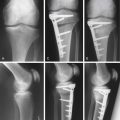
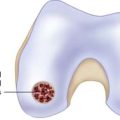
Chapter 9B Autologous Chondrocyte Implantation
Quality Assurance of Cells for Chondrogenic Implantation
Introduction
High-quality chondrocytes are essential for successful autologous chondrocyte implantation (ACI) outcomes. Cell therapy manufacturers must comply with good manufacturing practices (GMPs), which are enforced by national authorities in most countries. The goal of GMPs is to ensure that products are safe, pure, and effective. The GMPs require that manufacturers establish quality systems for the design, manufacture, packaging, labeling, and storage of products commercially distributed for human use. In many countries, regulatory agencies have also issued guidance documents that pertain specifically to additional requirements for cellular therapeutics. It is the responsibility of the manufacturer to validate the manufacturing process and establish product release specifications. Products cannot be shipped unless all release specifications have been met. At a minimum, product release assays should assess cell number and viability, test for adventitious agents, and evaluate purity (i.e., endotoxin). Recently, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) have begun requiring that cell therapy manufacturers also develop identity and potency assays for their products. Each of these assays is defined in the following section. For detailed descriptions of quality control and quality assurance systems for cell therapy manufacturing, the reader is referred to Kielpinski et al.1 and Mayhew et al.2
Quality Assessment of Chondrocytes
Viability
Viability is defined as the percentage of live cells in the product. Viability is easily measured for cells in suspension using trypan blue or other well-established assays.
The measurement of viable cells in a matrix or on a membrane requires a customized approach. For example, Wang et al.3 developed a novel assay to accurately and precisely measure viability in matrix-induced autologous chondrocyte implanation (MACI) implants because the collagen membrane caused interference in all conventional assay techniques that were tested.
Sterility
Sterility assays typically take 14 days to perform. Therefore, it is common to test prerelease samples of the product. A rapid microbial test, the BacT/Alert System (Genzyme Biosurgery, Cambridge, Mass.), has been validated for sterility testing of Carticel and MACI implants.4 This system usually provides test results in a matter of hours. Regardless of the system used, product samples must be negative for microbial growth. The aseptic process must be validated using media fills.
Identity
The purpose of an identity assay is to confirm the identity of a culture as consisting of chondrocytes and to assess heterogeneity, if any. Historically this has been done by assessing cell morphology. Recently, two novel assays that can distinguish cultured chondrocytes from closely related synoviocytes on the basis of genetic markers have been described.5,6 The assay developed by Rapko and colleagues has been validated as a chondrocyte identity assay and approved by the F&A as a Carticel lot release assay.5 This is the first genetic marker-based chondrocyte identity assay approved for use in the United States.
Potency
Potency assays are meant to measure the functional potential of cultured chondrocytes. This is an active area of investigation, and several approaches have been described.7,8,9 Although the potency assay requirement is relatively new, it is likely that potency assays will be used to evaluate chondrocytes produced for clinical use in the near future.
Quality Verification in the Operating Theater
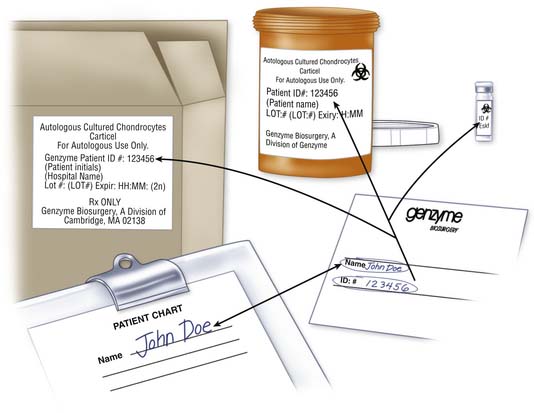
Verification of Contents
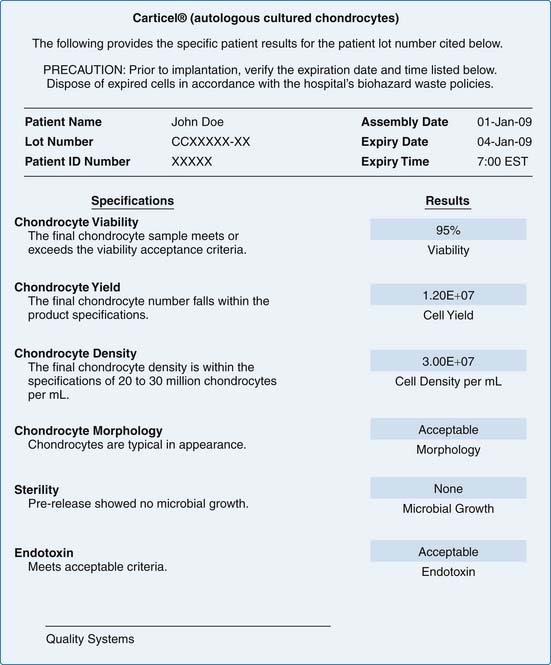
Certificate of Analysis
The results of product release testing are recorded on a certificate of analysis (C of A). Identifying information for the patient, and product expiration date and time are found at the header of the C of A. Testing requirements may differ from one country to another. The example in Figure 9B-2 is a C of A that conforms to U.S. FDA regulations.
Packaging Integrity
Inspect the shipping box for signs of damage to the exterior (Fig. 9B-3).
Open the box; it should contain insulating material and frozen or refrigerated gel packs to maintain the product at the appropriate temperature (Fig. 9B-4).
Inspect for leaking of the contents, such as gel packs or product shipping media.
Remove the secondary transport container. This could be a hard container or a bag. Inspect for damage and leaking (Fig. 9B-5).
Remove the primary product container.
Cell suspensions are typically packaged in vials (Fig. 9B-6), whereas membrane-based products are packaged in dishes (Fig. 9B-7).
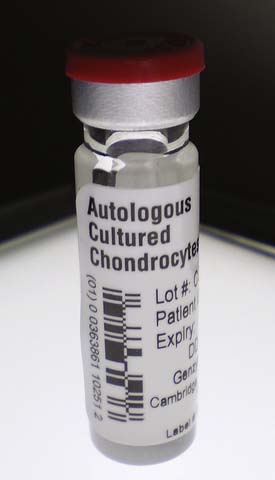
FIGURE 9B-6 Vial containing a cell suspension of chondrocytes. The rubber stopper and crimp seal must be intact.
Shipping media should be clear, not cloudy.
Ensure that the container seal has not been compromised.
If there are any questions regarding packaging integrity, contact the manufacturer immediately.
1. Kielpinski G., Kehinde O., Kaplan B.M., et al. Quality control for ex vivo cell therapy. Bio Pharm. 1997;10:34-40.
2. Mayhew T., Williams G.R., Senica M.A., et al. Validation of a quality assurance program for autologous cultured chondrocyte implantation. Tissue Eng. 1998;4:325-334.
3. Wang Y., Dono D., Duguid J., et al. A new method to evaluate viability of advanced cell therapy and tissue engineering products. International Cartilage Repair Society meeting. Miami: FL: Poster presentation; 2009. May 24 – 26
4. Kielpinski G., Prinzi S., Duguid J., et al. Roadmap to approval: use of an automated sterility test method as a lot release test for Carticel, autologous cultured chondrocytes. Cytotherapy. 2005;7:531-541.
5. Rapko S, Zhang M, Richards B, et al. Identification of the chondrocyte lineage using microfibril-associated glycoprotein-2, a novel marker which distinguishes chondrocytes from synovial cells. Tissue Eng, in press.
6. Rapko S., Baron U., Hoffmüller U., et al. DNA methylation analysis as novel tool for quality control in regenerative medicine. Tissue Eng. 2007;13:2271-2280.
7. Dell’Accio F., De Bari C., Luyten F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44:1608-1619.
8. Pelttari K., Lorenz H., Boeuf S., et al. Secretion of matrix mellatoproteinase 3 by articular chondrocytes as a predictor of ectopic cartilage formation capacity in vivo. Arthritis Rheum. 2008;58:467-474.
9. Parker A., Rapko S., Duguay S.J. Evaluation of gene markers to predict the potential for chondrogenesis of cells in MACI® implants. International Cartilage Repair Society meeting. Miami: FL: Poster presentation; 2009. May 24 – 26