Chapter 7 Autism
With contribution from Dr Jenny Altermatt
Introduction
Autism is a lifelong complex neurodevelopmental disorder that has its onset in infancy. It has a wide range of clinical presentations, mostly heralded by impairments in social interaction, and communication, and repetitive, stereotyped behaviours.1, 2 This spectrum of disorders dramatically affects the lives of patients and their families and the broader community.3
Children with Autism Spectrum Disorder (ASD) continue to have problems as adults, and experience challenges with independent living, mental health and social relationships, regardless of their intellectual capacity.4
Autism Spectrum Disorders is classified in the DSM-IV as 1 of 5 related pervasive developmental disorders, of which Asperger’s syndrome, Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS), Rett’s syndrome, and Disintegrative Disorder are the other 4 and these have different ages of onset and presentations.5, 1
Definition/diagnosis
A child may be brought into a general practice with the parents complaining that something is wrong. Listed below are established ‘red flags’ that alert the GP to possible concerns:5
The Checklist for Autism in Toddlers (CHAT) screening test is used by the Royal Children’s Hospital (Australia) and can be easily used by both parent and clinician to decide whether further, more involved testing is required. Figure 7.1 shows the CHAT screening test designed to be used in children from 18 months to 36 months to help identify autism.6, 7, 8
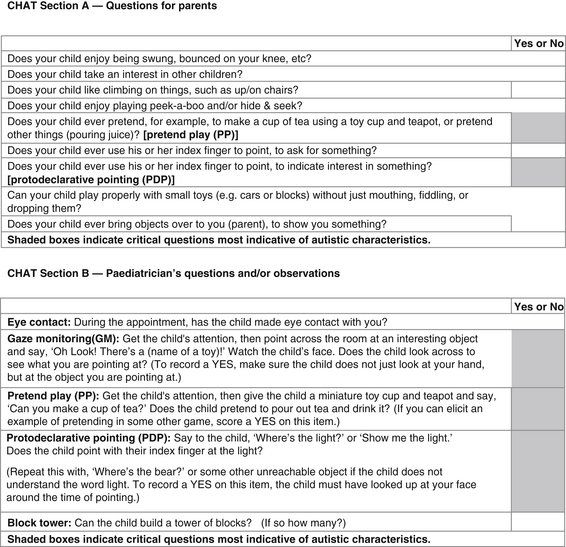
Figure 7.1 Checklist for Autism in Toddlers (CHAT) screening test, 18–36 months
(Source: Royal Children’s Hospital. Online: www.rch.au/genmed/clinical.cfm?doch_id=?497)
Overall the more ‘NOs’ in the CHAT test the higher the chance of autism.
While a diagnosis of Autism is generally formally made between the ages of 2 and 3 years1, recent published research (2008) using home video observations and parental interview have found anomalies in eye contact, smiling, sharing and interaction initiation on video as early as 12 months and reported by parents as early as 6 months.9
This has implications for age of surveillance screening and Australian researchers, including Professor Cheryl Dissanayake, developmental psychologist, who heads the newly established Olga Tennison Autism Research Centre at Latrobe University Melbourne (Australia), now believe that intense intervention should commence as soon as these children are detected. Research is suggesting that children detected early can make huge strides in improving connection, language and behaviour.10, 11 This early detection is supported by the American Academy of Paediatrics.4 Care can be coordinated with paediatricians and a large, hopefully integrated, team of psychologists, occupational therapists, speech therapists, and teachers to provide intense therapy and care and achieve improved outcomes.
Medical issues should be attended to concurrently, including childhood illnesses, sleep dysfunction, challenging behaviours and psychiatric conditions that these children may suffer. Bowel dysfunction has been found to be more common in autistic children, although this needs further research due to a lack of controls in studies.4
Incidence/prevalence
The number of people with ASD has dramatically increased over the past decade, and problem behaviours in autism are an increasing challenge to families, schools, physicians and other health care professionals.12, 13
Researchers are not certain whether this increase is due to broader diagnostic criteria, better detection or a true increase.14 This is likely to be the result of more precise diagnosis, better service availability and improved awareness of autism.15 Many studies have confirmed this increased incidence, but have reported differences in incidence depending on the study. It has been reported at 6.7 per 10 000 for autism16 and 25 per 10 000 for ASD17, but also as high as 13 per 10 000 for autism and 60–65 per 10 000 for ASD.18
US epidemiological data suggest that this increased rise in incidence is slowing in recent years.17 Prevalence in Australia is difficult to determine as there are inconsistencies in existing data. There is a need to improve data systems across the country.19
Autism rates were measured to be as low as 4–5 per 10 000 in WA and NSW in 2000.20
Risk factors and causes of autism
Genetic susceptibility, health, nutritional status, and environmental exposures might all contribute to the causation of this complex of disorders.5 There is unprecedented pressure for autism researchers to find a cause. This is difficult because of the multitude of brain deficits and genetic variants, and no integrated theory has emerged. Research going forward should assume that autism is an aggregation of myriad independent disorders of impaired sociality, social cognition, communication, and motor and cognitive skills.21
Genetic predisposition
Multiple genes are involved in inheritance, which demonstrate great phenotypic variation. If 1 sibling already has an ASD, there is approximately a 5–6% risk of a subsequent sibling having the disorder, and this is higher still with subsequent children.22
Monozygotic twin studies also show 60% concordance for Autism and 92% for ASD.5
Possible involved chromosomes are 2q, 7q, 16p and 19p, and cytogenetic abnormalities have also been found on chromosome 15 and in Fragile X Syndrome.1
New groundbreaking research published in Nature in March 2009 as an association study of 2 large cohorts (n = 3100 and 1200) of affected children and 6500 controls, found a genome-wide significant association of variants on chromosome 5 for susceptibility for ASD.23 Especially significant were genes coding for ‘neuronal cell adhesion molecules’, responsible for neuron to neuron communication. These abnormalities are especially seen in the areas of the brain that are structurally abnormal in autism and ASD: namely the frontal, temporal lobes and the amygdala (which is larger than normal in autistic children in the first 2 years of life). Crucially, this work was replicated in 2 other independent cohorts, and has implications for further research.
Medical causes
A specific medical cause is only found in 6–10% of cases. These include prenatal causes such as congenital rubella, untreated metabolic disorders, anticonvulsants during pregnancy, tuberous sclerosis and severe postnatal infections.1
Epilepsy
Epilepsy may be associated with autistic regression and impaired mental functioning in childhood autism.24 The incidence of seizures has been estimated to be between 11–39%, and is associated with severe global developmental delay, where the incidence is higher at 42%. With children with ASD and no mental retardation, the incidence is only 6–8%. There are 2 peaks, 1 in children less than 5 years of age, and another peak in adolescence. Electroencephalogram (EEG) abnormalities occur in 10–72% of affected children.25
Immune malfunction
Immune dysfunction has been associated with autism, and further research in this area might be productive. Antenatal levels of fetal protein IgG were found in some mothers who carried children who went on to develop ASD.26
While immune malfunction, deficiency and auto immunity also has some evidence to support causality, no theory is unified and complete.5
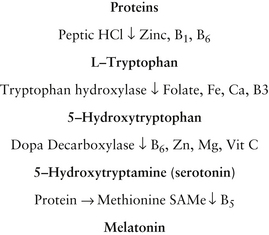
Nutritional deficiencies
One study of 45 children found lower levels of blood and red cell zinc in autistic children compared with controls.27 More research is required to test this hypothesis.
Environmental exposure to metals and chemicals
Research demonstrates biochemical abnormalities can occur in autism and include liver detoxification impairment via impaired sulfation pathways, mineral imbalances such as reduced zinc/excess copper,28 environmental insults such as heavy metal and organochlorine pesticide exposure,29, 30, 31 and gastro intestinal disturbances which are theorised to lead to excessive intestinal permeability and excessive absorption of breakdown products of certain foods leading to opioids intoxicating the brain.
It is well recognised that some industrial chemicals such as lead, methylmercury, polychlorinated biphenyls (PCBs), arsenic and toluene are toxic to brain development and lead to impaired brain function. A further 200 chemicals are neurotoxic to adults, and many more are toxic to laboratory animals.32 More careful precautionary regulation is required to protect fetal brains from possible exposure causing damage at far lower levels than adults.
Lead, mercury and PCBs have proven effects in attention, memory, learning, social behaviour and IQ at only background-population levels.33 Testing is usually lacking and not carried out for patients with neurodevelopmental problems.
Organochlorines and organophosphate exposure
Researchers in USA, California have identified a possible link between antenatal organochlorine pesticide exposure and increased risk of autism spectrum disorders.34 The study compared all births in the area (over 200 000) and compared these with all cases of ASD (465). They found that children had a 6-fold increased risk of ASD if their mothers lived within 500m of a field sprayed with organochlorines. Limitations to the study were that only a small proportion of mothers living in proximity to spraying in the critical early gestational period of neural development were found to be associated with increased risk (29 mothers, of whom 8 of their children had ASD). The authors comment that more studies are needed to confirm this hypothesis.
A review in 2008 confirmed these findings, reporting on in-utero organochlorine and organophosphate exposure and impaired neurodevelopment.35 It thus is logical to advise mothers to avoid exposure to these harmful substances if possible.
The incidence of ASD has been positively linked in the Pacific North West of the US to times of precipitation, implying possible fallout from airborne pollutants with rain.36
Research centering on the San Francisco Bay (USA) area studied 284 children with ASD and 657 controls and hazardous air pollutant concentrations.37 They found an increased association between autism and exposure to estimated airborne metal concentrations and possibly solvents, namely mercury, nickel, cadmium, trichloroethylene and vinyl chloride. They conclude that more refined exposure studies are required.
Mercury
Mercury is a heavy metal that has also been studied to identify any associations with ASD. A recent population study in Texas found that ASD incidence rises 2.6% per 1000lbs of industrial exposure release, and rises 3.7% with power plant emissions.38 These risks reduced with increasing distance from exposure, reducing to 2% and 1.6% respectively at 10 miles.
A prospective blinded study evaluating 28 children with severe ASD demonstrated that they had higher mercury intoxication-associated urinary porphyrins compared with less severe sufferers, and decreased plasma levels of reduced glutathione, cysteine and sulfate.39 They also had higher oxidised glutathione levels and there was an association with increased autism severity scores with these levels, and also the mercury associated porphyrins. The researchers implied from this that mercury intoxication is significantly associated with autistic symptoms, and that transsulfuration abnormalities observed indicate that this is associated with increased oxidative stress and decreased detoxification capacity.39
A small study from Arizona, USA, measuring levels of mercury, lead, and zinc in baby teeth of children with ASD compared with normal controls, found that autistic children had significantly higher levels of mercury only.40 They also had a higher use of oral antibiotics, which in rats inhibits excretion of mercury due to gut flora alteration. This is proposed as a possible mechanism for the higher mercury, and also for the increased incidence of chronic gastrointestinal problems in autistic children.
However, a case controlled study of 400 children compared with 410 controls that explored a possible association with risk of ASD and administration of anti-D globulin during pregnancy, which contains thimerosal, to Rh negative mothers. It showed no increased incidence.41
This lack of association was further confirmed in another US study conducted in Missouri.42
Environmental climate factors
Researchers in Louisiana have found that the prevalence of autism in offspring is related to exposure of mothers to severe hurricanes and electrical storms during middle and late gestation (p<0.001), complementing other research suggesting that antenatal events predispose children to having autism.43
Sunshine
There is currently an enormous interest in vitamin D as an important area of health research. Vitamin D supplementation is postulated to potentially reduce the risk of all-cause mortality by up to 7%.44
Research postulates that the apparent increase in autism is coincidental with widespread lowered vitamin D levels as a result of advice to avoid sun exposure to reduce risk of skin malignancies.45 This hypothesis needs to be supported by solid research. However, animal studies demonstrate that severe vitamin D deficiency during gestation leads to protein dysregulation and abnormal neurodevelopment and similar anatomical changes found to that of autistic children. Children with rickets have autistic markers that disappear with supplementation. Calcitriol is also involved, reducing inflammatory cytokines in the brain, which are increased in autism disorders. Calcitriol responds more to testosterone than oestrogen, so potentially explaining the differences in incidence in males. Dark skinned people also have a higher incidence of autism, and autism-related disorders increase with distance from the equator. This theory and hypothesis is worthy of more research.
Researchers describe 2 cases of scurvy and vitamin D deficiency in children who also had cognitive disorders, highlighting the importance of adequate good quality nutrition and exercise, and dietary histories in at-risk children.46
Maternal smoking
A single cross-sectional survey of 546 children and their mothers found that maternal smoking during pregnancy was associated with high fetal testosterone levels, and a low ratio of the length of the 2nd and 4th fingers, which is thought to be related to increased incidence of autism. The observation only held for boys.47
Fetal testosterone and autism traits
Professor Baron-Cohen has undertaken much work in the area of high fetal testosterone levels being related to an increased incidence of autism later on. He presents recent research to support this linking high fetal amniotic testosterone levels with autistic traits as determined by written tests completed by mothers about their children (n = 74).48
This supports earlier work identifying a link between high fetal testosterone and abnormal social development and the concept of an ‘extreme male brain’. Animal research studies have also identified an association.49 More prospective work is required in this controversial area.
Pregnancy
A review of articles on epidemiological studies of pre- and perinatal risk factors for ASD demonstrated increased paternal50 and maternal age, maternal place of birth outside of Europe or North America, low birth weight, duration of gestation, and intra partum hypoxia were associated with ASD.51 Further prospective studies were recommended.
Danish researchers found that maternal age and ‘medicine’ use during pregnancy, but not birth interventions or fetal distress, was associated with an increased risk of autism in offspring. Low birth weight babies and congenital malformations were also positively associated.52
Examination of obstetric data of mothers who have given birth to children with ASD found an association with a wide range of complications: threatened abortion, epidural anaesthesia use, induction of labour, precipitate labours of less than 1 hour, fetal distress, low Apgars of less than 6 at 1 minute and caesarean section were all positive risk factors.53 Because there is no single causal association the conclusion is that underlying genetic factors interact with the environment.
Prenatal stress
Researchers in Ohio tried to correlate prenatal stressors for mothers and subsequent incidence of autism, and found a positive correlation from stressors occurring between 21–32 weeks and an increase in ASD.54 It is thought to cause pathological changes in the cerebellum consistent with autism. Prospective studies are recommended.
Alcohol during pregnancy
Excessive alcohol exposure during pregnancy can increase the risk of ASD as well as other neurodevelopmental disorders.55
Pre-term infants
A study of 91 pre-term infants who were found to have a 26% rate for development of autism, and risk factors included low birth weight, male gender, chorioamnionitis, acute intra partum haemorrhage, illness severity on admission, and abnormal MRI studies.56
A population-based case-control study found that mothers who had assisted reproduction had offspring with a lower incidence of autism than matched controls.57 This could be due to their generally better health, and warrants further research and bigger studies.
Pregnancy and folic acid
A hypothesis put forward by researchers is that the increased incidence in autism is related to the supplementation of pregnant mothers with folic acid, due to the survival of fetuses who would otherwise miscarry, with a combination of polymorphism of 5-methylenetetrahydrofolate reductase and consequent high homocysteine levels.58 They are now surviving in the presence of high folate levels but then after delivery are suffering neurodevelopmental disorders including autism, when the folate levels are not maintained, due to lack of methylation during this critical period. They suggest that detection of these polymorphisms as well as other methionine cycle enzymes would be helpful in detection of at risk infants.
Exercise
Two small studies have found that children with ASD are less active compared with their more ‘normal’ counterparts.59 There was no consistency to their exercise pattern.60
Table 7.1 provides a summary of the possible risk factors for autism.
| Genetic susceptibility | Male gender |
| Medical causes |
∗ Care must be taken with how these findings are interpreted as more research is required to validate these findings.
Vaccination and autism
MMR Vaccine
Wakefield and his team achieved great notoriety when they suggested an association between the MMR vaccine and inflammatory bowel disease in 1993, and again in 1998 when they described a type of inflammatory bowel disease that was associated with developmental disorders such as autism.61 Criticisms of their work have been discredited worldwide and attributed to studies being of small sample size, unblinded, lacking no controls, and conducted on highly selected patients with gastrointestinal disease.62
The link between behavioural change and vaccination was based on parental recall, and the timing of vaccination coincides with the age at which parents became concerned about their child’s development anyway. Criticisms also included lack of scientific evidence of any benefit in giving the individual components separately and that it would in fact cause a resurgence of these childhood illnesses because of reduction in vaccination uptake. Any possible link between MMR vaccine and an increased risk of ASD has been refuted by many large epidemiological studies; for example, from the UK (n = 498)63 and France (n = 6100).64 A retrospective study of all children vaccinated in Denmark65 from 1991 to 1998 showed less risk if any of ASD in vaccinated children, similarly supported by a lack of increased incidence of autism with MMR vaccine coverage over time in California66 and in UK general practices.67 Finland failed to find 1 serious adverse event from MMR vaccination over 14 years.68
This supports other research published in 2001, again refuting a link with the MMR vaccine, and also refuting the link between MMR and gastric enterocolitis, as there is no proven increased incidence of this colitis in the face of almost universal vaccination.69
Complementary therapies
Parents are presenting to their doctors armed with a myriad of complementary treatment suggestions and doctors should have an open mind to discussing and knowing about these treatments to encourage objectiveness about what works and what doesn’t and the safety of the various therapies and treatment modalities. It is vital the medical practitioner coordinates care with the various health practitioners for the autistic child and families concerned.70
The use of complementary medicine (CM) administered by parents for their autistic child is widespread, but trials have been small and limited in number, lacking sound clinical evidence. There have been anecdotes of benefit, especially if coupled with intensive behavioural and education intervention.71, 2
A review of 3 private paediatrician practices in the eastern US demonstrated a ubiquitous use of CM and delay in diagnosis of 18 months amongst sample patients. Causes attributed to autism as thought by the parents included genetic risk, immunisations and environmental exposure. Fifty percent of the parents of autistic children interviewed thought the child had at least 1 gastrointestinal, neurological and/or allergic symptom, and one-third had immunological symtoms.72
Mind–body medicine
Cognitive behaviour therapy (CBT)
Cognitive behaviour therapy (CBT) should be considered and offered to autistic children and family members in addition to a comprehensive integrated behavioural program, which includes applied behaviour analysis, cognitive approaches, developmental therapy, and structured teaching.4
A study of 47 children with high functioning ASD and anxiety responded well to family based CBT after 12 weekly group sessions with significant reductions in symptoms of anxiety and 71% no longer suffering from an anxiety disorder as such compared with a control group.73
A small pilot study (n = 16) in Singapore showed that a CBT program over 16 sessions improved symptoms of anxiety in children with high functioning ASD or Asperger’s syndrome (mean age 11.5 years), and reduced parental and teacher stress.74
A small study of high functioning adults with ASD responded well to CBT compared with controls that had treatment as usual.75
Israeli researchers examined cognitive improvement in children (n = 81) with autism, and found that improvement is not predictable from the baseline severity of autism, but that with cognitive improvement comes improvement in social behaviour and communication.76
Music and sound therapy
A Cochrane systematic review published in 2004 identified 6 randomised controlled trials (RCTs) with 1 cross-over trial, of which half showed improvement in features of ASD, auditory processing, quality of life and adverse events after 3 months (n = 171) with sound therapy.77 The technique used was Auditory Integration Therapy to reduce abnormal sound sensitivity in sufferers of ASD. There is still insufficient evidence for its use but warrants further research.
An update of the 2004 Cochrane review in 2006 included 3 small studies (n = 24) and found that after 1 week of daily sessions of music therapy that verbal and gestural communication skills improved, but not behaviour.78
In a recent randomised control study, Californian researchers investigated the use of a specific sound therapy called ‘Tomatis Sound Method’ and showed that although there was improvement in language development, it was unrelated to autism itself.79
In 2009, a small (n = 12) controlled trial on 11-year old autistic students was conducted, and found that background music was helpful in helping these children understand their emotions, so crucial to social interactions.80
Sleep
Sleep disturbance is common in autistic children and can negatively impact behaviour. Poor sleep patterns are associated with impaired behaviour and social interaction in these children.82 Furthermore, sleep disturbance is also associated with impaired health such as vision problems, upper respiratory tract infections, poor appetite and poor growth.83
Numerous studies, both large and small, have documented sleep disturbances in children with autism and ASD with longer duration of getting to sleep and increased night-time waking than the control groups.84–88 Concurrent factors such as younger age, bedtime rituals, asthma, ADHD, medication use, co-sleeping, and family history of sleep problems were also contributory. Epilepsy contributed to daytime sleepiness.89
Canadian researchers found that sleep disturbances may be related to abnormal organisation of neural networks interfering with the microstructure of sleep in ASD sufferers.90
Understandably, parents reported marked increased stress levels when their children had sleep difficulties.91
Behavioural strategies for sleep
Intervention behavioural strategies to improve sleep can improve daytime functioning and behaviour enormously in autistic children.92–95 Strategies include bedtime routines, reinforcement, effective instructions, partner support and extinction (removing reinforcement to reduce a specific behaviour) maintained consistently over time.96
Sleep apnoea
Concurrent sleep apnoea also requires attention and treatment in children with autism.4
A case report of a 5-year old female with ASD described significant improvement of sleep patterns and daytime functioning such as social communication, attention, and repetitive behaviours following adenotonsillectomy for the treatment of the sleep apnoea.97
Melatonin
Melatonin is a pineal hormone that plays a central part in regulating bodily rhythms and sleep. Studies suggest melatonin treatment may be helpful for sleep problems in children with ASD and Fragile X Syndrome.
In a very small trial of 7 children with ASD, the children were randomised to melatonin or placebo. Sleep improved significantly with melatonin supplementation resulting in reduced wakings per night, earlier onset of sleep and increased duration of sleep.98
A 4-week randomised placebo-controlled cross-over trial included 12 children with ASD and/or Fragile X Syndrome (11 males; mean age 5.47 years) demonstrated when compared with placebo children taking melatonin 3mgs nocte, overall mean night sleep duration was longer by 21 minutes, sleep-onset patency was shorter by 28 minutes and sleep-onset time was earlier by 42 minutes.99
Researchers in Europe have found that autistic children have a defect in melatonin synthesis due to reduced activity of aceylserotonin methyltransferase, the final enzyme in the melatonin biosynthetic pathway, and may be a risk factor for ASD. Mutation abnormalities in gene coding for this were found on the autosomal region on the sex chromosome.100 This highlights the crucial function of melatonin in human cognition and behaviour.
Sunshine
Lack of sunshine exposure and vitamin D deficiency may be a risk factor for autism (see section under risk factors). Sunshine is the main source of vitamin D produced by the body in response to direct skin exposure to ultraviolet B (UVB). This means that no or minimal exposure to sun can contribute to vitamin D deficiency, as seen in community groups with dress codes (e.g. wearing veils), and those living in geographically prone areas, especially over winter (southern or northern latitudes), working indoors (e.g. office work), institutionalised patients, and those who are bed-bound and requiring prolonged hospitalisation, particularly in dark skinned people who need longer sun exposure for adequate vitamin D absorption. (See also Chapter 30, Osteoporosis.) Vitamin D deficiency is likely to be the commonest nutritional deficiency in Australia and many other countries worldwide. Vitamin D has a multiplicity of roles involving virtually every body system.
Physical activity
Environmental
Heavy metal detoxification
Chelation therapy
This form of heavy metal detoxification has been used by many quarters claiming benefit.
An RCT of chelation therapy using dimercaptosuccinic acid to treat lead-exposed children failed to show any benefit.102
Hepatotoxicity and anaphylaxis have been reported as a side-effect of chelation,103 including reports of cardiac arrests as a result of hypocalcaemia from the wrong chelating agent being used (Na EDTA).104 Its use therefore is not recommended.
Redox and metallothioneins
Redox active metals, such as copper and zinc can play an important role in the healthy functioning of the nervous system.105 Metallothioneins are physiological proteins that naturally help to regulate the levels of redox active metals within the body and they form the first line of defence against toxic heavy metals as their structure incorporates multiple sulfhydryl groups. Consequently, the use of ‘metallothionein promoters’ could be of benefit to autistic children, although there is a lack of solid evidence to support their use.
Of interest, a study of 66 people from families with autistic children found a 30% incidence of anti-metallothionein IgG; however these antibodies did not correlate with the autistic children in these families.106
Zinc and copper ratios were measured retrospectively in 230 children with ASD and found to be much lower than normal controls, implying that this might indicate metallothionein enzyme dysfunction, and also be a biomarker of heavy metal toxicity.107
Glutathione has been implicated with this, an antioxidant tripeptide involved in DNA and protein synthesis, enzyme and immune system function. Glutathione is theorised to reduce oxidative stress implicated in autism, but there is no research to confirm this.2
A clinical trial involving combined anti-androgen and anti-heavy metal therapy over a 4-month period demonstrated significant improvements in sociability, cognitive awareness, behaviour, and clinical symptoms/behaviours of hyperandrogenemia observed in children with ASDs, resulting in significant decreases in blood androgens and increases in urinary heavy metal concentrations compared with the baseline.108 Minimal drug adverse effects were identified with this treatment, warranting additional studies in this area.
Nutritional influences
Gastrointestinal symptoms
Gastrointestinal symptoms are also exceedingly common in children with autism and ASD. A prevalence of 24% of at least 1 gastrointestinal (GI) symptom was found in a sample size of 137 children in a specialised clinic in the US.109 However there was no association between GI symptoms and developmental regression.
A lack of case controls limits the validity of these studies. However a cross-sectional study of structured interviews asking about a lifetime history of gastrointestinal symptoms have found that 70% of ASD sufferers complain of abnormal stools, constipation, vomiting and abdominal pain, compared with 42% of children with other developmental disorders and 28% of normal controls.110
Diet
Gluten and casein free diets
A recent Cochrane review explored the efficacy of gluten and casein free diets for ASD.111 It found only 1 trial of adequate quality for assessment, and found that it helped in the important area in reduction in autistic traits, though there was huge variation in the cognition, linguistic and motor skills. This has been cited as an important area of research.
A small randomised control prospective trial found no change in urinary casein and gliadin peptide levels of children on such a diet compared with the controls, refuting the increased gut permeability or ‘leaky gut’ theory, and also demonstrated no statistically significant change in behavioural markers in these children.112
Care is required with dietary restrictions and elimination of food groups in autistic children as this may lead to further micronutrient deficiencies, as autistic children are prone to poor nutritional intake and deficiencies due to self-restriction of food intake, especially for fibre, calcium, iron, vitamin E and vitamin D.113, 114
Two further rigorous studies are being conducted with bigger numbers and for at least 12 weeks: a single-blind trial in Norway115 and a double-blind trial in the US116 to assess dietary interventions on children’s behaviour with autism. Results are awaited with interest.
A study of 109 children with ASD found an association between gastrointestinal symptoms and ingestion of cow’s milk protein, but not for casein or soy, and demonstrated marked production and elevated levels of pro inflammatory cytokines TNF-alpha and IL-12.117 However, biochemical abnormalities do not equate to clinical effects, and more research is needed.
Probiotics
Probiotics may help ASD children, especially if they have a history of multiple courses of antibiotics and suffer entercolitis.118, 119
Clostridium histolyticum has been found in larger numbers in these children than normal controls and Vancomycin has been found to help gastrointestinal symptoms in children with severe regressive symptoms of ASD.4,120 It may help limit any toxic effects of abnormal bacteria on the bowel and production of a so-called ‘leaky gut’.
Nutritional supplements
Vitamins
Multivitamins
One trial showed improvement in sleep and gastrointestinal symptoms in children with ASD (n = 20) supplemented with a multi-mineral and vitamin supplement.121
Vitamin B6
Pyridoxine is involved in the synthesis of several neurotransmitters. Nutritional supplementation is very popular for children with autism.2
Small trials have demonstrated that children with autism have high serum vitamin B6 levels and low levels of the active form of the vitamin, pyridoxal 5 phosphate. They have also shown that these children have low pyridoxal kinase activity, which is necessary for neurotransmitter formation and over 100 other enzymatic reactions.122, 123
Interestingly, a trial demonstrated children with autism have abnormally high plasma levels of vitamin B6 even when not taking supplements compared to controls not taking supplements.119
Despite the findings above a small trial (n = 8) found an increase in verbal IQ with B6 supplementation compared with placebo, with 2 other trials failing to show an effect (n = 10 and 15).124, 125
More rigorous research is needed before blanket recommendations can be given.
Folic acid
Researchers identified a subgroup of autistic patients who demonstrated folate receptor autoimmunity with consequent blocking of folate binding to choroid epithelial cells, thereby reducing folate transport across the blood brain barrier into the CSF. These patients had low 5-methyltetrahydrofolate levels, despite normal serum folate. Supplementing this group with folate led to ‘partial or complete clinical recovery after 12 months’.126
Further to this, researchers have found preliminary evidence for genetic polymorphisms of dihydrofolate reductase leading to possible interactions between folate and glutamate and abnormal neural excitation.127
A case report of a 6-year old girl with autistic features had documented low CSF levels of 5-methyltetrahydrofolate, normal peripheral blood folate and serum vitamin B12 levels.128 Treatment with folinic acid corrected low CSF abnormalities and improved motor skills.
Vitamin B12
An open label trial of autistic children (n = 40) demonstrated that methylcobalamin and folinic acid treatment resulted in improved levels of glutathione, cysteinyl-glycine, cysteine, and reduced oxidised disulfide form of glutathione, and an increased glutathione redox ratio.129 No mention in the trial was made of clinical improvement in these children, and more research is warranted to ascertain whether this intervention is indeed to be recommended.
Vitamin C
Vitamin C may play an important role in fetal brain development and neurohormonal production. It is a cofactor in the conversion of tyrosine to dopamine and tryptophan to serotonin, which is an antioxidant, important for moods and also an immune function regulator.130
A double-blind, placebo-controlled trial (1993) of a small number of English autistic children (n = 18) of megadose vitamin C therapy (8g/70kg/day) demonstrated a significant improvement in behaviour in the ascorbic acid arm compared with the placebo arm.131
Minerals
Magnesium and Vitamin B6
An update of a systematic review from 2002 of combined vitamin B6-magnesium supplementation for treating children with ASD, has found that only 3 studies could be included, and that due to the small size of the studies, and poor methodological quality, no recommendation could be given.132
Research published a year after the Cochrane review, of 33 children with ASD and 36 controls, measured baseline red blood cell magnesium (Mg) levels and supplemented with a Mg-vitamin B6 combined supplement.133 Children with ASD demonstrated significantly lower red blood cell magnesium levels, and following supplementation for 6 months, demonstrated improved social interaction, communication, stereotyped restricted behaviour and abnormal/delayed functioning compared with controls. After cessation of supplements the behaviours deteriorated after a few weeks.
Iron and zinc
A small (n = 33) controlled clinical trial found a correlation between low iron intake and sleep disturbance, which improved with supplementation, and also noted a high incidence of iron deficiency (69% of preschoolers and 35% of school age) in children with autism and related disorders.134
Other supplements
Omega-3 fatty acids
Omega-3 and omega-6 fatty acids are vital for neurodevelopmental growth.2
A review of randomised double-blind control trials demonstrated that DHA and EPA supplementation improved symptoms in autism disorder.135
Apart from this review, 4 small trials had mixed results, and 1 small double-blind placebo controlled-trial of 13 adolescents showed preliminary behavioural benefit especially in hyperactivity after 6 weeks of 1.5gram/day EFA supplementation.136 However, 2 small trials could not replicate these findings and demonstrate any benefit with omega-3 supplementation.137, 138
In fact, a very small study of 16 male children with high functioning autism showed a naturally occurring significant increase in DHA fatty acids, with an increase in the 3:6 ratio.139 The researchers hypothesise that 3-polyunsaturated fatty acids (PUFAs) may cause alterations in serotonin turnover and the immune response system, and advise caution regarding omega-3 supplementation.
A study of children with autism and Asperger’s syndrome demonstrated significant fatty acid deficiency but the children responded to supplementation in those with larger increases in blood levels of EPA and DHA than supplemented controls.140 Supplemented autistic children also had reduced pro-inflammatory phospholipase A2 levels than non-supplemented autistic children.
Physical therapies
Acupuncture
A small pilot study of 2 cases of autistic children administered intensive electroacupuncture over 8 weeks and found variable responses in certain aspects of social functioning and relatedness, but much more investigation is obviously required.141
Hyperbaric oxygen therapy
A recent multi-centre randomised control trial (RCT) of 62 children used hyperbaric oxygen therapy and found that 30% of children were ‘very much improved’ or ‘much improved’ compared with 8% of controls. Treated children had improved language, social skills and eye contact.142 The therapy used a pressure of 1.3 atmospheres and 24% oxygen for an hour and was administered 40 times over a month, and was very well tolerated. The authors speculated that hyperbaric oxygen works by decreasing brain inflammation and therefore swelling and improving function via increased oxygen delivery. PET scans confirmed this improved perfusion of oxygen supply and brain activity in these children.
More well-performed research is required to confirm these findings.
Massage
An open exploratory study of 14 parents and children involved in a ‘Training and Support Program’ demonstrated improvement in children’s’ sleep patterns, openness to being touched and relaxation.143 They were also able to undertake routine tasks such as dressing more easily. At the end of 16 weeks they were actually asking for further massage. This has implications for helping enhance the emotional bonds between parent and child.144, 145
An open design US study (n = 20) comparing autistic children who were massaged for 15 minutes every evening to another group being read Dr Seuss stories found that massage therapy improved social interactions, better sleep and less stereotyped behaviour compared with the reading group.146
One controlled study of 13 young autistic children in China treated with a form of qigong massage daily for 5 months showed that they had significantly improved socialisation, basic living skills, bowel and sleep normalisation, and reduction in sensory impairment.147
Conclusion
In view of the rising global trend in autism worldwide, it would appear that there is an urgent need for well-performed studies to explore risk factors, lifestyle factors and treatment options for children with autism and ASD. There are no lifestyle and complementary treatments that can effectively cure autism but many may be of benefit for improving social skills and behaviour patterns in an autistic child.
For example, exercise, restoring adequate sleep, treating sleep apnoea if necessary, sunshine exposure and dietary changes may show clinical benefit on behaviour patterns associated with ASD. Counselling and cognitive therapy for the high functioning child with ASD and for the parents who need substantial emotional support may be of benefit. Music and massage therapy, whilst not demonstrating high level evidence, are relatively safe and can help with sleep and behaviour patterns. Table 7.2 summarises the level of evidence for some CM therapies for ASD.
Table 7.2 Levels of evidence for lifestyle and complementary medicines/therapies in the management of Autism Spectrum Disorder (ASD)
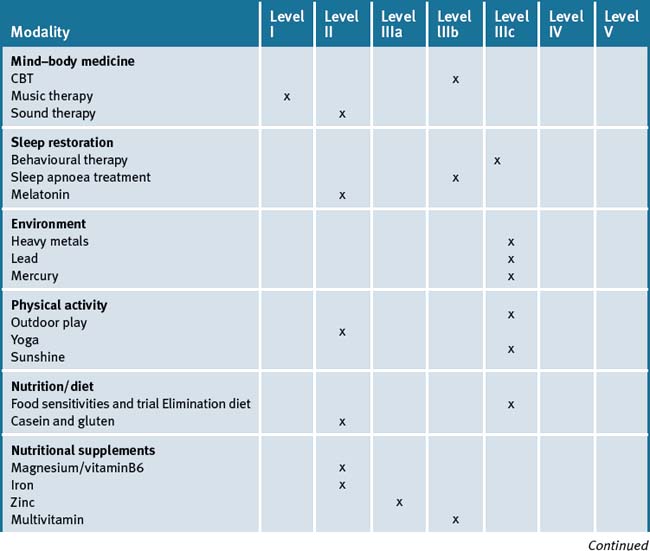
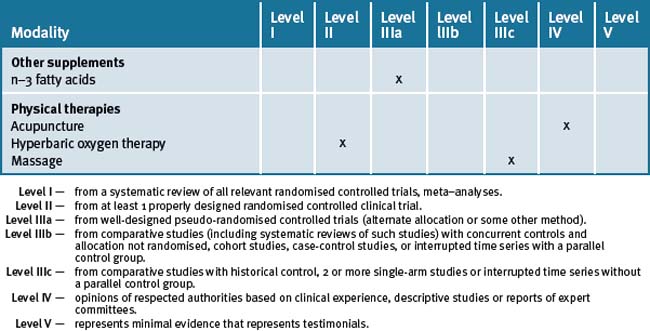
ASD is a condition that is difficult to treat, and challenges parents, family members, any health practitioner and teachers. The parents require support on many levels. The child with ASD requires coordinated care from a team of carers.
Clinical tips handout for patients — Autistic Spectrum Disorder (ASD)
1 Lifestyle advice
Sunshine
3 Mind–body medicine
4 Environment
5 Dietary changes
6 Nutritional supplementation
Probiotics
Dosage: 1–2 capsules before breakfast.
Side-effects: if using wrong probiotic, digestion symptoms may get worse.
Contra-indications: true dairy allergies if dairy in preparation.
Vitamin D3
Dosage: safe sunshine exposure of skin is the safest source of vitamin D.
Vitamin D (cholecalciferol 1000 international units).
Doctors should check blood levels and suggest supplementation if levels are low.
Children at risk: 200–400IU daily under medical supervision.
Pregnant and lactating women at risk: under medical supervision.
Vitamin B6
Do not provide as a single nutrient.
Indication: to reduce hyperactivity/anxiety and increase attention in combination with magnesium.
Dosage: up to 20 mg Pyridoxal-5-Phosphate (Activated B6).
Results: 1–3 months: always needs to be combined with B complex in long-term e.g. Multi B complex.
Contraindications: caution with amiodarone, phenobarbitone, phenytoin.
Magnesium
Zinc
Supplements that may be of assistance for children with digestive problems
Sleep disturbance
Melatonin
1 Baird G., Cass H., Slonims V. Diagnosis of autism BMJ. 2003;327:488-493.
2 Angley M., Semple S., Hewton C., et al. Children with Autism. Part 2–Management with Complementary Medicines and Dietary Interventions. Australian Family Physician. 2007 October;36(10):827-829.
3 Altevogt B.M., Hanson S.L., Leshner A.I.. Autism and the environment: challenges and opportunities for research. Forum on Neuroscience and Nervous System Disorders, 2001. Institute of Medicine, Washington, DC, USA. Online. Available baltevogt@nas.edu, 2001. (accessed 27-08-09)
4 Myers S.M., Johnson C.P. Management of Children With Autism Spectrum Disorders, the Council on Children With Disabilities. Pediatrics. 2007 November;120(5):1162-1182. doi:10.1542/peds.2007–2362
5 Angley M., Ellis D., Chan W. Children with Autism. Part 1-Recognition and Pharmacological Management. Australian Family Physician. 2007 September;36(9):741-744.
6 Baron Cohen S., Allen J., Gillberg C. Can autism be detected at 18 months? The needle the haystack and the CHAT. British Journal of Psychiatry. 1992;161:839-843.
7 Baron Cohen S., Wheelwright S., Cox A., et al. Early identification of autism by the Checklist for Autism in Toddlers (CHAT). J R Soc Med. 2000;93:521-525.
8 Baird G., et al. A screening instrument for autism at 18 months of age: a 6 year follow up study. Am.Acad. Child and Adolesc. Psychiatry. 2000;39:694-702.
9 Clifford S.M., Dissanayake C. The early development of joint attention in infants with autistic disorder using home video observations and parental interview. J Autism Dev Disord. 2008 May;38(5):791-805.
10 Clifford S.M., Dissanayake C.. The early development of Joint Attention in Infants with Autistic Disorder Using Home Video Observations and Parental Interview. Journal of Autism and Development Disorders, 2008 May;38(5):791-805 http://www.ncbi.nlm.nih.gov/pubmed/17917803, 2008 May. Online. Available(accessed 27–08–09)
11 Barbaro J.. Dissanayake C Autism Spectrum Disorders in infancy and toddlerhood: A review of evidence on early signs, early identification tools, and early diagnosis. Journal of Developmental and Behavioral Pediatrics. , 2009. Publisher, Forthcoming. http://journals.lww.com/jrnldbp/pages/default.aspx, 2009. Online. Available(accessed 27-08-09)
12 Hollander E., Phillips A.T., Yeh C- C. Targeted treatments for symptom domains in child and adolescent autism. Lancet. 2003 Aug 30;362(9385):732-734.
13 Barbaresi W.J., Katusic S.K., Colligan R.C., et al. The incidence of autism in Olmsted County, Minnesota, 1976–1997: results from a population-based study. Arch Pediatr Adolesc Med. 2005 Jan;159(1):37-44.
14 Baird G., Simonoff E., Pickles A., et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet. 2006 Jul 15;368(9531):210-215.
15 Barbaresi W.J., Katusic S.K., Colligan R.C., et al. The incidence of autism in Olmsted County, Minnesota, 1976–1997: results from a population-based study. Arch Pediatr Adolesc Med. 2005 Jan;159(1):37-44.
16 Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators. Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ. 9, 2007 Feb. 56(1):1-11
17 Newschaffer C.J., Falb M.D., Gurney J.G. National Autism Prevalence Trends From United States. Special Education Data. Pediatrics. 2005 Mar;115(3):e277-e282.
18 Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66:3-8.
19 Williams K., MacDermott S., Ridley G., et al. The prevalence of autism in Australia. Can it be established from existing data? J Paediatr Child Health. 2008 Sep;44(9):504-510. Epub 2008 Jun 28
20 Williams K., Glasson E.J., Wray J., et al. Incidence of autism spectrum disorders in children in two Australian states. Med J Aust. 2005 Feb 7;182(3):108-111.
21 Waterhouse L. Autism overflows: increasing prevalence and proliferating theories. Neuropsychol Rev. 2008 Dec;18(4):273-286. Epub 2008 Nov 18
22 Johnson C.P., Myers S.M. the Council on Children with Disabilities. Identification and Evaluation of Children With Autism Spectrum Disorders. Pediatrics. 2007;120:1183-1215. doi:10.1542/peds.2007–2361
23 Wang K, Zhang H, Ma D, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders Nature advance online publication 28 April 2009 doi:10.1038/nature07999; Received 28 November 2008; Accepted 18 March 2009; Published online 28 April 2009.
24 Hrdlicka M., Komarek V., Propper L., Kulisek R., Zumrova A., Faladova L., Havlovicova M., Sedlacek Z., Blatny M., Urbanek T. Eur Child Adolesc Psychiatry. 2004 Aug;13(4):209-213.
25 Myers S.M., Johnson C.P. the Council on Children With Disabilities. Management of Children With Autism Spectrum Disorders. Pediatrics. 2007 November;120(5):1162-1182. doi:10.1542/peds.2007–2362
26 Croen L.A., Braunschweig D., Haapanen L., et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008 Oct 1;64(7):583-588. Epub 2008 Jun 20
27 Yorbik O., Akay C., Sayal A., et al. Zinc status in autistic children. The Journal of Trace Elements in Experimental Medicine. 2004;17:101-107.
28 Angley M., Ellis D., Chan W., et al. Children with Autism. Part 1-Recognition and Pharmacological Management. Australian Family Physician. Setember 2007;36(9):741-744.
29 Roberts E., et al. Maternal Residence Near Agricultural Pesticide Applications and Autism Specrum Disorders amond Children in the California Central Valley. Environ Health Perspect. 2008 Apr;116(4):A155.
30 Windham G.C., Zhang L., Gunier R., et al. Autism Spectrum Disorders in Relation to Distribution of Hazardous Air Pollutants in the San Francisco Bay Area. Environmental Health Perspectives. Research Triangle Park. 2006 Sep;114(9):1438-1444. (7 pp.)
31 Fitzpatrick M. Autism and environmental toxicity. The Lancet Neurology. 2007 Apr. (1 pg)
32 Grandjean P., Landrigan P.J. Developmental neurotoxicity of industrial chemicals. The Lancet. London. 2006 Dec 16–22;368(9553):2167-2178. (12 pp.)
33 Stein J., Schettler T., Wallinga D., et al. In harm’s way: toxic threats to child development. J Dev Behav Pediatr. 2002 Feb;23(1 Suppl):S13-S22.
34 Roberts E., et al. Maternal Residence Near Agricultural Pesticide Applications and Autism Specrum Disorders amond Children in the California Central Valley. Environ Health Perspect. 2008 Apr;116(4):A155.
35 Rosas L.G., Eskenazi B. Pesticides and Child Neurodevelopment. Current Opinion Pediatric. 2008 April;20(2):191-197.
36 Waldman M., Nicholson S., Adilov N., et al. Autism prevalence and precipitation rates in California, Oregon, and Washington counties. Arch Pediatr Adolesc Med. 2008 Nov;162(11):1026-1034.
37 Gayle C Windham, Lixia Zhang, Robert Gunier, et al. Autism Spectrum Disorders in Relation to Distribution of Hazardous Air Pollutants in the San Francisco Bay Area. Environmental Health Perspectives. Research Triangle Park. 2006 Sep;114(9):1438-1444. (7 pp.)
38 Palmer R.F., Blanchard S., Wood R. Proximity to point sources of environmental mercury release as a predictor of autism prevalence. Health Place. 2009 Mar;15(1):18-24.
39 Geier D.A., Kern J.K., Garver C.R., et al. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009 May 15;280(1–2):101-108. Epub 2008 Sep 25
40 Adams J.B., Romdalvik J., Ramanujam V.M., et al. Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A. 2007 Jun;70(12):1046-1051.
41 Croen L.A., Matevia M., Yoshida C.K., et al. Maternal Rh D status, anti-D immune globulin exposure during pregnancy, and risk of autism spectrum disorders. Am J Obstet Gynecol. 2008 Sep;199(3):234. e1–e6. Epub 2008 Jun 13
42 Miles J.H., Takahashi T.N. Lack of association between Rh status, Rh immune globulin in pregnancy and autism. Am J Med Genet A. 2007 Jul 1;143A(13):1397-1407.
43 Kinney D.K., Miller A.M., Crowley D.J., et al. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008 Mar;38(3):481-488. Epub 2007 Jul 6
44 Cannell J.J., Hollis B.W. Use of vitamin D in clinical practice. Altern Med Rev. 2008 Mar;13(1):6-20.
45 Cannell J.J. Autism and vitamin D. Med Hypotheses. 2008;70(4):750-759. Epub 2007 Oct 24
46 Noble J.M., Mandel A., Patterson M.C. Scurvy and rickets masked by chronic neurologic illness: revisiting ‘psychologic malnutrition’. Pediatrics. 2007 Mar;119(3):e783-e790.
47 Rizwan S., Manning J.T., Brabin B.J. Maternal smoking during pregnancy and possible effects of in utero testosterone: evidence from the 2D:4D finger length ratio. Early Hum Dev. 2007 Feb;83(2):87-90. Epub 2006 Jun 30
48 Auyeung B., Baron-Cohen S., Ashwin E., et al. Fetal testosterone and autistic traits. Br J Psychol. 2009 Feb;100(Pt 1):1-22. Epub 2008 Jun 10
49 Knickmeyer R.C., Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J Child Neurol. 2006 Oct;21(10):825-845.
50 Reichenberg A., Gross R., Weiser M., et al. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63:1026-1032.
51 Kolevzon A., Gross R., Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007 Apr;161(4):326-333.
52 Maimburg R.D., Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr Scand. 2006 Oct;114(4):257-264.
53 Glasson E.J., Bower C., Petterson B., et al. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004 Jun;61(6):618-627.
54 Kinney D.K., Munir K.M., Crowley D.J., et al. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008 Oct;32(8):1519-1532. Epub 2008 Jun 13
55 Aronson M., Hagberg B., Gillberg C. Attention deficits and autistic spectrum problems in children exposed to alcohol during gestation: a follow-up study. Dev Med Child Neurol. 1997;39:583-587.
56 Limperopoulos C., Bassan H., Sullivan N.R., et al. Positive screening for autism in ex-pre-term infants: prevalence and risk factors. Pediatrics. 2008 Apr;121(4):758-765.
57 Maimburg R.D., Vaeth M. Do children born after assisted conception have less risk of developing infantile autism? Hum Reprod. 2007 Jul;22(7):1841-1843. Epub 2007 Apr 24
58 Rogers E.J. Has enhanced folate status during pregnancy altered natural selection and possibly Autism prevalence? A closer look at a possible link. Med Hypotheses. 2008 Sep;71(3):406-410. Epub 2008 Jun 2
59 Pan C.Y., Frey G.C. Physical activity patterns in youth with autism spectrum disorders. J Autism Dev Disord. 2006 Jul;36(5):597-606.
60 Pan CY. Objectively measured physical activity between children with autism spectrum disorders and children without disabilities during inclusive recess settings in Taiwan. J Autism Dev Disord. 2008 Aug;38(7):1292-1301. Epub 2007 Dec 18
61 Wakefield A.J., Anthony A., Murch S.H., et al. Enterocolitis in Children With Developmental Disorders. Am J Gastroenterol. 2000;95:2285-2295.
62 McIntyre P., McIntyre C.R. Editorial: MMR, autism and inflammatory bowel disease: responding to patient concerns using an evidence-based framework. There is no convincing evidence that the MMR vaccine is associated with autism or IBD. MJA. 2001;175:129-132.
63 Taylor B., Miller E., Farrington C.P., et al. Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. Lancet. 1999;353:2026-2029.
64 Fombonne E., Du Mazaubrun C., Cans C., et al. Autism and associated medical disorders in a French epidemiological survey. J Am Acad Child Adolesc Psychiatry. 1997;36:1561-1569.
65 Madsen K.M., Hviid A., Vestergaard M., et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002;347:1477-1482.
66 Dales L., Hammer S.J., Smith N.J. Time trends in autism and in MMR immunization coverage in California. JAMA. 2001;285:1183-1185.
67 Kaye J.A., del Mar Melero-Montes M., Jick H. Mumps, measles, and rubella vaccine and the incidence of autism recorded by general practitioners: a time trend analysis. BMJ. 2001;322:460-463.
68 Patja A., Davidkin I., Kurki T., et al. Serious adverse events after measles-mumps-rubella vaccination during a fourteen-year prospective follow-up. Pediatr Infect Dis J. 2000;19:1127-1134.
69 Fombonne E., Chakrabarti S. No Evidence for a New Variant of Measles-Mumps-Rubella–Induced Autism. Pediatrics. 2001 October;108(4):1-8.
70 Levy S.E., Hyman S.L. Use of complementary and alternative treatments for children with autistic spectrum disorders is increasing. Pediatr Ann. 2003 Oct;32(10):685-691.
71 Hanson E., Kalish L.A., Bunce E., et al. Use of complementary and alternative medicine among children diagnosed with autism spectrum disorder. J Autism Dev Disord. 2007 Apr;37(4):628-636.
72 Harrington J.W., Rosen L., Garnecho A., et al. Parental perceptions and use of complementary and alternative medicine practices for children with autistic spectrum disorders in private practice. J Dev Behav Pediatr. 2006 Apr;27(2 Suppl):S156-S161.
73 Chalfant A.M., Rapee R., Carroll L. Treating anxiety disorders in children with high functioning autism spectrum disorders: a controlled trial. J Autism Dev Disord. 2007 Nov;37(10):1842-1857. Epub 2006 Dec 15
74 Ooi Y.P., Lam C.M., Sung M., et al. Effects of cognitive-behavioural therapy on anxiety for children with high-functioning autistic spectrum disorders. Singapore Med J. 2008 Mar;49(3):215-220.
75 Turner-Brown L.M., Perry T.D., Dichter G.S., et al. Brief report: feasibility of social cognition and interaction training for adults with high functioning autism. J Autism Dev Disord. 2008 Oct;38(9):1777-1784. Epub 2008 Feb 2
76 Ben Itzchak E., Lahat E., Burgin R., et al. Cognitive, behavior and intervention outcome in young children with autism. Res Dev Disabil. 2008 Sep-Oct;29(5):447-458. Epub 2007 Oct 17
77 Sinha Y., Silove N., Wheeler D., et al. Auditory integration training and other sound therapies for autism spectrum disorders. Cochrane Database Syst Rev. (1):2004. CD003681
78 Gold C., Wigram T., Elefant C. Music therapy for autistic spectrum disorder. Cochrane Database Syst Rev. (2):2006 Apr 19. CD004381
79 Corbett B.A., Shickman K., Ferrer E. Brief report: the effects of Tomatis sound therapy on language in children with autism. J Autism Dev Disord. 2008 Mar;38(3):562-566.
80 Katagiri J. The effect of background music and song texts on the emotional understanding of children with autism. J Music Ther. 2009 Spring;46(1):15-31.
81 Sams M.J., Fortney E.V., Willenbring S. Occupational therapy incorporating animals for children with autism: A pilot investigation. Am J Occup Ther. 2006 May-Jun;60(3):268-274.
82 Malow B.A., Marzec M.L., McGrew S.G., et al. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep. 2006 Dec 1;29(12):1563-1571.
83 Gail Williams P., Sears L.L., Allard A. Sleep problems in children with autism. J Sleep Res. 2004 Sep;13(3):265-268.
84 Allik H., Larsson J.O., Smedje H. Sleep patterns in school-age children with Asperger syndrome or high-functioning autism: a follow-up study. J Autism Dev Disord. 2008 Oct;38(9):1625-1633. Epub 2008 Feb 22
85 Goodlin-Jones B.L., Tang K., Liu J., et al. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. J Am Acad Child Adolesc Psychiatry. 2008 Aug;47(8):930-938.
86 Krakowiak P., Goodlin-Jones B., Hertz-Picciotto I., et al. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J Sleep Res. 2008 Jun;17(2):197-206.
87 Bruni O., Ferri R., Vittori E., et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep. 2007 Nov 1;30(11):1577-1585.
88 Miano S., Bruni O., Elia M., et al. Sleep in children with autistic spectrum disorder: a questionnaire and polysomnographic study. Sleep Med. 2007 Dec;9(1):64-70. Epub 2007 Aug 28
89 Liu X., Hubbard J.A., Fabes R.A., et al. Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry Hum Dev. 2006 Winter;37(2):179-191.
90 Limoges E., Mottron L., Bolduc C., et al. Atypical sleep architecture and the autism phenotype. Brain. 2005 May;128(Pt 5):1049-1061. Epub 2005 Feb 10
91 Goodlin-Jones B.L., Tang K., Liu J., Anders T.F. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. J Am Acad Child Adolesc Psychiatry. 2008 Aug;47(8):930-938.
92 Johnson K.P., Malow B.A. Assessment and pharmacologic treatment of sleep disturbance in autism. Child Adolesc Psychiatr Clin N Am. 2008 Oct;17(4):773-785. viii
93 Polimeni M.A., Richdale A.L., Francis A.J. A survey of sleep problems in autism, Asperger’s disorder and typically developing children. J Intellect Disabil Res. 2005 Apr;49(Pt 4):260-268.
94 Johnson K.P., Malow B.A. Sleep in children with autism spectrum disorders. Curr Neurol Neurosci Rep. 2008 Mar;8(2):155-161.
95 Polimeni M.A., Richdale A.L., Francis A.J. A survey of sleep problems in autism, Asperger’s disorder and typically developing children. J Intellect Disabil Res. 2005 Apr;49(Pt 4):260-268.
96 Weiskop S., Richdale A., Matthews J. Behavioural treatment to reduce sleep problems in children with autism or fragile X syndrome. Dev Med Child Neurol. 2005 Feb;47(2):94-104.
97 Malow B.A., McGrew S.G., Harvey M., et al. Impact of treating sleep apnea in a child with autism spectrum disorder. Pediatr Neurol. 2006 Apr;34(4):325-328.
98 Garstang J., Wallis M. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child Care Health Dev. 2006 Sep;32(5):585-589.
99 Wirojanan J., Jacquemont S., Diaz R., et al. The Efficacy of Melatonin for Sleep Problems in Children with Autism, Fragile X Syndrome, or Autism and Fragile X Syndrome. Journal of Clinical Sleep Medicine. 2009;5(2):145-150.
100 Melke J., Botros H.G., Chaste P., et al. Abnormal melatonin synthesis in autism spectrum disorders. Molecular Psychiatry. 2008;13:90-98. doi:10.1038/sj.mp.4002016; published online 15 May 2007
101 Pitetti K.H., Rendoff A.D., Grover T., et al. The efficacy of a 9–month treadmill walking program on the exercise capacity and weight reduction for adolescents with severe autism. J Autism Dev Disord. 2007 Jul;37(6):997-1006.
102 Dietrich K.N., Ware J.H., Salganik M., et al. Effect of chelation therapy on the neuropsychological and behavioral development of lead exposed children after school entry. Pediatrics. 2004;114:19-26.
103 Angley M., Semple S., Hewton C., et al. Children and Autism. Part 2– Management with Complementary Medicines and Dietary Interventions Aust Fam Physician. 2007 Oct;36(10):827-830.
104 Beauchamp R.A., Willis T.M., Betz T.G., et al. Deaths Associated With Hypocalcemia From Chelation Therapy-Texas, Pennsylvania, and Oregon, 2003–2005. JAMA Chicago. 2006 May 10;295(18):2131-2133. (3 pp)
105 Angley M., Semple S., Hewton C., et al. Children and Autism. Part 2–Management with Complementary Medicines and Dietary Interventions. Aust Fam Physician. 2007 Oct;36(10):827-830.
106 Russo A.F. Anti-metallothionein IgG and levels of metallothionein in autistic families. Swiss Med Wkly. 2008 Feb 9;138(5–6):70-77.
107 Faber S., Zinn G.M., Kern J.C.2nd, et al. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers. 2009 May;14(3):171-180.
108 Geier D.A., Geier M.R. A clinical trial of combined anti-androgen and anti-heavy metal therapy in autistic disorders. Neuro Endocrinol Lett. 2006 Dec;27(6):833-838.
109 Molloy C.A., Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003 Jun;7(2):165-171.
110 Myers Scott M. Chris Plauché Johnson, the Council on Children With Disabilities. Management of Children With Autism Spectrum Disorders. Pediatrics. 2007 November;120(5):1162-1182. (doi:10.1542/peds.2007–2362)
111 Millward C., Ferriter M., Calver S., et al. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. (2):2004. CD003498. Update in: Cochrane Database Syst Rev 2008;(2):CD003498
112 Elder J.H. The gluten-free, casein-free diet in autism: an overview with clinical implications. Nutr Clin Pract. 2008 Dec-2009 Jan;23(6):583-588.
113 Herndon A.C., DiGuiseppi C., Johnson S.L., et al. Does nutritional intake differ between children with autism spectrum disorders and children with typical development? J Autism Dev Disord. 2009 Feb;39(2):212-222. Epub 2008 Jul 4
114 Goday P. Whey Watchers and Wheat Watchers: The Case Against Gluten and Casein in Autism. Nutrition in Clinical Practice. 2008 Dec;23(6):581-582.
115 Clinical Trails Registry. Scan Brit. Dietary Intervention in Autism. http://clinicaltrials.gov/ct2/show/NCT00614198 Accessed Aug 8 2008.
116 Clinical Trials Registry. Diet and Behaviour in Young Children with Autism. http://clinicaltrials.gov/ct/show/NCT00090428 Accessed Aug 8 2008.
117 Jyonouchi H., Geng L., Ruby A., et al. Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. J Pediatr. 2005 May;146(5):605-610.
118 Garvey J. Diet in autism and associated disorders. J Fam Health Care. 2002;12:34-38.
119 Horvath K., Perman J.A. Autism and gastrointestinal symptoms. Curr Gastroenterol Rep. 2002;4:251-258.
120 Finegold S.M., Molitoris D., Song Y., et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002 Sep 1;35(Suppl 1):S6-S16.
121 Adams J.B., Holloway C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J Altern Complement Med. 2004 Dec;10(6):1033-1039.
122 Adams J.B., Holloway C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J Altern Complement Med. 2004 Dec;10(6):1033-1039.
123 Adams J.B., George F., Audhya T. Abnormally high plasma levels of vitamin B6 in children with autism not taking supplements compared to controls not taking supplements. J Altern Complement Med. 2006 Jan-Feb;12(1):59-63.
124 Kuriyama S., Kamiyama M., Watanabe M., et al. Pyridoxine treatment in a subgroup of children with pervasive developmental disorders. Dev Med Child Neurol. 2002;44:284-286.
125 Angley M., Semple S., Hewton C., et al. Children and Autism Part 1–Recognition and Pharmacological Management. Aust Fam Physician. 2007 Oct;36(10):827-830.
126 Ramaekers V.T., Blau N., Sequeira J.M., et al. Folate receptor autoimmunity and cerebral folate deficiency in low-functioning autism with neurological deficits. Neuropediatrics. 2007 Dec;38(6):276-281.
127 Adams M., Lucock M., Stuart J., et al. Preliminary evidence for involvement of the folate gene polymorphism 19bp deletion-DHFR in occurrence of autism.Med Hypotheses. 2008;70(4):750-759. Epub 2007 Oct 24
128 Moretti P., Sahoo T., Hyland K., et al. Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology. 2005 Mar 22;64(6):1088-1090.
129 James S.J., Melnyk S., Fuchs G., et al. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr. 2009 Jan;89(1):425-430. Epub 2008 Dec 3
130 McEachin J.J., Smith T., Lovaas O.I. Long term outcomes for children with autism who received early intensive behavioral treatment. Am J Ment Retard. 1993;97:359-372.
131 Dolske M.C., Spollen J., McKay S., et al. A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog Neuropsychopharmacol Biol Psychiatry. 1993 Sep;17(5):765-774.
132 Nye C, Brice A. Combined vitamin B6–magnesium treatment in autism spectrum disorder. Cochrane Database Syst Rev 2005 Oct 19;(4):CD003497.Update of: Cochrane Database Syst Rev. 2002;(4):CD003497.
133 Mousain-Bosc M., Roche M., Polge A., et al. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6. II. Pervasive developmental disorder-autism. Magnes Res. 2006 Mar;19(1):53-62.
134 Dosman C.F., Brian J.A., Drmic I.E., et al. Children with autism: effect of iron supplementation on sleep and ferritin. Pediatr Neurol. 2007 Mar;36(3):152-158.
135 Kidd P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007 Sep;12(3):207-227.
136 Amminger G.P., Berger G.E., Schäfer M.R., et al. Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Biol Psychiatry. 2007 Feb 15;61(4):551-553. Epub 2006 Aug 22
137 Peregrin T. Registered dietitians’ insights in treating autistic children. J Am Diet Assoc. 2007 May;107(5):727-730.
138 Politi P., Cena H., Comelli M., et al. Behavioral effects of omega-3 fatty acid supplementation in young adults with severe autism: an open label study. Arch Med Res. 2008 Oct;39(7):682-685.
139 Sliwinski S., Croonenberghs J., Christophe A., et al. Polyunsaturated fatty acids: do they have a role in the pathophysiology of autism? Neuro Endocrinol Lett. 2006 Aug;27(4):465-471.
140 Bell J.G., MacKinlay E.E., Dick J.R., et al. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot Essent Fatty Acids. 2004 Oct;71(4):201-204.
141 Chen W.X., Wu-Li L., Wong V.C. Electroacupuncture for children with autism spectrum disorder: pilot study of 2 cases. J Altern Complement Med. 2008 Oct;14(8):1057-1065.
142 Rossignol D.A., Rossignol L.W., Smith S., et al. Hyperbaric treatment for children with autism: a multicenter, randomized, double-blind, controlled trial. BMC Pediatr. 2009 Mar 13;9:21.
143 Cullen L.A., Barlow J.H., Cushway D. Positive touch, the implications for parents and their children with autism: an exploratory study. Complement Ther Clin Pract. 2005 Aug;11(3):182-189.
144 Williams T.I. Evaluating effects of aromatherapy massage on sleep in children with autism: a pilot study. Evid Based Complement Alternat Med. 2006 Sep;3(3):373-377. Epub 2006 Apr 19
145 Cullen L., Barlow J. Kiss, cuddle, squeeze’: the experiences and meaning of touch among parents of children with autism attending a Touch Therapy Program. J Child Health Care. 2002 Sep;6(3):171-181.
146 Escalona A., Field T., Singer-Strunck R., Cullen C., Hartshorn K. Brief report: improvements in the behavior of children with autism following massage therapy. J Autism Dev Disord. 2001 Oct;31(5):513-516.
147 Silva L.M., Cignolini A., Warren R., et al. Improvement in sensory impairment and social interaction in young children with autism following treatment with an original qigong massage methodology. Am J Chin Med. 2007;35(3):393-406.