CHAPTER 28 Automated Percutaneous Lumbar Discectomy: Technique
INTRODUCTION
Automated percutaneous lumbar discectomy (APLD) was introduced in 1985 by Gary Onik et al.1–3 Since the 1960s, many different techniques for percutaneous removal of the nucleus pulposus or its protruding components have been proposed; they may achieve the goal in different ways, with different types of instruments, with or without fiberoscopic vision, or different types of energy (radiofrequency [RF], laser, coblation, etc.).4–12 The basic principle, shared by most percutaneous intradiscal decompressive procedures, including APLD, is that in an enclosed space a reduction in volume, even partial, confers a much greater reduction in pressure; this leads to decreased pressure upon the nerve root, and relief of sciatica, even without a radiographically evident reduction in total disc volume.13 After weeks or months, the partial vacuum causes the protruded portion of nucleus pulposus (or other disc material) to move away from the nerve root back towards the center of the disc, pushed by partially intact fibers and ligaments of the outer anulus; this process, along with regeneration of a more fibrous nucleus pulposus, favors restoration of the inner fibers of the anulus and decreases the tendency to further protrusion towards the spinal canal. The success of the procedure depends to a great extent on selecting lesions to treat: the protruding nucleus pulposus must be at least partially contained by the external fibers of the disc, without a large extrusion and migrated or sequestrated fragments.14–16
For decades, minimally invasive treatments for disc protrusions have been opposed by the surgical community, despite the high preference of patients to undergo a less intrusive intervention. APLD seems to have suffered the drawback of having been the first nonchemical, nonmanual procedure to be used worldwide, as the technique was met with fierce opposition. It would be fair to state that 20 years ago, surgeons were not ready to embrace percutaneous procedures. Now that the neurosurgical and orthopedic communities have accepted the concepts of intradiscal decompression and minimally invasive procedures,17 other techniques less effective than APLD dominate the field. In all likelihood, this relationship stems from the fact that APLD is still burdened with old, biased, and superficial judgments that are in part substantiated by poorly conducted studies.18,19 In most published series good results range from 60% to 85%,20–26 depending on patient selection criteria, while poor results are reported in the only two randomized and controlled studies.18,19 In 1993, Revel et al. reported a 37% success rate at 1 year in a study comparing APLD and chemonucleolysis.18 Chatterjee et al., 2 years later, found a 29% success rate with APLD when compared to open surgery.19 However these studies, like others reporting low percentages of good outcomes,27–29 have limitations and features that make the patient populations and technical conditions not really suitable for a comparison, and their results unreliable. First, the numbers of patients are low: 32 treated by one operator19 and 69 treated by many operators in a multicenter study.18 The authors do not state how experienced the operators were, i.e. how many APLD procedures each had already performed. The technical learning curve for APLD is longer than one might expect. It is only after many procedures are performed that the surgeon can obtain sufficient quantities of nucleus pulposus, and from the correct location of the disc. For example, the L5–S1 level is approached safely and reliably only by operators having performed a minimum of 40–50 procedures at higher levels. It is highly likely that the operators in the two studies mentioned above were much more experienced in open surgery or chemonucleolysis. As well, these investigators did not have access to some of the technique modifications that are described later in this chapter.
APLD achieves a very good compromise between low invasiveness and the need to obtain discal decompression. Its clinical results remain among the most satisfying when dealing with minimally invasive percutaneous treatments.
SAFETY
The mortality rate of the procedure is zero. Lesions of nerve roots, vessels, or the ureter are possible;30–32 however, as previously emphasized, with thorough knowledge of and attention to radiographic landmarks for proper probe positioning, vascular, neural, or dural injuries are very unlikely. The only major reported injury following APLD occurred in Mexico and resulted in a cauda equina direct lesion. It is quite likely that there was little if any attention directed to the radiographic landmarks that allow the surgeon to stay out of a potentially harmful pathway;33 moreover, the procedure was performed under general anesthesia, definitely contraindicated, for the reasons explained later.
A posteriorly placed colon can insinuate behind the psoas muscle.34,35 For this reason the preoperative imaging studies, both computed tomography (CT) or magnetic resonance imaging (MRI), must be carefully examined to exclude the presence of such an anatomical condition, since bowel in the path of the instruments could be perforated, with the risk of peritoneal or disc infection or local abscess formation. If not available, a planning CT scan of the whole abdomen through the disc space of interest with large field of view (FOV) must be obtained. When the L5–S1 disc is being removed, two scan slices (at the L4–5 and L5–S1 levels) should be obtained because the entry point for the L5–S1 placement is at the L4–5 level to avoid the iliac crest. In addition, this preoperative, planning CT can provide other valuable information. At the L5–S1 level, special attention should be paid to the bifurcation of the iliac vessels; at upper levels, the scan ensures that the lower pole of the kidney or the sulcus of the pleural space will not be traversed.
Beginning in June 1987, in our institution more than 1250 patients (accounting for more than 1450 discs) were treated. We observed and reported an overall complication rate of less than 0.9%.36 There were no injuries to nerve roots, dura mater, ureters, major vessels, or bowel. We suspect this extremely low complication rate stemmed from our singular use of only local anesthesia, with or without light sedation, and the avoidance of general anesthesia. There was one acute hematoma in the iliopsoas that occurred following injury to a small artery and which resolved without sequelae in approximately 1 month. Among the side effects observed were two cases of discitis, resulting in a rate of 0.16 %, similar to the rate published in large series of discography.37–39 Since discitis is a major complication, special care must be taken during skin prep and draping. We also use prophylactic antibiotics and typically give 2 g of intravenous cephalosporin to cover Streptococcus epidermidis.
INSTRUMENTATION
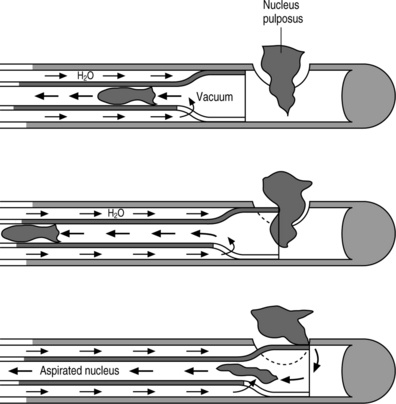
Automated percutaneous lumbar discectomy utilizes a probe called Nucleotome® (Fig. 28.1), manufactured by Clarus Medical, LLC, for removal of the nucleus pulposus. The probe tip, excluding the handle, is 20.2 cm long and has an outer diameter of 2.2 mm. The blunt tip is an extremely important safety feature. Once the probe is inserted, the lack of a sharp end prevents it from piercing through the outer limits of the disc, even with an inadvertent hard push. This feature is unique and not a component of other instruments such as laser, RF probes, or manual biopsies, thus essentially removing the risk of lesion of vessels or other abdominal structures. The negative pressure for aspiration is generated by a console. A vacuum is created that draws nuclear material into the side port, which is located a few millimeters proximal to the distal tip of the probe. The cutting blade for fragmentation of nucleus pulposus aspirated through the port, works with a reciprocal, not rotatory motion. This type of movement is a safety feature because the ‘guillotine’ blade is contained within the probe. Consequently, only the nuclear material that is drawn into the port can be cut. The blade is pneumatically driven by a pressure pulse, generated by the same console that creates the vacuum that draws nuclear material into the side port. The console also controls the cut rate and the flow of irrigation fluid to the probe. Internal irrigation with sterile saline is a vehicle for easy aspiration. The reciprocal movement of the internal cutting blade also sequences the introduction of liquid inside the disc, to prevent accumulation of nuclear material and consequent clogging inside the probe, or an excess infusion of fluid within the disc. The cutting rate knob on the console allows for adjustment of between 60 and 180 cuts per minute. At the beginning of the procedure, the maximum cutting rate should be used to cut smaller pieces of disc and prevent the instrument from clogging. As the decompression proceeds, the amount of disc material aspirated diminishes, allowing the surgeon to ratchet down the cutting rate. This will allow more time for the negative pressure to draw disc material through the port before it is resected.
PATIENT POSITIONING AND SELECTION OF ENTRY ROUTE
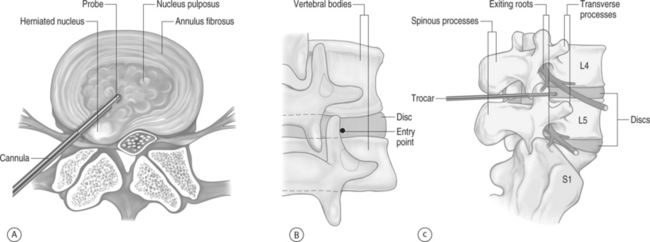
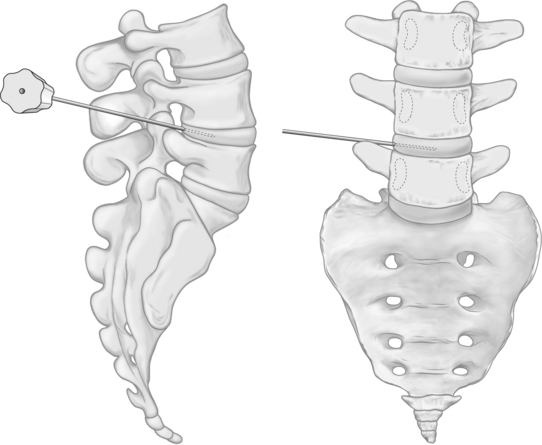
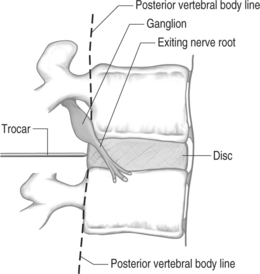
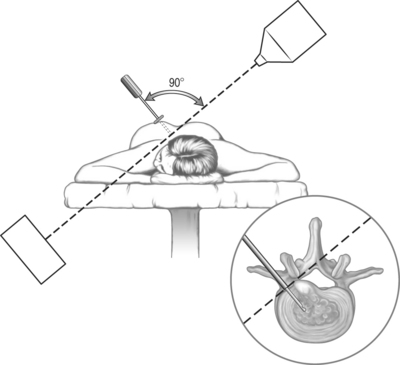
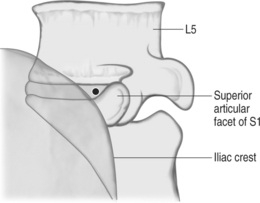
The entry route, as usual with every lumbar percutaneous approach to the disc space, is posterolateral (Fig. 28.2). Correctly positioning the guiding trocar is crucial to the result. The trocar must be placed with its tip in the midline in frontal view, at the junction of the middle and posterior thirds of the disc in lateral view, where the normal nucleus lies (Fig. 28.3). This location corresponds to the initial working position of the probe. In cases of large, posterior protrusions invading the spinal canal, it is preferable to aim for a more posterior position of the probe.
The best and safest way to accurately place the trocar is to choose a path that passes anteriorly and laterally tangent to the posterior zygapophyseal complex, i.e. with the trocar touching the anterolateral surface of the superior articular process (see Fig. 28.2). The usual skin entry point for such a route is 10 cm from the spinous processes. The operator can use the preoperative CT scan to accurately and easily geometrically derive the proper skin entry site. Once the skin target has been determined, a skin wheal is raised. Immediately thereafter, a 22–25-gauge spinal needle is inserted more posteriorly than the anticipated route to the disc, with the aim to touch the posterior facets. Local anesthetic is injected at this location and the needle is gradually withdrawn toward the skin, allowing for anesthetization of the underlying spinal musculature. It is critical that local anesthetic is not deposited anterior to the articular processes, which can anesthetize the exiting root. General anesthesia is contraindicated for this procedure since the chance of nerve root injury is much greater under those circumstances.
Which side to begin the procedure depends upon the side of the patient’s symptoms. We usually select the side of the patient’s symptoms. There are two compelling reasons to do so: first, to place the nucleotome probe as close to the herniation as possible, particularly when the herniation is lateralized; second, to avoid the possibility of bilateral symptoms in the event of complications. In rare circumstances a contralateral approach is preferred. First, if a correct trocar positioning inside the disc becomes impossible. That situation can arise when the trocar repeatedly abuts the exiting nerve root, precluding safe advancement into the anulus. Anatomic circumstances that can lead to this scenario are a conjoined nerve root, flattened root compressed by the herniation, and in particular at L5–S1, the normal position of the exiting L5 root. When a conjoined nerve root is present, both roots can exit from a single foramen. The superior root takes a more caudal and inferior course, which places it in the line of a correctly placed trocar. In addition, in case of a far lateral herniation, it is possible that the nerve root is in an abnormal position and pushed posteriorly to close the space between the nerve and the anterior surface of the facet. The second reason to use the contralateral side is when the patient does not tolerate lying with the ipsilateral side superior. A third factor is when an anatomical variant precludes entry. For instance, an L5–S1 approach is not possible from the side of a monolateral transitional vertebra, with sacralization of the transverse process, or if there is a risk of posteriorly placed colon, behind the psoas muscle, as shown by the preoperative CT scan with large field of view (FOV). In the latter case, considering that the left-sided sigmoid colon is more mobile, the surgeon can let gravity assist, positioning the patient on his/her right side; under this condition the left colon falls away from the entry route of the instruments. It must be emphasized that the prone position increases the risk of piercing posteriorly placed bowel.
The crucial radiographic rules are as follows:
NUCLEOTOME PLACEMENT AND ASPIRATION OF THE NUCLEUS PULPOSUS
As with the needle for local anesthesia, the operator should start, in lateral view, with a posterior trajectory until the trocar hits the bone of the articular facets; then, the trocar is worked anteriorly with tiny movements, until it slides along the anterior surface of the articular complex (actually, over the anterolateral surface of the superior articular facet) (see Fig. 28.2). The closer to this superior articular process the instrumentation is, the less likely the operator is to touch the nerve root. Then, the trocar is pushed toward the posterior profile of the disc, corresponding to the posterior somatic walls, always parallel to the vertebral endplates. When the tip of the trocar reaches the posterior profile of the disc, a gritty, hard but elastic (definitely different from bone and soft tissues) tactile sensation is felt, corresponding to the anulus fibrosus (Fig. 28.5). If the operator does not feel the anulus, the trocar must be withdrawn and replaced more posteriorly; under no circumstances should the operator push the trocar anterior to the posterior profile of the disc. In this situation, the path of the trocar would be too anterior, rendering it impossible to achieve correct trocar placement. Moreover, the risk of injuring nerve root, bowel, inferior vena cava, aorta, or iliac arteries would be escalated. If one attempts to position the trocar more posteriorly, but is precluded because of the articular complex, then a more lateral skin entry point must be used.
The described trajectory brings the needle and eventually the nucleotome dorsal to the nerve, which is coursing from the upper portion of the foramen anteriorly and inferiorly (see Fig. 28.5). If the patient is going to experience radicular pain from the needle placement, it will usually occur when the needle is placed too high in the foramen or anterior to the posterior vertebral body’s line. If the nerve root is touched, the patient experiences radicular symptoms, usually a sensation described as a sudden ‘electrical shock’ which may be experienced as distal as the foot, depending on the root that has been abutted. In contrast, the pain originating directly from the nociceptive fibers of the external anulus is less intense and never goes down distal to the knee. If the patient describes a radicular sensation, the trocar must be withdrawn and replaced, even if that requires the skin entry point be modified. In general, moving the entry point 1–1.5 cm in either direction is enough to change the trajectory and obtain a painless trocar placement. All major redirections of the trocar require that it be withdrawn into the subcutaneous space before readvancement, because the fascial planes create a point of fixation that does not allow for major path corrections. Consequently, if the trocar is not withdrawn a sufficient distance, further attempts of trocar placement will only result in trocar bending.
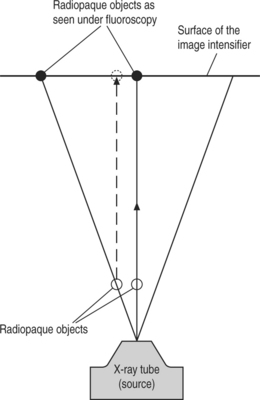
When the anulus is reached, as determined by its tactile quality, and the 18-gauge trocar touches the posterior vertebral body’s line and is midway between and parallel to the vertebral body endplates, the anteroposterior view should be checked (Fig. 28.6) and this represents a major safety feature. It confirms that the 18-gauge trocar is outside the spinal canal and consequently will not traverse the thecal sac as it is advanced into the disc space. In this anteroposterior view, the tip of the trocar should be lateral to a vertical line connecting the medial borders of the pedicles, thereby confirming its correct position outside the spinal canal. Once confirmed, the trocar can be safely advanced into the center of the disc using this anteroposterior view. The fluoroscope is then repositioned to obtain a lateral view, allowing the surgeon to confirm that the trocar’s tip is correctly placed on both anteroposterior and lateral views (see Fig. 28.3). If the trajectory is too anterior, the trocar tip is visible in the center of the disc on the anteroposterior view, but extends ventral to the center of the disc on the lateral view. Since we want to be as close as possible to the disc herniation, no placement that has an anterior trajectory is acceptable.
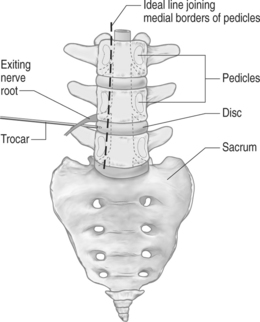
Fig. 28.6 In the anteroposterior view, after the trocar has touched the anulus at the posterior vertebral body line, as shown in Figure 28.5, the trocar’s tip must absolutely lie lateral to the vertical line joining the medial borders of the pedicles; this confirms that the trocar is outside the spinal canal and it will not traverse the thecal sac when pushed to advance into the disc space.
Once the trocar is in the correct position, the dilator and cannula are placed over the trocar and advanced until the anulus is reached. The dilator is then removed, and the cannula is pushed the extra few millimeters to rest on the anulus. At this point, the biopsy stop is brought down to the skin. Later in the procedure, this marker will indicate whether the cannula has been withdrawn or pushed into the anulus. The fluoroscopic beam is now reoriented perpendicular to the cannula, in an oblique view, to confirm that the cannula has reached the anulus (Fig. 28.7). Due to the oval shape of the disc, the tip of the cannula could overlap the margins of the disc on the anteroposterior and lateral views and still not be against the anulus. In this circumstance, if the trephine is used and the cannula is not absolutely against the anulus, the adjacent nerve root could be inadvertently injured. When the oblique view confirms that the cannula is resting on the anulus, the dilator is exchanged with the trephine while keeping cannula and trocar in place. During this maneuver the cannula must be pushed firmly against the anulus to prevent the root from insinuating itself between the cannula and the anulus. The trephine is placed against the anulus, which is then incised. Just prior to using the trephine, lightly tapping the anulus with the trephine ensures that a portion of nerve root has not been trapped by the cannula. Patients with a chronic disc disease may experience intense, nonradicular pain when the cannula and the trephine are pushed against the annulus, probably because of sprouting of nociceptive fibers that can accompany degeneration of a disc. If the patient cannot tolerate such pain, before incision with the trephine, a long, 22-gauge needle is inserted in the space between cannula and trocar, and 0.5 ml of 1% plain lidocaine are injected at the site of incision of the anulus.
Once the aspiration probe is seen to be in the correct position in anteroposterior and lateral views, the port of the instrument is rotated toward the area of the herniation, the nucleotome console is turned on, the footswitch pressed, and the disc aspiration is initiated. As much disc material as possible is aspirated, while gradually moving the probe back and forth. Once no more additional disc material can be retrieved with the port in the direction of the herniation, turn the port to a new area and repeat the back-and-forth motions along the entire length of the nucleus. The cannula can be angled to obtain material from other areas of the disc. During the initial 5–7 minutes, use the straight cannula to aspirate centrally and anteriorly. A curved cannula should be utilized for the rest of the aspiration phase of the procedure, which usually does not take more than 12–15 minutes. The curved cannula that is provided with the surgical kit is particularly helpful in reaching the L5–S1 disc, especially when the approach is partially covered by the iliac crest. It also enhances the range of movement of the probe inside a disc, resulting in the aspiration of greater amounts of nucleus pulposus. This advantage is extremely important when in the more posterior or posterolateral positions, where the nerve root is compressed. For this reason the curved cannula is used at every discal level. During aspiration, the probe is constantly moved in a double rotation-type maneuver, therefore creating successively larger and larger arcs of motion. During this action, the port is maintained in a position that is external to these virtual circles. Ultimately, at least two large tunnels are excavated in the disc.
When treating discogenic back pain, aspiration is never stopped before blood starts coming from the disc (from granulation tissue or endplate cartilage). Observing blood in the tube suggests the possibility of subsequent scarring. It is always possible to achieve this result when using the ‘double rotation’ technique. It is likely the good results of APLD for discogenic pain are achieved by lowering the pressure within the disc and by stimulating scar formation. The latter ultimately reinforces the anulus with more fibrous tissue and diminishes the motion in cases of symptomatic microinstability. It is reasonable to assume that discogenic pain can be improved or cured thanks to removal of nodules of innervated, painful ‘granulation’ tissue. These areas may be evident on MRI as zones of high signal, usually in the posterior annulus;40 since granulation tissue is vascularized, early bleeding occurs during aspiration. Bleeding is not, however, necessary when treating patients for relief of root compression; aspiration is stopped when the amount of disc material coming from the probe greatly decreases. When treating radicular pain, at the end of the procedure, the side port of the probe is positioned close to the protruding parts of the disc in the neural foramen in an attempt to aspirate the protruding material itself. This ‘topographical’ criterion must guide the aspiration. It is important to keep in mind that the probe is not positioned to work only in the center of the disc, but should be directed to look for the main bulk of the nucleus pulposus and its protruding components. As long as the preprocedural MRI or multiplanar reformatted CT scan indicate that the height of the disc is preserved, at least a moderate amount of nuclear material must be removed. In general, the less degenerated, i.e. yellowish, the nucleus material appears, the more quantity must be aspirated. If, after an apparently correct first positioning of the probe inside the disc, nuclear material does not come freely, the probe must be moved to locate it. Treatment proceeds until nucleus is found and removed.
PERCUTANEOUS DISCECTOMY AT THE L5–S1 LEVEL
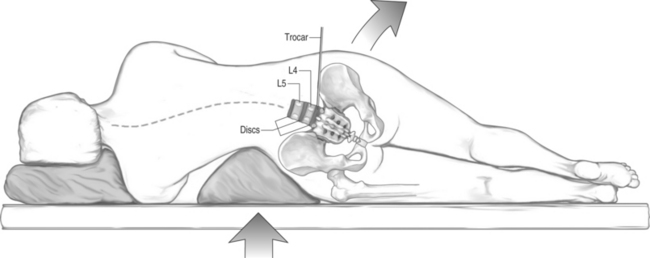
Performing the procedure with the patient lying in the lateral decubitus position increases the probability of correctly entering the L5–S1 disc. A soft silicon gel cushion or other similar prop wedged just superior to the iliac crest will laterally flex and lower the iliac crest on the entry side, thus opening an access trajectory to the L5–S1 disc (Fig. 28.8).
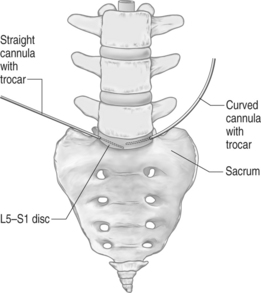
Although placement of the curved cannula is technically demanding, it offers a palpable benefit. When oriented correctly, the curve of the cannula allows the trocar and the probe to enter the plane of the disc (i.e. parallel to the endplates), despite an angled approach from above of the devices (Fig. 28.9).
Once the trocar is correctly placed, the dilator is removed and the trephine is placed over the trocar and through the cannula. The trephine and trocar are then both removed and the probe is placed through the cannula into the disc. If the curved cannula was required to deposit the probe parallel with the endplates, it can be subsequently used to reach posterior aspects of the disc (Fig. 28.10). Indeed, the reach it achieves is superior to that offered by a straight cannula. It is sometimes difficult to pass the curved cannula through the lumbar fascia, because of the larger diameter and bigger step down from the edge of the cannula to the dilator. If so, it may be necessary to dilate the tract first with the straight cannula and dilator.
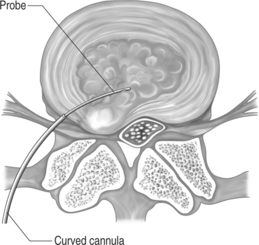
Fig. 28.10 After positioning of the probe in the L5–S1 disc space as described in the text and in Figure 28.9, the curved cannula can be turned with an anterior convexity, so that the probe will be directed to work and aspirate in a position more posterior than it would be allowed by a straight cannula.
In favorable anatomy, an alternative method for placement of the trocar in the L5–S1 can be used. The path to the disc is found centering the X-ray beam over the L5–S1 disc space in the anteroposterior view. The fluoroscopic unit is then angled in the cephalocaudal direction until the endplates of the disc are visible as single superior and inferior entities, indicating that the entry angle is parallel to the endplates. The X-ray beam is then angled toward the lateral view, and as it is moved in an oblique orientation, the L5–S1 facet joint moves across the disc space and the iliac crest starts to overlap the anterior portion of the disc. When the beam is at approximately a 45° angle, a triangular window at the center of the disc space is seen (Fig. 28.11). This triangle is bounded laterally by the iliac crest, medially by the anterior surface of the superior articular process of S1, and superiorly by the inferior endplate of the L5 vertebra. Once this view is obtained and centered on the screen, the trocar, with use of a holder, is inserted in bull’s-eye fashion through the middle of the triangle. When done properly the trocar will appear as a single dot. The trocar should then be advanced using real-time imaging until it touches the outer annular fibers.
POSTOPERATIVE CARE
We routinely perform APLD on an outpatient basis. At the conclusion of the procedure, observation is needed for about 2 hours before discharging the patient home. Prescriptions are provided for a 2-week supply of a nonsteroidal antiinflammatory agent, and for diazepam at bedtime for 2–3 days. Patients are encouraged to move, stand, and walk on day three. Driving or prolonged sitting is proscribed for a 2–3-week interval.
1 Onik G, Helms CA, Ginsburg L, et al. Percutaneous lumbar diskectomy using a new aspiration probe. Am J Neuroradiol. 1985;6:290-293.
2 Maroon JC, Onik G. Percutaneous automated discectomy: a new method for lumbar disc removal. J Neurosurg. 1987;66:143-146.
3 Onik G, Maroon JC, Helms CA, et al. Automated percutaneous discectomy: initial patient experience. Radiology. 1987;162:129-132.
4 Smith L, Garvin PJ, Jennings RB. Enzyme dissolution of nucleus pulposus. Nature. 1963;198:1398-1400.
5 Smith L. Enzyme dissolution of nucleus pulposus in humans. JAMA. 1964;187:137-140.
6 Choy DS. Percutaneous laser disc compression (PLDD): twelve years’ experience with 752 procedures in 518 patients. J Clin Laser Med Surg. 1998;16:325-331.
7 Friedman WA. Percutaneous discectomy. An alternative to chemonucleolysis? Neurosurgery. 1983;13:542-547.
8 Hijikata S, Yamagishi M, Nakayama T, et al. Percutaneous nucleotomy: a new treatment method for lumbar disk herniation. J Toden Hosp. 1975;5:5-13.
9 Hijikata S. Percutaneous nucleotomy: a new concept technique and 12 years’ experience. Clin Orthop. 1989;238:9-23.
10 Kambin P, Gellmann H. Percutaneous lateral discectomy of the lumbar spine. A preliminary report. Clin Orthop. 1983;174:127-132.
11 Schreiber A, Suezawa Y. Transdiscoscopic percutaneous nucleotomy in disc herniation. Orthop Rev. 1986;15:75-78.
12 Yeung AT. The evolution of percutaneous spinal endoscopy and discectomy: state of the art. Mt Sinai J Med. 2000;67:327-332.
13 Shea M, Takeuchi TY, Wittenberg RH, et al. A comparison of the effects of automated percutaneous diskectomy and conventional diskectomy on intradiskal pressure, disk geometry, and stiffness. J Spinal Disord. 1994;7:317-325.
14 Milette PC. The proper terminology for reporting lumbar intervertebral disk disorders. Am J Neuroradiol. 1997;8:1859-1866.
15 Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26:E93-E113.
16 Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. American Society of Neuroradiology, 2003.. www.asnr.org.
17 Haines SJ, Jordan N, Boen JR, et al. Discectomy strategies for lumbar disc herniation: results of the LAPDOG trial. J Clin Neurosci. 2002;9:411-417.
18 Revel M, Payan C, Vallee C, et al. Automated percutaneous lumbar discectomy versus chemonucleolysis in the treatment of sciatica. A randomized multicenter trial. Spine. 1993;18:1-7.
19 Chatterjee S, Foy PM, Findlay GF. Report of a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation. Spine. 1995;20:734-738.
20 Castro WH, Jerosch J, Hepp R, et al. Restriction of indication for automated percutaneous lumbar discectomy based on computed tomographic discography. Spine. 1992;17:1239-1243.
21 Moon CT, Cho J, Chang SK. Availability of discographic computed tomography in automated percutaneous lumbar discectomy. J Korean Med Sci. 1995;10:368-372.
22 Dullerud R, Amundsen T, Lie H, et al. CT-diskography, diskomanometry and MR imaging as predictors of the outcome of lumbar percutaneous automated nucleotomy. Acta Radiol. 1995;36:613-619.
23 Dullerud R, Amundsen T, Lie H, et al. Clinical results after percutaneous automated lumbar nucleotomy. A follow-up study. Acta Radiol. 1995;36:418-424.
24 Grevitt MP, McLaren A, Shackleford IM, et al. Automated percutaneous lumbar discectomy. An outcome study. J Bone Joint Surg [Br]. 1995;77:626-629.
25 Sortland O, Kleppe H, Aandahl M, et al. Percutaneous lumbar discectomy. Technique and clinical result. Acta Radiol. 1996;37:85-90.
26 Bernd L, Schiltenwolf M, Mau H, et al. No indications for percutaneous lumbar discectomy? Int Orthop. 1997;21:164-168.
27 Shapiro S. Long-term follow-up of 57 patients undergoing automated percutaneous discectomy. Neurosurgery. 1995;83:31-33.
28 Lee SH, Lee SJ, Park KH, et al. Comparison of percutaneous manual and endoscopic laser diskectomy with chemonucleolysis and automated nucleotomy. Orthopäde. 1996;25:49-55.
29 Ramberg N, Sahlstrand T. Early course and long-term follow-up after automated percutaneous lumbar discectomy. J Spinal Disord. 2001;14:511-516.
30 Schreiber A, Suezawa Y, Leu H. Does percutaneous nucleotomy with discoscopy replace conventional discectomy? Eight years of experience and results in treatment of herniated lumbar disc. Clin Orthop. 1989;238:35-42.
31 Stern MB. Early experience with percutaneous lateral discectomy. Clin Orthop. 1989;238:50-55.
32 Flam TA, Spitzenpfeil E, Zerbib M, et al. Complete ureteral transection associated with percutaneous lumbar disk nucleotomy. J Urol. 1992;148:1249-1250.
33 Onik GM, Maroon JC, Jackson R. Cauda equina syndrome secondary to an improperly placed nucleotome probe. Neurosurgery. 1992;30:412-415.
34 Hopper KD, Sherman JL, Luethke JM, et al. Retrorenal colon in the supine and prone patient. Radiology. 1987;162:443-446.
35 Prassopoulos P, Raissaki M, Daskalogiannaki M, et al. Retropsoas positioned bowel: incidence and clinical relevance. J Comput Assist Tomogr. 1998;22:304-307.
36 Bonaldi G. Automated percutaneous lumbar discectomy: technique, indications and clinical follow-up in over 1000 patients. Neuroradiology. 2003;45:735-743.
37 Collis JSJr, Gardner WJ. Lumbar discography. Analysis of 600 degenerated disks and diagnosis of degenerative disk disease. JAMA. 1961;178:67-70.
38 Collis JSJr, Gardner WJ. Lumbar discography. An analysis of one thousand cases. J Neurosurg. 1962;19:452-461.
39 Feinberg SB. The place of discography in radiology as based on 2320 cases. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1275-1281.
40 Aprill C, Bobduk N. High intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.