6 Atrioventricular Septal Defect
Echocardiographic Assessment
Background
Key Points
Anatomy
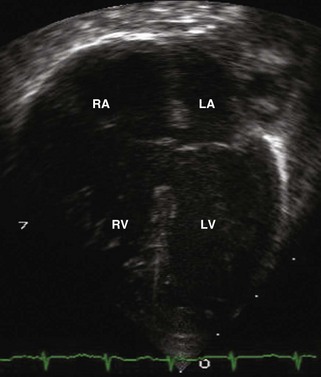
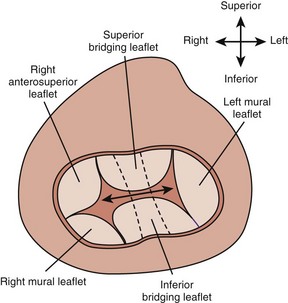
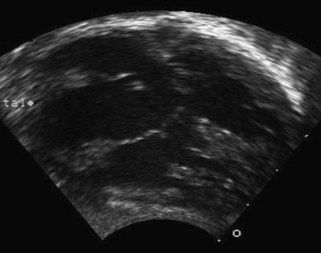
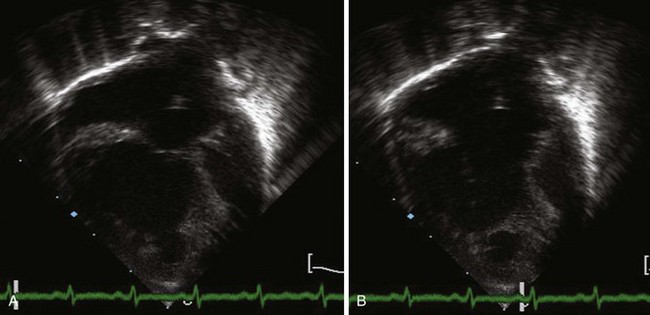
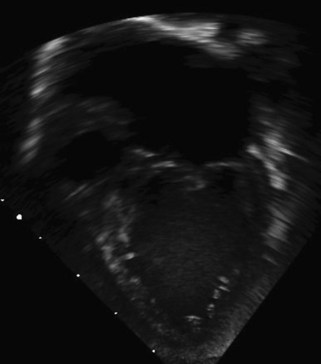
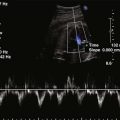
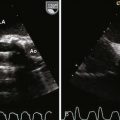
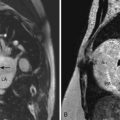
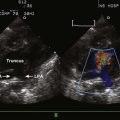
A complete AVSD has a common AV junction, a primum ASD, an IVSD, and a common AVV (Fig. 6-1). The primum ASD is anterior and inferior to the fossa ovalis, adjacent to the AVVs. The AVV consists of five leaflets: superior and inferior bridging leaflets, a left mural leaflet, a right mural leaflet, and a right anterosuperior leaflet (Fig. 6-2).
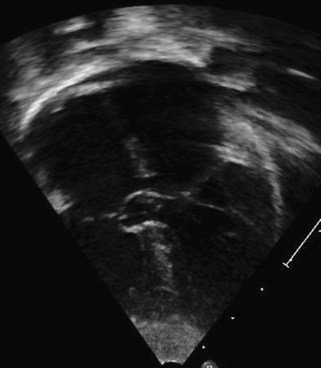
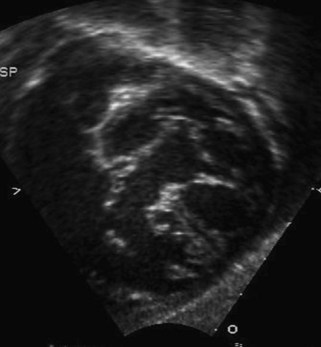
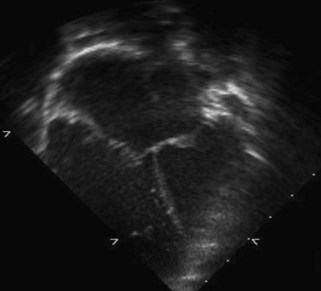
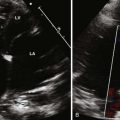
A partial AVSD usually has a primum ASD and two separate AVVs, with a cleft in the anterior leaflet of the left-sided AVV (mitral valve [MV]) (Fig. 6-3). The cleft in the left-sided AVV usually results in some degree of regurgitation of the valve. There are rare instances of partial AVSDs with only an IVSD and no primum ASD.
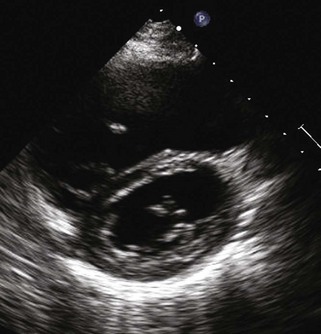
Figure 6-3 Parasternal short axis view showing a cleft in the anterior leaflet of the left-sided AVV.
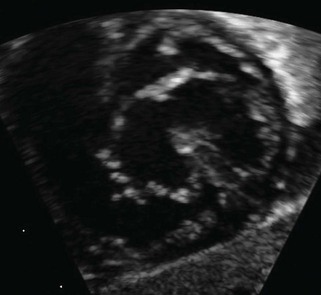
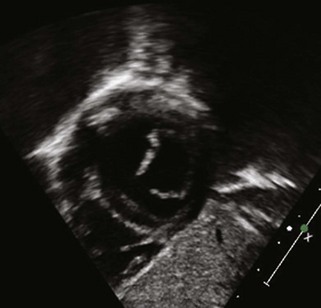
The term transitional AVSD has been used to refer to complete AVSDs in which the IVSD is predominantly occluded by chordal tissue from the AVV, resulting in minimal or no ventricular level shunting (Fig. 6-4). In transitional defects, there are two separate AVV orifices with a cleft in the left-sided AVV.
Overview of the Echocardiographic Approach
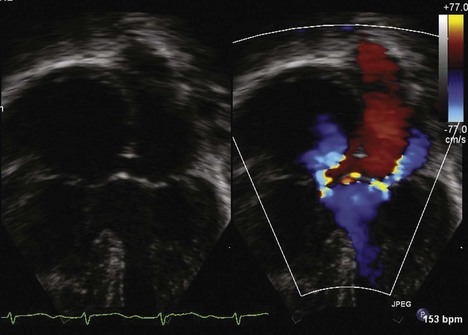
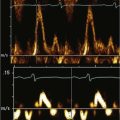
The echocardiogram (echo) should fully evaluate all the components of the AVSD and assess for any associated abnormalities. Images should define the size of the atrial and ventricular components and demonstrate the direction of shunting. The AVV attachments need to be defined, and function of the AVV should be assessed by pulsed wave (PW) Doppler and color flow Doppler (CFD). The balance of the AVV above the ventricles should be evaluated along with flow across the valve to demonstrate the flow into both ventricles. Additional ASDs or ventricular septal defects (VSDs) can be present, and careful assessment of the atrial septum (AS) and ventricular septum (VS) should be performed. The outflow tracts should be interrogated, looking for any obstruction (Box 6-1).
Goals of Echocardiographic Examination
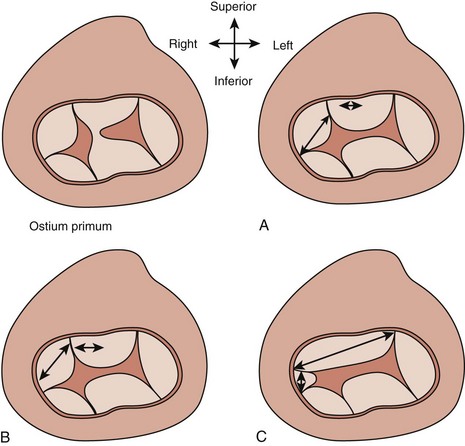
AVSDs are described by Rastelli classification, which is based on papillary muscle configuration, specifically attachments of the superior bridging leaflet (Box 6-2 and Fig. 6-5):
Anatomic Imaging
Subcostal views allow evaluation of the primum ASD to determine size and direction of flow (Fig. 6-6). Subcostal short axis view allows an en face view of the AVV and can be used to assess the balance of the valve to the ventricles and look for attachments of the superior bridging leaflet to determine the Rastelli classification (Figs. 6-7 and 6-8).
The aortic valve, which is usually wedged between the MV and tricuspid valve (TV) annuli, is anteriorly displaced or “unwedged” in AVSDs, resulting in elongation of the left ventricular outflow tract (LVOT). LVOTO may occur in all types of AVSDs but is more common in the partial defects. An apical five-chamber (5C) view allows assessment of the LVOT for possible obstruction (Boxes 6-3 and 6-4).
Overview of Imaging Windows
Partial AVSDs will have two separate AVVs noted to be at the same level. A cleft in the anterior leaflet of the left-sided AVV usually results in AVVR. There is also a primum ASD. Rarely a partial AVSD will have no primum ASD, but will have only an IVSD. The best views for evaluation include the subcostal long axis and short axis views to look at the AS and the AVV, the apical 4C view for the AVVs and ventricular shunting, and the parasternal short axis for the VS and the cleft in the MV (see Box 6-3).
A transitional AVSD refers to a complete AVSD in which there are chordal attachments of the AVV to the crest of the VS, resulting in a restrictive VSD or no shunting at the ventricular level. There is a single AVV and a primum ASD. Imaging views are the same as for complete AVSDs (see Box 6-3).
Key Points
Acquisition
Complete and Transitional Atrioventricular Septal Defect
Step 1: Assess the Atrioventricular Valve
Step 3: Assess the Primum Atrial Septal Defect
Partial Atrioventricular Septal Defect
Step 1: Assess the Atrioventricular Valves
Step 2: Assess the Atrial Septum
Physiologic Data
Pitfalls
After surgery, patients will require lifelong follow-up. Echocardiography can be helpful in assessing for residual atrial or ventricular level shunting. The right- and left-sided AVVs should be assessed not only for regurgitation, but also for stenosis. Severe left AVVR may occur in as many as 20% immediately postoperatively, and 10% to 15% of patients will require reoperation. LVOTO may occur in 10% to 15% of patients after repair and is more common in partial AVSDs. Reoperation is required in 5% to 10% of patients to relieve the obstruction. If the repair was performed later in life, patients may be at risk of the development of PHTN and should be assessed for this possibility (Box 6-5).
Alternative Approaches
1 Backer CL, Stewart RD, Mavroudis C. Overview: History, anatomy, timing, and results of complete atrioventricular canal. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007:3-10.
A review of the history of surgical repair of AVSD, timing of repair, and recent outcomes.
2 Bakhtiary F, Takacs J, Cho MY, et al. Long-term results after repair of complete atrioventricular septal defect with two-patch technique. Ann Thorac Surg. 2010;89:1239-1243.
3 Cohen MS. Common atrioventricular canal defects. In: Lai WW, Mertens LL, Cohen MS, Geva T, editors. Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult. Hoboken, NJ: Wiley-Blackwell; 2009:230-248.
A chapter with a discussion of echocardiographic findings in AVSDs.
4 Cohen GA, Stevenson JG. Intraoperative echocardiography for atrioventricular canal: Decision-making for surgeons. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007:47-50.
5 Craig B. Atrioventricular septal defect: from fetus to adult. Heart. 2006;92:1879-1885.
Review of AVSDs including fetal diagnosis.
6 Ebels T, Elzenga N, Anderson RH. Atrioventricular septal defects. In: Anderson RH, Baker EJ, Redington A, et al, editors. Paediatric Cardiology. 3rd ed. Philadelphia: Elsevier; 2010:553-589.
A chapter with a thorough review of anatomy, pathophysiology, evaluation, and treatment discussions.
7 Espinola-Zavaleta N, Munoz-Castellanos L, Kuri-Nivon M, Keirns C. Understanding atrioventricular septal defect: Anatomoechocardiographic correlation. Cardiovasc Ultrasound. 2008;6:33.
8 Lim DS, Ensing GJ, Ludomirsky A, et al. Echocardiographic predictors for the development of subaortic stenosis after repair of atrioventricular septal defect. Am J Cardiol. 2003;91:900-903.
9 Mahle WT, Shirali GS, Anderson RH. Echo-morphological correlates in patients with atrioventricular septal defect and common atrioventricular junction. Cardiol Young. 2006;16:43-51.
A good review of echocardiographic imaging of AVSDs and the limitations of imaging.
10 Sittiwangkul R, Ma RY, McCrindle BW, et al. Echocardiographic assessment of obstructive lesions in atrioventricular septal defects. J Am Coll Cardiol. 2001;38:253-261.