Chapter 6 Attention deficit hyperactivity disorder (ADHD)
With contribution from Dr Lily Tomas
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) — characterised by attention-deficit, impulsivity and hyperactivity — is one of the most common neurobehavioral disorders affecting children and adolescents today.1, 2, 3 The incidence of ADHD is rising with the annual prevalence in Australia in 2001 being estimated at 11% (diagnosed by DSM-IV criteria), equating to a 7.5% prevalence in people aged 6–17 years.4
There has been a concomitant rise in the use of stimulant medications, namely phenylmethidate and dexamphetamine, despite a lack of studies regarding long-term social and psychological effects, and cardiovascular and neurophysiological clinical effects. Although stimulants are very helpful in 60–70% of patients, many families seek alternatives because of adverse reactions, lack of compliance and the fact that stimulants cannot be given late in the day, limiting the benefits largely to school hours.5
The use of complementary and alternative medicines (CAM) for ADHD has increased, both by parents and health care providers.6 Parents are especially drawn to CAM interventions in order to avoid or decrease the use of psychotropic medications.7, 8 Because of the wide-ranging disruptive impact on the lives of both patients and their families, an integrative approach to management simply reflects the multifactorial aetiology and nature of this disorder.
A recent Australian survey demonstrated that the most common CAM therapies used include dietary modification, nutritional supplementation, aromatherapy and chiropractic. It has been advised that doctors should always inquire about the use of CAM and use available resources to help guide families in their therapeutic choices.9
The exact aetiology of ADHD is unknown and, indeed, may differ from individual to individual. Genetics and genetic polymorphisms certainly play a role, however, major aetiological contributors may also include adverse responses to food additives, intolerances of foods, differing biochemical pathways and nutritional deficiencies, sensitivities to environmental chemicals and exposure to neurodevelopmental toxins such as heavy metals and tobacco smoke.10–15
ADHD is a complicated condition that requires multidimensional treatment strategies.16 It is imperative to understand that the aetiology and hence the management of ADHD may be different for each individual. One must attempt to elucidate all possible contributory factors and eliminate or treat each respectively.
The PFC, especially the right hemisphere, is crucial to the regulation of behaviour and attention. It is extremely sensitive to its neurochemical environment, with either too much or too little catecholamine release weakening the individual’s cognitive control of both behaviour and attention.17
Individuals with ADHD are known to have depleted levels of dopamine and noradrenaline most likely as a result of dysfunctional transporter systems.18 The role of other neurotransmitters such as histamine, acetylcholine, glutamine and serotonin in modulating catecholamine pathophysiology in ADHD is yet to be elucidated.19
Lifestyle
Most of the lifestyle factors associated with ADHD are covered under the appropriate headings below.
Mind–body medicine
Neurobiofeedback
Electroencephalogram (EEG) biofeedback is a promising alternative treatment for patients with ADHD.20 It is a form of behavioural training aimed at developing skills for the self-regulation of brain activity.21 Most individuals with ADHD, as compared to matched peers, show abnormal functioning of their anterior cingulate cortex with excess slow-wave (theta) activity and reduced fast-wave (beta) activity during tasks of selection attention.22, 23
In particular, it is well documented that hyperactive behaviour in many children with ADHD is due to abnormally enhanced 4-8Hz Theta activity in both frontal and central cortical areas of the brain.24 In the last decade there has been a multitude of clinical trials and literature reviews that demonstrate the positive effects of biofeedback on these children with clinical improvement being primarily directly related to declining theta/beta ratios and/or amplitudes over the frontal/central cortex.25–42 These have largely been good quality studies that have tended to overcome the methodological shortcomings of earlier studies.21
Research findings published to date indicate a positive clinical response (reduced hyperactivity and impulsivity, improved attention, IQ, processing speed and music performance) in approximately 75% of patients treated in controlled group studies. The Association for Applied Psychophysiology and Biofeedback and the International Society for Neuronal Regulation deem EEG biofeedback to be ‘probably efficacious’ for the treatment of ADHD, particularly for those patients who do not respond to medications.30, 36, 38
During biofeedback, individuals are taught to increase their beta activity and suppress their theta activity over a period of usually 30 or more sessions.29, 30, 32, 33 This enables the child to become an active agent of their own coping strategies and thus increase their internal locus of control.43 EEG biofeedback therapy works by rewarding scalp EEG frequencies that are associated with relaxed attention and suppressing those frequencies associated with under or over-arousal.20 It provides immediate feedback to the individual about his/her brain-wave activity in the form of a video/computer game, the action of which is influenced by the individual meeting predetermined threshold of brain activity.22 Obviously, the child must be at an age where they are able to play such games — most studies include children aged 6 years and above.28, 29, 30, 33
There are many different forms of biofeedback available. The regulation of cortical excitation thresholds are also considered to be impaired in children with ADHD and the training of slow cortical potentials addresses the regulation of cortical excitability. It has been suggested that the regulation of fronto–central negative slow cortical potentials affects the cholinergic-dopaminergic balance, allowing children to adapt to task requirements with more flexibility.21, 25, 30
Recently published studies using quantitative EEG (QEEG) techniques indicate that power spectral analysis and event-related cortical potentials may be useful in differentiating ADHD from other disorders, such that QEEGs may be used clinically in the assessment, diagnosis and treatment of ADHD.25, 35 In particular, a deficit in low frequency wave (approximately 1 Hz) activity associated with levels of hyperactivity and impulsivity has been demonstrated in both children and adolescents with ADHD. This marker is evident across a range of tasks and may, indeed, be specific to ADHD.25
Several recent neuro-feedback trials have demonstrated comparable results with Methylphenidate (Ritalin) in terms of increased attention span and reduced problem behaviour (impulsivity and hyperactivity) in children with ADHD without the side-effects often associated with medication.20, 22, 31, 37, 39, 44
It is also important to note that in those studies of children using both biofeedback and methylphenidate, only those children who had received biofeedback sustained these gains when reassessed without Ritalin. Furthermore, Quantitative Electroencephalographic Scanning Process (QESP) studies show a significant reduction in persistent cortical slowing only in those patients who underwent EEG biofeedback.39, 45 This is confirmed by parent and teacher evaluations who report significant behavioural and cognitive improvements for at least 6 months after the cessation of treatment.29
Psychosocial and/or cognitive behaviour therapy (CBT)
Although pharmacological treatments have traditionally been considered the first-line therapy for ADHD, many individuals continue to experience major functional impairment or choose not to use such medications. Behavioural school interventions and parent training have been supported by empirical evidence.1, 46 It is important to note, however, that the children respond to such behavioural interventions only when they are appropriately implemented both at home and in the classroom setting.47
Psychosocial therapies, especially behavioural modification techniques, should be considered for children with ADHD and oppositional behaviours whilst cognitive behaviour therapy (CBT) may be useful for adolescents and adults.48, 49 When behavioural therapies have been combined with medication, improvements in function have been demonstrated and the amount of stimulant reduced.50
A recent Cochrane review has confirmed that both behavioural and CBT interventions are highly effective, however, access to these treatments is limited due to length of consultations and expense, with significant behavioural improvements taking up to 2 hours of therapist time.51
It has been hypothesised that family therapy without medication may help to develop family structure and may help to manage children’s behaviour. A 2005 Cochrane review has deemed that further research is necessary to determine whether family therapy is an effective intervention for children with ADHD.52
Available data supports the use of group and individual structured, skills-based psychosocial interventions for adults with ADHD.53, 54
Meditation and relaxation training
Mindfulness meditation may also improve behavioural and neurocognitive impairments in adolescents and adults with ADHD. One recent study has demonstrated improved attention and cognition with reduced anxiety and depression after an 8 week mindfulness training program.55
A range of studies have suggested that relaxation training can help children with ADHD to learn to relax, thereby decreasing their autonomic activity.7, 56 Reductions in problem behaviour, increased attention span and greater internal locus of control are other potential benefits of relaxation therapy. It should be noted, however, that these skills need to be practised regularly for continued effect.57–60
In the mainstream literature there have been no published studies on the potential application of meditation for ADHD, however, the Royal Hospital for Women, Sydney, has devised a pilot clinic aimed at developing meditative strategies, using Sahaja Yoga Meditation, to help these children (www.sesiahs.health.nsw.gov.au/rhw/). The clinic exclusively accepted children with a formal diagnosis of ADHD and whose usual supervising health professional had permitted their involvement. Both the child and at least 1 parent were required to attend classes and practice daily meditation. The results were very promising. Of the 16 children who completed the program, 6 were able to decrease and 3 were able to stop their medication whilst maintaining completely normal behavioural traits. All parents reported feeling generally better, less stressed and more relaxed and most felt the program had benefited their children.
About half the parents said their child was less restless with improved sleep and that they were experiencing a better relationship with them. Whilst this is not a randomised controlled study, it is at the moment the best available evidence for meditation’s potential role in ADHD and clearly suggests that Sahaja Yoga meditation may be particularly beneficial for this condition. More rigorously designed studies are planned in order to achieve a more conclusive understanding of this radically different approach.3
Sound therapy
Recent studies have demonstrated that children with ADHD, upon performing visual discrimination tasks, were less attentive than controls when exposed to distracting novel sounds. Event-related brain potentials correspondingly displayed significant differences over the fronto-central left hemisphere and the left parietal scalp region, revealing low control of involuntary attention that may further underlie their abnormal distractibility.61
Systems such as the ‘Tomatis Method’, ‘Integrated Listening System’ (ILS) and Dr Guy Berard’s (ENT physician) Auditory Integration Training (www.integratedlistening.com) have been designed around the brain’s ability to form new neural connections throughout life, changing the way the brain processes auditory information. ILS stimulates both cerebellar activity, in order to strengthen these neural connections, and the vestibular system, in order to improve balance, posture and hand/eye coordination. Such therapies are used by many patients who experience auditory processing difficulties and hypersensitivity to specific auditory frequencies. There are currently no well-designed studies in the literature regarding such therapies, however, anecdotal reports are very promising.
The Moderate Brain Arousal model suggests that dopamine levels modulate how much noise is required for optimal cognitive performance. Studies have shown that individuals with low dopamine levels (ADHD) need more background noise than controls for optimal cognitive performance. This positive effect of noise may be explained by the phenomenon of Stochastic Resonance (SR), whereby external noise is relayed as internal noise into the neural network subsequently affecting neurotransmitter levels.62 This method aims to teach children with ADHD to focus and intently listen to specific sounds, subsequently helping with behaviour modification, cognitive development and concentration.
Normally, high dopamine down-regulates stimuli-evoked phasic dopamine responses through autoreceptors, however, abnormally low extracellular dopamine in ADHD up-regulates these receptors so that stimuli-evoked phasic dopamine is boosted. It is postulated that these boosted phasic responses create hypersensitivity to environmental stimuli in ADHD. Empirical data supports the concept that more noise is required for SR to occur in dopamine-deprived neural systems in ADHD.17
There is also some evidence to show that music therapy may contribute to a reduction in a range of ADHD symptoms in the classroom.43, 63
Sleep and behaviour
It is important to note that, even in children that do not suffer with ADHD, sleep problems during school transitions are common, and are associated with poor outcomes. Future RCTs could determine if sleep interventions can reduce the prevalence and impact of such detrimental sleep problems.64
Sleep restoration/melatonin
There is a clear correlation between ADHD and sleep difficulties with substantial evidence that ADHD psychopathology and sleep/wake regulation share common neurobiological mechanisms. Furthermore, there may even be an overlap between ADHD and sleep disorders such as obstructive sleep apnoea and restless leg syndrome (RLS).65 Anecdotal evidence strongly suggests that magnesium assists in the treatment of RLS.
Approximately 25% of children suffering with ADHD also experience some form of sleep disorder. Unfortunately, in contrast with adults, these often go undetected. Diagnosis of these patients is critical.66 Therefore, all children with ADHD should be fully assessed for sleep disturbances because adequate treatment is often associated with improvement of symptoms and a decreased requirement for stimulant medications.65, 66
The circadian rhythm of melatonin secretion from the pineal gland is reflective of mechanisms that are in control of the sleep/wake cycle. In those individuals with primary insomnia, nocturnal plasma melatonin levels tend to be lower than in healthy controls. Melatonin has been used successfully to treat insomnia in children with ADHD.67 Several randomised double-blind placebo-controlled trials using 3–6mg melatonin for 1 month demonstrated enhanced total time asleep in children with ADHD and chronic sleep onset insomnia. Melatonin is shown to be a safe and effective treatment for sleep disorders, however this had no observable effect on other ADHD symptoms.68, 69
Environment
Outdoor play
Research on children with ADHD demonstrates significant improvement in symptoms such as inattention and impulsivity after exposure to natural views or settings. Four hundred and fifty-two parents or guardians from across the US with ADHD or Attention Deficit Disorder (ADD) children aged 5–18 years enrolled in a study to assess the benefits of playing outdoors in a natural setting.70 Compared with baseline results, outdoor activities conducted in natural environments significantly improved ADHD symptoms (difficulty in remaining focused, completing tasks, listening and following directions, and in resisting distractions) compared to activities conducted indoors or those in built outdoor settings, such as parking lots.
Heavy metals and chemicals
Lead
Lead is a common environmental contaminant such that in the year 2000, nearly 1 million preschool-aged children in the US alone were shown to have elevated blood levels (>10ug/dL).71
In 1991, the US Centres for Disease Control and Prevention (CDC) established 10ug/dL as the lowest concern for children’s blood lead levels. However, in recent years, there has been a wealth of evidence-based clinical trials demonstrating that levels below 10ug/dL may impair neurological development. In fact, there is now sufficient and compelling scientific evidence to call for the CDC to lower the blood lead action level in children to a level as low as 2ug/dL.72, 73, 74
Indeed, no level of lead exposure appears to be safe with multiple studies now demonstrating reduced IQ and academic deficits in otherwise ‘healthy’ children. There appears to be an inverse relationship between lead levels and IQ levels, particularly at levels <10ug/dL.75, 76, 77 At lower levels of toxicity, a child may have no specific individual symptoms but may certainly be affected sub-clinically.78 For this reason, health care practitioners should obtain a thorough environmental history on all children they examine.74
Having adjusted for covariates, children with 5–10ug/dL have been shown to have 5 points lower IQ scores compared to children with blood lead levels of 1–2ug/dL. Verbal IQ appears to be more negatively associated than performance IQ, as does reading and maths composite scores. Working memory and attention were also shown to be lowered with increasing lead levels.79, 80
In particular, there have been several studies associating ADHD with elevated lead levels. Individuals with ADHD are more likely to have been exposed to lead during childhood, such that ADHD may now be deemed an additional deleterious outcome of lead exposure, even when levels are <10ug/dL.81 Its effects may be mediated by less effective cognitive control, consistent with a route of influence via striatal-frontal neural circuits.82
Chelation therapy is advised for children with blood level concentrations of >44ug/dL, however, there are no evidence-based clinical trials for other gentler chelation treatment options for children with levels less than this. Because lead absorption is partially related to nutritional status, micronutrient supplements may be a possible solution for combating low-level chronic lead exposure.71, 80 Zinc, in particular, is 1 supplement that has shown some results in effectively reducing oppositional, hyperactive, cognitive problems and other ADHD symptoms in most individuals.80, 83
Mercury
Numerous studies report positive correlations between the number of dental amalgams and urinary mercury concentrations in non-occupationally exposed individuals.84, 85, 86 Experimental evidence consistently demonstrates that mercury is released from dental amalgams and is absorbed by the human body.87 However, there is much controversy regarding the effects of mercury (from dental amalgams and vaccinations) on neurodevelopment, renal and immune function.
One of the latest randomised controlled studies has confirmed that treatment of children with amalgam restorations leads to increased, albeit low level, exposure to mercury. Amalgam exposure resulted in small, transient immune deficits 5–7 days post treatment, however, it did not cause overt immune defects. The authors concluded that these changes ‘most likely did not need to be of concern to practitioners considering the use of this restorative dental material’.88 It is important to note, however, the history of what initially constituted toxic lead levels and how this has changed in recent years with accumulated evidence-based studies.
In a similar manner, mean urinary mercury concentrations have been found to be greater in children with amalgams rather than composite dental restorations.89, 90 Children treated with mercury amalgams did not ‘on average’ have statistically significant differences with respect to neuropsychological function. ‘Although it is possible that very small IQ effects cannot be ruled out’, thus far evidence-based trials largely demonstrate that dental amalgam is not associated with an increase in children’s risk of experiencing neuropsychological dysfunction.75, 90, 91, 92,
These results support the concept that some healthy children may, indeed, be out of the bell-curve, being more predisposed to toxic effects of mercury at lower levels than others. This is further highlighted by a recent trial specifically concerning children suffering with ADHD. In this study of Chinese children, a significant difference in blood mercury levels was noted between children with ADHD compared with controls, after adjustments for age, gender and parental occupations. The geometric mean blood mercury level was also significantly higher in children with inattentive and combined subtypes of ADHD. In fact, children with a blood mercury level >29nmol/L were found to have 9.69 times higher risk of having ADHD after adjustment for confounding variables. The researchers concluded that high blood mercury levels were associated with ADHD.93
Manganese/aluminium
Manganese (Mn) is an essential trace element, however, it has also been shown to be toxic at high doses. Animal studies have recently shown that intra-nasally administered Mn actually circumvents the Blood-Brain-Barrier and passes directly into the brain via olfactory pathways. Long-term exposure to inhaled Mn from shower water as a significant risk for central nervous system (CNS) neurotoxicity is currently being investigated. Similarly, existing Mn drinking water standards may also need to be revised.94
A recent study of Canadian children found that those who were exposed to drinking water that was naturally high in Mn had greater scores of hyperactivity and oppositional behaviour (Revised Connor’s Rating Scale) than controls. All children with T scores >65 had hair Mn higher than 3.0ug/g.95
It is postulated that both aluminium and Mn toxicity may potentiate oxidative and inflammatory stress, subsequently leading to impaired neurological function.96
Industrial chemicals
Available data up to 2007 show that at least 202 widely-used industrial chemicals, (including lead, methylmercury, polychlorinated biphenyls, arsenic and toluene) can damage the human brain, the researchers concluding that chemical pollution may have harmed the brains of millions of children worldwide.97, 98 The specific role these chemicals may play in the development of ADHD is not certain, but a detailed environmental history should be taken in all of those with neurodevelopmental disorders.99
Tobacco smoke, air pollution, pesticides
Environmental tobacco smoke, air pollution and pesticides have also been shown to have adverse effects on fetal growth and child neurodevelopment.100, 101, 102
Tobacco smoke
A recent systematic review has demonstrated that both prenatal and possibly postnatal tobacco smoke exposure are significantly associated with increased rates of behaviour problems and ADHD.11, 12, 14, 15 If causally linked, it is estimated that prenatal tobacco exposure may account for at least 270 000 excess cases of ADHD in American children today.13
Like stimulant medication, nicotine has been shown to lower the availability of the dopamine transporter, a significant factor in dopamine metabolism.18
Physical activity
Exercise
Exercise is considered an important part of the management of the child with ADHD as it not only increases coordination skills (that many children with ADHD lack) but it provides opportunities for social interaction.7 A recent study determining the effects of exercise on children with ADHD suggests that vigorous exercise may provide a dopaminergic adjuvant in the management of behavioural features of ADHD.103
It has also been demonstrated that adolescents with ADHD have frontal lobe deficits, particularly on the right sides of their brain. Animal studies were subsequently designed which showed that ‘rough-and-tumble’ play therapy was able to reduce hyperactivity and excessive playfulness, concluding that this may be a useful new treatment for ADHD.104
Another pilot study on Therapeutic Eurythmy — a holistic movement developed by Rudolph Steiner — has also reported shifts in the concentration and motor skills of children with ADHD.105
Yoga
Randomised controlled trials (RCTs) on the effectiveness of body-oriented therapies such as yoga for children with ADHD are lacking. The effects of yoga were recently compared to the effects of conventional motor exercises in children with ADHD. It was found that yoga was an effective complementary or concomitant treatment for children with ADHD. It should be noted that the training was especially effective for children also taking medications.106 Another study of boys with ADHD practicing yoga confirmed this finding, where yoga was particularly effective in the evening when the effects of medication were absent.107
Dietary modifications
Food elimination regimes
There has been much discussion over the last 30 years regarding the possible links between diet and behaviour of the individual with ADHD. In 1975, Benjamin Feingold, an allergist, hypothesised that an intake of salicylates in artificial flavours, colours and preservatives and/or natural salicylates may induce hyperactive behaviour and learning disabilities in children. Although Feingold demonstrated that 50% of children with ADHD improved after eliminating these substances from their diet, this has not been successfully repeated until recent years.43
The use of food elimination diets in the management of individuals with ADHD is now well documented.108–112 In fact, a strictly supervised elimination diet is considered to be a valuable instrument in determining whether dietary factors are contributing to ADHD symptoms. In a recent RCT, the number of criteria on the ADHD rating scale showed a scale reduction of 69.4%. Furthermore, comorbid symptoms of oppositional defiant disorder (ODD) also showed a significantly greater decrease in the intervention than the control group.113
There is an accumulating body of evidence that many children with behavioural problems, including ADHD, are sensitive to 1 or more food components that can negatively impact upon their behaviour.114 In 1 study, 19 of 26 children with ADHD improved dramatically after eliminating artificial colours, corn, wheat, milk, soy and oranges from their diet. It is interesting that most of the children who responded to such dietary changes had atopic histories implying that atopic children are more likely to benefit from a restricted diet.112 Other risk factors associated with a beneficial dietary response were a family history of migraine and young age.109 Another study also demonstrated a significant improvement in behaviour in 62% of 40 children with ADHD after a 2-week diet based solely on rice, turkey, pear and lettuce.115 This diet is clearly too restrictive for any child but it does demonstrate that nutrition can influence behaviour.
For children showing behaviour problems such as hyperactivity, the use of dietary manipulation tends to be a more acceptable approach to treatment than the use of drugs.116 If parents strongly suspect a specific dietary item, a trial of elimination is warranted.117 However, there are various regimes; usually this would consist of avoiding the item for 3 weeks then reintroducing it in a step-wise fashion — a little the first day, then challenging the body with a higher dose during the second day if no obvious reaction has already occurred. Depending upon the age of the child, multiple foods may be eliminated simultaneously and reintroduced separately in a similar manner under strict supervision. In conducting such dietary modifications, it is mandatory that all practitioners be aware of the dangers potentially associated with unsupervised restriction diets with children.116, 118
In general, dietary modification plays a major role in the management of ADHD and should be routinely considered as part of the treatment protocol.114 Many children with ADHD have associated digestion problems — including diarrhoea, constipation, abdominal bloating, excess burping and/or flatulence, reflux/indigestion or abdominal pain/discomfort. These are generally symptoms of deficient beneficial gut flora (probiotics) and/or food intolerances which may serve as triggers for abnormal behaviour.
A plethora of studies have now shown that food additives have also been shown to increase hyperactivity symptoms in children.119, 120 This includes a recent systematic review, which concluded that an additive-free elimination diet was considered Level II evidence with respect to its current level of evidence.121
Although the use of single food additives at their regulated concentrations are believed to be relatively safe in terms of their neuronal development, their combined effects remain unclear. Four common food additives, brilliant blue, L-glutamic acid, Quinolone yellow and aspartame were observed in combination. Neurotoxicity (measured as inhibition of neurite outgrowth) was found at concentrations of additives theoretically achievable in plasma by the ingestion of a typical snack and drink.122
Symptoms that are due to, or exacerbated by, specific food additives usually involve non-IgE mediated mechanisms that are usually less severe than those induced by food allergy.123 A recent RCT demonstrated that artificial colours and/or a sodium benzoate preservative in the diet results in increased activity in children aged 3 or 8–9 years old.124 This confirmed an earlier study where the adverse behaviour of 3-year olds from the same additives was detectable by parents but not by a simple clinical assessment.125
Despite a plethora of anecdotal reports that sugar increases hyperactivity and disruptive behaviour in the child with ADHD, there are as yet no consistent clinical trials to support this allegation.126
Like most neuropsychiatric disorders, there is evidence that ADHD is associated with increased oxidative stress and therefore increased lipid peroxidation. For this reason, it is postulated that individuals with ADHD may benefit from a whole-food, plant-based diet that is high in antioxidants and devoid of refined carbohydrate products.127
Several recent studies have also reported a possible association of coeliac disease with ADHD. In fact, ADHD-like symptomatology is markedly over represented amongst individuals with untreated coeliac disease. A gluten-free diet can result in a significant improvement of such symptoms within a short time period, such that coeliac disease (as well as gluten intolerance) should be included in the list of diseases associated with ADHD-like symptomatology.128, 129 Furthermore, a high frequency (57%) of antigliadin antibodies has been demonstrated in adult patients with neurological dysfunctions of unknown cause. In a study of children with various neurological disorders, including ADHD, 13% (compared with 9% of controls) showed positive for IgG antigliadin antibodies but negative for IgA and endomysial antibodies.130
Nutritional supplementation
Nutritional supplementation is widely used to help ameliorate the symptoms of ADHD. Many nutrients, (vitamins, minerals, essential amino acids, essential fatty acids) have direct effects on the structure and function of the human brain.131 Indeed, the role of nutrition in the prevention and management of ADHD is vital, cost-effective and extremely safe.132
Magnesium/vitamin B6
Magnesium deficiency has long been known to cause hyperexcitability with seizures in animal studies, effects that have been successfully reversed by treatment with magnesium. Significantly decreased plasma and red blood cell magnesium with concomitant decreases in Mg(2+) ATPase activity have been identified in children with ADHD.133–136 Magnesium is required for more than 350 different biochemical metabolism pathways in the human body, including oxidation/reduction and ionic regulation.131
Vitamin B6 is essential to the synthesis of many neurotransmitters, particularly dopamine, noradrenaline GABA, etc (see Figure 6.1).131
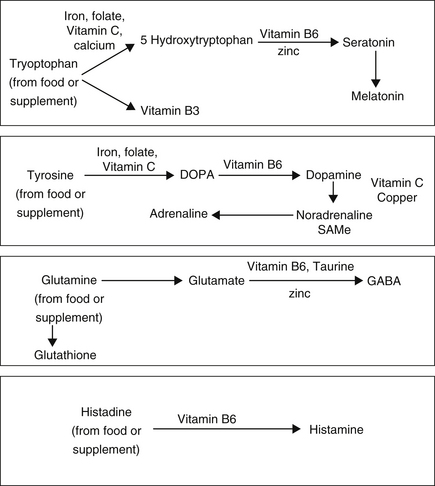
Figure 6.1 Central Nervous system biochemical pathways and cofactors required for neurotransmitter and hormone production132
Several European trials have demonstrated an improvement in the symptoms of ADHD with a combination of magnesium and vitamin B6. Children prescribed a magnesium/B6 regimen (6mg/kg/day Mg, 0.6mg/kg/day B6) for at least 8 weeks displayed a significant reduction in their symptoms of hyperactivity and aggressiveness whilst their attention at school was improved. When the supplementation was ceased, clinical symptoms of the disease reappeared within a few weeks, as did their original lower red blood cell Mg concentrations.137
After 1 month of supplementation of magnesium and B6, magnesium homeostasis was again normalised and there were noticeable improvements in behaviour and attention whilst levels of anxiety and aggression were reduced. Thus, it has been postulated that the determination of plasma and red blood cell Mg can be used to detect deficits and monitor the efficiency of treatment.135, 136
An earlier study found that even though 32 out of 50 children with ADHD demonstrated low red blood cell Mg levels, all patients showed an improvement in symptoms (hyperexcitability, physical aggressiveness, instability, attention, hypertony, spasm, myoclony) after 1–6 months of treatment with magnesium.133 Similarly, children with ADHD receiving 200mg/day magnesium showed a significant decrease in hyperactivity compared to a non-supplemented control group.138
Iron
Iron is necessary to ensure oxygenation, produce energy in the cerebral parenchyma (via cytochrome oxidase) and for the synthesis of both neurotransmitters and myelin. Iron concentrations in the umbilical artery are critical for the development of the fetus and are specific to the IQ/cognition of the child, playing a major role in both brain structure and function.131, 132
Furthermore, the lower the serum ferritin levels, the more severe the general ADHD (hyperactivity) symptoms.139 Available data is conflicting as to the relationship of low serum ferritin specifically to cognitive deficits.140, 141
As stated previously, there is a documented significant comorbidity between ADHD and RLS. Iron is a cofactor in dopamine production and patients with restless legs have lower levels of dopamine in their substantia nigra.142 Thus iron deficiency may, indeed, be one of the underlying common pathophysiological mechanisms in individuals with both ADHD and RLS. Thus, it is suggested that physicians assess children with ADHD for RLS, a family history of RLS and iron deficiency.139
A recent trial of iron supplementation (ferrous sulfate 170mg/day) resulted in an improvement of ADHD symptoms in those children with low serum ferritin levels. Iron therapy was well tolerated with effectiveness comparable to stimulant medication.143 However, an earlier Cochrane study has demonstrated that there is no clear evidence that iron treatment in children less than 3 years of age with iron deficiency anaemia will improve psychomotor development after 5–11 days of treatment.144 There is a need for future investigations with larger controlled trials.
Zinc
Zinc is an important co-factor that is needed for the metabolism of free fatty acids, neurotransmitters, prostaglandins and melatonin. It plays a role in the both the structure and function of the brain and indirectly affects the metabolism of dopamine (by inhibiting the dopamine transporter), known to be intricately involved in ADHD.18, 132, 145 Furthermore, plasma zinc levels have recently been found to have a direct effect on information processing in children with ADHD through event related potentials. In particular, the latencies of ‘N2’ waves in both the frontal and parietal regions of children with zinc deficiency and ADHD are significantly longer. N2 wave changes may reflect a different inhibition process and further studies are deemed warranted to investigate the effects of zinc on the inhibitory process in children with low zinc and non-low zinc ADHD.146
Numerous controlled studies report cross-sectional evidence that mean plasma and tissue zinc levels have been found to be significantly lower in children with ADHD than controls suggesting that zinc deficiency may play a substantial role in the aetiopathogenesis of ADHD.146, 147, 148 Previous reports regarding this have come mainly from countries with differing diets and socioeconomic status, however, recent studies also show that zinc deficiency is common, for example, in middle-class Americans. Although there are mixed results, these studies suggest that inattention symptoms are more prominent with lower zinc levels.149
A statistically significant correlation has been found between zinc and serum free fatty acids (FFA) in children with ADHD.147, 150 A study involving 48 children with ADHD demonstrated a mean serum FFA level of 0.176+/-0.102 mEq/L compared with 0.562+/-0.225 mEq/L in the control group and a mean serum zinc level of 60.6 +/-9.9 microg/dL compared with 105.8 +/-13.2 microg/dL in the control group. These findings indicate that zinc deficiency may be a significant factor in the aetiopathogenesis of ADHD. It is yet to be determined whether FFA deficiency is one of the primary causal factors of ADHD or if this is actually secondary to zinc deficiency.150
Several recent trials have demonstrated that zinc supplementation is effective in reducing symptoms of hyperactivity, impulsivity and impaired socialisation in patients with ADHD.147, 149, 151, 152 In 1 double-blind randomised placebo-controlled study, 150mg/day zinc sulfate was administered to children with ADHD. It was determined that the reduction of the above symptoms were more significant in patients of older age, higher BMI score with low zinc and low free fatty acid levels. Zinc sulfate was well tolerated with a low rate of side-effects.147
Several trials have documented a synergistic effect when zinc is used in combination with drug therapy methylphenidate.149, 153, 154 In a recent 6-week placebo-controlled double-blind RCT children with ADHD were given either medication Methylphenidate plus Zinc sulfate 55mg/day (15mg elemental zinc) or placebo. The parent and teacher rating scale scores improved significantly in the group receiving zinc. There was no difference in side-effects.153
A recent double-blind placebo-controlled study determining the relationships between zinc, essential fatty acids and d-amphetamine found that responses to d-amphetamine improved in a linear fashion with zinc levels. 145, 155
Essential fatty acids (EFAs)
There have been numerous studies of late demonstrating a definitive link between essential fatty acids (EFAs) and neurodevelopment. It has been shown that many children suffering from ADHD have significantly lower concentrations of plasma and red blood cell omega-3 and omega-6 EFAs.156 Both omega-3 and omega-6 long chain polyunsaturated fatty acids (LCPUFAs) are critical for brain development and function.157 They function exclusively via cell membranes, in which they are anchored by phospholipids.
Docosahexaenoic acid (DHA, an omega-3 EFA) is a major structural component of neuronal membranes and is essential to pre- and postnatal brain development whereas eicopentanoic acid (EPA, an omega-3 EFA) appears more influential on behaviour and mood.158 Both generate metabolites that are neuroprotective.159 Increasing evidence also indicates that LCPUFA imbalance or deficiencies may be associated with ADHD through involvement in the dopaminergic cortico-striatal metabolism.160
The fatty acid composition of neuronal cell membrane phospholipids directly reflects dietary intake. Changes in the fatty acid composition of neuronal membranes subsequently leads to functional changes in the activity of receptors and other proteins embedded in the phospholipid membrane.158 The ratio of omega-3 to omega-6 fatty acids also influences various aspects of serotoninergic and catecholaminergic neurotransmission and prostaglandin formation processes that are essential in the maintenance of normal brain function. This, again, can be modulated by dietary intake.161
There is a direct association between EFA deficiency or imbalance with a variety of behavioural disorders including ADHD.132, 157, 162, 163, 164 Indeed, it has been demonstrated that children with lower concentrations of omega-3 fatty acids display significantly more maladaptive behaviours, hyperactivity, temper tantrums, learning, health and sleep difficulties than those with higher concentrations of omega-3 EFAs.165, 166
It is not known exactly why these children have lower concentrations of EFAs, however, several theories have been postulated, including low dietary intake, decreased conversion of EFAs to LCPUFAs and increased metabolism of EFAs.165 It has also been suggested that deficiencies of DHA may be responsible for abnormal signal transduction associated with learning disabilities and cognitive deficits. Such abnormalities in this signal transduction process have been shown to be partially corrected by supplementation with DHA.167
In many randomised placebo-controlled double-blind trials, EPA and DHA combinations have been shown to benefit ADHD, amongst many other neuropsychiatric disorders. 151, 157–160, 168–175. For instance, 132 Australian children with ADHD recently participated in a 15-week placebo-controlled double-blind RCT where they received PUFAs, PUFAs + micronutrients or placebo. Improvements in hyperactivity, impulsivity and inattention were recorded in both PUFA groups, with no additional effects being found with micronutrient supplementation.172 Different doses have been used in each study up to a maximum of 16.2g EPA/DHA daily. In this study of high-dose EPA/DHA, there was also a significant correlation between the reduction in the AA:EPA ratio and global severity of illness scores.172
Supplementation with omega-6 EFAs has demonstrated mixed results regarding effects on the symptoms of ADHD. Correct dosage may be critical for optimal effectiveness and further studies are warranted.158, 176, 177
Given that supplementation with EFAs is safe and well-tolerated compared with existing pharmacological interventions, results from such studies strongly support the case for further investigations.178, 179
Essential amino acids
Functional and morphological studies in children with ADHD demonstrate a prefrontal cortex (PFC) dysfunction. This region of the brain is regulated by dopaminergic, noradrenergic, cholinergic, serotonergic, glutamatergic and histaminergic pathways. Currently, there is a wealth of evidence showing that those with ADHD have depleted levels of dopamine and noradrenaline, however, there is still much to be learnt regarding other neurotransmitter systems and how they interact with each other.180–190
The pharmaceutical Methylphenidate primarily affects the PFC and striatum, increasing dopamine and noradrenaline release through multiple means.191, 192, 193 Recent animal studies are now demonstrating that high-dose intranasal dopamine reduces hyperactivity and intermediate-dose improves attention.194 Likewise, further studies have now indicated that there is an inverse relationship between 5-hydroxytryptophan (5HT) and aggression in adolescents with ADHD.195, 196, 197 There is also new evidence implicating the glutamatergic prefrontal-striatal pathway in the pathogenesis of ADHD.190, 191 CNS histamine in the PFC is closely linked with cognition and it has only recently become known that Methylphenidate also enhances cortical histamine in animal studies. The newer non-stimulant drugs primarily work as selective noradrenaline re-uptake inhibitors and by increasing extracellular levels of histamine in the PFC.198, 199 Modulation of the H3 histamine receptor can affect cognition via the release of several other neurotransmitters, including acetylcholine and noradrenaline.200, 201, 202
It is imperative to remember that all neurotransmitters are synthesised from amino acids and all require various vitamins and minerals, primarily zinc and vitamin B6, as cofactors. Deficiencies or imbalances in amino acids, vitamins and minerals can therefore profoundly influence neurotransmitter synthesis and breakdown. Supplements that contain the essential amino acids and nutrients can significantly reduce symptoms by their conversion to specific neurotransmitters.203
To date, there is a paucity of studies regarding specific amino acid supplementation and its effects on the symptoms of ADHD. There is increasing evidence for oxidative stress mechanisms underlying the pathophysiology of ADHD, which offers new treatment targets in oxidation biology systems. Of these, the glutathione system has the most favourable theoretical foundation as it is the most generic of all cellular antioxidants. Several studies have shown the efficacy of N-Acetylcysteine, a glutathione precursor, in the treatment of various psychiatric conditions of oxidative stress, indicating that glutathione itself may be a promising therapeutic target.204
Carnitine is another amino acid that has been shown to exert positive effects on the symptoms of ADHD. Acetyl-L-Carnitine (ALC) is essential for energy metabolism and essential fatty acid anabolism. A 16-week placebo-controlled RCT demonstrated that 500–1500mg bd L-carnitine was superior to placebo in treating inattention-type symptoms.205 This confirms earlier studies which have reported significant benefits in ADHD symptoms. Another study has shown significantly reduced attention problems and aggressive behaviour in boys with ADHD supplemented with carnitine.206Animal studies have demonstrated that ALC increases noradrenaline and the 5HIAA/5HT ratio in the cingulated cortex, subsequently decreasing behavioural impulsivity.207 As ALC is safe with no psychostimulant properties, more studies are warranted, particularly for possible significant benefits in the inattentive type.207
Homeopathy
An increasing number of parents are turning to homeopathy to treat their hyperactive child.208 There have been several placebo-controlled double-blind RCTs demonstrating mixed results for the efficacy of homeopathy in the treatment of ADHD.208–211 A 2007 Cochrane review, however, has deemed that there is currently little evidence to support the use of homeopathy, with the recommendation that optimal treatment protocols be developed prior to further RCT being undertaken.212
It is important to note, however, that homeopathy focuses on the individual characteristics of each patient’s experience and symptoms and uses this information to determine the appropriate prescription for each patient.213 For this reason, RCTs are very difficult to conduct and the results, difficult to interpret.213, 214
One study of children with ADHD receiving individualised homeopathic treatment demonstrated a 75% response to treatment, reaching a clinical improvement rating of 73% and an amelioration of the Connors Global Index (CGI) of 55%. In comparison, clinical improvement under Methylphenidate was 65% with a lowering of the CGI to 48%. Both treatments appeared to be similar in efficacy and it was concluded that in cases where treatment is not urgent, homeopathy is a valuable alternative to methylphenidate.215
Herbal medicines
St John’s wort
There has been only 1 recent study on the effects of Hypericum perforatum on the symptoms of ADHD. No differences were observed between St John’s wort or placebo.216
Ginkgo biloba + ginseng
A pilot study has recently been conducted on a herbal product containing American ginseng extract, panax quinquefolium (200mg) and ginkgo biloba extract (50mg). One capsule administered twice daily for only 1 month resulted in significant improvements in behaviour of many children with ADHD, warranting further research in this area.217
Traditional Chinese medicine (TCM)
There have been several clinical trials conducted in China with promising results in the treatment of ADHD. One RCT involving 100 children compared a combination of Bupleurum chinense, Scutellaria baicalensis, Astragalus membranaceus, Codonopsis pilosula, Ligustrum lucidum, lophatherum gracile and thread of ivory with 5–15mg Ritalin twice daily administered for 1–3 months. In the TCM group, 23 cases were ‘cured’ (measured by clinical symptoms disappearing, an increase of 10 IQ units, a normalised EEG and no recurrence of symptoms 6 months post-treatment). Four cases were improved (symptoms and signs markedly improved, an increase of 4 IQ units and normalising EEG) and 11 cases ineffective. In the group taking Ritalin, 6 cases were ‘cured’, 12 cases improved and 2 cases were ineffective. Although there was no significant difference regarding efficacy between the 2 groups in this study, the side-effects of TCM were considerably less.218
Double-blind studies comparing the effects of Duodongining (DDN) with Ritalin have also shown similar clinical responses with a marked reduction of side-effects in those taking DDN.219
These effects have once again been seen with Tiaoshen liquor. Its therapeutic mechanism is thought to be related to improvements in information transmission through cholinergic neuron synapses and enhancement of hypoxia tolerance of cerebral tissues.220
A further randomised controlled study involving 200 children assessed the effects of Ritalin, Yihzi mixture (YZM) and a combination of both, for a period of 3 months. By assessment with multiple questionnaires, it was shown that the therapeutic effect of the combined treatment was better than either Ritalin or YZM alone. Furthermore, only the YZM and combined groups showed significant improvement in both soft nerve signs and EEGs, again with less side-effects than Ritalin.221
An interesting study of Jiangqian granules (JQG) has also shown significant clinical improvements when compared with Ritalin. In this particular study, blood lead concentrations were measured and found to be significantly higher in the ADHD group compared with the control group. These parameters were remeasured at the end of the 3-month study. Although there were reductions in blood lead concentrations in both groups, the lowering was significantly more in the group treated with JQG and this is believed to be its prime mechanism of action.222
Pycnogenol
Oxidative stress has been implicated in the pathogenesis of many chronic diseases, including ADHD, with correspondingly new targets for the development of different therapeutic interventions.223
Pycnogenol, an extract from the bark of the French maritime pine, is a potent polyphenol complex that contains phenolic acids, cetechin, taxifolin and procyanidins. It acts as a highly powerful antioxidant and chelating agent that stimulates the activity of other antioxidants such as SOD (superoxide dismutase) and eNOS (endothelial nitric oxide synthase).224–228
Concentrations of catecholamines have been found to be higher in the urine of patients with ADHD compared with healthy children. Furthermore, adrenaline and noradrenaline concentrations positively correlate with plasma levels of oxidised glutathione.224
Several placebo-controlled, double-blind RCTs have shown that pycnogenol reduces symptoms of hyperactivity and improves attention, visual-motor co-ordination and concentration in children with ADHD. One month of pycnogenol (1mg/kg/day) significantly reduced oxidised glutathione and increased reduced glutathione compared with placebo. Urinary catecholamines (dopamine, adrenaline and noradrenaline) were also decreased. Thus, pycnogenol reduces oxidative damage to DNA, normalising total antioxidant status of ADHD children.224, 225, 229 At 1 month post-cessation of Pycnogenol, a relapse of ADHD symptoms was noted.226
Physical therapies
Massage
Massage intervention is known to benefit childhood mental wellbeing. For instance, a meta-analysis of the literature demonstrated massage intervention can be of benefit in promoting mental and physical wellbeing in any child under the age of 6 months by improving the mother–infant interaction, sleeping and crying, and on hormones influencing stress levels which may impact positively in children’s behaviour in later life.230
Massage has been shown to increase serotonin levels which may modulate dopamine levels in children with ADHD. One study demonstrated increased concentration and decreased hyperactivity after children with ADHD received 15 minute massages for 10 consecutive school days.231 More recently, massage for 20 minutes twice weekly for 1 month benefited students with ADHD by improving short-term mood state and longer-term classroom behaviour.232
Chiropractic
In 1 small study, chiropractic manipulation has been shown to decrease autonomic nervous system activity and improve behaviour in children with ADHD, thus warranting further investigation in this area.233
There is currently a placebo-controlled, double-blind RCT underway in Australia investigating the effects of Neuro Emotional Technique (NET), a branch of chiropractic, on children with ADHD. The control group are continuing their existing medical regime whilst the intervention and placebo group have the addition of NET and sham NET protocols added to their regime, respectively. These NET/sham NET protocols are performed twice weekly for the first month and then monthly for the next 6 months. This study should provide good evidence as to the efficacy of NET as an adjunct therapy to conventional medical therapy.234
Conclusion
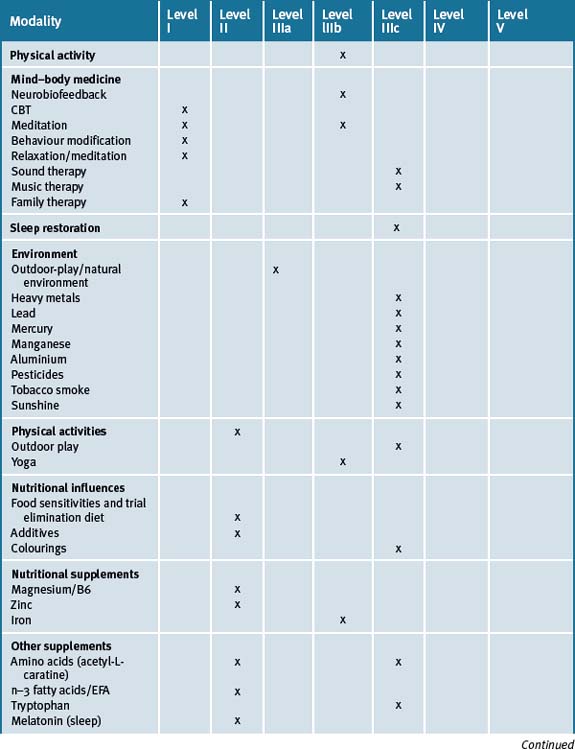
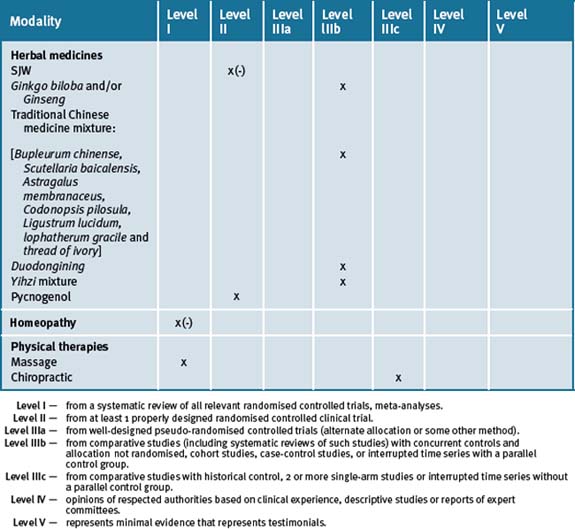
ADHD is a chronic, complex and multifactorial illness that has become one of the most common cognitive and behavioural disorders diagnosed among children of school age today. Current conventional treatment includes the use of stimulant medications which significantly influence catecholamine concentrations. Not all children respond to these medications and the risk of side-effects combined with concerns regarding the safety of the long-term use of such medications makes CAM therapies an attractive option. There is a wealth of evidence for the use of many of these therapies and an holistic approach to the management of our future generations is well warranted. Management of ADHD should include various behavioural and lifestyle changes that include avoidance of chemicals and smoking, sleep restoration, dietary changes, exercise outdoors in a natural setting and relaxation strategies. Table 6.1 summarises the best evidence for CAM therapies for ADHD.
Clinical tips handout for patients — attention deficit hyperactivity disorder (ADHD)
1 Lifestyle advice
2 Physical activity/exercise
3 Mind–body medicine
Counselling/psychotherapy
4 Environment
5 Dietary changes
6 Supplementation
Probiotics
Digestive enzymes
Magnesium
Vitamin B6
Zinc
L–Tyrosine
1 Kaiser N.M., Hoza B., Hurt E.A. Multimodal treatment for childhood ADHD. Expert Rev Neurother. 2008;8(10):1573-1583.
2 Hassler F., Duck A., Reis O., et al. Alternative agents used in ADHD. Z Kinder Jugendpsychiatr Psychother. 2009;37(1):13-24.
3 ADHD Tomas L. J Comp Med. 2004;3(2):24-30.
4 J Am Acad Child Adolesc Psychiatry, 2001.
5 Appl Psychophysiology and Biofeedback, 2003.
6 Weber W., Newmark S. Complementary and alternative medical therapies for ADHD and autism. Pediatr Clin North Am. 2007;54(6):983-1006.
7 Chan E. The role of CAM in ADHD. J Dev Behave Pediatr. 2002;23(1)(suppl.):S37-S45.
8 Sawni A. ADHD and CAM. Adolesc Med State Art Rev. 2008;19(2):313-326.
9 Sihna D., Efron D. CAM use in children with ADHD. J Pediatr Child Health. 2005;41(1–2):23-26.
10 Kidd P.M. ADHD in children: rationale for its integrative management. Altern Med Rev. 2000;5:402-428.
11 Herrmann M., King K., Weitzman M. Prenatal tobacco smoke and postnatal second hand smoke exposure and child neurodevelopment. Curr Opin Pediatr. 2008;20(2):184-190.
12 Braun J.M., Froelich T.E., Daniels J.L., et al. Association of environmental toxicants and conduct disorder in US children: NHANES 2001–2004. Environ Health Perspect. 2008;116(7):956-962.
13 Braun J.M., Kahn R.S., Froelich T., et al. Exposures to environmental toxicants and ADHD in US children. Environ Health Perspect. 2006;114(12):1904-1909.
14 Banerjee T.D., Middleton F., Faraone S.V. Environmental risk factors for ADHD. Acta Pediatr. 2007;96(9):1269-1274.
15 Linnet K.M., Dalsgaard S., Obel C., et al. Maternal lifestyle factors in pregnancy risk of ADHD and associated behaviours: review of the current evidence. Am J Psychiatry. 2003;160(6):1028-1040.
16 Patel K., Curtis L.T. A comprehensive approach to treating autism and ADHD; a prepilot study. J Altern Complement Med. 2007;13(10):1091-1097.
17 Brennan A.R., Arnsten A.F. Neuronal mechanisms underlying ADHD: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236-245.
18 Krause J. SPECT and PET of the dopamine transporter in ADHD. Expert Rev Neurother. 2008;8(4):611-625.
19 Wilens T.E. Effects of Methylphenidateon the catecholaminergic system in ADHD. J Clin Psychopharmacol. 2008;28(3)(suppl. 2):S46-S53.
20 Friel P.N. EEG biofeedback in the treatment of ADHD. Altern Med Rev. 2007;12(2):146-151.
21 Heinrich H., Gevensleben H., Strehl U. Annotation: Neuro-feedback-train your brain to train your behaviour. J Child Psycho Psychiatry. 2007;48(1):3-16.
22 Butnik S.M. Neuro-feedback in adolescents and adults with ADHD. J Clin Psychol. 2005;61(5):621-625.
23 Levesque J., Beauregard M., Mensour B. Effect of neuro-feedback training on the neural substrates of selective attention in children with ADHD: a functional MRI study. Neurosci Lett. 2006;394(3):216-221.
24 Thompson L., Thompson M. Neuro-feedback combined with training in metacognitive strategies; effectiveness in students with ADD. Appl Psychophysiol Biofeedback. 1998;23(4):243-263.
25 Monastra V.J. QEEG and Adhd: Implications for clinical practice. Curr Psychiat Rep. 2008;10(5):432-438.
26 Hou J.H., Zhang Y., Xu C. EEG Biofeddback for the treatment of ADHD in children. Zhongguo Dang Dai Er Ke Za Zhi. 2008;10(6):726-727.
27 Doehnert M., Brandeis D., Straub M., et al. Slow cortical potential neuro-feedback in ADHD: is there neurophysiological evidence for specific effects? J Neural Transm. 2008;115(10):1445-1456.
28 Alexander D.M., Hermens D.F., Keage H.A., et al. Event-related wave activity in the EEG provides new marker of ADHD. Clin Neurophysiol. 2008;119(1):163-179.
29 Leins U., Goth G., Hinterberger T., et al. Neuro-feedback for children with ADHD: a comparison of SCP and Theta/Beta protocols. Appl Psychophysiol Biofeedback. 2007;32(2):73-88.
30 Strehl U., Leins U., Goth G., et al. Self-regulation of slow cortical potentials: a new treatment for children with ADHD. Pediatrics. 2006;118(5):e1530-e1540.
31 Holtmann M., Stadler C. EEG Biofeedback for the treatment of ADHD in children and adolescence. Expert Rev Neurother. 2006;6(4):533-540.
32 Beauregard M., Levesque J. Functional MRI investigation of the effects of neuro-feedback training on the neural bases of selective attention and response inhibition in children with ADHD. Appl Psychophysiol Biofeedback. 2006;31(1):3-20.
33 Xiong Z., Shi S., Xu H. A controlled study of the effectiveness of EEG biofeedback training on children with ADHD. J Huazhong Univ Sci Technolog Med Sci. 2005;25(3):368-370.
34 Fox D.J., Tharp D.F., Fox L.C. Neuro-feedback: an alternative and efficacious treatment for ADHD. Appl Psychophysiol Biofeedback. 2005;30(4):365-373.
35 Loo S.K., Barkley R.A. Clinical utility of EEG in ADHD. Appl Neuropsychol. 2005;12(2):64-76.
36 Gruzelier J., Wegner T. Critical validation studies of neuro-feedback. Child Adolesc psychiatr Clin N Am. 2005;14(1):83-104.
37 Rossiter T. the effectiveness of neuro-feedback and stimulant drugs in treating ADHD: part 11. Replication. Appl Psychophysiol Biofeedback. 2004;994(2):233-243.
38 Monastra V.J. EEG biofeedback as a treatment for ADHD: rationale and empirical foundation. Child Adolesc Psychiatr Clin N Am. 2005;14(1):55-82.
39 Holtmann M., Stadler C., Leins U., et al. Neuro-feedback for the treatment of ADHD in childhood and adolescence. Z Kinder Jugendpsychiatr Psychother. 2004;32(3):187-200.
40 Nash J.K. Treatment of ADHD with Neurotherapy. Clin EEG. 2000;31(1):30-37.
41 Linden M., Habib T., Radojevic V. A controlled study of the effects of EEG biofeedback on cognition and behaviour of children with ADD and learning disabilities. Biofeedback and Self-regulation. 1996;21:35-49.
42 Lubar J.F., Swartwood M.O., Swartwood J.N., et al. Evaluation of the effectiveness of EEG neuro-feedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioural ratings and WISC-R performance. Biofeedback and Self-regulation. 1995;20(1):83-99.
43 Baumgaertal A. Alternative and controversial treatments for ADHD. Paediatr Clin of North Am. 1999;46(5):977-992.
44 Fuchs T., Birbaumer N., Lutzenberger W., et al. Neuro-feedback treatment for ADHD in children: a comparison with methylphenidate. Appl Psychophysiology and Biofeedback. 2003;28(1):1-12.
45 Monastra V.J., Monastra D.M., George S. The effects of stimulant therapy, EEG biofeedback and parenting style of ADHD. Appl Psychopys and Biofeedback. 2002;27(4):231-249.
46 Knight L.A., Rooney M., Chronis-Tuscano A. Psychosocial treatments for ADHD. Curr Psychiatry Rep. 2008;10(5):412-418.
47 Daly B.P., Creed T., Xanthpoulos M., et al. Psychosocial treatments for children with ADHD. Neuropsychol Rev. 2007;17(1):73-89.
48 Waxmonsky J.G. Non-stimulant therapies for ADHD in children and adults. Essent Psychopharmacol. 2005;6(5):262-276.
49 Toplak M.E., Connors L., Shuster J., et al. review of Cognitive, CBT and neural-based interventions for ADHD. Clin Psychol Rev. 2008;28(5):801-823.
50 Brown R.T., Amler R.W., Freeman W.S., et al. Treatment of ADHD: Overview of the evidence. Pediatrics. 2005;115(6):e749-e757.
51 Montgomery P., Bjornstad G., Dennis J. Media-based behavioural treatments for behavioural problems in children. Cochrane Database Syst Rev. (1):2006. CD002206
52 Bjornstad G., Montgomery P. Family therapy for ADD or ADHD in children and adolescents. Cochrane Database Syst Rev. (2):2005. CD005042
53 Knouse L.E., Cooper-Vince C., Sprich S., et al. Recent developments in the psychosocial treatment of ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
54 Ramsay J.R. Current status of CBT as a psychosocial treatment for adults with ADHD. Curr Psychiatry Rep. 2007;9(5):427-433.
55 Zylowski L., Ackerman D.L., Yang M.H., et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746.
56 Goldbeck L., Schmid K. Effectiveness of autogenic relaxation training in children and adolescents with behavioural and emotional problems. J Am Acad Child Adolesc Psychiatry. 2003;42(9):1046-1054.
57 Klein S.A., Deffenbacher J.L. Relaxation and exercise for hyperactive impulsive children. Percept Mot Skills. 1977;45:1159-1162.
58 Donney V.K., Poppen R. Teaching parents to conduct behavioural relaxation training with their hyperactive children. J Behav Ther Exp Psychiatry. 1989;20:319-325.
59 Raymer R., Poppen R. Behavioural relaxation training with hyperactive children. J Behav Ther Exp Psychiatry. 1985;16:309-316.
60 Dunn F.M., Howell R.J. Relaxation training and its relationship to hyperactivity in boys. J Clin Psychology. 1982;38:92-100.
61 Gumenyuk V., Korzyukov O., Escera C., et al. Electrophysiological evidence of enhanced distractability in ADHD children. Neurosci Lett. 2005;374(3):212-217.
62 Soderlund G., Sikstrom S., Smart A. Listen to the noise: Noise is beneficial for cognitive performance in ADHD. Child Psychol Psychiatry. 2007;48(8):840-847.
63 Rickson D.J. Intstructional and improvisational models of music therapy with adescents who have ADHD: a comparison of the effects on motor impulsivity. J Music Ther. 2006;43(1):39-62.
64 Quach J., Hiscock H., Canterfield L., et al. Outcomes of child sleep problems over the school transition period: Australian Population Longitudinal Study. Pediatrics. 2009;123(5):1287-1292.
65 Dominguez-Ortega L., de Vincente-Colomina A. ADHD and sleep disorders. Med Clin (Barc). 2006;126(13):500-506.
66 Betancourt-Fursow D.E., Jimenez Y.M., Jimenez-Leon J.C., et al. ADHD and sleep disorders. Rev Neurol. 2006;42(suppl.2):S37-S51.
67 Pandi-Perumal S.R., Srinivasan V., Spence D.W., et al. Role of the Melatonin system in the control of sleep: therapeutic implications. CNS Drugs. 2007;21(12):995-1018.
68 Van der Heijden K.B., Smits M.G., Van Someren E.J., et al. Effect of melatonin on sleep, behaviour and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry. 2007;46(2):233-241.
69 Weiss M.D., Wasdell M.B., Bomben M.M., et al. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J Am Acad Child Adolesc Psychiatry. 2006;45(5):512-519.
70 Kuo F.E., Taylor A.F. A potential natural treatment for attention-deficit/hyperactivity disorder: evidence from a national study. American Journal of Public Health. 2004;94(9):1580-1586.
71 Markowitz M. Lead poisoning: a disease for the next millennium. Curr Probl Pediatr. 2000;30(3):62-70.
72 Gilbert S.G., Weiss B. A Rationale for lowering the blood lead action level from 10 to 2ug/dL. Neurotoxicology. 2006;27(5):693-701.
73 Woolf A.D., Goldman R., Bellinger D.C. Update on the clinical management of childhood lead poisoning. Pediatr Clin North Am. 2007;54(2):271-294.
74 Binns H.J., Campbell C., Brown M.J., et al. Interpreting and managing blood lead levels of <10ug/dL in children and reducing childhood exposure to lead: recommendations of the Centres for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Pediatrics. 2007;120(5):e1285-e1298.
75 Bellinger D.C. Very low lead exposures and children’s neurodevelopment. Curr Opin pediatr. 2008;20(2):172-177.
76 Lanphear B.P., Hornung R., Khoury J., et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894-899.
77 Canfield R.L., Henderson C.R.Jr., Cory-Slechta D.A., et al. Intellectual impairment in children with blood lead concentrations <10ug/dL. N Engl J Med. 2003;348(16):1517-1526.
78 Guidotti T.L., Ragain L. Protecting children from toxic exposure: 3 strategies. Pediatr Clin North Am. 2007;54(2):227-235.
79 Surkan P.J., Zhang A., Trachtenberg F., et al. Neuropsychological function in children with blood levels <10ug/dL. Neurotoxicology. 2007;28(6):1170-1177.
80 Rico J.A., Kordas K., Lopez P., et al. Efficacy of iron and/or zinc supplementation on cognitive performance of lead-exposed Mexican school-children: a RCT. Pediatrics. 2006;117(3):e518-e527.
81 Wang H.L., Chen X.T., Yang B., et al. Case-control study of blood lead levels and ADHD in Chinese children. Environ Health Perspect. 2008;116910):1401-1406.
82 Nigg J.T., Knottnerus G.M., Martel M.M., et al. Low blood levels associated with clinically diagnosed ADHD and mediated by weak cognitive control. Biol Psychiatry. 2008;63(3):325-331.
83 Kordas K., Stoltzfus R.J., Lopez P., et al. Iron and zinc supplementation does not improve parent or teacher ratings of behaviour in first Grade Mexican children exposed to lead. J Pediatr. 2005;147(5):632-639.
84 Dye B.A., Schober S.E., Dillon C.F., et al. Urinary mercury concentrations assoc with dental restorations in adult women aged 16–49 years: US 1999–2000. Occup Environ Med. 2005;62(6):368-375.
85 Nylander M., Friberg L., Lind B. Mercury concentrations in the human brain and kidneys in relation to exposure from dental amalgam fillings. Swed Dent J. 1987;11(5):179-187.
86 Kingman A., Albertini T., Rown L.J. Hg concentrations in urine and whole blood assoc with amalgam exposure in a US military population. J Dent Res. 1998;77(3):461-471.
87 Brownawell A.M., Berent S., Brent R.L., et al. The potential adverse effects of dental amalgam. Toxicol Rev. 2005;24(1):1-10.
88 Shenker B.J., Maserejian N.N., Zhang A., et al. Immune function effects of dental amalgam in children: a RCT. J Am Dent Assoc. 2008;139(11):1496-1505.
89 DeRouen T.A., Martin M.D., Leroux B.G., et al. Neurobehavioral effects of dental amalgam in children — a Randomised clinical trial. JAMA. 2006;295(15):1784-1792.
90 Bellinger D.C., Trachtenberg F., Daniel D., et al. Dental amalgam restorations and children’s neuropsychological function: the New England Children’s Amalgum Trial. Environ Health Perspect. 2007;115(3):440-446.
91 Bellinger D.C., Trachtenberg F., Barregard L., et al. Neuropsychological and renal effects of dental amalgam in children- a Randomised clinical trial. JAMA. 2006;295(15):1775-1783.
92 Bellinger D.C., Trachtenberg F., Daniel D., et al. A dose-effect analysis of children’s exposure to dental amalgam and neuropsychological function: the New England Children’s Amalgum Trial. J Am Dent Assoc. 2007;138(9):1210-1216.
93 Cheuk D.K., Wong V. ADHD and blood Mercury level: a case-control study in Chinese children. Neuropediatrics. 2006;37(4):234-240.
94 Elsner R.J., Spangler J.G. Neurotoxicity of Inhaled Mn: public health danger in the shower? Med Hypotheses. 2005;65(3):607-616.
95 Bourchard M., Laforest F., Vandelac L., et al. Hair Mn and hyperactive behaviours: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115(1):122-127.
96 Halatek T., Sinczuk-Walczak H., Rydzynski K. Early neurotoxic effects of inhalation exposure to Al and/or Mn assessed by serum levels of phospholipid-binding Clara cells protein. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;43(2):118-124.
97 Labie D. Developmental neurotoxicity of industrial chemicals. Med Sci (Paris). 2007;23(10):868-872.
98 Grandjean P., Landrigan P.J. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167-2178.
99 Hussain J., Woolf A.D., Sandel M., et al. Environmental evaluation of a child with developmental disability. Pediatr Clin North Am. 2007;54(1):47-62.
100 Perera F.P., Rauh V., Whyatt R.M., et al. A summary of recent findings on birth outcomes and development effects of prenatal ETS, PAH and pesticide exposures. Neurotoxicology. 2005;26(4):573-587.
101 Landrigan P.J. Pesticides and PCBs: an analysis of the evidence that they impair children’s neurobehavioral devt. Mol Genet Metab. 2001;73(1):11-17.
102 Brondum J. Environmental exposures and ADHD. Environ Health Perspect. 2007;115(8):A398.
103 Tantillo M., Kesick C.M., Hynd G.W., et al. The effects of exercise on children with ADHD. Med Sci Sports Exerc. 2002;34(2):203-207.
104 Panskepp J., Burgdorf J., Turner C., et al. Modelling ADHD-type arousal with unilateral cortex damage in rats and beneficial effects of play therapy. Brain Cogn. 2003;52(1):97-105.
105 Complement Ther Nurs Midwif, 2004;10(1):46-53.
106 Haffner J., Roos J., Goldstein N., et al. The effectiveness of body-oriented methods in the treatment of ADHD: results of a controlled pilot study. Z Kinder Jugendpsychiatr Psychotherapy. 2006;491(3):37-47.
107 Jensen P.S., Kenny D.T. The effects of Yoga on the attention and behaviour of boys with ADHD. J Atten Disord. 2004;7(4):205-216.
108 Sinn N. Nutritional and dietary influences on ADHD. s.l. Nutr Rev. 2008;66(10):558-568.
109 Breakey J. The role of diet and behaviour in childhood. J Paedietric Clin Health. 1997;33:190-194.
110 Bellisle F. Effects of diet on behaviour and cognition in children. s.l. Br J Nutr. 2004;92(suppl.2):S227-S232.
111 Chaves-Carbello E. Diet therapy in the treatment of neuropaeditric disorders. s.l. Rev Neurol. 2003;37(3):267-274.
112 Boris M., Mandel F.S. Foods and additives are common causes of ADHD in children. Ann Allergy. 1994;72(5):462-468.
113 Peisser L.M., Frankena K., Toorman J., et al. A RCT into the effects of food on ADHD. Eur Child Adolesc Psychiatry. 2009;18(1):12-19.
114 Schnoll R., Burshteyn D., Cea-Aravena J. Nutrition in the treatment of ADHD: a neglected but important aspect. s.l. Appl Psychophysiol Biofeedback. 2003;28(1):63-75.
115 Pelsser L.M., Buitelaar J.K. Favourable effect of a standard elimination diet on the behaviour of young children with ADHD: A pilot study. Nederlands Tijdschrift voor Geneeskunde. 2002;146(52):2543-2547.
116 Stevenson J. Dietary influences on cognitive development and behaviour in children. s.l. Proc Nutr Soc. 2006;65(4):361-365.
117 Cruz N.V., Bahna S.L. Do food or additives cause behaviour disorders? s.l. Pediatr Ann. 2006;35(10):744-745. 748–54
118 Lees of MH. Food Intolerances and Allergy — a review. QJ Med. 1983;52(206):111-119.
119 Silfverdal S.A., Hernall O. Food additives can increase hyperactivity in children. Results from a British study confirm the connection t. s.l. Lakartidningen. 2008;105(6):354-355.
120 Wallis C. Hyper kids? Check their diet. Reserach confirms a long-suspected link between hyperectivity and food additives. s.l. Time. 2007;170(13):68.
121 Ghuman J.K., Arnold L.E., Anthony B.J. Psychopharmacological and other treatments in preschool children with ADHD: Current evidence and practice. s.l. J Child Adolesc Psychopharmacol. 2008;18(5):413-447.
122 Lau K., McLean W.G., Williams D.P., et al. Synergistic interactions between community used food additives in a developmental neurotoxicity test. s.l. Toxicol Sci. 2006;90(1):178-187.
123 Cardinale F., Mangini F., Berardi M., et al. Intolerance to food additives: an update. s.l. Minerve Pediatr. 2008;60(6):1401-1409.
124 McCann D., Barrett A., Cooper A., et al. Food additives and hyperactive behaviour in 3 year old and 8/9 year old children in the community: a Randomised Double-Blinded Placebo-controlled Trial. s.l. Lancet. 2007;370(9598):1560-1567.
125 Batemen B., Warner J.O., Hutchinson E., et al. The effects of a double-blind, placeb-controlled, artificial food colourings and benzoate preservatives challenge on hyperactivity in a general population sample of preschool children. s.l.:. Arch Dis Child. 2004;89(6):506-511.
126 Krummel D., Seligson F.H., Guthrie H.A. Hyperactivity: Is candy causal? Critical Reviews in Food Science and Nutrition. 1996;36(1, 2):31-47.
127 Tsaluchidu S., Cocchi M., Tonello L., et al. Fatty acids and oxidative stress in psychiatric disorders. s.l.: BMC Psychiatry. 2008;8(suppl.)1:S5.
128 Niederhofer H., Pittschieler K. A preliminary investigation of ADHD symptoms in persons with coeliac disease. s.l.:. J Atten Disord. 2006;10(2):200-204.
129 Pynnonen P.A., Isometsa E.T., Verkasalo M.A., et al. Gluten-free diet mey alleviate depressive and behavioural symptoms in adolescents with coeliac disease: a prospective follow-up acse-series. s.l. BMC Psychiatry. 2005;5:14.
130 Lahlat E., Broide E., Leshem M., et al. Prevalence of coeliac antibodies in children with neurological disorders. s.l. Pediatr Neurol. 2000;22(5):393-396.
131 Bourre J.M. Effects of nutrients (food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Ageing. 2006;10(5):377-385.
132 Ramakrishnan U., Imhoff-Kunsch B., DiGirolamo A.M. Role of DHA in maternal and child health. Am J Clin Nutr. 2009;89(3):958S-962S.
133 Mousain-Bosc M., Roche M., Rapin J., et al. M6 / Vit B6 intake reduces CNS hyperexcitability in children. s.l. J Am Coll Nutr. 2004;23(5):545S-548S.
134 Nogovitsina O.R., Levitina E.V. Neurological aspects of the clinical features, pathophysiology and corrections of impairments in ADHD. Neurosci Behav Physiol. 2007;37(3):199-202.
135 Nogovitsina O.R., Levitina E.V. Effect of MAGNE-B6 on the clinical and biochemical manifestations of the syndrome of attention deficit and hyperactivity in children. Eksp Klin Farmacol. 2006;69(1):74-77.
136 Nogovitsina O.R., Levitina A.V. Diagnostic value of examination of ther Mg homeostasis in children with ADHD. Klin Lab Diagn. 2005;5:P17-P19.
137 Mousain-Bosc M., Roche M., Polge A., et al. Improvement of neurobehavioral disorders in children supplemented with magnesium-Vitamin B6. ADHD. Magnes Res. 2006;19(1):46-52.
138 Starobrat-Hermilin B., Kozielec T. The effects of Mg physiological suppl on hyperactivity in children with ADHD. Positive response to Mg oral loading test. Magnes Res. 1997;10(2):149-156.
139 Konofal E., Lecendreux M., Arnulf I., et al. Iron deficiency in children with ADHD. Arch Pediatr Adolesc Med. 2004;158(12):1113-1115.
140 Oner O., Alkar O.Y., Oner P. Relation of ferritin levels with symptom ratings and cognitive performance in children with ADHD. Pediatr Int. 2008;50(1):40-44.
141 Burden M., Westerlund A., Armony-Sivan R., et al. An event-related potential study of attention and recognition memory in infants with iron deficiency anaemia. Pediatrics. 2007;!20(2):336-345.
142 Patrick L.R. Restless legs syndrome: pathophysiology and the role of iron and folate. Altern Med Rev. 2007;12(2):101-112.
143 Konofal E., Lecendreux M., Deron J., et al. Effects of iron suppl on ADHD in children. Pediatr Neurol. 2008;38(1):20-26.
144 Martins S, Logan S, Gilbert R. Cochrane Database of Syst Rev 2006; CD001444.
145 Arnold L.E., Pinkham S.M., Votolato N. Does zinc moderate essential fatty acid and amphetamine treatment for ADHD? J Child & Adolescent Psychopharmacology. 2000;10(2):111-117.
146 Yorbik O., Ozdag M.F., Olgun A., et al. Potential effects of zinc on information processing in boys with ADHD. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):662-667.
147 Bilici M., Yildrim F., Kandil S., et al. Double-blind, placebo-controlled study of zinc sulfate in the treatment of ADHD. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):181-190.
148 Arnold L.E., DiSilvestro R.A. Zinc in ADHD. J Child Adolesc Psychopharmacol. 2005;15(4):619-627.
149 Arnold L.E., Bozzolo H., Hollway J., et al. Serum zinc correlates with parent and teacher-rated inattention in children with ADHD. J Child Adolesc Psychopharmacol. 2005;15(4):628-636.
150 Bekaroglu M., Aslan Y., Gedik Y., et al. Relationships between serum free fatty acids and zinc, and ADHD: A research note. J Child Psychology & Psychiatry & Allied Disciplines. 1996;37(2):225-227.
151 Rucklodge J.J., Johnstone J., Kaplan B.J. Nutrient supplementation approaches in the treatment of ADHD. s.l. Expert Rev Neurother. 2009;9(4):461-476.
152 Uckardes Y., Ozmert E.N., Unal F., et al. Effects of zinc supplementation on parent and teacher behaviour rating scores in low socioeconomic level Turkish primary school children. Acta Paediatr. 2009;98(4):731-736.
153 Akhondzadeh S., Mohammadi M.R., Khademi M. Zinc sulfate as an adjunct to Methylphenidate for the treatment of ADHD in children: a double-blind and randomized trial. BMC Psychiatry. 2004;4:9.
154 DiGirolamo A.M., Ramirez-Zea M. Role of zinc in maternal and child mental health. Am J Clin Nutr. 2009;89(3):940S-945S.
155 Arnold L.E., Votolato N.A., Kleykamp D., et al. Does hair zinc improve amphetamine improvement of ADD/Hyperactivity? Int J Neurosci. 1990;50(1–2):103-107.
156 Stevens L.J., Zentall S.S., Deck J.L., et al. Essential fatty acid metabolism in boys with ADHD. Am J Clin Nut. 1995;62(4):761-768.
157 Germano M., Meleleo D., Montorfano G., et al. Plasma, RBC phospholipids and clinical evaluation after long chain n-3 supp. in children with ADHD. Nutr Neurosci. 2007;10(1–2):1-9.
158 Peet M., Stokes C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. 2005;65(8):1051-1059.
159 Kidd P.M. Omega-3 DHA and EPA for cognition, behaviour and mood: clinical findings and structural functional synergies with cell membrane phospholipids. Altern Med Rev. 2007;12(3):207-227.
160 Frohlich J., Dopfner M. The treatment of ADHD with PUFAs-an effective treatment alternative? Z Kinder Jugendpsychiatr Psychother. 2008;36(2):109-116.
161 Haag M. Essential fatty acids and the brain. Can J Psychiatry. 2003;48(3):195-203.
162 Antalis C.J., Stevens L.J., Campbell M., et al. Omega-3 fatty acids in ADHD. Prostaglandins Leuko essent Fatty acids. 2006;75(4–5):299-308.
163 Richardson A.J. LCPUFAs in childhood developmental and psychiatric disorders. Lipids. 2004;39(12):1215-1222.
164 Young G.S., Conquer J.A., Thomas R. Effect of randomized supplementation with high dose olive, flax or fish oil on serum phospholipid fatty acid levels in adults with ADHD. Reprod Nutr Rev. 2005;45(5):549-558.
165 Burgess J.R., Stevens L., Zhang W., et al. Long chain polyunsaturated fatty acids in children with ADHD. Am J Clin Nut. 2000;71(1)(suppl.):327S-330S.
166 Mitchell E.A., Aman M.G., Turbott S.H., et al. Clinical characteristics and serum EFA levels in hyperactive children. Clin Paediatr. 1987;26(8):406-411.
167 Farooqu A.A., Horrocks L.A. Plasmalogens, phospholipase A2 and DHA turnover in brain tissue. J Mol Neurosci. 2001;16(2–3):263-272. discussion 279–284
168 Riediger N.D., Othman R.A., Suh M., et al. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109(4):668-679.
169 Sinn N., Bryan J., Wilson C. Cognitive effects of PUFAs in children with ADHD symptoms: a RCT. Prostaglandins Leukot Essent Fatty Acids. 2008;7894–5:311-326.
170 Vaisman N., Kaysar N., Zaruk-Adasha Y., et al. correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n-3 fatty acids containing phospholipids. Am J Clin Nutr. 2008;87(5):1170-1180.
171 Sinn N., Bryan J. Effect of supplementation with PUFAs and micronutrients on learning and behaviour problems associated with child ADHD. J Dev Behav Pediatr. 2007;28(2):82-91.
172 Sorgi P.J., Hallowell E.M., Hutchins H.L., et al. Effects of an open-label pilot study with high dose EPA/DHA concentrates on plasma phospholipids and behaviour in children with ADHD. Nutr J. 2007;6:16.
173 Ross B.M., Seguin J., Sieswerda L.E. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.
174 Richardson A.J. Omega-3 fatty acids in ADHD and related neurodevelopmental disorders. Int Rev Psychiatry. 2006;18(2):155-172.
175 Richardson A.J., Montgomery P. The Oxford-Durham study: a RCT of dietary supp with fatty acids in children with development coordination disorder. Pediatrics. 2005;115(5):1360-1366.
176 Arnold L.E., Kleykamp D., Votolato N.A., et al. Gamma-linolenic acid for ADHD: Placebo-controlled comparison to D-Amphetamine. Biol Psychiatry. 1989;15;25(2):222-228.
177 Aman M.G., Mitchell E.A., Turbott S.H. The effects of EFA supplementation in hyperactive children. J Abnorm Child Psychology. 1987;15(1):75-90.
178 Richardson A.J., Puri B.K. A Randomised double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(2):233-239.
179 Richardson A.J., Puri B.K. The Potential role of fatty acids in ADHD. Prostaglandins,. Leukotrienes and EFA’s. 2000;63(1–2):79-87.
180 Viggiano D., Ruocco L.A., Arcieri S., et al. Involvement of NAd in the control of activity and attention processes in animal models of ADHD. Neural Plast. 2004;11(1–2):133-149.
181 Prince J. Catecholamine dysfunction in ADHD: an update. J Clin Psychopharmacol. 2008;28(3)(suppl. 2):S39-S45.
182 Spencer T.J., Biederman J., Madras B.K., et al. Further evidence of dopamine transporter in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059-1061.
183 Levy F. What do dopamine transporter and COMT tell us about ADHD? Pharmacogenomic implications. Aust NZ J Psychiatry. 2007;41(1):10-16.
184 Staller J.A., Faraone S.V. Targeting the dopamine system in the treatment of ADHD. Expert Rev Neurother. 2007;7(4):351-362.
185 Walitza S., Melfson S., Herhaus G., et al. Association of Parkinson’s disease with symptoms of ADHD in childhood. J Neural Transm (suppl. 2007;72:311-315.
186 Spencer T.J., Biederman J., Madras B.K., et al. In vivo neuroreceptor imaging in ADHD: a focus on the dopamine transporter. Biol Psychiatry. 2005;57(11):1293-1300.
187 Juciate A., Fernell E., Halldin C., et al. Reduced midbrain dopamine transporter binding in male adolescents with ADHD: association between striatal dopamine markers and motor hyperactivity. Bio psychiatry. 2005;57(3):229-238.
188 Bellgrove M.A., Domachke K., Hawi Z., et al. The methionine allele of the COMT polymorphism impairs prefrontal cognition in children and adolescents with ADHD. Exp Brain Res. 2005;163(3):352-360.
189 Perlov E., Philipsen A., Hesslinger B., et al. Reduced cingulated glutamate/glutamine to creatine ratios in adult patients with ADHD — a magnet resonance spectroscopy study. J Psychiat Res. 2007;41:934-941.
190 Carrey N.J., MacMaster F.P., Gaudet L., et al. Striatal creatine and glutamate/glutamine in ADHD. J Child Adolesc Psychopharmacol. 2007;17(1):11-17.
191 Pliszka S.R. The neuropsychopharmacology of ADHD. Biol Psychiatry. 2005;57(11):1385-1390.
192 Arnsten A.F. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacol. 2006;31(11):2376-2383.
193 Madras B.K., Miller G.M., Fischman A.J. The dopamine transporter and ADHD. Biol Psychiatry. 2005;57(11):1397-1409.
194 Ruocco L.A., de Souza Silva M.A., Topic B., et al. Intranasal application of dopamine reduces activity and improves attention in Naples High Excitability rats that feature the mesocortical variant of ADHD. Eur Neuropsychopharmacol. 2009 Mar 27. [Epub ahead of print]
195 Stadler C., Zepf F.D., Demisch L., et al. Influence of rapid tryptophan depletion on laboratory-provoked aggression in children with ADHD. Neuropsychobiology. 2007;56(2–3):104-110.
196 Zepf F.D., Stadler C., Demisch L., et al. Serotonergic functioning and trait-impulsivity in ADHD boys: influence of rapid tryptophan depletion. Hum Psychopharmacol. 2008;23(1):43-51.
197 Zepf F.D., Wockel L., Poustka F., et al. Diminished 5–HT functioning in CBCL pediatric bipolar disorder-profiled ADHD patients versus normal ADHD: susceptibility to rapid tryptophan depletion influences reaction time performance. Hum Psychopharmacol. 2008;23(4):291-299.
198 Liu L.L., Yang J., Lei G.F., et al. Atomoxetine increases histamine release and improves learning deficits in an animal model of ADHD: the spontaneously hypertensive rat. Basic Clin Pharmacol Toxicol. 2008;102(6):527-532.
199 Prasad S., Steer C. Switching from neurostimulant therapy to atomoxetine in children and adolescents with ADHD: clinical approaches and review of current available evidence. Paediatr Drugs. 2008;10:39-47.
200 Stocking E.M., Letavic M.A. Histamine H3 antagonists as wake-promoting and pro-cognitive agents. Curr Top Med Chem. 2008;8(11):988-1002.
201 Esbenshade T.A., Browman K.E., Bitner R.S., et al. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol. 2008;154(6):1166-1181.
202 Esbenshade T.A., Fox G.B., Cowart M.D. Histamine H3 receptor antagonists: preclinical promise for treating obesity and cognitive disorders. Mol Interv. 2006;6(2):77-88. 59
203 Lakhan S.E., Vleira K.F. Nutritional therapies for mental disorders. s.l. Nutr J. 2008;7:2.
204 Berk M., Ng F., Dean O., et al. Glutathione: a novel treatment target in psychiatry. Trends Pharmacol Sci. 2008;29(7):346-351.
205 Arnold L.E., Amato A., Bozzolo H., et al. ALC in ADHD: a multi-site, placebo-controlled pilot trial. s.l. J Child Adolesc Psychopharmacol. 2007;17(16):791-902.
206 Van Oudheusden L.J., Scholte H.R. Efficacy of carnitine in the treatment of children with ADHD. Prostaglandins Leukot Essental fatty Acids. 2002;67(1):33-38.
207 Adriana W., Rea M., Baviera M., et al. ALC reduces impulsive behaviour in adolescent rats. Psychopharmacology (Berl.). 2004;176(3–4):296-304.
208 Frei H., Everts R., von Ammon K., et al. Homeopathic treatment of children with ADHD: a randomized, double-blind, placebo-controlled trial. Eur J Pediatr. 2005;164(12):758-767.
209 Altunc U., Pittler M.H., Ernst E. Homeopathy for childhood and adolescence ailments: systematic review of RCTs. Mayo Clin Proc. 2007;82(1):69-75.
210 Jacobs J., Williams A.L., Girard C., et al. Homeopathy for ADHD: a pilot RCT. J Altern Complement Med. 2005;11(5):799-806.
211 Pintov S., Hochman M., Livne A., et al. Bach flower remedies used for ADHD in children- a prospective double-blind controlled study. Eur J Paediatr Neurol. 2005;9(6):395-398.
212 Coulter M.K., Dean M.E. Homeopathy for ADHD or hyperkinetic disorder. Cochrane Database Syst Rev. (4):2007. CD005648
213 Frei H., Everts R., von Ammon K., et al. RCTs of homeopathy in hyperactive children: Treatment procedure leads to an unconventional study design. Experience with open-label homeopathic treatment preceding the Swiss ADHD placebo- controlled, randomized, double-blind cross-over trial. Homeopathy. 2007;96(1):35-41.
214 Frei H., von Ammon K., Thurneyson A. Treatment of hyperactive children: increased efficacy through modifications of homeopathic diagnostic procedure. Homeopathy. 2006;95(3):163-170.
215 Frei H., Thurneysen A. Treatment for hyperactive children: homeopathy and Methylphenidate compared in a family setting. Br Homeopath J. 2001;90(4):183-188.
216 Weber W., Vander Stoep A., McCarty R.L., et al. Hypericum perforatum for ADHD in children and adolescents: a RCT. JAMA. 2008;299922:2633-2641..
217 Lyon M.R., Cline J.C., Totosy de Zepetnek J., et al. Effect of the Herbal Extract combination Panax quinquefolium and Ginkgo biloba on ADHD: A Pilot study. J Psychiatry Neurosci. 2001;26(3):221-223.
218 Zhang H., Huang J. Preliminary study of TCM treatment of minimal brain dysfunction: analysis of 100 cases. Zhong Xi Yi He Za Zhi. 1990;10(5)(260):278-279.
219 Li X., Chen Z. Clinical comparative observation on Duodongining and Ritalin in treating child hyperkinetic syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1999;19(7):410-411.
220 Wang L.H., Li C.S., Li G.Z. Clinical and experimental studies on tiaoshen liquor for infantile hyperkinetic syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15(6):337-340.
221 Ding G.A., Yu G.H., Chen S.F. Assessment on effect of treatment for children hyperkinetic syndrome by combined therapy Yizhi mixture and Ritalin. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22(4):255-257.
222 Chen J., Chen Y.Y., Wang X.M. Clinical study on treatment of children ADHD by Jiangqian granules. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22(4):258-260.
223 Ng F., Berk M., Dean O., et al. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851-876.
224 Dvorakova M., Jezova D., Blazicek P., et al. Urinary catecholamines in children with ADHD: modulation by a polyphenolic extract from pine bark (Pycnogenol). Nutr Neurosci. 2007;10(3–4):151-157.
225 Dvorakova M., Sivonova M., Trebaticki J., et al. The effect of polyphenolic extract from pine bark, Pycnogenol on the level of glutathione in children suffering from ADHD. Redox Rep. 2006;11(4):163-172.
226 Trebaticka J., Kopasova S., Hradecna Z., et al. treatment of ADHD with French maritime pine bark extract. Pycnogenol Eur Child Adolesc Psychiatry. 2006;15(6):329-335.
227 Rohdewald P.A. Review of the French maritime pine bark extract, Pycnogenol, a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40(4):158-168.
228 Packer L., Rimbach G., Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritime) bark. Pycnogenol. Free Radic Biol Med. 1999;27(5–6):704-724.
229 Chovanova Z., Muchova J., Sivonova M., et al. Effect of polyphenolic, Pycnogenol, on the level of 8–oxoguanine in children suffering from ADHD. Free Radic Res. 2006;40(9):1003-1010.
230 Underdown A., Barlow J., Chung V., Stewart-Brown S. Massage intervention for promoting mental and physical health in infants aged under six months. The Cochrane Database of Systematic Reviews. (4):2006.
231 Field T., Quintino O., Hernandez-Reif M., et al. Adolescents with ADHD benefit from massage therapy. Adolescence. 1998;33(129):103-108.
232 Khilnani S., Field T., Hernandez-Reif M., et al. Massage therapy improves mood and behaviour of students with ADHD. Adolescence. 2003;38(152):623-638.
233 Giesen J.M., Center D.B., Leach R.A. An evaluation of chiropractic manipulation as a treatment of hyperactivity in children. J Manipulative Physiol Ther. 1989;12(5):353-363.
234 F. Karpouzis, H. Pollard, R. Bonello. A RCT of NET for ADHD: a protocol. Trials. 10(6), 2009.