Autologous Chondrocyte Implantation
Karen Hambly, Kai Mithoefer, Holly J. Silvers and Bert R. Mandelbaum
Since its first description by Brittberg in 1994,1 chondrocyte implantation (also known as chondrocyte transplantation) has been performed in more than 15,000 patients worldwide and many studies have clinically validated this technique as a reliable method for biologic restoration of injuries of articular cartilage surfaces.2–12 Based on the recent evolution and better understanding of the treatment of articular cartilage defects and cell-based implantation techniques, this challenging surgical technique has undergone several technical modifications. These recent developments can help to reduce patient morbidity and technique-specific complications as chondrocyte implantation techniques continue to evolve using modern tissue engineering technologies.
Preoperative Considerations
History and Clinical Symptoms
Obtaining a thorough history in patients with chondral defects presents a critical first step in the treatment process and selection of patients that are appropriate candidates for chondrocyte implantation. Articular cartilage defects may present acutely after joint trauma such as knee ligament tears, or more chronically as part of a degenerative process. Pain is often present with weight bearing and impact activities. Joint effusion is frequently reported, particularly after demanding impact activities. Cartilage defects of the femoral condyles may produce point tenderness over the femoral condyle rather than the joint line. Catching and locking sensation can occur from cartilage flaps or larger cartilage defects but are nonspecific and may also result from other knee pathology such as meniscal tears. Defects of the patella or trochlea usually lead to pain when ambulating stairs, driving a car, or getting out of a chair or squatting position. Symptoms of patellar instability may be reported. Patients considered for chondrocyte implantation techniques may have undergone prior cartilage repair procedures and a detailed evaluation of the prior surgical procedures should be performed. The time since the initial injury and the type of previous surgeries should be recorded since these factors have been shown to affect the outcome after chondrocyte implantation.
Physical examination includes the evaluation of the patient’s gait pattern and lower extremity alignment. Hip, knee, and ankle range of motion (ROM) should be assessed and any joint effusion noted. Since articular cartilage lesions are frequently found in patients with acute hemathrosis, acute or chronic ligamentous instability, patellar dislocation or maltracking, or lower extremity malalignment, these factors should be routinely evaluated. Depending on the defect’s location and size, mechanical symptoms may or may not be present and may overlap with meniscal tests. The patient’s body mass index should be assessed because it has been shown to correlate with functional outcome after articular cartilage repair.
Diagnostic Imaging
Plain radiographs including weight-bearing anterior/posterior and lateral views, Rosenberg and tunnel views, long-leg films, and Merchant views can help to identify osteochondral lesions, joint space narrowing, patellar maltracking, or lower extremity malalignment. Cartilage sensitive magnetic resonance imaging (MRI) presents a sensitive, specific, and accurate tool for noninvasive diagnosis of articular cartilage injury.13,14 Cartilage sensitive MRI provides useful information about meniscal and ligamentous status, subchondral bone, lesion size, location, and depth. Because of the pathologic changes in the surrounding cartilage, the final size of the defect intraoperatively usually is larger than defect size measured on a preoperative MRI. Besides its use for preoperative diagnosis, cartilage sensitive MRI can be very helpful for postoperative evaluation of cartilage repair. Routine postoperative MRI is recommended to evaluate the cartilage repair tissue and detect potential complications such as graft hypertrophy. If available, newer technologies such as T2 mapping, delayed gadolinium enhanced MRI of cartilage, or T1 rho relaxation mapping can be used to obtain additional detail about cartilage morphology before and after surgery.14
Indications and Contraindications
Chondrocyte implantation is indicated for symptomatic, high-grade chondral and osteochondral defects of the knee in active patients that are physiologically too young for arthroplasty. This technique can be successfully used as a first-line treatment for lesions more than 2 cm2 of femoral condylar, trochlear, and patellar lesions or used as a revision technique for failed prior cartilage repair procedures.2,10–12 The technique can be successfully used for isolated, multiple, and kissing cartilage defects. Prerequisites for successful autologous chondrocyte implantation (ACI) include adequate ROM, appropriate axial alignment or patellar tracking, ligamentous stability, and the ability to comply with the postoperative rehabilitation. Adjuvant procedures may be indicated to correct coexisting knee pathology and can be performed simultaneously with chondrocyte implantation without negative effects of these complex procedures on the postoperative functional outcome and activity level.
Absolute contraindications for ACI include generalized osteoarthritis, inflammatory arthropathies, or joint sepsis. Patients unable to comply with the postoperative rehabilitation and weight-bearing protocol should also not be treated with this technique. A body mass index greater than 30 kg/m2 has been associated with limited improvement after knee articular cartilage repair and presents a relative contraindication. Patients with severe meniscal deficiency should be considered for meniscal allograft transplantation at the time of chondrocyte implantation.
Preoperative Patient Counseling
Based on the history, physical examination, and radiologic information, the indication for ACI or adjuvant procedures is discussed with the patient. In-depth preoperative patient counseling is critically important to determine the patient’s demands, to assess his or her ability to comply with the postoperative rehabilitation plan, and to create realistic expectations and goals for postoperative knee function and activity level.
Surgical Technique
Stage 1: Chondrocyte Harvest
Step 1: Diagnostic Joint Arthroscopy
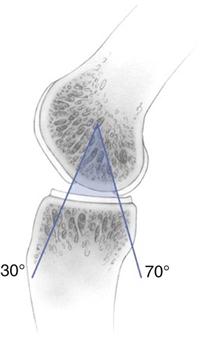
ACI of the knee presents a two-stage procedure. The first stage involves a complete arthroscopic evaluation. Besides identification of the index cartilage defect, the entire articular cartilage surface is inspected to rule out any additional cartilage pathology. During the first-stage arthroscopy, the cartilage defect is identified and existing cartilage flaps are débrided back to a stable and healthy peripheral margin. Following thorough débridement, the size of the articular cartilage lesion is measured and its containment documented. The knee is taken through a full ROM and the arc of motion, during which the lesion articulates with the opposing joint surface, is carefully recorded (Fig. 25-1). The opposing joint surface should always be carefully inspected for the presence of a kissing lesion or signs of low-grade cartilage injury. Partial meniscectomy and meniscal repair can be performed because it minimally affects the course after the initial arthroscopy and decreases operative time during the second-stage procedure. However, surgical treatment of meniscal deficiency or ligamentous pathology should be performed simultaneously with ACI to avoid repetitive operative joint trauma and prolonged rehabilitation.
Step 2: Chondrocyte Harvesting
Following initial arthroscopic evaluation, 200 to 300 mg of normal articular cartilage is obtained from a lesser weight-bearing area of the injured knee, generally the medial or lateral superior ridge of the femoral condyle or intercondylar notch. The intercondylar notch is preferred for patellofemoral lesions. Using cartilage that has been débrided from the cartilage defect as graft is not recommended because chondrocyte quality from this area has been shown to be inferior. The grafted cartilage tissue is sent for standardized commercial isolation and culturing of chondrocytes. Implantation of cultured chondrocytes is performed after 2 to 4 weeks when a sufficient number of cells have been obtained for the size of the defect (range, 4 to 12 million cells). Chondrocyte viability is routinely tested and should be more than 95% before implantation.
Stage 2: Chondrocyte Implantation
Step 1: Adjuvant Procedures
Ligamentous instability, meniscal injury and deficiency, or malalignment have been shown to contribute to the development of articular cartilage lesions, and surgically addressing these concomitant pathologies is critical for an effective and durable articular cartilage repair.10–12 Concomitant ligament reconstruction, meniscal allograft, or osteotomy should be performed before ACI. Isolated or combined adjuvant procedures do not negatively affect postoperative activity levels after ACI.8 Concomitant rather than staged adjuvant procedures avoid prolonged rehabilitation, promote postoperative activity, and provide a significant cost benefit.
Step 2: Autologous Chondrocyte Implantation
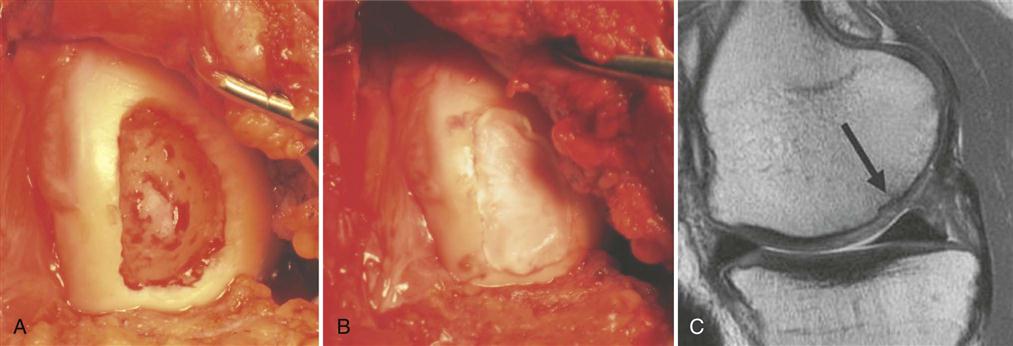
Following parapatellar arthrotomy, the cartilage defect is débrided back to a healthy cartilage margin and the calcified cartilage and intralesional osteophytes are carefully removed without violating the subchondral bone (Fig. 25-2, A). If bleeding is encountered, hemostasis of the defect bed can be achieved by application of thrombin or fibrin glue. A template of the defect is created to then harvest an appropriately sized periosteal flap from the proximal medial border of the tibia. When using periosteum, the tissue patch should be slightly larger than the defect because of a tendency of the periosteum to contract. Any adherent fatty or connective tissue needs to be carefully removed to minimize the potential for graft hypertrophy. The periosteal flap is then sutured flush to the articular cartilage defect using interrupted 6-0 Vicryl* with the cambium layer facing into the defect. As an alternative to the periosteum, a type I/III collagen membrane can be used to cover the defect (Fig. 25-2, B and C). This allows for smaller incisions, reduces patient morbidity, and minimizes the potential for graft hypertrophy. The rim of the periosteal flap or collagen membrane is sealed watertight with fibrin glue except for one corner, where the implanted chondrocytes are injected into the defect. Following cell injection, the remaining corner of the periosteal flap is secured with sutures and sealed with fibrin glue. Drains are not normally used to prevent injury to the periosteal cover or fibrin seal of the defect from direct abrasion by the drain during postoperative joint mobilization. A compression dressing is placed over the involved joint and cryotherapy is used routinely. Bracing is not indicated for isolated chondrocyte transplantations but may be used if simultaneous adjuvant procedures require postoperative protection.
Deep Chondral or Osteochondral Defects
In patients with lesions deeper than 1 cm, autologous bone grafting should be performed in combination with ACI. Implantation of chondrocytes in these cases is performed using the “sandwich technique.” The osseous defect is filled with a cancellous bone graft from the iliac crest or proximal tibia to the level of the subchondral plate. One periosteal flap or collagen membrane—sized for the defect—is then anchored using fibrin glue applied between the patch and the bone graft. A second sized periosteal flap or membrane is then placed facing into the defect and sutured to the surrounding cartilage. The rim is again sealed with fibrin glue and the cultured chondrocytes are then implanted between the two periosteal flaps or collagen membranes (sandwich technique).
Postoperative Management
Complications
Complications after chondrocyte implantation include stiffness and adhesions in up to 10% of patients. Graft hypertrophy is seen in 25% to 63% of postoperative magnetic resonance images but described as clinically symptomatic in only 13% to 15%.1–12 Symptomatic hypertrophy can be effectively treated by arthroscopic chondroplasty. Hypertrophy may lead to partial detachment or graft delamination. Substituting the periosteum with collagen membranes or second generation matrix-associated chondrocyte implantation (MACI) effectively reduce the risk for periosteal hypertrophy.15 Graft failure has been described in 6% to 7% of cases. Grafts usually fail between 12 to 24 months after surgery and frequently show central degeneration. Treatment with revision chondrocyte implantation has been shown to be effective in many cases.12 All patients with graft failure should be carefully evaluated for the presence of subtle instability, axial malalignment, or patellar maltracking, which has been shown to lead to lower success rates after ACI.
Surgical Pearls and Pitfalls
Pearls
Pitfalls
Surgical Summary
ACI has been successfully used for hyaline-like restoration of full-thickness articular cartilage lesions in the knee with long-term durability of functional improvements of up to 11 years.10,11 Good to excellent results were found in 92% of isolated femoral condyle lesions, 89% of osteochondritis dissecans, 75% of femoral condyle lesions with concomitant anterior cruciate ligament reconstruction, 67% of patients with multiple lesions, and in 65% of patella lesions.1–12 Return to sport is possible in 33% to 96%, with best return rates in competitive and adolescent athletes. Patients with single lesions, younger age, and short preoperative intervals have the best results.8,9 Correcting axial malalignment, patellar maltracking, and ligamentous laxity is critical for the functional improvement. Limitations of this technique include its invasiveness, long postoperative rehabilitation, and periosteal hypertrophy, which may lead to acute graft delamination. This cartilage repair technique provides significant functional improvement with high return rates to demanding sports and excellent durability even under high athletic demands both in the primary and revision setting.
Scaffold-associated second-generation autologous cartilage implantation techniques use three-dimensional biodegradable scaffolds to temporarily support the chondrocytes until they are replaced by matrix components synthesized from the implanted cells. MACI has been used with promising results in Europe and Australia but is not routinely available for use in the United States.15,16 The use of the biomatrix seeded with chondrocytes reduces surgical invasiveness and has the theoretic advantages of less chondrocyte leakage, more homogeneous chondrocyte distribution, and less graft hypertrophy. Arthroscopic MACI has been described with improvement of knee function up to 90%.16 Future developments are aimed at improving cellular matrix production by using more productive “characterized” chondrocytes and more sophisticated bioactive scaffolds that include growth factors and stimulate a more natural spatial distribution of chondrocytes within the repair cartilage.17,18 Other promising future approaches include identification and selective expansion of specific chondrocyte subpopulations capable of producing more hyaline-like repair cartilage tissue or implantation of neocartilage tissue produced in specifically designed bioreactors.19,20
Therapy Guidelines for Rehabilitation
Rehabilitation has been identified as an important component in articular cartilage repair with the potential to influence both the patient outcome and the quality of repair tissue.21 In the last 10 years, the fastest evolving surgical repair of articular cartilage has been ACI. The first published review of ACI postoperative care and rehabilitation was in 2006.22
The goal of any ACI rehabilitation program is to provide a mechanical environment for the local adaptation and remodeling of the repaired tissue that will enable the patient to safely return to the optimal level of function. The challenge is to construct an individualized rehabilitation program that matches mechanical loading to the status of the repair tissue at any postoperative time point. Successful ACI rehabilitation is reliant on a collaborative environment, with thorough communication between the surgeon, therapist, and patient. A graded rehabilitation program incorporating preoperative counseling, progressive weight bearing,30 and controlled exercise31 is generally recommended following ACI. An understanding of applied clinical biomechanics and appreciation of the forces and loads that will be exerted on the repair tissue are essential in the design of an ACI rehabilitation program. This chapter focuses on the principles behind effective ACI rehabilitation and the rationale behind the components of the rehabilitation.
There are a number of elements that are essential in the designing of an individualized ACI rehabilitation program, including the following:
2 Recognition of factors that affect functional outcome after ACI
3 Respect for the biologic timeframes for healing
4 Application of exercise programming knowledge
5 Establishment of criteria for modification or progression of exercise
6 Acknowledgement and incorporation of the expectations and goals of the patient
![]() The three main components required within an ACI rehabilitation program are progressive weight bearing; restoration of ROM; and enhancement of muscle control, proprioception, and strength.22–25 The ACI-specific evidence base to directly support the frequency, intensity, type, and timing of exercise modalities during rehabilitation is limited. Studies have advocated avoidance of certain ranges of knee movement, for example, active knee flexion between 40° and 70° in the early stages after patellofemoral ACI.22 However, virtually all exercise activities, including common activities such as walking, cycling, and rowing, involve a knee flexion/extension pattern within this range.31 The incorporation of exercises into the ACI rehabilitation program may be better considered in terms of minimizing joint stress as opposed to the complete avoidance of specific ranges of movement. This can be achieved through the selection, introduction, and progression of exercise activities that are appropriate for the graft status, size, and location. Exercises should complement and not replace functional movement retraining.
The three main components required within an ACI rehabilitation program are progressive weight bearing; restoration of ROM; and enhancement of muscle control, proprioception, and strength.22–25 The ACI-specific evidence base to directly support the frequency, intensity, type, and timing of exercise modalities during rehabilitation is limited. Studies have advocated avoidance of certain ranges of knee movement, for example, active knee flexion between 40° and 70° in the early stages after patellofemoral ACI.22 However, virtually all exercise activities, including common activities such as walking, cycling, and rowing, involve a knee flexion/extension pattern within this range.31 The incorporation of exercises into the ACI rehabilitation program may be better considered in terms of minimizing joint stress as opposed to the complete avoidance of specific ranges of movement. This can be achieved through the selection, introduction, and progression of exercise activities that are appropriate for the graft status, size, and location. Exercises should complement and not replace functional movement retraining.
The guidelines that follow are designed to help guide therapists through the rehabilitation process. It should be noted that these guidelines are for first-generation ACI using either periosteal flap or collagen membrane and that there is currently considerable variation in rehabilitation practice between clinical centers. The therapist should evaluate each patient on an individual basis and select appropriate rehabilitation content and criteria for progressions for the individual, working around any restrictions imposed by the surgeon.
Initial Evaluation
Several factors have been associated with superior functional outcome following cartilage repair surgery.26 These include:
• Shorter preoperative duration of symptoms
• Fewer number of previous surgeries
• Smaller size of defect (cm2)
• Medial femoral condyle repairs do better than patellofemoral repairs
• Isolated defects do better than multiple and/or kissing defects.
• The correction of mechanical malalignment before surgery
• Professional/competitive athletes do better than recreational athletes.
• Participants of low impact sports do better than participants of high-impact sports.
A preoperative evaluation should be made for each patient. Overt muscle imbalances should be noted and addressed in a home exercise program before surgery. The patient’s occupational demands and their access to rehabilitation facilities and modalities is extremely useful information when designing an individualized rehabilitation program.
Preoperative Management
A preoperative session is beneficial for both patient and therapist. To maximize the benefits of ACI surgery, it is essential for patients to understand and consequently comply with their ACI rehabilitation program. Preoperative assessment should include ROM, muscle strength, proprioception, function, gait, and an assessment of problematic activities of daily living (ADL). A preoperative therapy session provides an ideal opportunity to explain the rehabilitation program; teach ambulation with crutch(es); and demonstrate early home exercises to the patient.
Postoperative Rehabilitation
The repair site is at its most vulnerable during the first few months after ACI. Although chronologic time frames have been established for the rehabilitation phases, it is more important that the patient achieves the qualitative goals of each phase. An important rehabilitation principle is program individualization. Patients may progress through rehabilitation at different paces because of multiple factors, including lesion size, lesion location, preoperative duration of symptoms, preoperative baseline physical condition, age, and patient motivation. When a patient achieves all the functional goals of a particular rehabilitation phase, they may advance to the next phase of the rehabilitation.
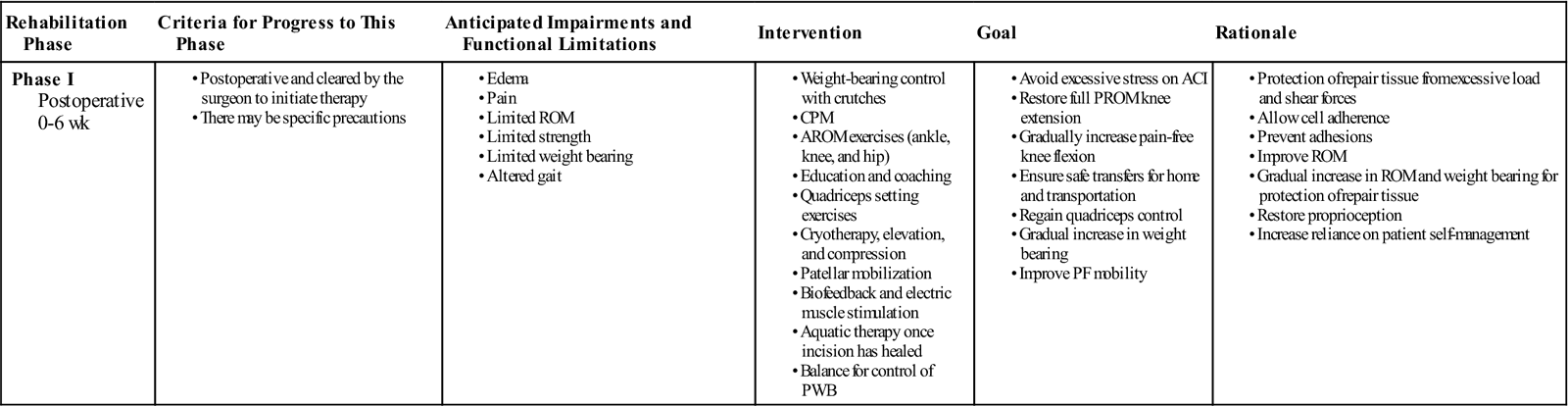
Phase I: Recovery and Protection
TIME: 0 to 6 weeks
GOALS: Protection of the repair tissue, restoration of joint homeostasis, and improving ROM (Table 25-1)
TABLE 25-1
Autologous Chondrocyte Implantation
< ?comst?>
| Rehabilitation Phase | Criteria for Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase I Postoperative 0-6 wk |
• CPM
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
ACI, Autologous chondrocyte implantation; AROM, active range of motion; CPM, continuous passive motion; PF, patellofemoral; PROM, passive range of motion; PWB, partial weight bearing; ROM, range of motion.
Exercises: Lateral hip (abduction) side-lying, hip extension work (glut activation) without knee flexion, isometric glut squeezes.
The evidence basis for optimal timing of return to full weight bearing (FWB) following ACI is limited. Historically, as with most new surgical interventions, rehabilitation has been conservative and has included lengthy periods of non–weight bearing and partial weight bearing (PWB). Newly emerging research indicates that it is possible to accelerate weight-bearing loads in certain patient populations with good clinical and functional outcome after 2 years without jeopardizing the graft.27,28 Research indicates that moderate dynamic compression and shear loading are beneficial to extracellular matrix biosynthesis; chondrocyte proliferation; and repair tissue maturation, whereas static compression and immobilization are associated with adverse effects.29,32 ![]() High shear stress may lead to mechanical failure of ACI grafts in the early postoperative rehabilitation phase and it is therefore necessary to implement a graded increase in loading. Weight-bearing status should be predicated based on the location of repair, secondary to the biomechanical differences between ACI to a tibiofemoral joint surface and a patellofemoral joint surface.
High shear stress may lead to mechanical failure of ACI grafts in the early postoperative rehabilitation phase and it is therefore necessary to implement a graded increase in loading. Weight-bearing status should be predicated based on the location of repair, secondary to the biomechanical differences between ACI to a tibiofemoral joint surface and a patellofemoral joint surface.
• Toe-touch weight bearing for 1 to 2 weeks at minimal body weight (<10%) and as tolerated
• Begin heel-toe touch down weight bearing (approximately 10 to 15 kg) at week 2
• Progress to PWB (approximately 30% of body weight) at week 4
For trochlea and patella defects:
• Progress to 50% body weight in full extension at week 2
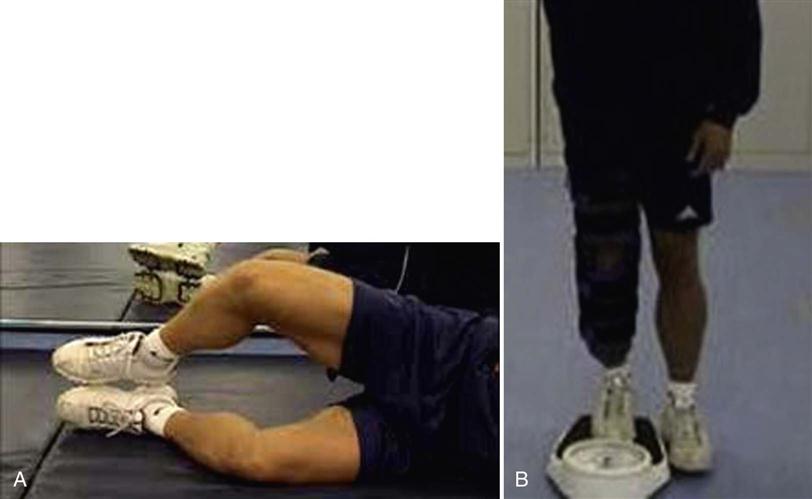
It is important to note that patients are not completely reliable in PWB and tend to overestimate weight in the early rehabilitative stages even after instruction.33 The accuracy of weight bearing can be practically assessed, taught, and reinforced with patients using two pairs of identical scales (Fig. 25-3).22 This is also a useful technique for controlling weight shift exercises and for the correction of body posture, and any residual unloading of the involved limb later in the rehabilitation process.
Continuous passive motion (CPM) is reported to increase the quality of chondral repair tissue34–36 and stimulate the metabolism of proteoglycan 4.37 Consequently, CPM is a standard inclusion in ACI rehabilitation in many centers.25 However, for patients with small, isolated defects of the femoral condyle, results may not be negatively affected by the replacement of CPM with graded weight bearing and active ROM (AROM); however, the evidence base is limited.38,39 The recommendation for the use of CPM is currently based on basic science, usual practice, case series, and disease-orientated evidence.25 Once again, there is a need to differentiate the rehabilitation based on the defect location:
• Initiate CPM day 1 for a total of 6 to 8 hours per day at 0° to 60°
• Progress CPM by 5° to 10° per day as tolerated
• Continue CPM for a total of 6 to 8 hours per day for 6 weeks
• Knee flexion ROM goal is 90° by week 2; 105° by week 3; 115° by week 4; and 125° by week 5.
For trochlea and patella defects:
• Initiate CPM day 1 for a total of 6 to 8 hours per day at 0° to 40°
• Progress CPM by 5° to 10° per day as tolerated
• Continue CPM for a total of 6 to 8 hours per day for 6 weeks
• Knee flexion ROM goal is 90° by week 3; 105° by week 4; and 120° by week 6.
• No active open kinetic chain (OKC) knee extension exercises during this phase.
CPM is not consistently used across cartilage repair centers and is often not available to patients. Where CPM is not available, it can be substituted by 500 active assisted heel slides performed three times per day with the same ROM progressions and goals as indicated for CPM (Fig. 25-4). ![]() ROM exercises should progress through a controlled increase in ROM through passive, active-assisted, and then active movements. Repetitive dynamic movement through the available ROM provides mechanical stimulation to chondrocytes, as well as increasing synovial fluid flow over the graft.40,41
ROM exercises should progress through a controlled increase in ROM through passive, active-assisted, and then active movements. Repetitive dynamic movement through the available ROM provides mechanical stimulation to chondrocytes, as well as increasing synovial fluid flow over the graft.40,41
In addition to weight bearing, CPM, and ROM guidance, most rehabilitation guidelines will provide further information regarding exercise and therapeutic modalities. In the early postoperative time period, the application of cryotherapy, compression, and elevation are important to lower tissue temperature, slow metabolism, decrease secondary hypoxic injury, and reduce edema formation.42 Gentle patellar mobilizations in all directions 4 to 6 times per day are important to prevent adhesions and arthrofibrosis (Fig. 25-5).43–46 Knee surgery results in proprioceptive deficits47 that should be addressed at the earliest postoperative opportunity. Proprioception can be initiated in phase I of rehabilitation as long as weight-bearing restrictions are applied. This may require adaptation of exercises to PWB. Gait training focuses on crutch walking to minimize soft tissue constrictions (especially tightness in hamstrings, gastrocnemius, and soleus muscles) and increasing the acceptance of load on the involved leg through controlled weight shifting. Aquatic therapy to enhance gait training and improve lower extremity strength/ROM can commence once the surgical incision has healed and the patient is able to safely transfer in and out of the pool. The water depth should reflect the weight-bearing status of the individual. Stationary cycling can be introduced with minimal resistance once knee flexion ROM is at least 100°.22 There is currently no evidence-based consensus to support or refute the use of postoperative bracing for ACI. Some centers will use bracing for patellofemoral ACI repairs for the first 4 to 6 weeks, especially if the defects are large or kissing or if there is a quadriceps lag. Neuromuscular electrical stimulation can be introduced and is a valuable adjunct to the program, especially where voluntary control of the quadriceps mechanism is limited.
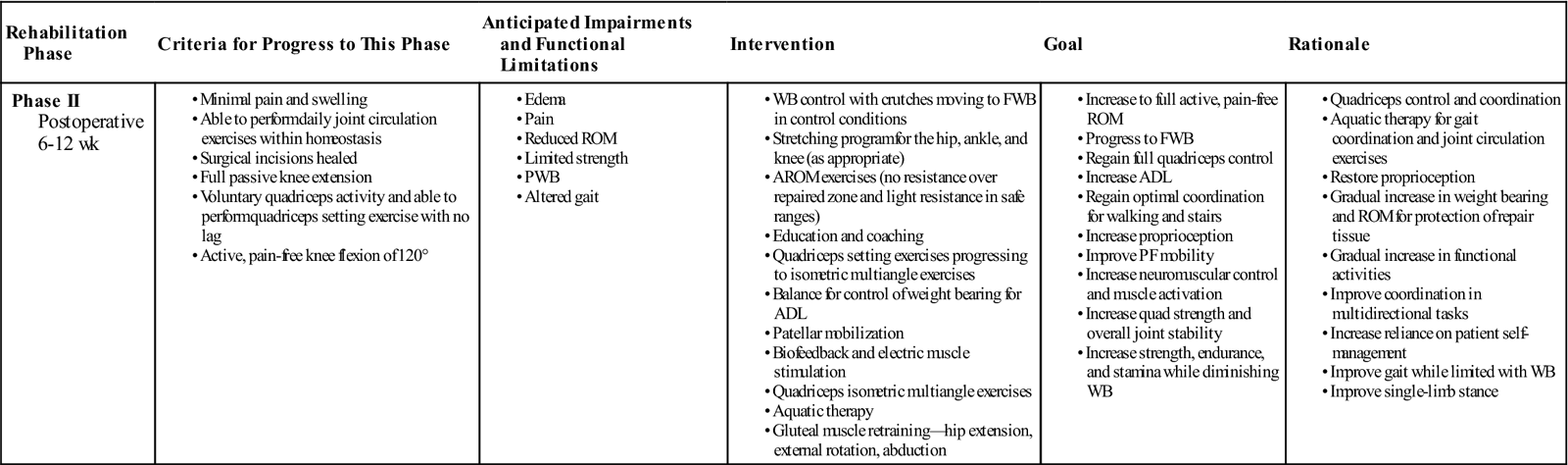
Phase II: Transition
TIME: 6 to 12 weeks
GOALS: Inauguration of the repair tissue, restoration of full ROM, and initiation and progression of muscle strengthening (Table 25-2)
TABLE 25-2
Autologous Chondrocyte Implantation
< ?comst?>
| Rehabilitation Phase | Criteria for Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase II Postoperative 6-12 wk |
|
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
ADL, Activities of daily living; AROM, active range of motion; FWB, full weight bearing; PF, patellofemoral; PWB, partial weight bearing; ROM, range of motion; WB, weight bearing.
Exercises: Gluteal strength: standing hip extension (with appropriate knee angle), standing abduction—use mild resistance (i.e., Thera-Band) to address gluteus medius and gluteus maximus deficiencies.
During the transition phase, many of the interventions from phase I are continued and incorporated into a maintenance program. AROM exercises can be progressed to light resistance in “safe ranges” while simultaneously maintaining no resistance over the repaired area. Safe ranges will be dictated by the articulation surfaces, contact area, and size and location of the graft. For example, as the posterior aspect of the medial femoral condyle contacts the tibia between 90° and 120°,48 light resistance in the range from 0° to 80° may be appropriate. Several research articles provide detailed information on the clinical biomechanics of the tibiofemoral and patellofemoral joints.11,49–52 Once FWB has been restored, forward lunges and forward and lateral step-ups can be introduced and treadmill walking can be initiated within safe ranges as dictated by the repair location and size.
Quadriceps setting exercises can be progressed from isometric multiangle exercises. Gluteal muscle retraining is an important component of ACI rehabilitation, especially where patients have altered lower extremity kinematics.53 Gluteus medius and minimus play an important role in the neuromuscular and valgus control of the knee54 and consequently normal posture and gait patterns, so exercises should be targeted at these muscles (Fig. 25-6, A and B).55,56 Proprioception and balance exercises can be progressed in line with increased weight-bearing status. The therapist must monitor any progressions in exercises and check that symptoms are not increased.
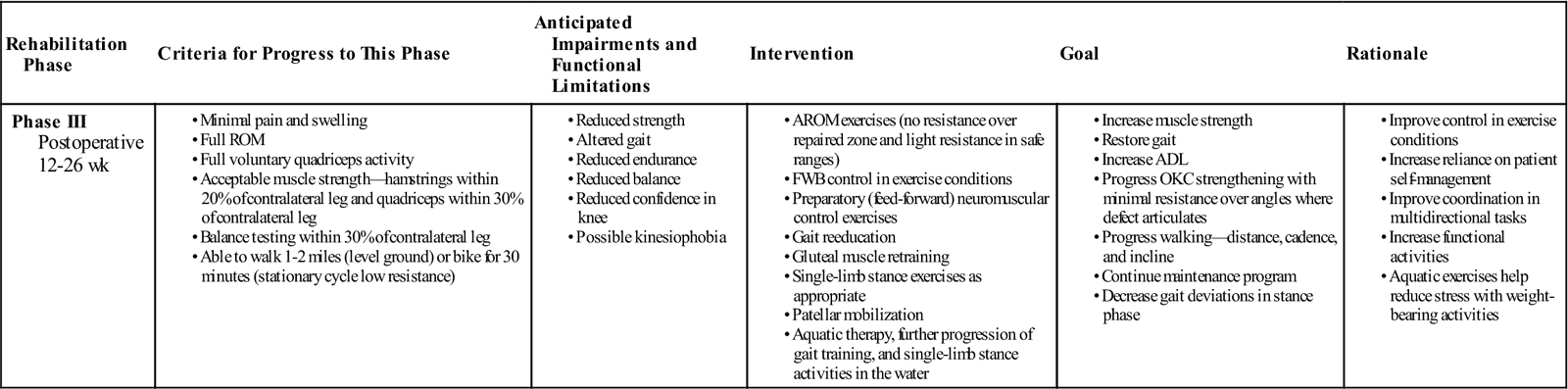
Phase III: Remodeling
TIME: 12 to 26 weeks
GOALS: Improvement of muscle strength and endurance and reintroduction of functional activities (Table 25-3)
TABLE 25-3
Autologous Chondrocyte Implantation
< ?comst?>
| Rehabilitation Phase | Criteria for Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase III Postoperative 12-26 wk |
• Full ROM
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
ADL, Activities of daily living; AROM, active range of motion; FWB, full weight bearing; OKC, open kinetic chain; ROM, range of motion.
Exercises: Increase progressive resistive therapeutic exercise for quadriceps (knee extension with appropriate knee angles), knee flexion, hip extension/flexion/abduction/adduction in standing with resistance at ankle joint, supine core/trunk stability work (i.e., Pilates), physioball (supine and prone), and proprioceptive work on unstable surface (wobble boards, Bosu, Airex mat).
Phase III continues with the maintenance program of exercises within phase II with appropriate progressions in resistance, repetitions, sets, and frequency, with close monitoring of patient response. Proprioception and balance training can be progressed to involve multidirectional functional tasks. Feed-forward muscular control exercises, such as lunges and unilateral step up/downs (Fig. 25-7) in a controlled therapy environment will not only assist in strengthening and restoring dynamic control but will also help to enhance the patient’s confidence in the involved knee. Gait analysis allows the therapist to assess the patient dynamically, identifying asymmetries in pressure transfer, compensatory mechanisms, and movement-related problems during the gait cycle (Fig. 25-8). Cardiovascular and muscular endurance can be increased with the introduction of low impact exercise activities, such as swimming, rowing, or elliptical training, and through the progression of the walking program with increases in distance, cadence, and incline.
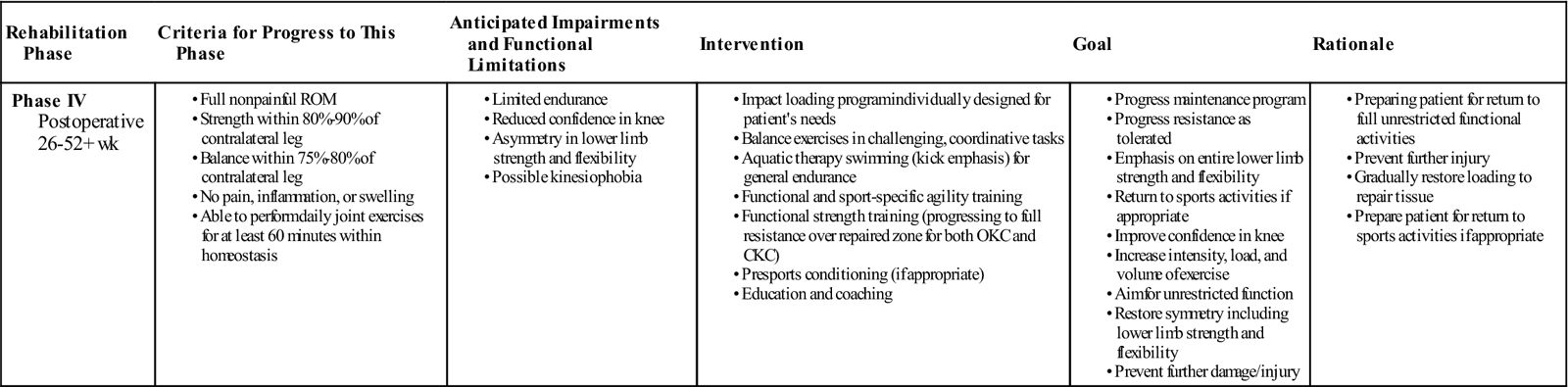
Phase IV: Maturation
TIME: 26 weeks onward
GOALS: Returning the patient to full unrestricted activity (Table 25-4)
TABLE 25-4
Autologous Chondrocyte Implantation
< ?comst?>
| Rehabilitation Phase | Criteria for Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase IV Postoperative 26-52+ wk |
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
CKC, Closed kinetic chain; OKC, open kinetic chain; ROM, range of motion.
Exercises: Address any pathokinematics during joint loading (i.e., dynamic valgus). Improve single-leg balance with combined balance/strength activities. Increase intensity, duration, and resistance of therapeutic exercise for core/trunk, hip, knee, and ankle joints. Increase time duration for supine plank work. Aerobically (cardiovascular), incorporate interval work for incline and speed to return to prior level of fitness.
During the maturation phase, return to work times will depend on the demands of the occupation. Sedentary work can be resumed earlier than manual labor. A phased return to work allows for a more controlled return, with initial recovery time for the body to adapt. Return to driving times will depend on which leg is involved and whether the vehicle is manual or automatic transmission.
Return to sport times will depend on the type of sport that the individual wants to return to and the ability of the individual to accept the demands of that sport without short- or long-term adverse effects. Low impact exercise activities such as swimming, cycling, and rowing can be started at 4 to 6 months. Higher impact exercise activities, such as jogging, running, and aerobics, may be performed from 8 months. Jogging can be introduced earlier for athletes by using a positive air pressure treadmill at reduced body weight (Fig. 25-9). High impact sports such as basketball, ice hockey, badminton, tennis, football, and soccer are allowed at 12 to 18 months. Return to sport rates after ACI from published clinical studies average 67%, but there is a considerable range (33% to 96%).26 Average time to return to sport is reported to be 18 months (range, 12 to 36 months), varying on the individual factors and the nature of the sport.26 It is important to note that not all individuals will return to sport after ACI and this will be key to managing patient expectations.
Rehabilitation Pearls and Pitfalls
Pearls
1 Agree on realistic expectations and goals with the patient.
2 Obtain details on the surgery, size, location, and articulation of the defect.
3 Address the faulty pathokinematics that may have led to the defect in the first place.
4 Focus on what the patient can do rather than the restrictions.
5 Closely monitor clinical signs of pain or swelling; modify or regress rehabilitation as appropriate.
7 Provide emotional support for the patient through the rehabilitation process.
8 Recognize that not all patients will return to sports activities.
Pitfalls
Suggested Home Maintenance for the Postsurgical Patient
The rehabilitation following ACI is a lengthy process that requires considerable input from the patient. The home maintenance suggested for the postsurgical ACI patient is integral within the general rehabilitation guidelines as presented earlier. The therapist needs to provide guidance on the progression and the exercise parameters (intensity, frequency, repetitions, duration) based on the individual patient’s age, needs, and rehabilitative status. Where particular facilities and modalities are not available to a patient, the therapist should recommend suitable alternatives that allow the patient to achieve the rehabilitation goal. Individualization of the rehabilitation program is critical to patient compliance.
Troubleshooting
Mechanical complications are fairly common after ACI.57 Graft failure and delamination accounted for almost half of the adverse events reported to the United States Food and Drug Administration up until 2003.57 Tissue hypertrophy was shown to be one of the more common adverse events,57 but this is predominantly associated with periosteal ACI. The introduction of second generation ACI has resulted in lower levels of hypertrophy.15 One of the complications of ACI surgery that potentially has the most impact on the patient is the development of arthrofibrosis.43–46 ACI rehabilitation is lengthy and noncompliance to the rehabilitation program can be an issue with some patients. Education and counseling regarding the rehabilitation program and why restrictions are imposed can be useful.
The rehabilitation guidelines provided in this chapter should provide a framework for ACI rehabilitation but should not be viewed as strict protocols. Monitoring of patient status and progression throughout the rehabilitation process is critical and referral back to the surgeon should be considered where appropriate. Signs that the rehabilitation program should be reviewed include the following:
• Increased levels of pain, especially pain after cessation of activity
• Sustained plateau or reduction in ROM, weight bearing, or strength
If concomitant surgical procedures such as ACL reconstruction or meniscus repair are performed, the rehabilitation program should be revised on an individual basis to incorporate the requirements of the concomitant procedure in concert with the ACI requirements.
Summary
This chapter has focused on the principles behind effective ACI rehabilitation and the factors in developing an individualized rehabilitation program. A general rehabilitation guideline has been provided for first generation ACI. The emergence of newer, cell-based articular cartilage repair technologies and variations of ACI surgery are likely to require modification to the existing rehabilitation guidelines.