Chapter 34 Attention deficit and hyperactivity disorder (ADHD)
EPIDEMIOLOGY, AETIOLOGY AND CLASSIFICATION
The worldwide prevalence rate of attention deficit and hyperactivity disorder (ADHD) has been estimated at between 2 and 29%.1 The rate at which ADHD is diagnosed and treated in both children and adults has dramatically increased since the syndrome was first recognised as a specific disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM) in the 1970s. In the United States of America as many as 10% of males and 4% of females have been diagnosed with ADHD.2 An objective epidemiological or scientific basis for the rapidly increasing prevalence of ADHD in general and the higher incidence of the syndrome in boys compared to girls is highly controversial and may reflect social issues and changes in diagnostic criteria more than actual changes in prevalence rates.3
The causes of ADHD are multifactorial. Data from twin studies show that ADHD is a highly heritable disorder4 and the risk of developing this disorder is probably influenced by genes that affect CNS transport of dopamine and serotonin.5 Other causes of ADHD include problems associated with premature birth, birth trauma, childhood illness and environmental toxins.6 Increased risk of ADHD is associated with in-utero exposure to alcohol, tobacco smoke and lead. Up to 20% of ADHD cases are probably caused by brain injury around the time of birth. Certain food preservatives exacerbate the symptoms of ADHD, but probably do not actually cause the disorder.7 Some cases of ADHD may be associated with delayed development of certain areas of the frontal and temporal lobes and relatively rapid maturation of motor areas of the brain.8 Children diagnosed with ADHD frequently experience disturbed sleep including restlessness, sleep walking, night terrors and restless leg syndrome; however, a causal relationship between sleep disorders and ADHD has not been clearly established.9 Early childhood neglect or abuse probably also increase the risk of developing ADHD. Most cases of ADHD probably result from multiple genetic, developmental, physiological, environmental and psychosocial factors.10
Three subtypes of ADHD are recognised in the DSM, depending on the type and severity of symptoms:
Symptoms of inattention, impulsivity or hyperactivity must cause clinically significant impairment in at least two spheres including social, academic or occupational functioning. Neuropsychological testing is frequently employed to assess inattention, processing speed and neurocognitive deficits. A diagnosis of ADHD should be made in childhood only after other childhood disorders, including pervasive developmental disorders, learning disorders and anxiety disorders, have been ruled out.11 Many children diagnosed with ADHD also meet criteria for conduct disorder. When evaluating adults a thorough medical history is important to rule out medical or psychiatric disorders that mimic symptoms or functional impairments that resemble ADHD. These include, for example, bipolar disorder, absence seizures, hypothyroidism, obsessive-compulsive disorder and chronic sleep deprivation.12
RISK FACTORS
The hyperactivity of ADHD often resolves by late adolescence or adulthood; however, symptoms of distractibility may not lessen with age. It has been estimated that fewer than one-fifth of adults with ADHD have been correctly diagnosed and appropriately treated, resulting in significant social and occupational risk. ADHD is highly comorbid with oppositional-defiant disorder and learning disorders in children, and with major depressive disorder, anxiety disorders and substance abuse in adults.13 It has been estimated that almost half of individuals diagnosed with ADHD never graduate from high school and fewer than 5% complete a 4-year university degree program.14 The high prevalence rate of ADHD significantly affects employment statistics. A large population survey of US adults found that a diagnosis of ADHD was associated with 35 days of lost work on average. Extrapolating these findings to the population suggests that US$19 billion in lost productivity and 120 million lost work days annually are attributable to ADHD.15
CONVENTIONAL TREATMENT
Stimulant medications are the standard Western treatment of ADHD; however, SSRIs and other antidepressants are also used with varying degrees of success. Extended-release forms of stimulants are better tolerated and less often lead to abuse. Approximately one-third of children and adolescents who take stimulants experience significant adverse effects, including abdominal pain, decreased appetite and insomnia, and 10% experience serious adverse effects.16 Because stimulants are classified as scheduled or restricted medications (depending on the country), prescriptions are usually limited to a short supply; this can result in treatment interruptions and transient symptomatic worsening when refills are not obtained on time. One-third of all individuals who take stimulants for ADHD report significant adverse effects, including insomnia, decreased appetite and abdominal pain.16 Sporadic cases of stimulant-induced psychosis have been reported.17 Neurotoxic effects associated with long-term stimulant use have not been fully elucidated; however, chronic amphetamine use in childhood is associated with slowing in growth. Stimulants and other conventional treatments of adulthood ADHD may be only half as effective as they are in children.13
Short-acting stimulants are the most prescribed conventional treatments of adult ADHD. Controlled-release stimulants, buproprion and the SSRI antidepressants are being increasingly used in the adult ADHD population; however, research findings suggest these medications may not be as efficacious as fast-released stimulants.18 The non-stimulant medication atomoxetine has less potential for abuse, but may not be as efficacious as stimulants.19 Although atomoxetine is the only non-amphetamine medication approved by the US Food and Drug Administration for the treatment of childhood ADHD, there are growing concerns about its adverse effects, including hypertension, tachycardia, nausea and vomiting, liver toxicity and possibly increased suicide risk.20,21 In Australia atomoxetine is registered for use by the Therapeutic Goods Administration.
Growing concerns about inappropriate or over-prescribing by physicians of stimulant medications and incomplete understanding of risks associated with their long-term use have led to increasing acceptance of emerging non-conventional therapies.22,23 In addition to conventional prescription medications, behavioural modification is a widely used conventional treatment of ADHD in children. Psychotherapy and psychosocial support help reduce the anxiety and feelings of loss of control that frequently accompany ADHD.
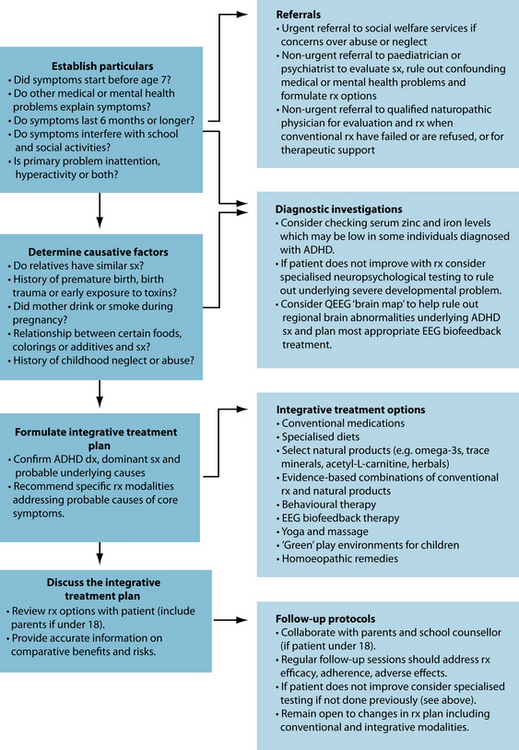
INTEGRATIVE MEDICAL DIAGNOSIS AND TREATMENT OPTIONS
Many individuals diagnosed with ADHD use alternative therapies alone or adjunctively with conventional pharmacological treatments.24 Over half of parents of children diagnosed with ADHD treat their children’s symptoms using one or more CAM therapies, including vitamins, dietary changes and expressive therapies, but few disclose this to their child’s paediatrician.25 Most CAM therapies for ADHD are supported by limited evidence; however, when any herbal or other naturopathic therapy is used to treat ADHD it is regarded as the primary treatment over 80% of the time.25 Appropriate CAM and integrative treatment strategies for ADHD will depend on the subtype of ADHD that is being addressed, symptom severity, previous treatment outcomes using conventional or CAM modalities, adverse effect issues, psychiatric or medical comorbidities, patient preferences, the availability of qualified CAM practitioners and access to reputable brands of specific supplements. Dietary modifications, including reduced sugar and caffeine intake and specialised restrictive diets, are reasonable first-line strategies in ADHD-diagnosed children who are predominantly hyperactive. There are no contraindications to taking stimulant medications while following a restrictive diet; however, parents of ADHD children should first consult their child’s paediatrician before initiating a strict dietary regimen, and ideally with a nutritionist who can provide them with expert guidance. Preliminary findings suggest that omega-3 essential fatty acids in doses up to 16 g/day are effective adjuvants when combined with stimulants for both hyperactivity and inattention; however, more studies are needed to confirm this. Preliminary findings suggest that a standardised extract of the bark of the French maritime pine tree may be beneficial in some cases of ADHD; however, it is not clear whether this product has adjunctive benefits when combined with stimulants. Zinc supplementation may enhance the efficacy of prescription stimulants permitting reductions in stimulant doses in some cases, and preliminary findings suggest that acetyl-L-carnitine at doses up to 1500 mg/day may significantly ameliorate symptoms of inattention, but not hyperactivity. Other reasonable integrative treatment strategies for ADHD combine specific EEG biofeedback protocols with restrictive diets, the above supplements and stimulants. When EEG biofeedback training is pursued on a regular basis, effective doses of conventional stimulants can sometimes be reduced, resulting in fewer adverse effects, improved treatment adherence and better outcomes.
INTEGRATIVE MEDICAL TREATMENT AIMS
Dietary modification
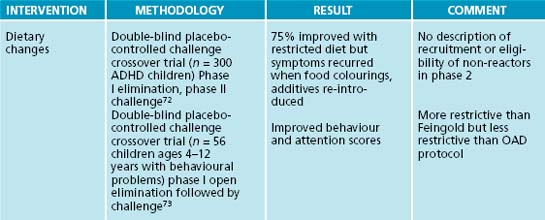
Early studies on a restrictive diet that eliminates all processed foods reported promising findings in children with ADHD;26 however, a review of controlled studies failed to support these findings.27 The oligoantigenic diet (OAD) is a highly restrictive multiple elimination diet that excludes food colourings and additives, in addition to dairy products, sugar, wheat, corn, citrus, eggs, soy, yeast, nuts and chocolate. Most OAD research protocols consist of two lean meats, two fruits, two sources of complex carbohydrates, some vegetables, water and salt. Studies involve several phases requiring many weeks to complete. During phase I, which typically lasts 4 weeks, specific food items are withheld from the diet and the patient is monitored using standardised symptom rating scales. In cases where symptoms improve during the initial treatment phase, specific foods are gradually re-introduced in phase II. A third phase follows a placebo-controlled crossover design in which the patient is randomised to a food item that initially caused symptoms or an acceptable placebo for 1 week, followed by a washout period, and subsequently exposed to either placebo or a specific food item or additive for an additional week.
THE PUTATIVE ROLE OF SUGAR IN ADHD
Several studies on the OAD regimen reported significant reductions in hyperactivity in children diagnosed with ADHD when specific food items were eliminated from the diet using the above protocol.28,29,30 In all of these studies behavioural symptoms improved during the elimination and placebo phases and recurred when children were subsequently challenged with the eliminated food item following a blinded protocol. Although these results are promising they cannot be used to develop general ADHD treatment protocols because of study design flaws, including heterogeneity of patient populations, absence of standardised outcome measures, high drop-out rates and, in some studies, non-blinded researchers.31
EEG biofeedback
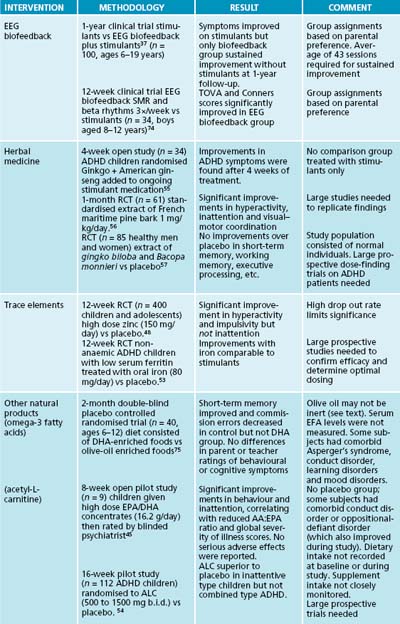
Many individuals diagnosed with ADHD have abnormal patterns of brain electrical activity, including ‘under-arousal’ in frontal and midline cortical regions and frontal ‘hyper-arousal’ that is more frequent in stimulant non-responders.36 Electroencephalographic (EEG) biofeedback is aimed at normalising EEG activity in order to correct the brain’s state of relative under-arousal and improve cognitive and behavioural functioning.37 Two EEG biofeedback protocols have been extensively evaluated as treatments of ADHD. Sensorimotor rhythm (SMR) training reinforces EEG activity in the faster ‘beta’ frequency range (16–20 Hz) in the midline cortical regions with the goal of reducing symptoms of impulsivity and hyperactivity. ‘Theta suppression’ reduces EEG activity in the slower ‘theta’ frequency range (4–8 Hz) and is primarily used to treat symptoms of inattention. Controlled studies comparing EEG biofeedback to a stimulant medication versus a waitlist report positive clinical effects and EEG normalisation with select protocols have been conducted; however, it has not yet been established whether improved alertness is associated with increased or decreased alpha activity (12–18 Hz).38,39 The significance of research findings is limited by small study sizes, heterogeneous populations, absence of a control group, inconsistent outcome measures and limited or absent follow-up. Large studies in which patients are randomly allocated to true versus sham biofeedback are needed to rule out positive group expectation effects. The use of sophisticated QEEG analysis with reference to a normative database may help future clinicians select the most efficacious treatment protocols for a particular ADHD symptom pattern.
Nutritional medicines
Children diagnosed with ADHD have lower plasma concentrations of certain essential fatty acids compared to the average population.40 Findings to date on controlled trials of essential fatty acids in ADHD are inconsistent. One study of EFAs as an adjunctive therapy to stimulants found no differential benefit of essential fatty acids compared to stimulants plus a placebo.41 Another adjunctive study found only modest improvements over placebo in disruptive behaviour and attention.42 In a placebo-controlled trial on EFAs as a stand-alone treatment of ADHD, parents of children in the treatment group reported more improvement than parents of children receiving a palm oil placebo.43 This study has been criticised because a high drop-out rate biases findings in a positive direction.33 The use of olive oil as a placebo may mask the beneficial clinical effects of essential fatty acids because an active constituent of olive oil is converted into oleamide, which is known to affect brain function.44 The short durations and low doses of essential fatty acids used in most studies may not be adequate to result in the long-term changes in neuronal membrane structure required for clinical improvement.41 The issue of dosing has been addressed by a small open-label study (n = 9) in which ADHD children were supplemented with high dose EPA/DHA concentrates (16.2 g/day) while continuing on stimulant medications. Most children were rated by a blinded psychiatrist as having significant improvements in both inattention and hyperactivity that correlated with reductions in the AA:EPA ratio at the end of an 8-week treatment period.45 Large prospective trials are needed to replicate these findings.
Some children diagnosed with ADHD may have abnormally low plasma zinc levels, which may interfere with optimal information processing and result in difficulties maintaining attention.46,47 Findings of studies on zinc in ADHD are inconsistent. In a large 12-week prospective controlled randomised trial (PCRT) (n = 400), children and adolescents randomised to zinc (150 mg/day) experienced significant improvements in hyperactivity and impulsivity, but not inattention, over placebo.48 A high drop-out rate limits the significance of findings. In another study adding zinc to a stimulant resulted in greater improvement than stimulant alone.49 Large prospective studies are needed to replicate these preliminary findings and confirm the optimum dosing of zinc sulfate.50
Abnormally low serum ferritin levels may be associated with hyperactivity in non-anaemic ADHD children, but not with deficits in cognitive performance.51 In an open trial non-iron-deficient children given oral iron for 1 month were perceived as less hyperactive and distractible by teachers but not by parents.52 In a small 12-week randomised PCRT non-anaemic ADHD children with abnormally low serum ferritin levels randomised to oral iron (ferrous sulfate 80 mg/day) showed progressive improvements in ADHD symptoms over placebo that were comparable to improvements obtained with stimulants.53
Acetyl-L-carnitine (ALC) is required for energy metabolism and synthesis of fatty acids. Findings of one small study suggested that L-carnitine significantly reduces the severity of ADHD symptoms; however, design flaws limit the significance of these findings.54 In a multi-site 16-week pilot study 112 ADHD children were randomised to placebo versus ALC (500 to 1500 mg b.i.d). Children in the ALC group with predominantly inattentive type ADHD experienced greater improvement over placebo, but there was no differential benefit in children with combined type ADHD. Significant adverse effects were not reported. Large prospective trials are needed to replicate this finding before ALC can be recommended for ADHD.
Herbal medicines
In a 4-week open study involving 36 children diagnosed with ADHD, a herbal preparation containing Ginkgo biloba and Panax quinquefolium was added to their existing ADHD medication.55 Beneficial effects were observed in children taking the herbal combination after 4 weeks. It should be noted, however, that the absence of a placebo group (or a stimulant-only group) and the small size of the study limit the significance of findings. Findings of open studies suggest that a standardised extract of Pinus pinaster bark is beneficial in ADHD; however, only one PCRT has been published to date. Sixty-one children and adolescents randomised to a standardised bark extract (Pycnogenol™) 1 mg/kg/day for 1 month experienced significant improvements in hyperactivity, inattention and visual–motor coordination over placebo; these returned to pretreatment baseline levels after a 1-month washout.56 Only one case of mild gastric discomfort was reported. These findings should be regarded as preliminary pending replication by large prospective studies. Bacopa monnieri is an Ayurvedic herbal used as a tonic and memory enhancer. In one randomised controlled trial (RCT) (n = 85) healthy men and women randomised to a extract containing G. biloba and B. monnieri did not perform better than a placebo group in tests of short-term memory, working memory, executive processing, planning, problem solving and information processing speed.57 These findings cannot be generalised to ADHD; however, they suggest that this herbal formula does not ameliorate the core symptoms of ADHD.
Homoeopathic remedies
A systematic review of PRCTs on homoeopathic remedies in ADHD found no evidence of beneficial effects of homoeopathy on symptom severity, core symptoms or the course of ADHD.58 These findings have been criticised because conventional PCRT study designs may not permit the demonstration of measurable clinical effects of homoeopathic remedies for ADHD. Long-term studies that include an initial open-label phase are needed to determine the ‘optimal’ homoeopathic remedy for each unique patient over several months. In a subsequent placebo-controlled phase individuals could then be randomised to their ‘optimum’ remedy versus a randomly selected homoeopathic preparation.59
Yoga and massage
In a small pilot study ADHD children randomised to yoga experienced greater improvement over time compared to children who exercised. Children who continued on stimulants while practising yoga experienced the greatest improvements.60 Two small controlled studies suggest that yoga and regular massage therapy may reduce the severity of ADHD symptoms.61,62
Therapeutic considerations
Stimulant abuse is an established risk factor in long-term prescription stimulant use among adults; however, a meta-analysis of six controlled trials found that when stimulants are used to treat childhood ADHD the risk of subsequent substance abuse actually decreases.65 Although the American Academy of Paediatrics endorses the use of stimulants as safe and effective, considerable controversy surrounds their widespread use, especially
‘GREEN’ PLAY ENVIRONMENTS
among children, and their long-term safety has not been clearly established.66 Several cases of sudden cardiac death in children and adults taking mixed amphetamine salts resulted in the temporary withdrawal of this stimulant from the Canadian marketplace in 2005, and led to American Heart Association guidelines recommending a baseline electrocardiogram (ECG) and monitoring of blood pressure and pulse before starting stimulant therapy and during the course of therapy in children, adolescents and adults.67
ADHD remains a highly controversial diagnosis among mental health professionals and parents because of many unresolved issues, and some claim that the disorder does not even exist.68,69 In spite of considerable research there is still no strong evidence of a genetic or physiological basis for the disorder.70 Diagnostic criteria required to diagnose ADHD continue to evolve, suggesting that there is no consensus on the symptoms that constitute ADHD. Finally, although there is probably little abuse potential when stimulants are used to treat childhood ADHD, this remains a central concern among parents.71
Example treatment
In the above example case, the use of EEG biofeedback, dietary and lifestyle modifications, and nutritional treatments may be effective in enhancing her focus and improving her mood. If her serum ferritin level is in the low range, iron supplementation may be
beneficial. The supplementation of EFAs and ALC may provide an adjuvant effect on ameliorating her ADHD symptoms. EEG biofeedback training on a weekly basis using a protocol aimed at suppressing theta activity in frontal brain regions may also be beneficial. If these protocols are found to be effective, she may be able to cut back on her psychostimulant medication. This may reduce her headaches and improve her sleep.
Expected outcomes and follow-up protocols
KEY POINTS
Biederman J., Faraone S.V. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
Lake J., Spiegel D., editors. Complementary and alternative treatments in mental health. Washington: American Psychiatric Press Inc, 2007.
Sears W., Thompson L. The A.D.D. Book. USA: Little, Brown and Company; 1998.
Shannon S.M. Please don’t label my child. USA: Rodale Inc; 2007.
1. Barkley R.A. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment, 2nd edn. New York: Guilford Press; 1998.
2. Centers for Disease Control. National Health Interview survey. Atlanta: Centers for Disease Control; 2002. 2004
3. Biederman J., Faraone S.V. The Massachusetts General Hospital studies of gender influences on attention-deficit/hyperactivity disorder in youth and relatives. Psychiatr Clin North Am. 2004;27(2):225-232.
4. Biederman J., Faraone S.V. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
5. Wallis D., et al. Review: Genetics of attention deficit/hyperactivity disorder. J Pediatr Psychol. 2008;33(10):1085-1099.
6. Swanson J.M., et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17(1):39-59.
7. McCann D., et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet. 2007;370(9598):1560-1567.
8. Brennan A.R., Arnsten A.F. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236-245.
9. Silvestri R., et al. Sleep disorders in children with attention-deficit/hyperactivity disorder (ADHD) recorded overnight by video-polysomnography. Sleep Med. 2009 Jun 13. [Epub ahead of print]
10. Di Michele F., et al. The neurophysiology of attention-deficit hyperactivity disorder. Int J Psychophysiol. 2005;58(1):81-93.
11. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edn. Arlington: American Psychiatric Association; 2000.
12. Pearl P., et al. el al. Medical mimics of ADHD. In: Wasserstein J., editor. ADHD in adults: brain mechanisms and behavior. Ann NY Acad Sci; 2001:99-111.
13. Newcorn J.H., et al. The complexity of ADHD: diagnosis and treatment of the adult patient with comorbidities. CNS Spectr. 2007;12(8 Suppl 12):1-14.
14. Cimera R. Making ADHD a gift: teaching Superman how to fly. Lanham: Scarecrow Press, Inc.; 2002. 16
15. Kessler R.C., et al. The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med. 2005;47(6):565-572.
16. Schachter H.M., et al. How efficacious and safe is short-acing methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165(11):1475.
17. Berman S.M., et al. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2009;14(2):123-142.
18. Slatkoff J., Greenfield B. Pharmacological treatment of attention-deficit/hyperactivity disorder in adults. Expert Opin Investig Drugs. 2006;15(6):649-667.
19. Findling R.L. Evolution of the treatment of attention-deficit/hyperactivity disorder in children: a review. Clin Ther. 2008;30(5):942-957.
20. Miller M.C. What is the significance of the new warnings about suicide risk with Strattera? Harv Ment Health Lett. 2005;22(6):8.
21. Nissen S.E. ADHD drugs and cardiovascular risk. N Engl J Med. 2006;354(14):1445-1448.
22. Stubberfield T., Parry T. Utilization of alternative therapies in attention-deficit hyperactivity disorder. J Paediatr Child Health. 1999;35(5):450-453.
23. Anderson S.L., Navalta C.P. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Deve Neurosci. 2004;22(5–6):40-423.
24. Bussing R., et al. Use of complementary and alternative medicine for symptoms of attention-deficit hyperactivity disorder. Psychiatr Serv. 2002;53(9):1096-1102.
25. Chan E., et al. Complementary and alternative therapies in childhood attention and hyperactivity problems. J Dev Behav Pediatr. 2003;24(1):4-8.
26. Feingold B. Why your child is hyperactive. New York: Random House; 1975.
27. Wender E.H. The food additive-free diet in the treatment of behavior disorders: a review. J Dev Behav Pediatr. 1986;7(1):35-42.
28. Egger J., et al. Controlled trial of hyposensitization in children with food-induced hyperkinetic syndrome Lancet. 1992;339:1150-1153.
29. Carter C.M., et al. Effects of a new food diet in attention-deficit disorder. Arch Dis Child. 1993;69:564-568.
30. Schmidt M.H., et al. Does oligoantigenic diet influence hyperactive/conduct-disordered children: a controlled trial. Eur Child Adolesc Psychiatry. 1997;6:88-95.
31. Rojas N.L., Chan E. Old and new controversies in the alternative treatment of attention-deficit hyperactivity disorder. Ment Retard Dev Disabil Res Rev. 2005;11(2):116-130.
32. Wolraich S., et al. Effects of diets high in sucrose or aspartame on the behavior and cognitive performance of children. N Engl J Med. 1994;330(5):301-307.
33. Weber W., Newmark S. Complementary and alternative medical therapies for attention-deficit/hyperactivity disorder and autism. Pediatr Clin North Am. 2007;54(6):983-1006. xii
34. Hoover D.W., Milich R. Effects of sugar ingestion expectancies on mother-child interactions. J Abnorm Child Psychol. 1994;22(4):501-515.
35. Cormier E., Elder J.H. Diet and child behavior problems: fact or fiction? Pediatr Nurs. 2007;33(2):138-143.
36. Butnik S.M. Neurofeedback in adolescents and adults with attention deficit hyperactivity disorder. J Clin Psychol. 2005;61(5):621-625.
37. Monastra V.J., et al. The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Appl Psychophysiol Biofeedback. 2002;27(4):231-249.
38. Monasstra V.J., et al. Electroencephalographic biofeedback in the treatment of attention-deficit/hyperactivity disorder. Applied Psychophysiology and Biofeedback. 2005;30(2):95-114.
39. Ramirez P., et al. EEG biofeedback treatment of ADD: a viable alternative to traditional medical intervention? Ann NY Acad Sci. 2001;931:342-358.
40. Gedik Y., et al. Relationships between serum free fatty acids and zinc, and attention deficit hyperactivity disorder: a research note. J Child Psychol Psychiatry. 1996;37:225-227.
41. Voigt R.G., et al. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr. 2001;139(2):189-196.
42. Stevens L.J., et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62(4):761-768.
43. Sinn N., Bryan J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr. 2007;28(2):82-91.
44. Richardson A.J., Puri B.K. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:233-239.
45. Sorgi P.J., et al. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutrition Journal. 2007;6:16.
46. Yorbik O., et al. Potential effects of zinc on information processing in boys with attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):662-667.
47. Arnold L.E., et al. Serum zinc correlates with parent- and teacher-rated inattention in children with attention-deficit/hyperactivity disorder. Child Adolesc Psychopharmacol. 2005;15(4):628-636.
48. Bilici M., et al. Double-blind, placebo-controlled study of zinc sulfate in the treatment of attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):181-190.
49. Akhondzadeh S., et al. Zinc sulfate as an adjunct to methylphenidate for the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial [ISRCTN64132371]. BMC Psychiatry. 2004;4(1):9.
50. Arnold L.E., DiSilvestro R.A. Zinc in attention-deficit/hyperactivity disorder. Child Adolesc Psychopharmacol. 2005;15(4):619-627.
51. Oner O., et al. Relation of ferritin levels with symptom ratings and cognitive performance in children with attention deficit-hyperactivity disorder. Pediatr Int. 2008;50(1):40-44.
52. Sever Y., et al. Iron treatment in children with attention deficit hyperactivity disorder: a preliminary report. Neuropsychobiol. 1997;35:178-180.
53. Konofal E., et al. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr Neurol. 2008;38(1):20-26.
54. Van Oudheusden L.J., Scholte H.R. Efficacy of carnitine in the treatment of children with attention-deficit hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2002;67(1):33-38.
55. Lyon M.R., et al. Effect of the herbal extract combination Panax quinquefolium and Ginkgo biloba on attention-deficit hyperactivity disorder: a pilot study. J Psychiatry Neurosci. 2001;26(3):221-228.
56. Trebaticka J., et al. Treatment of ADHD with French maritime pine bark extract PycnogenolR. Eur Child Adolesc Psychiatry Sept. 2006;15(6):329-335.
57. Nathan P.J., et al. Effects of a combined extract of Ginkgo biloba and Bacopa monnieri on cognitive function in healthy humans. Hum Psychopharmacol. 2004;19(2):91-96.
58. Coulter M.K., Dean M.E. Homoeopathy for attention deficit/hyperactivity disorder or hyperkinetic disorder. Cochrane Database System Reviews. (4):2007;. CD005648
59. Frei H., et al. Randomised controlled trials of homoeopathy in hyperactive children: treatment procedure leads to an unconventional study design. Experience with open-label homoeopathic treatment preceding the Swiss ADHD placebo controlled, randomised, double-blind, cross-over trial. Homoeopathy. 2007;96(1):35-41.
60. Haffner J., et al. The effectiveness of body-oriented methods of therapy in the treatment of attention-deficit hyperactivity disorder (ADHD): results of a controlled pilot study]. Z Kinder Jugendpsychiatr Psychother. 2006;34(1):37-47.
61. Jensen P.S., Kenny D.T. The effects of yoga on the attention and behavior of boys with attention-deficit/hyperactivity disorder (ADHD). J Atten Disord. 2004;7(4):205-216.
62. Khilnani S., et al. Massage therapy improves mood and behavior of students with attention-deficit/hyperactivity disorder. Adolescence. 2003;38(152):623-638.
63. Kuo F.E., Taylor A.F. A potential natural treatment for attention-deficit/hyperactivity disorder: evidence from a national study. Am J Public Health. 2004;94(9):1580-1586.
64. Canu W., Gordon M. Mother nature as treatment for ADHD: overstating the benefits of green. Am J Clin Nutr. 2005;95:371.
65. Faraone S.V., Wilens T. Does stimulant treatment lead to substance use disorders? J Clin Psychiatry. 2003;64(Suppl 11):9-13.
66. Lerner M., Wigal T. Long-term safety of stimulant medications used to treat children with ADHD. Pediatric Annals. 2008;37(1):37-45.
67. Vetter V.L., et al. Cardiovascular monitoring of children and adolescents with heart disease receiving stimulant drugs: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
68. Mayes R., et al. ADHD and the rise in stimulant use among children. Harv Rev Psychiatry. 2008;16(3):151-166.
69. Foreman D.M. Attention deficit hyperactivity disorder: legal and ethical aspects. Arch Dis Child. 2006;91(2):192-194.
70. US Department of Health and Human Services. Treatment of attention-deficit/hyperactivity disorder. US Department of Health and Human Services; 1999.
71. Jadad A.R., et al. The treatment of attention-deficit hyperactivity disorder: an annotated bibliography and critical appraisal of published systematic reviews and metaanalyses. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 1999;44(10):1025-1035.
72. Rowe K.S., Rowe K.J. Synthetic food coloring and behavior: a dose response effect in a double-blind, placebo-controlled, repeated-measures study. J Pediatr. 1994;125(5 Pt 1):691-698.
73. Dengate S., Ruben A. Controlled trial of cumulative behavioural effects of a common bread preservative. J Paediatr Child Health. 2002;38(4):373-376.
74. Fuchs T., et al. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: a comparison with methylphenidate. Appl Psychophysiol Biofeedback. 2003;28(1):1-12.
75. Hirayama S., et al. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder—a placebo-controlled double-blind study. Eur J Clin Nutr. 2004;58(3):467-473.