fibers.
Junctional receptors
Sympathetic junctional receptors (adrenoceptors) (Figure 13.5)
Two kinds of α adrenoceptor and two kinds of β adrenoceptor have been identified for norepinephrine:
Box 13.1 Innervation of the heart
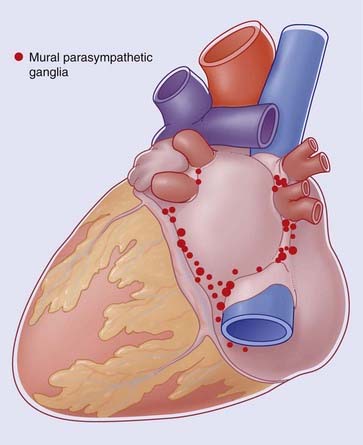
Experimental evidence indicates that the preganglionic parasympathic supply originates in neurons occupying the caudal ventrolateral medulla oblongata. The fibers descend within the trunk of the vagus and synapse within mural ganglia on the posterior walls of the atria and in the posterior atrioventricular groove (Figure Box 13.1.1). Postganglionic cholinergic fibers supply the same tissues as those of the sympathetic, although the direct supply to ventricles and coronary arteries is slight.
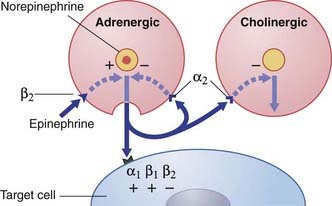
There is a high level of autonomic interaction where innervation is dense, notably within nodal tissue, in the modes shown in Figures 13.5 and 13.7. Many sympathetic nerve endings also release neuropeptide Y, which binds to a specific receptor on cholinergic terminals with adjuvant inhibitory effect on ACh release.
A fourth set of neurons is afferent in nature. Unipolar somas in the inferior ganglion of the vagus provide stretch-sensitive nerve endings close to the endocardium – notably in the right atrium where distension produces reflex slowing of the heart rate by way of a central pathway to the dorsal vagal nucleus via the solitary nucleus (Ch. 24).
The sinuatrial node is highly responsive to emotional states having their seat of origin in the right, ‘emotional’ hemisphere (Ch. 34). The descending pathways concerned are largely ipsilateral and polysynaptic, prior to reaching the lower autonomic nervous system centers of medulla and cord. Sympathetic overactivity, in response to ‘approach’ emotions of sexual or combative nature, may cause the heart to ‘miss a beat’ (extrasystole) or the ‘pulse to race’ (tachycardia). Parasympathetic overactivity, in response to ‘withdraw’ (aversive) emotions, usually of olfactory or visual origin, may cause bradycardia – or even cardiac arrest.
Prejunctional β2 receptors on adrenergic terminals promote release of norepinephrine.
The effects of drugs on the sympathetic system are considered in Clinical Panel 13.2.
Clinical Panel 13.2 Drugs and the sympathetic system
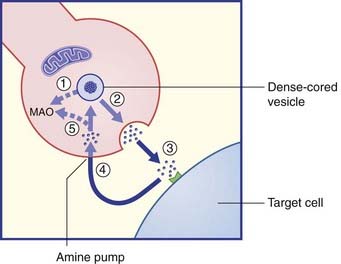
Considerable scope is offered for pharmacological interference at sympathetic nerve endings. Drugs which cross the blood–brain barrier (Ch. 5) may exert their effects upon central rather than peripheral adrenoceptors. Potential sites of drug action are numbered in Figure CP 13.2.1.
Parasympathetic junctional receptors
Parasympathetic junctional receptors are called muscarinic because they can be mimicked by application of the drug muscarine (Figure 13.7). Parasympathetic stimulation produces the following muscarinic effects:
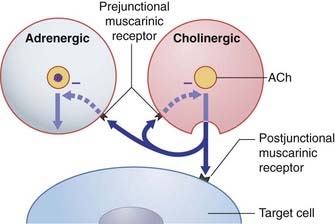
In addition to the above postjunctional effects, prejunctional muscarinic receptors located on sympathetic varicosities inhibit release of norepinephrine (Figure 13.6).
The effects of drugs on the parasympathetic system are considered in Clinical Panel 13.3. Drugs having muscarinic effects are described as cholinergic. Drugs that prevent access of ACh to junctional receptors are anticholinergic.
Clinical Panel 13.3 Drugs and the parasympathetic system
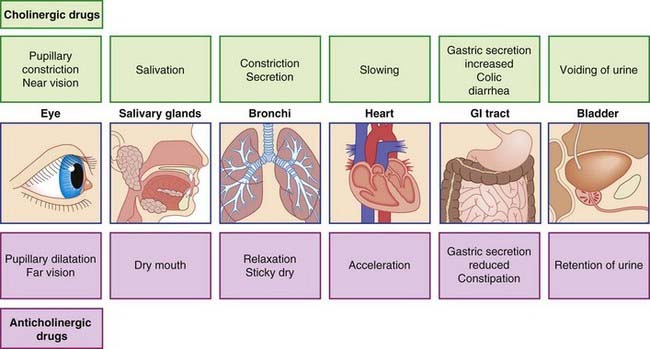
Possible peripheral effects of cholinergic and anticholinergic drugs are listed in Figure CP 13.3.1. Some success has been achieved in the search for organ- or tissue-specific drugs. For example, the contribution of the vagus nerve to acid secretion in the stomach involves activation of a muscarinic receptor (M1) which is distinct from the receptor type (M2) found in the heart or on smooth muscle. An M1-receptor blocker is available for patients suffering from peptic ulcer, for the specific purpose of reducing gastric acidity.
Regional Autonomic Innervation
Box 13.1 describes the autonomic innervation of the heart, Box 13.2 the enteric nervous system, Box 13.3 lower-level bladder controls, and Box 13.4 the functional innervation of the genital tract.
Box 13.2 Enteric nervous system
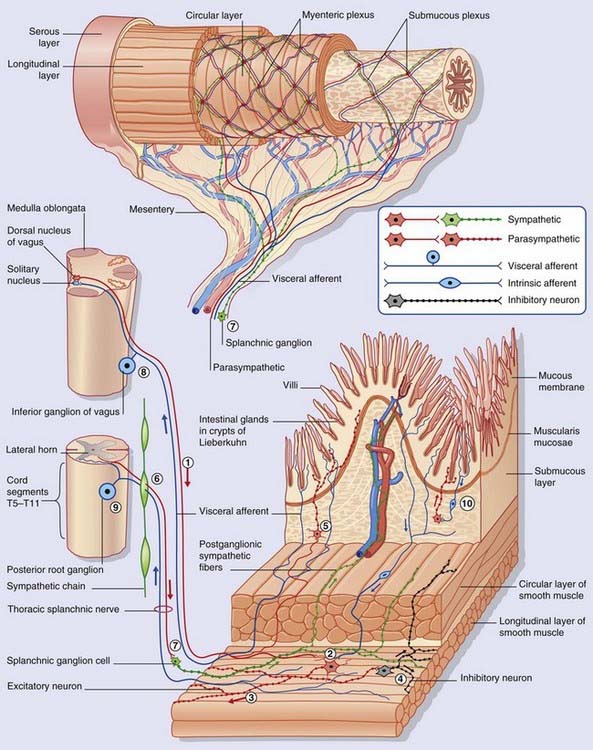
The enteric nervous system (ENS) shown in Figure Box 13.2.1 extends from the midregion of the esophagus all the way to the anal canal. Throughout the length of this tube, it controls peristaltic activity, glandular secretion, and water and ion transfer. In addition, the ENS supplies the pancreas, liver, and gall bladder. The number of intrinsic neurons in the wall of the gastrointestinal tract has been reckoned about the same as in the entire spinal cord. The ENS is sometimes referred to as the ‘gut brain’ on account of its size and relative functional independence.
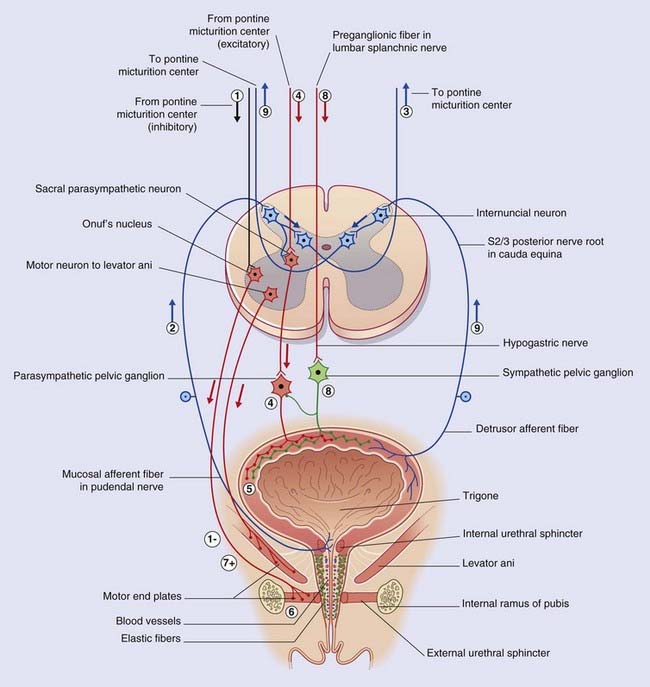
Box 13.3 Lower-level bladder controls
The female bladder is selected for this description, and also for higher-level bladder controls in Chapter 24.
Relevant anatomic details
The micturition cycle (Figure Box 13.3.1)
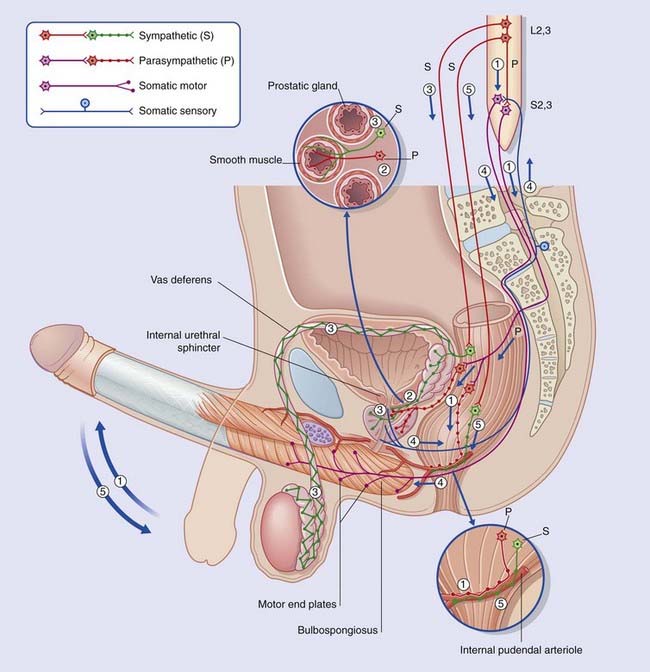
Box 13.4 Functional innervation of male genital tract (Figure Box 13.4.1)
The sympathetic system and psychogenic impotence
In addition to the prevertebral supply of the vas deferens mentioned above (3), a second, ‘paravertebral’ sympathetic pathway relays in the sacral sympathetic chain to supply the trabecular tissue, rich in β2 adrenoreceptors. The resting, flaccid state of the penis depends upon tonic activity in this pathway. In this context, the corpus cavernosum resembles a well-muscled artery. For erection to take place, the sympathetic supply must be switched off while the parasympathetic, relaxant supply is switched on. Both events may be coordinated at the level of the hypothalamus. Failure to switch off tonic sympathetic activity is regarded as the commonest immediate cause of psychogenic impotence, defined as impotence in the presence of intact anatomical pathways. Damage to reflex arcs, e.g. by spinal cord injury (Ch. 16), may cause reflexive impotence.
Visceral Afferents
Visceral pain
There are three fundamental types of visceral pain:
Pain and the mind
Although visceral pain has well-established causative mechanisms (inflammation, spasm of smooth muscle, ischemia, distension), thoracic or abdominal pain may be experienced in the complete absence of visceral disease. Pain that recurs or persists over a long period (months), and is not accounted for by standard investigational procedures, is more likely to have a psychological rather than a physical explanation. This is not to deny that the pain is real, but to imply that it originates within the brain itself. An example is the abused child whose abdominal pains represent a cry for help. In adults, recurrent and rather ill-defined pains are a common manifestation of major depression (see Ch. 26).
Irritable bowel syndrome is a very common disorder usually arising in the third or fourth decade. In this syndrome there is evidence of abnormality at intestinal cellular level, but alterations of bowel behavior appear to be heightened by a disorder of the brain–gut axis (Clinical Panel 13.4).
Clinical Panel 13.4 Irritable bowel syndrome
The overall situation is generally accepted as one of dysfunction of the brain–gut axis.
Figure 34.10 shows the position of the emotional nociceptive area within the cingulate gyrus. This area is activated by aversive (unpleasant) painful stimulation of any body part, as revealed by PET (positron emission tomography). In IBS patient volunteers it is activated by baloon distension of the distal colon at a lower balloon volume than in healthy controls. Heightened sensitivity to intestinal events seems to some extent centrally rather than peripherally generated. It is now believed that the preganglionic neurons of the parasympathetic system synapse mainly on internuncial neurons in the intestinal wall, rather than on the ‘traditional’ postganglionic motor neurons shown in Figure Box 13.2.1. The central drive of the parasympathetic may be an expression of stress, and because internuncial neurons may activate nociceptive afferents as well as motor neurons, heightened sensitivity may be maintained or even increased through this feedback loop.
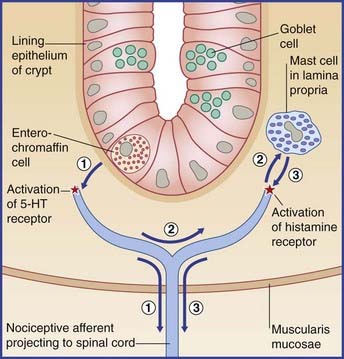
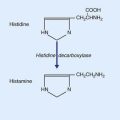
At peripheral level, biopsies taken from ileum and colon indicate that heightened sensitivity may be the outcome of an immune response generated by earlier gastrointestinal infection or food allergy, as evidenced by proliferation of enterochromaffin cells in the wall of intestinal crypts, and/or mast cells in the lamina propria (Figure CP 13.4.1). The peptide granules of enterochromaffin (chromate-staining) cells collectively contain more serotonin (5-HT) than does the entire brain. Serotonin liberated in response to intestinal distension has a double effect: it activates 5-HT3 receptors on smooth muscle cells, thereby promoting peristaltic contractions; and it activates nociceptors on nearby visceral afferents, thereby causing mast cells to liberate histamine, which in turn may potentiate the local effect of serotonin.
Note on vascular afferents
Two vascular sets of unipolar neurons are customarily included in descriptions of the visceral afferent system. One supplies the carotid sinus and aortic arch with stretch receptors involved in the maintenance of the systemic blood pressure (Ch. 24); the other supplies the carotid body with chemoreceptors and is involved in respiratory control (Ch. 24). There is a progressive tendency to acknowledge all vascular afferents as being visceral, because those on peripheral blood vessels are morphologically and functionally the same as those serving the heart. They all contain substance P, are ‘silent’ in health, and subserve pain in the presence of disease or injury – as witness, the ‘dragging’ leg pains accompanying varicose veins, or the stab of pain when a clumsily inserted antecubital venipuncture needle strikes the brachial artery. The pathway to the posterior nerve roots is still uncertain, but it appears that (to an approximation) perivascular fibers above elbow and knee send impulses by the sympathetic route (but in the reverse direction), and that more peripheral perivascular fibers send messages in company with cutaneous nerves (and in the same direction). The notion of visceral afferents running in cutaneous nerves is reminiscent of their same service with respect to nerve fibers terminating in Golgi tendon organs at wrist and ankle.
Core Information
Freeman R. Autonomic peripheral neuropathy. Neurol Clin. 2007;25:277-301.
Gai WP, Blessing WW. Human brainstem preganglionic parasympathetic neurons localized by markers for nitric oxide synthesis. Brain. 1996;119:145-1152.
Griffiths D, Tadic SC, Schaefer W, Resnic NM. Cerebral control of the bladder in normal and urge incontinent women. Neuroimage. 2007;37:1-7.
Vernino S, Sandrroni P, Singer W, Low PA. Autonomic ganglia: target and novel therapeutic tool. Neurology. 2008;70:1926-1932.
Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999-1003.
Mach T. The brain-gut axis in irritable bowel syndrome – clinical aspects. Med Sci Monit. 2004;10:RA125-131.
Yale SH, Musana AK, Kieke A, Hayes J, Glurich I, Chyou P-H. Applying case definition criteria to irritable bowel syndrome. Clin Med Res. 2008;6:9-16.