Chapter 58 Auditory Implants for the Central Nervous System
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
General technical and theoretical considerations of central auditory implantation and stimulation have been reviewed elsewhere.1,2
PATIENT SELECTION
Patients originally received the ABI under a protocol monitored by the FDA. The criteria for implantation are listed in Table 58-1. The device originally was designed for patients with NF-2 manifesting bilateral vestibular schwannomas, although others with compromised auditory nerves were considered to be eventual ABI candidates. At least 90 per cent of NF-2 patients exhibit bilateral eighth nerve neuromas.3 An unpublished review of patients with NF-2 seen at the House Ear Clinic revealed that two thirds had bilateral internal auditory canal–cerebellopontine angle (CPA) tumors alone or with one other tumor as the only central nervous system manifestation of their disease. The patients were young (average age, 28 years). With improvements in medical care and surgical techniques, the life span of most of these patients has been significantly prolonged. Restoration of even rudimentary auditory function can enhance their quality of life and ability to function in a hearing world. Our results have shown that the multichannel ABI has the potential of offering even greater benefit.
| Evidence of bilateral seventh and eighth cranial nerve tumors |
| involving the internal auditory canal or cerebellopontine angle |
| Language competency |
| Age 12 years or older |
| Psychologic suitability |
| Willingness to comply with research follow-up protocol |
| Realistic expectations |
The management of bilateral acoustic neuromas should be highly individualized.4 Hearing preservation remains an ideal goal in the management of these tumors in patients with NF-2, and early identification and treatment have permitted this in a number of cases. An intact auditory system is highly desirable in preference to an artificial means of restoring hearing. Therefore, preserving as much of the patient’s own hearing as possible is paramount. Patients meeting the criteria listed in Table 58-2 may be considered and observed accordingly. The availability of the ABI provides an alternative to a desperate attempt to preserve nonserviceable hearing when large tumors are removed and hearing conservation is unlikely.
TABLE 58-2 Criteria for Observation in Auditory Brainstem Implant Candidacy in Patients with Neurofibromatosis Type 2
| Second tumor in an only-hearing ear |
| Any tumor in a hearing ear that measures >2 cm in the largest diameter (hearing preservation unlikely with removal) |
| Short life expectancy due to other tumors, medical problems, or advanced age |
| Serviceable hearing with a tumor that shows no significant growth by sequential magnetic resonance scans and stable hearing by serial audiograms |
DEVICE
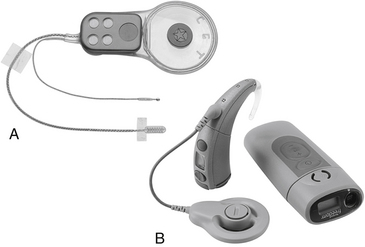
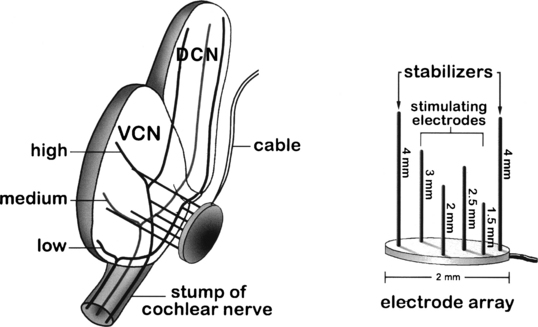
The ABI hardware has evolved through a number of modifications since the original ball electrode was inserted by Drs. William Hitselberger and William House in 1979.1,5 Significant design changes have involved transitioning from a percutaneous connector to a transcutaneous coil link to the implant, converting from ribbon electrodes to .7-mm-diameter disk electrodes, and fabrication of a semiflexible silicone electrode carrier (2.5 × 8.5 mm) with a specialized mesh backing to stabilize placement. The first 25 ABI recipients were fitted with a single-channel sound processor and a 2-or 3-electrode array. From 1992 until about 2000, the electrode array used in most patients employed eight platinum disks in a perforated silicone and mesh carrier connected to an implantable receiver/stimulator. From about 2000 to the present, the 21 electrode Nucleus ABI24 (see Fig. 58-1) has been used. The external device in the 8 electrode patients consisted of a post-auricular microphone, a transcutaneous transmitter coil, and a sound processor (Spectra Model, Cochlear Corporation). Signal-processing strategies have evolved in an effort to improve performance.6 Present patients receive a 21-electrode array (Fig. 58-1A and B) interfaced with the Freedom post-auricular sound processor and ear-level or body-worn controller (Cochlear Corporation, Englewood, CO).
Since 2003, use of a hybrid ABI array consisting of a version of the present surface electrode and a 10-electrode penetrating array also has been studied in an FDA clinical trials. Implementation of this system in laboratory animals originally demonstrated the capability for improved microstimulation of auditory neurons7 and possibly improved perceptual performance in humans.
ANATOMIC CONSIDERATIONS
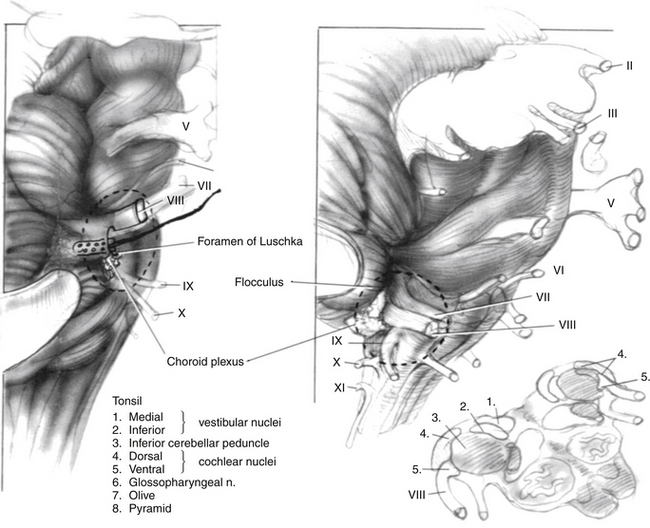
The target of the ABI electrodes is the cochlear nucleus complex—dorsal and ventral cochlear nuclei. In humans, the cerebellar peduncle that forms the base of the pons covers the auditory nuclei. This means that the nuclei are not visible to the surgeon and must be located from surface landmarks. Figure 58-2 illustrates the major structures of the pontomedullary junction region with the translabyrinthine approach surgical field of view within the dashed lines. The terminus of the sleeve-like lateral recess forms the foramen of Luschka. Just inferior to the foramen is the root of the glossopharyngeal (ninth) nerve. Superior to the foramen lie the root entry and exit zones of the vestibulocochlear and facial nerves. This area is frequently distorted by the tumor.
The cochlear nuclei come closest to the surface of the brainstem within the medial and superior aspect of the lateral recess.8,9 The main target for stimulation is the ventral cochlear nucleus, which forms the main relay for cochlear nerve input and the greater part of the ascending auditory pathway. Placement of the electrodes within the recess has resulted in the fewest side effects and preserves auditory stimulation even though some part of the electrode array lies adjacent to the dorsal cochlear nucleus.2 Also, the disadvantage of lack of exposure is partially offset by positional stability provided to the electrode carrier by the limited space in the lateral recess.
SURGICAL CONSIDERATIONS
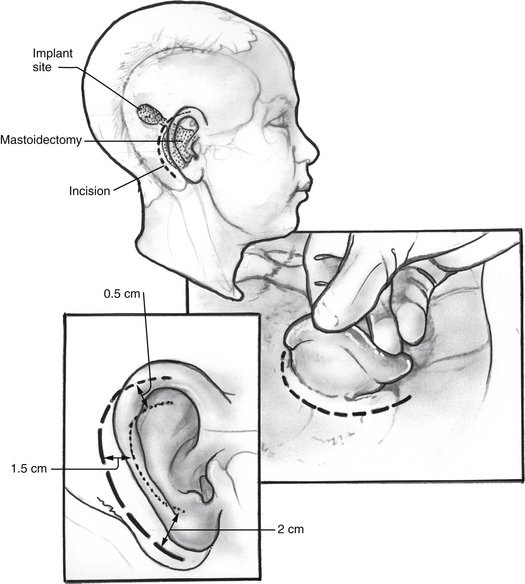
The surgical approach for tumor removal in ABI cases at HEI has been exclusively via translabyrinthine craniotomy (see Chapter 50). The translabyrinthine route has been found to provide the most direct access to the lateral recess and surface of the cochlear nuclei.10 Until the actual placement of the device, the surgery proceeds as in any other translabyrinthine acoustic neuroma excision, with the following exceptions. Electrodes are placed for recording electrically evoked auditory brainstem responses (EABRs) and for monitoring cranial nerves VII and IX. The postauricular incision used is shown in Figure 58-3. It is important that the incision not cross the receiver/stimulator package.
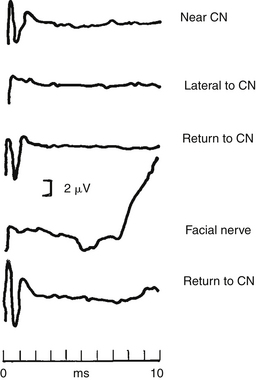
Electrophysiologic monitoring is performed during implantation to help ensure that the electrode array placement is correct for activating the auditory system, and also to detect possible activation of nonauditory brainstem structures. There may be considerable uncertainty about the correct position for the electrode array when a large tumor has distorted the anatomic landmarks at the brainstem. To aid in placing the electrode array, EABRs are recorded. A repeatable EABR indicates that stimulation of the auditory system is occurring. Intraoperative EABRs obtained with electrical stimulation of the cochlear nucleus differ considerably from brainstem responses routinely recorded with acoustic stimulation (ABRs) in normal-hearing individuals.11 An experienced electrophysiologist interprets these waveforms at the time of implantation based on data collected from previously implanted patients (Figure 58-4).
Electrophysiologic monitoring also helps determine the electrode array position that minimizes nonauditory side effects. In addition to monitoring the facial nerve in standard manner,12 bipolar electrodes are inserted in the ipsilateral pharyngeal (soft palate) muscles to monitor activation of cranial nerve IX. If the electromyographic recordings reveal activation of nonauditory centers during stimulation through the implant, or if a muscle evoked potential is seen in the averaged waveform, the electrode array is repositioned.
IMPLANTATION TECHNIQUE
Tumor dissection proceeds normally via a translabyrinthine craniotomy. After tumor removal and hemostasis, an area of cortical bone posterior to the mastoid is flattened, and a trough to accept the wires from the electrodes to the receiver/stimulator is created in a manner similar to that of cochlear implantation. Using a replica of the receiver/stimulator as a guide, a circular area of bony cortex posterosuperior to the mastoid defect is drilled with cutting burrs (Fig. 58-5). A specially designed butterfly bit or other cylindrical bits associated with high-speed drills may be employed. Using a replica of the receiver/stimulator as a guide, the surgeon drills four holes into the bone to accept the tiedown suture. The receiver/stimulator is fixed with nylon suture prior to electrode array positioning so that the manipulation of the leads does not alter the electrode placement (Fig. 58-6). Because only bipolar cautery may be used after the electrode array is inserted to minimize the risk of current shunting through the device into the brainstem, meticulous hemostasis of the entire wound and CPA is ensured prior to implantation.
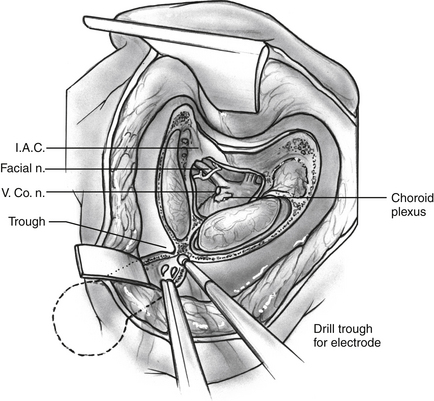
FIGURE 58-5 Surgical view of completed translabyrinthine craniotomy, trough for wires, and receiver/stimulator site.
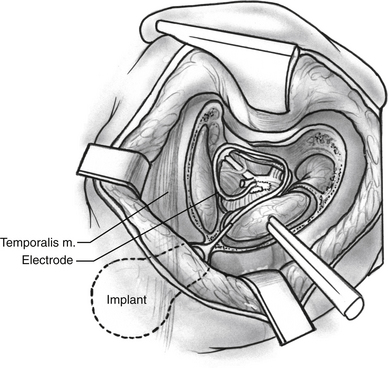
After identifying the foramen of Luschka, a Rosen needle is used to insert the electrode array into the lateral recess with the electrodes facing superiorly (Fig. 58-7). With experience, we have found that the system functions better, with fewer side effects, when the entire electrode array is placed just inside the lateral recess.2 After placement, selected electrodes in the array are activated to confirm their position over the nucleus. They are tested for the presence of EABRs, stimulation of adjacent cranial nerves (VII and IX), and changes in vital signs. The position of the electrode array usually needs some adjustment to maximize the EABRs and minimize electromyographic responses from the other nerves. If stimulation of the IX nerve occurs, the electrode is separated from it with teflon felt.
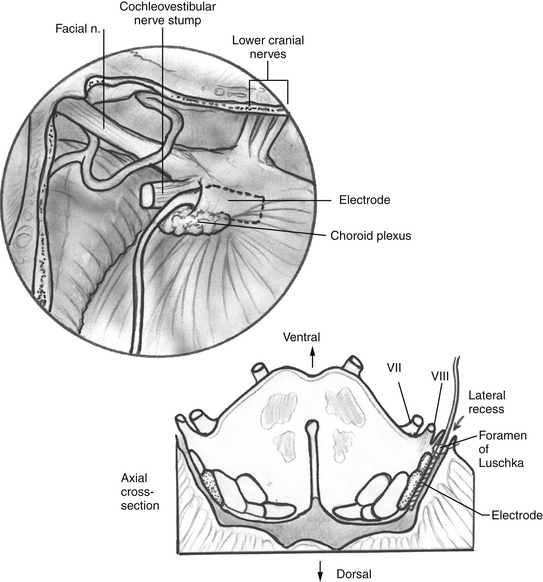
FIGURE 58-7 Surgical view of the electrode array placed into the lateral recess (magnified view of the dashed area in Figure 58-6) and cross-section through the brainstem in the region of the electrode array.
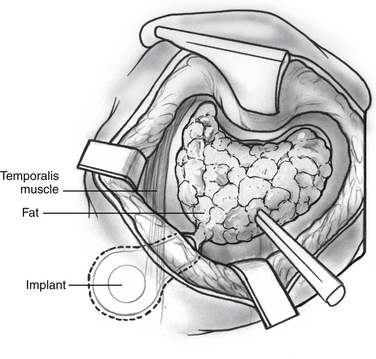
The electrode array is secured by a small piece of Teflon felt packed into the meatus of the lateral recess. Fibrous tissue eventually stabilizes the array in position. The wires are positioned in the mastoid cavity and the bony trough previously drilled (Fig. 58-8). Abdominal fat obliterates the mastoid defect. The incision is closed in three layers, and care is taken not to disturb the wires. The wound is not drained routinely. A large mastoid-type dressing is left in place for 4 days.
POSTOPERATIVE CARE
The postoperative care after implantation shares many of the features of routine translabyrinthine tumor resections (see Chapter 50). A similar schedule for advancing patient activity and decreasing the level of intensity of nursing care is maintained. A mastoid dressing should remain in place for at least 4 days. Careful attention to any moisture on the bandages allows prompt identification of cerebrospinal fluid leak through the postauricular wound. Intravenous antibiotics are administered prophylactically 1 day preoperatively and continued through the fifth day postoperatively.
RESULTS
Over 200 patients with NF-2 have been implanted with the Nucleus multichannel ABI system at HEI between 1992 and the present. Data from the earliest group of implantees comprised a large portion of a clinical trials submission to the FDA.13 In this chapter, we present results from speech and environmental sounds testing of a large group of patients experienced in using their ABIs. Since performance with the ABI improves more gradually than in cochlear implants, results from experienced users are more representative of longer term benefit. Early results on many of these patients have been presented elsewhere.6 Even 2 years of experience should not be considered sufficient to reach asymptomatic performance. Although improvements are generally greatest during the first year, many patients have continued to improve even after 10 years of use. All patients used the SPEAK (spectral maxima) speech processing strategy.14
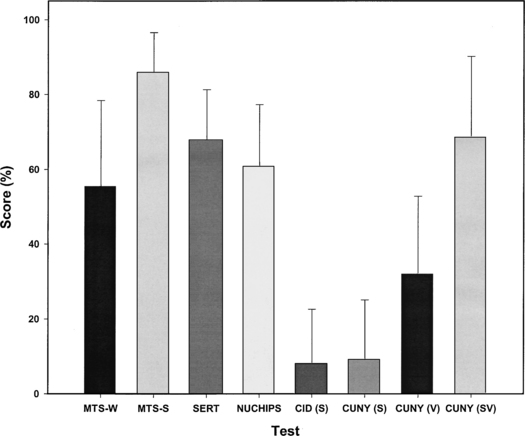
Figure 58-9 shows mean scores on a portion of the multichannel ABI perceptual test battery. The lowest scores shown are for the CID and City University of New York (CUNY) sentence tests, which are presented in sound only. These tests are difficult for the majority of ABI recipients, and they reflect the generally limited capability of the ABI in the absence of lipreading cues. The CUNY sentence scores in lipreading alone and in sound plus lipreading modes are higher than those administered with sound alone because of the visual cues provided. Obviously lipreading cues are highly important in face-to-face communication, and a large average increase (39 per cent) in sentence recognition occurs when ABI sound is added to lipreading as indicated by the lipreading only and sound plus lipreading CUNY scores.
With notable exceptions, these results indicate that ABI performance generally has not reached the high levels typically seen with multichannel cochlear implants. At least five patients have shown high levels (≥50 per cent) of open-set speech recognition ability on sound-only sentence tests, and about 16% of our ABI recipients have shown significant (at least 20 per cent correct) ability in this area. There is reason to be hopeful that ABI performance in general will improve. Many ABI recipients experience electrode-specific pitch sensations similar to cochlear implant recipients, and it may be possible to increase these cues with improved microstimulation systems such as the penetrating ABI (PABI). Capitalizing on these cues by carefully assessing auditory percepts from ABI stimulation can be time consuming, but is a necessary part of programming the speech processor to optimize performance.13
Speech processing for brainstem stimulation has profited from research in cochlear implants. Interestingly, similar strategies used in cochlear implants also have worked well with the brainstem implant.6 Flexibility of the ABI programming system has been essential to accommodate anatomic variations and the range of auditory and nonauditory sensations that can result from stimulation. Proper assessment and use of this information in configuring ABI speech processors can have a significant effect on speech perception performance. Poor selection or misalignment of frequency bands to electrode channels in speech processor programming can limit performance.13 Particularly in patients with a greater incidence of nonauditory sensations, experience and flexibility in the clinician’s approach can sometimes mean the difference between use and nonuse of a device. Future studies regarding stimulation rates, frequency assignment of channels, and methods of coding speech cues will contribute to improvements in speech processing strategies for the ABI.
OTHER RESEARCH
Niparko and associates15 demonstrated the feasibility of implanting and stimulating within the substance of the cochlear nucleus in guinea pigs. In a related study, el-Kashlan and colleagues16 compared the effectiveness of surface electrodes with those placed into the nucleus. They found lower thresholds and a wider dynamic range in animals with penetrating electrodes than in those with surface placement. These results were influential in the development of the penetrating ABI system that employs an array of needle microelectrodes. McCreery and coworkers7 demonstrated the efficacy of such a system for activating discrete populations of tonotopically tuned neurons within the substance of the cochlear nucleus. This was achieved without significant risk to tissue or blood supply in longer term preparations with properly constructed and inserted electrodes. The needle-type electrodes with a somewhat blunt-tip configuration (Fig. 58-10) were atraumatically inserted on-axis with a specialized spring-powered tool.
Results Using the Penetrating Electrode ABI
It is clear that placement of the penetrating electrode array is more critical than the surface electrode array and requires considerable accuracy. A slight deviation (only a mm or so) from the target region can result in no auditory responses on penetrating electrodes. The electrical ABR monitoring that is used intraoperatively to assist with placement of the surface ABI array has been of little use in penetrating electrode placement because the microelectrodes do not typically generate a sufficient neural response for detection by scalp monitoring electrodes. Also, neural response telemetry (NRT), that has been useful in the near-field detection of cochlear nerve action potentials generated by cochlear implants, does not appear to be useful in ABI (or PABI) implantation because of difficulty differentiating auditory from non-auditory action potentials. In postoperative testing with awake patients, NRT waveform morphology often appeared the same regardless of whether patients reported hearing sensations, non-auditory side effects, or no sensations at all.17
1. Brackmann D.E., Hitselberger W.E., Nelson R.A., et al. Auditory brainstem implant: I. Issues in surgical implantation. Otolaryngol Head Neck Surg. 1993;108:624-633.
2. Shannon R.V., Fayad J., Moore J.K., et al. Auditory brainstem implant: II. Postsurgical issues and performance. Otolaryngol Head Neck Surg. 1993;108:634-642.
3. Riccardi V.M. Neurofibromatosis. Neurol Clin North Am. 1987;5:337-349.
4. Briggs R.J., Popovic E.A., Brackmann D.E. Recent advances in the treatment of neurofibromatosis type II. Adv Otolaryngol Head Neck Surg. 1995;9:227-245.
5. Hitselberger N., House W.F., Edgerton B.S., Whitaker S. Cochlear nucleus implant. Otolaryngol Head Neck Surg. 1984;92:52-54.
6. Otto S.R., Shannon R.V., Brackmann D.E., et al. The multichannel auditory brainstem implant (ABI): Results in 20 patients. Otolaryngol Head Neck Surg. 1998;118:291-303.
7. McCreery D.G., Shannon R.V., Moore J.K., et al. Accessing the tonotopic organization of the ventral cochlear nucleus by intranuclear microstimulation. IEEE Trans Rehabil Eng. 1998;4:1-9.
8. Terr L.I., Edgerton B.J. Surface topography of the cochlear nuclei in humans: Two-and three-dimensional. Hear Res. 1985;17:51-59.
9. Sinha V.K., Terr L.I., Galey F.R., Linthicum F.H. Computer-aided threedimensional reconstruction of the cochlear nerve root. Otolaryngol Head Neck Surg. 1987;113:651-655.
10. Monsell E.M., McElveen J.T., Hitselberger W.E., House W.F. Surgical approaches to the human cochlear nucleus complex. Am J Otol. 1987;8:450-455.
11. Waring M.D. Refractory properties of auditory brainstem responses evoked by electrical stimulation of human cochlear nucleus: Evidence of neural generators. Electroenceph Clin Neurophysiol. 1998;108:331-344.
12. Niparko J.K., Kileny P.R., Kemink J.L., et al. Neurophysiologic intraoperative monitoring: II. Facial nerve function. Am J Otol. 1989;10:55-61.
13. Otto S.R., Ebinger K., Staller S.J. Clinical trials with the auditory brainstem implant. In: Waltzman S.B., Cohen N.L., editors. Cochlear Implants. New York: Thieme; 2000:357-365.
14. McDermott H.J., McKay C.M., Vandali A.E. A new portable sound processor for the University of Melbourne/Nucleus multielectrode cochlear implant. J Acoust Soc Am. 1992;91:3367-3371.
15. Niparko J.K., Altschuler R.A., Xue X.L., et al. Surgical implantation and biocompatibility of central nervous system auditory prostheses. Ann Otol Rhin Laryngol. 1989;98:965-970.
16. El-Kashlan H.K., Niparko J.K., Altschuler R.A., Miller J.M. Direct electrical stimulation of the cochlear nucleus: Surface versus penetrating stimulation. Otolaryngol Head Neck Surg. 1991;105:533-543.
17. Otto S.R., Waring M.D. Kuchta, J: Neural response telemetry and auditory/non-auditory sensations in 15 recipients of auditory brainstem implants. J Am Acad Audiol. 2005;16:219-227.