Chapter 21 Autonomic Nervous System
The autonomic nervous system represents the visceral component of the nervous system. It consists of neurones located within both the central nervous system (CNS) and the peripheral nervous system (PNS) and is concerned with control of the internal environment through innervation of secretory glands and with cardiac and smooth muscle. The term ‘autonomic’ is a convenient rather than appropriate title, because the functional autonomy of this part of the nervous system is illusory. Rather, its functions are normally closely integrated with changes in somatic activities, although the anatomical bases for such interactions are not always clear.
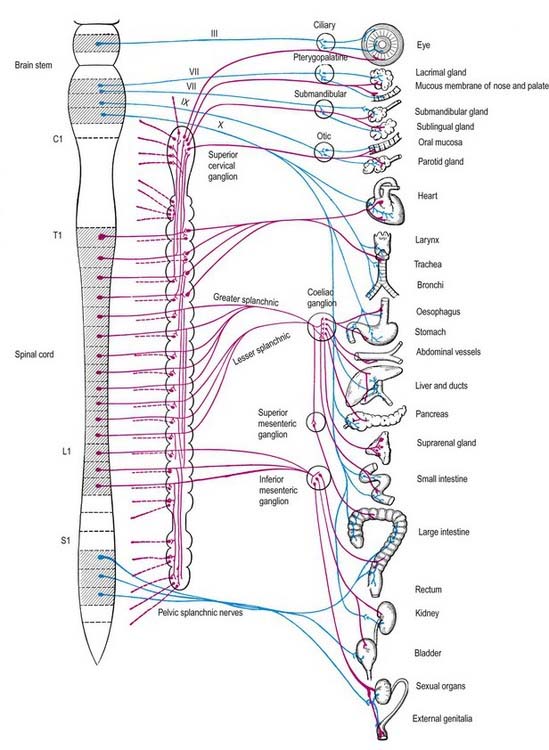
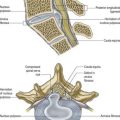
Visceral efferent pathways differ from their somatic equivalents, in that the former are interrupted by peripheral synapses; there is a sequence of at least two neurones between the CNS and the target structure (Fig. 21.1). These are referred to as preganglionic and postganglionic neurones. The somata of preganglionic neurones are located in the visceral efferent nuclei of the brain stem and in the lateral grey columns of the spinal cord. Their axons, which are usually finely myelinated, exit from the CNS in certain cranial and spinal nerves and then pass to peripheral ganglia, where they synapse with the postganglionic neurones. The axons of postganglionic neurones are usually non-myelinated. Postganglionic neurones are more numerous than preganglionic ones; one preganglionic neurone may synapse with 15 to 20 postganglionic neurones, which permits the wide diffusion of many autonomic effects.
Sympathetic Nervous System
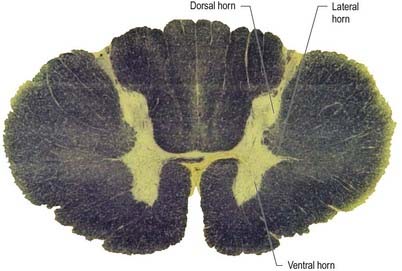
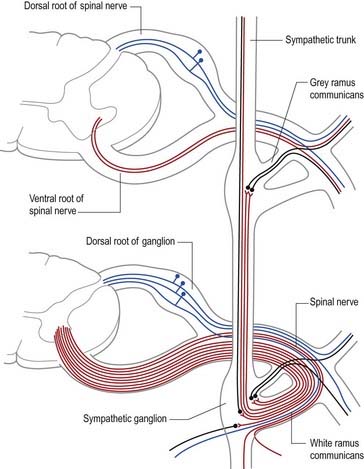
The cell bodies of preganglionic sympathetic neurones are located in the lateral horn of the spinal grey matter of all thoracic segments and the upper two or three lumbar segments (Fig. 21.2). Their axons are myelinated, with diameters of 1.5 to 4 µm. These leave the cord in corresponding ventral nerve roots and pass into the spinal nerves, but they soon leave in white rami communicantes to join the sympathetic trunk (Fig. 21.3). Neurones like those in the lateral grey column exist at other levels of the cord above and below the thoracolumbar outflow, and small numbers of their fibres leave in other ventral roots. Preganglionic sympathetic neurones release acetylcholine as their principal neurotransmitter.
On reaching the sympathetic trunk, preganglionic fibres may behave in one of several ways (see Fig. 21.3). They may synapse with neurones in the nearest ganglion or traverse the nearest ganglion and ascend or descend in the sympathetic chain to end in another ganglion. A preganglionic fibre may terminate in a single ganglion or, through collateral branches, synapse with neurones in several ganglia. Preganglionic fibres may traverse the nearest ganglion, ascend or descend and, without synapsing, emerge in one of the medially directed branches of the sympathetic trunk to synapse in the ganglia of autonomic plexuses (situated mainly in the midline, such as around the coeliac and mesenteric arteries). More than one preganglionic fibre may synapse with a single postganglionic neurone. Uniquely, the suprarenal gland is innervated directly by preganglionic sympathetic neurones that traverse the sympathetic trunk and coeliac ganglion without synapse.
The somata of sympathetic postganglionic neurones are located mostly in ganglia of the sympathetic trunk or ganglia in more peripheral plexuses. Therefore, the axons of postganglionic neurones are generally longer than those of preganglionic neurones; an exception is some of those that innervate pelvic viscera. The axons of ganglionic cells are non-myelinated. They are distributed to target organs in various ways. Those from a ganglion of the sympathetic trunk may return to the spinal nerve of preganglionic origin through a grey ramus communicans, which usually joins the nerve just proximal to the white ramus; they are then distributed through ventral and dorsal spinal rami to blood vessels, sweat glands, hairs and so forth in their zone of supply. Segmental areas vary in extent and overlap considerably. The extent of innervation of different effector systems (e.g. vasomotor, sudomotor) by a particular nerve may not be the same. Alternatively, postganglionic fibres may pass in a medial branch of a ganglion directly to particular viscera, or they may innervate adjacent blood vessels or pass along them externally to their peripheral distribution. They may ascend or descend before leaving the sympathetic trunk. Many fibres are distributed along arteries and ducts as plexuses to distant effectors.
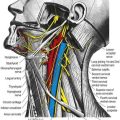
Cervical Sympathetic Trunk
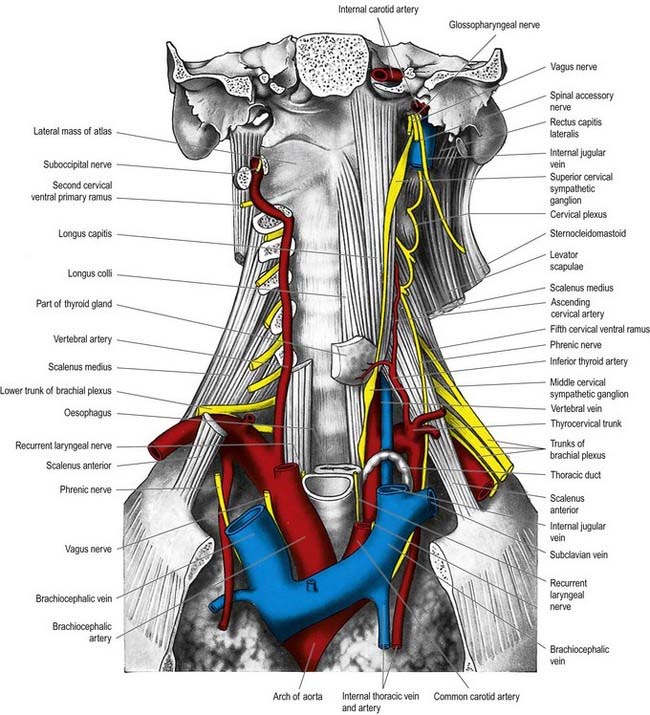
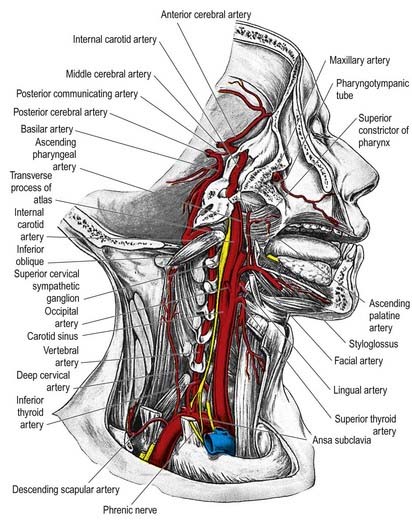
The cervical sympathetic trunk (Figs 21.4, 21.5) lies on the prevertebral fascia behind the carotid sheath and contains three interconnected ganglia: the superior, middle and inferior (stellate or cervicothoracic). However, there may occasionally be two or four ganglia. The cervical sympathetic ganglia send grey rami communicantes to all the cervical spinal nerves but receive no white rami communicantes from them. Their spinal preganglionic fibres emerge in the white rami communicantes of the upper five thoracic spinal nerves (mainly the upper three) and ascend in the sympathetic trunk to synapse in the cervical ganglia. In their course, the grey rami communicantes may pierce longus capitis or scalenus anterior.
Superior Cervical Ganglion
The superior cervical ganglion is the largest of the three ganglia. It lies on the transverse processes of the second and third cervical vertebrae and is probably formed from four fused ganglia, judging by its grey rami to C1–4. The internal carotid artery within the carotid sheath is anterior, and longus capitis is posterior (see Fig. 21.4). The lower end of the ganglion is united by a connecting trunk to the middle cervical ganglion. Postganglionic branches are distributed in the internal carotid nerve, which ascends with the internal carotid artery into the carotid canal to enter the cranial cavity, and in lateral, medial and anterior branches. They supply vasoconstrictor and sudomotor nerves to the face and neck, dilator pupillae and smooth muscle in the eyelids and orbitalis.
Middle Cervical Ganglion
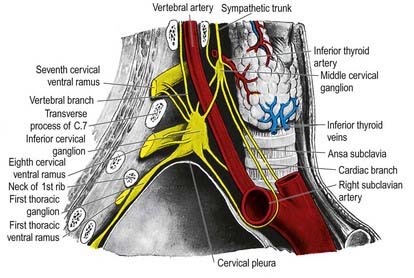
The middle cervical ganglion (Figs 21.6, 21.7) is the smallest of the three; it is occasionally absent, in which case it may be replaced by minute ganglia in the sympathetic trunk or fused with the superior ganglion. It is usually found at the level of the sixth cervical vertebra, anterior or just superior to the inferior thyroid artery, or it may adjoin the inferior cervical ganglion. It probably represents a coalescence of the ganglia of the fifth and sixth cervical segments, judging by its postganglionic rami, which join the fifth and sixth cervical spinal nerves (but sometimes also the fourth and seventh). It is connected to the inferior cervical ganglion by two or more very variable cords. The posterior cord usually splits to enclose the vertebral artery, whereas the anterior cord loops down anterior to, and then below, the first part of the subclavian artery, medial to the origin of its internal thoracic branch, and supplies rami to it. This loop, the ansa subclavia, is frequently multiple, lies in close contact with the cervical pleura and typically connects with the phrenic nerve and sometimes with the vagus.
The middle cervical ganglion gives off thyroid and cardiac branches. The thyroid branches accompany the inferior thyroid artery to the thyroid gland. They communicate with the superior cardiac, external laryngeal and recurrent laryngeal nerves and send branches to the parathyroid glands. Fibres to both glands are largely vasomotor, but some reach the secretory cells. The cardiac branch, the largest sympathetic cardiac nerve, arises either from the ganglion itself or, more often, from the sympathetic trunk cranial or caudal to it. On the right side it descends behind the common carotid artery, in front of or behind the subclavian artery, to the trachea, where it receives a few filaments from the recurrent laryngeal nerve before joining the right half of the deep (dorsal) part of the cardiac plexus. In the neck, it connects with the superior cardiac and recurrent laryngeal nerves. On the left side, the cardiac nerve enters the thorax between the left common carotid and subclavian arteries to join the left half of the deep (dorsal) part of the cardiac plexus. Fine branches from the middle cervical ganglion also pass to the trachea and oesophagus.
Inferior (Cervicothoracic Stellate) Ganglion
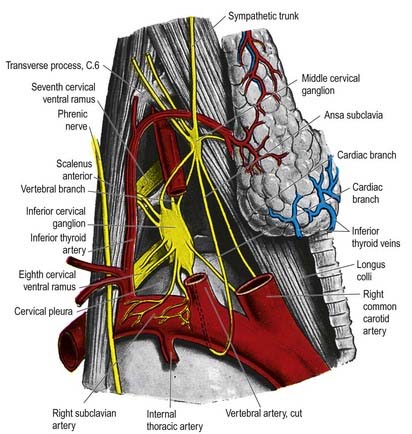
The inferior cervical (cervicothoracic stellate) ganglion is irregular in shape and much larger than the middle cervical ganglion (see Figs 21.6, 21.7). It is probably formed by a fusion of the lower two cervical and first thoracic segmental ganglia, sometimes including the second and even the third and fourth thoracic ganglia. The first thoracic ganglion may be separate, leaving an inferior cervical ganglion above it. The sympathetic trunk turns backward at the junction of the neck and thorax, so the long axis of the cervicothoracic ganglion becomes almost anteroposterior. The ganglion lies on or just lateral to the lateral border of longus colli between the base of the seventh cervical transverse process and the neck of the first rib (which are both posterior to it). The vertebral vessels are anterior, and the ganglion is separated from the posterior aspect of the cervical pleura inferiorly by the suprapleural membrane. The costocervical trunk of the subclavian artery branches near the lower pole of the ganglion, and the superior intercostal artery is lateral.
The cardiac branch descends behind the subclavian artery and along the front of the trachea to the deep cardiac plexus. Behind the artery it connects with the recurrent laryngeal nerve and the cardiac branch of the middle cervical ganglion (the latter is often replaced by fine branches of the inferior cervical ganglion and ansa subclavia).
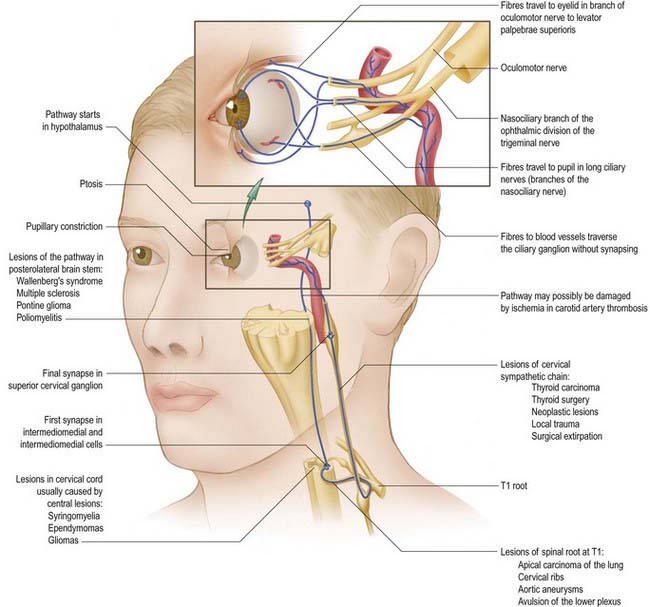
Horner’s Syndrome
Any condition or injury that destroys the sympathetic trunk ascending from the thorax through the neck into the face results in Horner’s syndrome (Fig. 21.8), characterized by a drooping eyelid (ptosis), sunken globe (enophthalmos), narrow palpebral fissure, contracted pupil (miosis), vasodilatation and lack of thermal sweating (anhidrosis) on the affected side. Classically, this occurs when a bronchial carcinoma invades the sympathetic trunk (see Ch. 18, Case 2). It also occurs as a complication of cervical sympathectomy or radical neck dissection.
Parasympathetic Nervous System
Preganglionic parasympathetic neurone cell bodies are located in certain cranial nerve nuclei of the brain stem (see Fig. 5.6) and in the grey matter of the second to fourth sacral segments of the spinal cord. Efferent fibres, which are myelinated, emerge from the CNS only in certain cranial nerves (oculomotor, facial, glossopharyngeal, vagus) and the second to fourth sacral spinal nerves. The preganglionic parasympathetic neurones are cholinergic.
Oculomotor preganglionic parasympathetic fibres originate in the Edinger–Westphal nucleus of the midbrain and travel in the nerve along its branch to the inferior oblique, reaching the ciliary ganglion, where they synapse. Postganglionic fibres, which are thinly myelinated, travel in the short ciliary nerves that pierce the sclera to run forward in the perichoroidal space to the ciliary muscle and sphincter pupillae. Their activation mediates accommodation of the eye to near objects and pupillary constriction.
The facial nerve contains preganglionic parasympathetic axons of neurones with their somata in the superior salivatory nucleus (see Ch. 10). The fibres emerge from the brain stem in the nervus intermedius, leave the main facial nerve trunk above the stylomastoid foramen and travel in the chorda tympani, which subsequently joins the lingual nerve (see Ch. 11). In this way, preganglionic fibres are conveyed to the submandibular ganglion, where they synapse on ganglionic neurones. Postganglionic fibres innervate the submandibular and sublingual salivary glands and are said to travel in the lingual nerve. Some preganglionic fibres may synapse around cells in the hilum of the submandibular gland. Stimulation of the chorda tympani dilates the arterioles in both glands, in addition to having a direct secretomotor effect. The facial nerve also contains efferent parasympathetic lacrimal secretomotor axons, which travel in its greater petrosal branch and then via the nerve of the pterygoid canal, to relay in the pterygopalatine ganglion. Postganglionic axons are thought to travel by the zygomatic nerve to the lacrimal gland and by ganglionic branches to the nasal and palatal glands.
Visceral Afferent Pathways
Visceral afferents that enter the spinal cord through spinal nerve roots terminate in the spinal grey matter. The central processes of vagal and glossopharyngeal afferent fibres end in the vagal nucleus or the nucleus solitarius of the medulla. Visceral afferents establish connections within the CNS that mediate autonomic reflexes. In addition, afferent impulses probably mediate visceral sensations such as hunger, nausea, sexual excitement, vesical distension and so forth. Visceral pain fibres may follow these routes. Although viscera are insensitive to cutting, crushing or burning, excessive tension in smooth muscle and some pathological conditions produce visceral pain. In visceral disease, vague pain may be felt near the viscus itself (visceral pain) or in a cutaneous area or other tissue whose somatic afferents enter spinal segments receiving afferents from the viscus—a phenomenon known as referred pain. If inflammation spreads from a diseased viscus to the adjacent parietal serosa (e.g. the peritoneum), somatic afferents will be stimulated, causing local somatic pain, which is commonly spasmodic. Referred pain is often associated with local cutaneous tenderness.
Benarroch E.E. The autonomic nervous system: basic anatomy and physiology. CONTINUUM Lifelong learning in neurology. 2007;1(6):13-32.
Benarroch E.E. Enteric nervous system: functional organization and neurologic implications. Neurology. 2007;69:1953-1958.
Björklund, Hökfelt, Owman, 1988Björklund A., Hökfelt T., Owman C. The Peripheral Nervous Systems: Handbook of Chemical Neuroanatomy. Vol. 6. 1988. Elsevier. Amsterdam.
Description of sensory–motor nerves.
Burnstock, 1990Burnstock G. Co-transmission: The fifth Heymans lecture. Arch. Int. Pharmacodyn. Ther.. 304:1990;7-33.
Review of neurotransmission in the autonomic nervous system.
Burnstock, 1992–1995Burnstock G. The Autonomic Nervous System. Vols 1–14. 1992–1995. Harwood Academic Publishers. Switzerland.
Extensive material on the neurotransmitters of the autonomic nervous system.
Freeman R., Kaufmann H. Disorders of orthostatic tolerance—orthostatic hypotension, postural tachycardia syndrome, and syncope. CONTINUUM Lifelong learning in neurology. 2007;1(6):50-88.
Kaufmann H., Goldstein D.S. Autonomic failure in neurodegenerative disorders. CONTINUUM Lifelong learning in neurology. 2007;1(6):111-142.
Maggi C.A. The pharmacology of the efferent function of sensory nerves. J. Auton. Pharmacol.. 1991;11:173-208.
Vernino S., Freeman R. Peripheral autonomic neuropathies. CONTINUUM Lifelong learning in neurology. 2007;1(6):89-110.
Appenzeller O (volume ed), Vinken P.J., Bruyn G.W., editors. The Autonomic Nervous System. Part 1. Normal Functions. Amsterdam and London: Elsevier Science Publishers, 1999.
Appenzeller O (volume ed), Vinken P.J., Bruyn G.W., editors. The Autonomic Nervous System. Part 2. Dysfunctions. Amsterdam and London: Elsevier Science Publishers, 2000.
Wasner G., Maag R., Ludwig J., Binder A., Schattschneider J., Stingele R., Baron R. Harlequin syndrome: one face of many etiologies. Neurology. 2005;1:54-59.