Chapter 42 Atrial Fibrillation
Epidemiology
Incidence and Prevalence
Atrial fibrillation (AF) is the most common sustained arrhythmia, with the incidence of 3.1 cases in men and 1.9 cases in women per 1000 person-years in the population younger than 64 years, rising to 19.2 per 1000 person-years in those 65 to 74 years, and as high as 31.4 to 38 in octogenarians.1 A rising proportion of the older population, markedly improved survival from previously fatal cardiovascular conditions, and a recently observed trend toward a continuous increase in the incidence of AF among younger age groups will result in a considerable increase in the number of patients with AF over the next 4 decades. The Framingham Study in the United States and the Rotterdam Study in Europe have estimated lifetime risk for development of AF to be 1 in 4 for men and women aged 40 years and older.2,3 Projected data from national databases have predicted that the number of patients with AF in the United States is expected to reach 5.6 to 15.9 million by 2050, particularly if the incidence of AF continues to rise (Figure 42-1).4–6 A similar increase in the proportion of population with AF is likely to be seen in Western Europe.3,7 However, even these projections may represent conservative estimates because of silent and short transient episodes of AF, which are likely to be diagnosed more frequently because of increased awareness of the arrhythmia, improved diagnostic tools, and a wider use of implantable rhythm-control or rhythm-monitoring devices.8
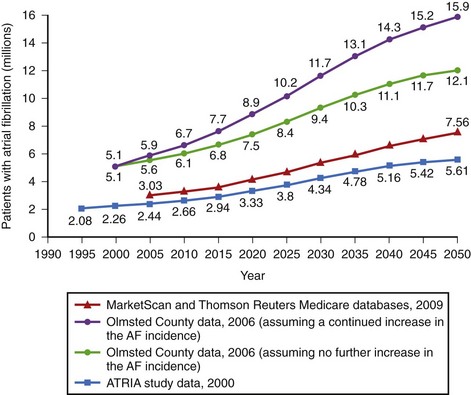
FIGURE 42-1 Estimates of the number of individuals with atrial fibrillation in the United States by 2050.
(Data from Go AS, Hylek EM, Phillips KA, et al: Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention. The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study, JAMA 285:2370–2375, 2001; Miyasaka Y, Barnes ME, Gersh BJ, et al: Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence, Circulation 114:119–125, 2006; and Naccarelli GV, Varker H, Lin J, Schulman KL: Increasing prevalence of atrial fibrillation and flutter in the United States, Am J Cardiol 104:1534–1539, 2009.)
Age, Gender, and Ethnicity
A series of epidemiologic studies have identified age to be one of the major determinants of risk of developing AF, with odds of 1.1 to 5.9 depending on the initial age and morbidity of a cohort studied and the duration of follow-up.9–12 In the Framingham Heart Study, the risk of AF was increased approximately twofold per decade of age in both men and women.11 Increased rates of AF related to age are attributed to inherent changes in the atria occurring with aging (e.g., fatty metamorphosis, myocyte degeneration, fibrosis), accumulation of risk factors, and progression of the underlying disease.
Men are affected almost twice as often and are diagnosed with AF at a younger age compared with women, but recent analyses have suggested that women with AF have more comorbidities, higher mortality rate, and higher risk of stroke and treatment-related complications (e.g., proarrhythmia or bleeding) compared with their male counterparts.11–15
The prevalence of AF is higher in white populations.16–19 In the Atherosclerosis Risk in Communities (ARIC) Study, age-adjusted incidence rates of AF were highest in white men and lowest in black women (7.45 and 3.67 per 1000 person-years, respectively).17 In the health survey of 664,754 U.S. veterans, white men were significantly more likely to have AF compared with all other races except Pacific Islanders (odds ratio [OR] compared with blacks, 1.84; vs. Hispanics, 1.77; vs. Asians, 1.41; vs. Native Americans, 1.15; P < .001).18 The prevalence and distribution of associated risk factors also depended on ethnicity. Thus white men were more likely to have valvular heart disease, coronary artery disease (CAD), and congestive heart failure (CHF); blacks had the highest prevalence of hypertension; and Hispanics had the highest prevalence of diabetes. Racial differences remained after adjustment for age, body mass index, and underlying pathology. In a meta-analysis of seven randomized clinical trials in patients with acute coronary syndrome (ACS), the prospectively collected information on the development of AF indicated that the rates of AF were lower in Asian patients than in white patients (4.7% vs. 7.6%; OR, 0.65; 95% confidence interval [CI], 0.50 to 0.84; P = .001).19 This contradicts the finding of a greater prevalence of unfavorable risk factors for AF in nonwhite populations.20 Nonwhite races have also been reported to be at higher risk of intracranial hemorrhage as a complication of anticoagulation therapy (hazard ratio [HR], with whites as referent, 4.06 for Asians, 2.06 for Hispanics, and 2.04 for blacks).21 Thus the link between ethnicity and risk of AF as well as the therapy for AF remains to be further investigated.
Associated Disease and Risk Factors
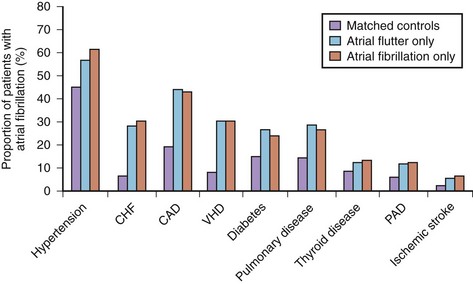
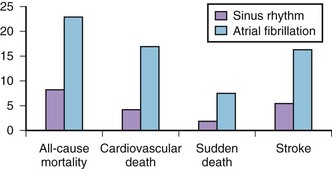
AF is commonly associated with underlying cardiovascular or endocrine pathology (Figure 42-2). The primary pathologies underlying or promoting the occurrence of AF vary more than for any other cardiac arrhythmia, ranging from autonomic imbalance through organic heart disease to metabolic disorders (Table 42-1). Hypertension, heart failure, and ischemic and valvular heart disease, particularly mitral stenosis, are the most common associations and risk factors for the development and progression of AF. Valvular heart disease of rheumatic etiology, one of the main causes of AF, with an odds of 2 to 3 and greater in early studies, no longer holds its leading role but is still important in the developing countries or in the very old.9–11
Table 42-1 Risk Factors and Associations of Atrial Fibrillation
| CARDIAC | NONCARDIAC | INTERVENTIONS |
|---|---|---|
| STRUCTURAL HEART DISEASE | PATHOLOGIC | CARDIAC |
| Valvular heart disease, particularly mitral | Diabetes mellitus | Coronary artery bypass grafting |
| Hypertension or increased pulse pressure | Thyrotoxicosis or subclinical hyperthyroidism | Valvular repair or replacement |
| Congestive heart failure and diastolic dysfunction | Chronic obstructive pulmonary disease | Surgical correction for congenital heart disease |
| Myocardial infarction | Lung cancer | NONCARDIAC |
| Ischemic heart disease | Chronic renal disease | Thoracic surgery, particularly pulmonary |
| Hypertrophic cardiomyopathy | Obesity (BMI) | Surgical procedures requiring general anesthesia |
| Dilated cardiomyopathy | Metabolic syndrome | SUBSTANCE USE AND ABUSE |
| Atrial septal defect | Sleep apnea | Alcohol excess |
| Myocarditis or pericarditis | Pheochromocytoma | Caffeine (trigger) |
| ARRHYTHMIAS | Electrolyte disturbances | Smoking |
| Sick sinus syndrome | PHYSIOLOGICAL | Thyroxine |
| Atrial tachycardia and atrial flutter | Male gender | Cytotoxic agents |
| WPW syndrome (accessory pathway) | Aging | Recreational drugs (cannabis, MDMA) |
| Long P-R interval | Tall stature | Anabolic steroids |
| GENETIC | Endurance training | |
| Familial lone AF | BIOMARKERS | |
| Short QT syndrome | Atrial natriuretic peptide, brain natriuretic peptide | |
| Brugada syndrome | Cross-reactive protein, tumor necrosis factor-α, interleukin-6 | |
| Long QT syndrome (rare) |
AF, Atrial fibrillation; BMI, body mass index; MDMA, 3,4-methylenedioxymethamphetamine (“ecstasy”); WPW, Wolff-Parkinson-White.
Heart Failure
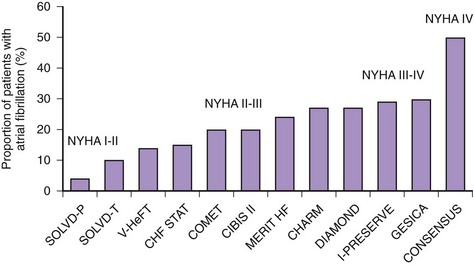
An increasing number of surviving patients with left ventricular impairment has led to CHF emerging as one of the leading risk factors for AF associated with a threefold to almost sixfold increase in the risk of AF.10,12,13 The prevalence of AF associated with CHF varies from 4% to 50%, depending on New York Heart Association (NYHA) class (Figure 42-3).22 The occurrence of CHF in middle age confers an 8% risk of developing AF over a 10-year period if the patient’s age at time of CHF diagnosis was 55 to 64 years, which rises to more than 30% if CHF was diagnosed at age 45 to 54 years.13
Diastolic left ventricular dysfunction with subsequent increases in filling pressures, which is common in older adults, mediates atrial remodeling and is associated with a 5.26-fold increased risk of AF.23 The incidence of AF in patients with CHF and preserved left ventricular systolic function is 20% to 30%.24
Hypertension
Hypertension is the most prevalent condition associated with AF, with a rate of approximately 70% (see Figure 42-2). It is a firmly established risk factor for the development of AF, as shown by many epidemiologic surveys, and increases the risk of AF by a factor of 1.5 to 2.10–13 The risk has been shown to rise proportionally to the level of blood pressure, although iatrogenic hypotension has been linked to AF, suggesting a J-shaped relationship between blood pressure and AF.12,25,26
Myocardial Infarction
AF is a common complication of ACS, occurring de novo in 6% to 15% of patients with myocardial infarction (MI); the incidence is higher (10% to 20%) if patients with a history of atrial tachyarrhythmias are included.27 Patients with ST-segment elevation MI (STEMI) are more likely to develop AF compared with patients with other types of ACS (8% vs. 6.4%), and the presence of early left ventricular systolic dysfunction and CHF has been consistently shown to increase the likelihood of AF by a factor of 1.58 to 3.28.27,28
Previous MI was associated with an increased risk of subsequent AF by a factor of 1.4 to 3.62, but this association is less well established for CAD without MI because these two entities have not been adequately distinguished in epidemiologic studies.9,10,12 It is reasonable to consider CAD, particularly with evidence of reversible ischemia, a risk factor because it may promote formation of the substrate for AF (e.g., due to atrial ischemia).
Diabetes
Diabetes mellitus has been identified as a risk factor for AF. In the Framingham Heart Study, diabetes mellitus was associated with a 1.4-fold increased risk of AF in men and 1.6-fold increase in women.10 Its relevance as a risk factor has been confirmed in recent population-based surveys and meta-analyses of randomized controlled studies. A report from the Veterans Health Administration Hospitals database with 845,748 participants has shown that type 2 diabetes increases the risk of AF by a factor of 2.13.29 A systematic review of 11 studies involving 108,703 cases of AF in more than 1.6 million individuals has revealed that patients with diabetes have a 40% greater risk of AF compared with their nondiabetic counterparts.30
The cause-and-effect association between diabetes and AF has not been fully elucidated as, for example, no substantial evidence that type 1 diabetes increases risk of AF exists. Patients with type 2 diabetes mellitus often have other comorbidities and risk factors that may predispose to AF, such as older age, hypertension, CHF, CAD, obesity, and sleep apnea, and the effect of confounding is significant. Although hyperglycemia may directly affect the electrophysiological properties of the atria, causing intra-atrial conduction delay and promoting structural remodeling by activation of the AGE-RAGE (advanced glycation end product–receptor for AGE) system and upregulation of circulating tissue growth factors, insulin resistance associated with type 2 diabetes may also be an important contributor.31,32
Cardiomyopathies
AF occurs in association with hypertrophic cardiomyopathy in 18% to 28% of patients, with an annual incidence of 2%.33,34 The prevalence of AF is higher in patients older than 50 years with evidence of left ventricular systolic dysfunction, and its occurrence is associated with limiting symptoms and increased morbidity from CHF (a threefold increase) and stroke (an 8- to10-fold increase) and an almost quadrupled mortality rate. A genetic predisposition to AF is seen in some forms of hypertrophic cardiomyopathy: an Arg663His mutation in the β-cardiac myosin heavy chain gene has been identified in patients with a specific phenotype of familial hypertrophic cardiomyopathy presenting with moderate septal left ventricular hypertrophy, predominantly localized in the proximal segment of the interventricular septum, and a 47% prevalence of AF.35
Dilated cardiomyopathy (DCM) can promote AF via the same mechanisms as CHF of ischemic etiology; that is, atrial overload, stretch, and fibrosis. Some types of mutations in the lamin A/C gene linked to DCM have also been shown to be associated with increased risk of progressive conduction system disease, atrial myopathy, and AF.36
Pre-excitation Syndrome
AF is found in 30% of patients with Wolff-Parkinson-White (WPW) syndrome and is associated with increased risk of sudden cardiac death (SCD).37 Unlike the atrioventricular (AV) node, accessory pathways usually exhibit rapid, nondecremental conduction, and if the effective refractory period of an accessory pathway is short (<250 ms), this may result in a rapid ventricular response during AF, with subsequent degeneration to ventricular fibrillation (VF). In some patients with an accessory pathway or a dual AV node, AV re-entrant tachycardia (AVRT) and AV node re-entrant tachycardia (AVNRT) can trigger AF.
Thyrotoxicosis
Thyrotoxicosis has a classic, but relatively minor, association with AF, whereas latent hyperthyroidism, which has been shown to be associated with an almost threefold increase in the age-adjusted incidence of AF in the Framingham Heart Study, is likely to have a greater impact in the general population.38 In the prospective population-based Rotterdam Study in older individuals, free thyroxine levels within the normal range showed a graded association with risk of AF (HR for highest vs. lowest quartile, 1.62).39 Convincing evidence that hypothyroidism may increase risk of AF does not exist.6
Smoking
Smoking as a risk factor has been debated, but recent analyses from the Atherosclerosis Risk in Communities (ARIC) and the Rotterdam studies have provided evidence linking smoking to the risk of AF in the general population, with a hazard ratio of 1.51 to 2.05 in current smokers and 1.32 to 1.49 in former smokers.40,41 In the highest tertile of accumulated smoking amount (>675 cigarette-years), the incidence of AF was 2.1 times greater than in those who never smoked.
Alcohol
High alcohol intake has been consistently associated with risk of AF, but the effects of light or moderate consumption are less clear, and some have suggested a neutral or even protective effect. A recent systematic review has reported a monotonic dose-response causal relationship between alcohol and onset of AF.42 Compared with nondrinkers, individuals consuming 24, 60, and 120 g of alcohol daily had relative risk of 1.08, 1.44, and 2.09, respectively (men), and 1.07, 1.42, and 2.02, respectively (women). Recent meta-analysis of 14 studies has shown that for each 10-mg increase per day in alcohol intake, the risk of AF increased by 1.08 (95% CI, 1.05 to 1.10; P < .001).43
Caffeine
In addition to a series of case reports, recent analyses from the Framingham Heart Study, the Danish Diet, Cancer, and Health Study, and the Women’s Health Study found no association between caffeine intake and the incidence of AF.44–46 In a canine model with enhanced AF inducibility caused by the stimulation of the ganglionated plexi, the presence of caffeine reduced the propensity for AF.47 It is possible that excessive caffeine consumption may trigger AF in individuals with a predominantly adrenergically mediated mechanism of the arrhythmia, but this association could not be detected in the epidemiologic studies.
Postoperative Atrial Fibrillation
AF may occur in about 30% of patients after isolated coronary artery bypass grafting (CABG), 40% of patients after valve surgery, and 50% of patients after combined coronary artery and valvular surgery.48 Most cases of AF occur during the first 3 to 4 days, and more than 90% have a paroxysmal form. Postoperative AF is associated with increased morbidity and mortality rates, largely because of stroke and circulatory failure and longer hospital stay. Atrial flutter (AFL) and atrial tachycardias (ATs), including multi-focal AT, are also common.
Risk factors include conventional clinical variables such as age, hypertension, left ventricular dysfunction, chronic obstructive pulmonary disease (COPD), renal impairment, and specific surgical aspects such as inadequate cardioprotection and hypothermia, right coronary artery grafting, and a longer bypass time.49 The incidence of postoperative atrial tachyarrhythmias is lower with minimally invasive techniques.
Other Risk Factors
Pneumonia is a one of the reversible causes of AF, whereas COPD, which is observed in 10% to 15% of patients with AF, increases the propensity to AF, such as by inflammatory mechanisms; therefore it has been identified as a risk factor for AF, although its exact impact is unknown.50 The presence of COPD can contribute to progression of AF to a more sustained form.51
Chronic renal dysfunction has recently been linked to increased risk of AF. In the ARIC study, reduced glomerular filtration rate (GFR), particularly in association with macroalbuminuria, increased risk of developing AF over 1 decade by 13-fold.52 In a population-based cohort of African-American and white American adults 45 years or older enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, individuals with chronic renal failure stage 1 to 2, 3, and 4 to 5 had the age-, race-, and gender-adjusted odds ratios for AF of 2.67, 1.68, and 3.52, respectively.53
Emerging Risk Factors
New risk factors such as obesity (relative risk [RR], 1.2 to 2.3), metabolic syndrome, a prolonged P-R interval on an electrocardiogram (ECG) (RR, 1.2 to 2.7), P-wave duration (1.15 per standard deviation increase), sleep apnea (RR, 2.2 to 3), psychological stress and negative emotions, endurance athletic training (RR, 5.3), visceral adipose tissue (pericardial fat) detected by computer tomography (CT) (RR, 1.11 to 1.18), and tall stature (RR, 1.7 for height >180 cm) have all been reported in association with AF.54 A series of biomarkers of inflammation and oxidative stress (cross-reactive protein, interleukin [IL]-6, intercellular adhesion molecule (ICAM)-1, tumor necrosis factor [TNF]-α), hemodynamic stress (atrial natriuretic and brain natriuretic peptides [ANP and BNP]), and cardiac damage (troponin) have emerged as predictors of incident AF.54
Genetic Factors
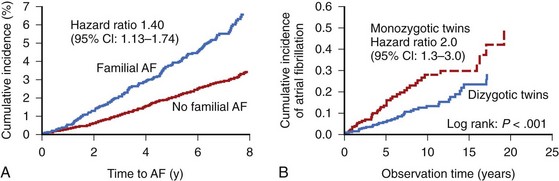
Among many risk factors for AF, genetic predisposition to AF or specific genetically predetermined forms of the arrhythmia have also been identified. Among the participants in the Framingham Heart Study, a parental history of AF increased the risk of AF in the offspring by a factor of 1.4 after adjustment for AF risk factors and AF-related genetic variants (Figure 42-4).55 Genetic variation may play even a greater role in the pathogenesis of lone AF (see below). AF in association with known genetic conditions such as Brugada syndrome and short QT interval syndrome is highly prevalent. The genetic substrate for AF ranges from a large kindred with monogenic forms of AF with rare genetic mutations and high penetrance to common genetic polymorphisms in a variety of different genes in general populations.
Monogenic mutations associated with AF have been described in the number of genes encoding several potassium channels (e.g., KCNQ1 and KCNE2, which encode α- and β-subunits, respectively, of the IKs channel; KCNA5 and KCNJ2, encoding Kv1.5 and Kir 2.1 channels underlying the IKur and IK1 currents, respectively), and α- and β-subunits of the sodium channels (SCN5A and SCN1/2B) as well as a number of non–ion channel mutations in the nucleoporin gene (NUP155), connexin-40 gene (GJA5), and ANP gene.56 However, monogenic forms of AF are rare and, at the population level, AF heritability is likely to be determined by aggregate effects of multiple common genetic variants interacting with environmental factors.
Population-based genome-wide association studies (GWASs) have identified a variety of single-nucleotide polymorphisms near the PITX2 (paired-like homeodomain 2) gene on chromosome 4q25 that almost double the susceptibility to AF in general populations of European and Asian descents.57,58 Other common variants include ZFHX3 (zinc finger homeobox 3) on chromosome 16q22, and KCNN3 (a calcium-activated, small conductance potassium current, which is also involved in atrial repolarization) on chromosome 1p21.
Lone Atrial Fibrillation
Lone AF poses a higher risk of heritability. Relatives of patients with lone AF are at a substantially increased risk of developing AF compared with the general population, suggesting a Mendelian genetic contribution to the etiology of this common trait. Analysis of the Danish Twin Study has revealed an increased risk of AF among monozygotic twins compared with dizygotic twins (22% vs. 12%), with the heritability of AF estimated at 62% (compared with 80% heritability of height and 35% heritability of the QT interval).58,59
GWASs have linked an SNP in KCNN3 to a greater risk of lone AF with an odds ratio of 1.52.60 The angiotensin-converting enzyme (ACE) insertion/deletion (I/D) polymorphism was associated with electrical remodeling in patients with lone AF.61
Vagally Mediated and Adrenergically Mediated Atrial Fibrillation
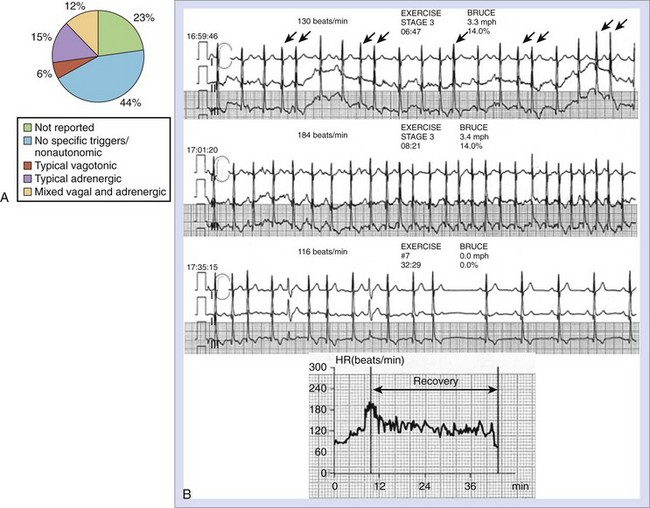
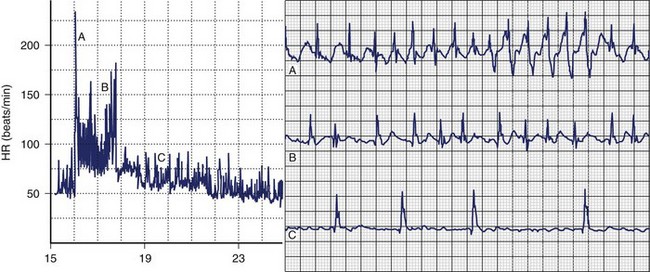
The balance between sympathetic and vagal influences plays an important role both in the initiation and prediction of AF. Two types of autonomic-induced AF exist. Adrenergically mediated episodes of AF are typically triggered by exercise and emotional stress (Figure 42-5), commonly associated with polyuria, and occur mainly during the day. Vagally mediated AF is characterized by male predominance, younger age, minimal tendency to progress to permanent AF, and onset at rest or at night. Episodes of vagal AF are typically preceded by progressive bradycardia. β-Blockers are treatment of choice in AF with a predominant adrenergic component but may worsen symptoms in patients with vagally mediated AF.
However, patients who can be classified in terms of sympathetic or adrenergic forms of AF typically represent the extreme of either sympathetic or parasympathetic influence, and the overall prevalence of typical vagally or adrenergically mediated AF is relatively low. In 1517 patients with paroxysmal AF included in the Euro Heart Survey, vagally mediated AF was found in 6% and adrenergically mediated AF was found in 15%.62
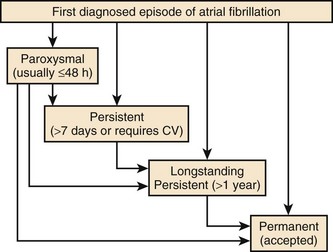
Classification
The 2010 European Society of Cardiology (ESC) guidelines introduced a longstanding persistent category for AF, which typically lasts for 1 year or more before a rhythm control intervention is attempted (Figure 42-6).63 This allows for a permanent form to be redesignated as longstanding persistent AF, that is, amenable to restoration and maintenance of sinus rhythm, mainly because of the increased success of ablation therapies.
Progression of Atrial Fibrillation
AF is a progressive disease because of the continuous remodeling of the atria caused by the AF itself, changes associated with aging, progression of underlying heart disease, and genetic and environmental factors. Progression from first-diagnosed or recurrent paroxysmal AF to persistent or permanent AF occurs, on average, at the rate of 5% to 15% per year, depending on a number of factors such as age at presentation and the presence of underlying heart disease (Table 42-2).64,65
Table 42-2 Rates of Progression of Paroxysmal Atrial Fibrillation to Persistent or Permanent Atrial Fibrillation
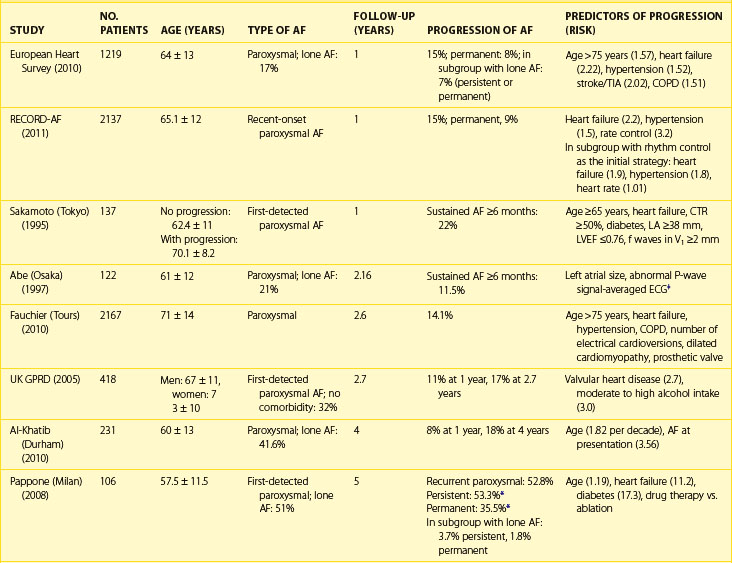
Factors, independently associated with the progression of AF in the European Survey on AF, formed a HATCH score to predict progression of AF (hypertension, age >75 years, transient ischemic attack [TIA] or stroke, COPD, history of stroke or heart failure). CHF and stroke were assigned 2 points each and the remaining factors 1 point each. Almost half the patients with a high HATCH score of 6 to 7 had AF progression after 1 year compared with only 6% of those with a HATCH score of 1. Rates of progression are higher in patients who presented with first-onset persistent AF, with 35% to 40% developing permanent arrhythmia by the end of the first year since diagnosis.66 Pursuing the aggressive rhythm control strategy with antiarrhythmic drugs, pulmonary vein ablation or both and, when necessary, cardioversion can alter the natural course and prevent or delay progression of AF.
Progression of AF is linked to structural atrial remodeling, accumulation of electrically silent fibrotic tissue, and transformation of AF from a primary electrical disorder requiring specific triggers such as atrial premature beats (APBs), pulmonary vein tachycardia, and neurohumoral stimuli (trigger-dependent AF) into a substrate-dependent variety (Figure 42-7). Patients with lone AF have been reported to have the lowest progression rates of approximately 1% to 2% per year.67 Atrial remodeling occurs in both lone AF and AF associated with cardiovascular disease, but it is thought to be more pronounced in the latter. However, recent studies employing delayed enhancement magnetic resonance imaging (MRI) techniques have demonstrated the presence of extensive fibrosis primarily accumulated in the left atrial posterior wall in patients with lone AF compared with healthy individuals without AF.68 Patients with lone AF were found to have a similar amount of fibrosis of the left atrium as those with associated cardiovascular and metabolic disease.
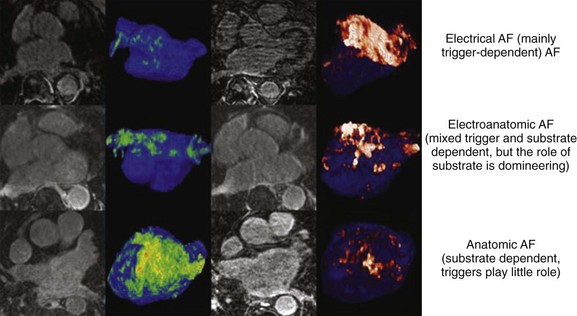
FIGURE 42-7 Stages of progression of atrial fibrillation assessed by delayed-enhancement magnetic resonance imaging.
(From Mahnkopf C, Badger TJ, Burgon NS, et al: Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: Implications for disease progression and response to catheter ablation, Heart Rhythm 7:1475–1481, 2010.)
Consequences of Atrial Fibrillation
The clinical significance of AF lies predominantly in high risk of ischemic stroke and heart failure and increased subsequent death. The occurrence of AF is commonly associated with worsening of pre-existing cardiovascular disease. New-onset AF in CHF has been linked to clinical deterioration and poor prognosis.22 Patients with concomitant AF and hypertension had a two- to threefold greater risk of developing stroke and CHF or dying.69 AF is associated with increased in-hospital and long-term mortality rates in patients with MI.27,70 AF leads to more hospital admissions than any other arrhythmia and presently costs approximately 1% of the health care budget in the Western countries.
Stroke
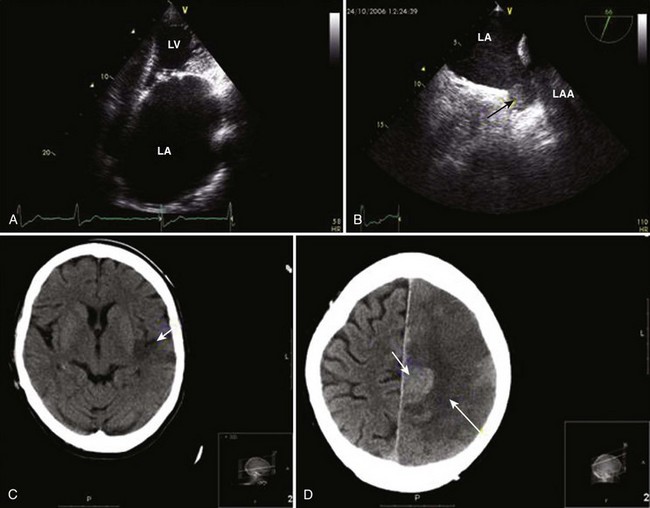
The presence of AF has been estimated to increase the risk of stroke by about fivefold.71 Whereas the effects of hypertension, CAD, and CHF on the risk of stroke become progressively weaker with advancing age, the impact of AF remains significant. In the Framingham Study, the annual risk of stroke attributable to AF among patients aged 50 to 59 years is 1.5% and increases to 23.5% in those older than 80 years.71 Approximately 15% of neurologically asymptomatic patients enrolled in the Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation (SPINAF) study had evidence of one or more previously undiagnosed cerebral infarcts on CT images of the brain.72 The risk is considerably higher in patients with previous stroke or TIA.
Strokes associated with AF are usually more severe and confer a high risk of subsequent morbidity, mortality, and poor functional outcome independent of the underlying heart disease. Risk of recurrent stroke is high, particularly within the first year, because of hemostatic abnormalities following the index event. The report from the Framingham Study has revealed that stroke associated with AF was nearly twice as likely to be fatal, and functional deficits were more likely to be severe among survivors.73 Nearly three quarters of stroke victims with AF were severely dependent in activities of daily living (ADLs) compared with about one third of their counterparts with sinus rhythm.
Dementia
Dementia as a consequence of AF has been only recently recognized. Evidence from the large ongoing prospective Intermountain Heart Collaborative Study database, AF was independently associated with all dementia types, and the risk was the highest in the younger group (<70 years), with an HR of 2.2 for vascular dementia, 3.34 for senile dementia, and 2.3 for Alzheimer disease after adjustment for age and comorbidities.74 According to data from the Olmsted County, Minnesota, in patients with a mean age of 71 years, the rate of dementia associated with AF was 2.7% at 1 year and 10.5% at 5 years.75 The diagnosis of dementia in the presence of AF was associated with a marked increased risk of subsequent mortality by factor of 3.49 in men and 3 in women.
Tachycardia-Induced Cardiomyopathy
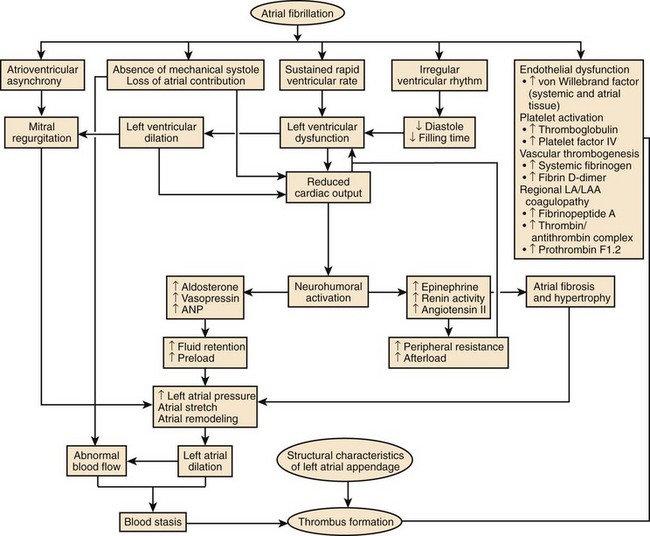
AF may compromise left ventricular systolic function and worsen CHF because of fast, uncontrolled ventricular rates, irregularity of ventricular response, and loss of atrial systolic input. Loss of AV synchrony is associated with impaired diastolic filling, reduced stroke volume, and elevated diastolic atrial pressure, resulting in an approximately 20% reduction in cardiac output.22 An irregular ventricular response further decreases cardiac output and increases right atrial pressure and pulmonary capillary wedge pressure independent of rate. Increased adrenergic stimulation to maintain adequate cardiac output in the setting of heart failure will facilitate AV conduction and favor the progression of cardiomyopathy. AF has been reported to confer a threefold risk of worsening CHF.76 In patients with CHF secondary to diastolic dysfunction, the occurrence of AF may be particularly hazardous.
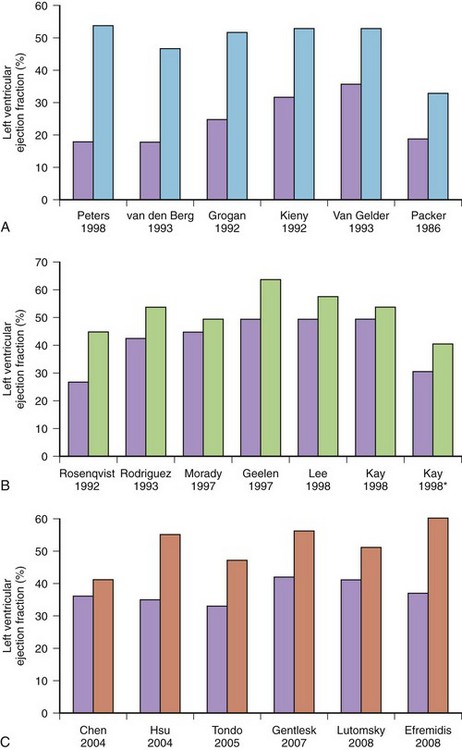
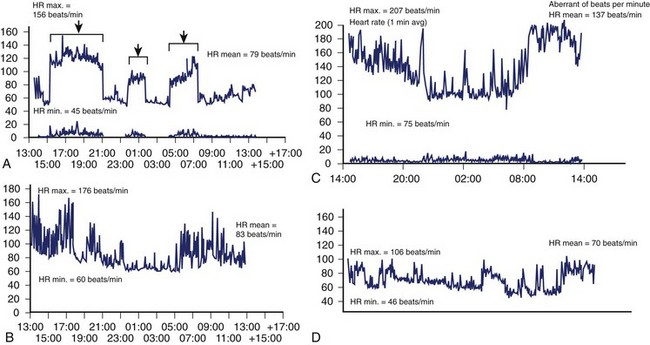
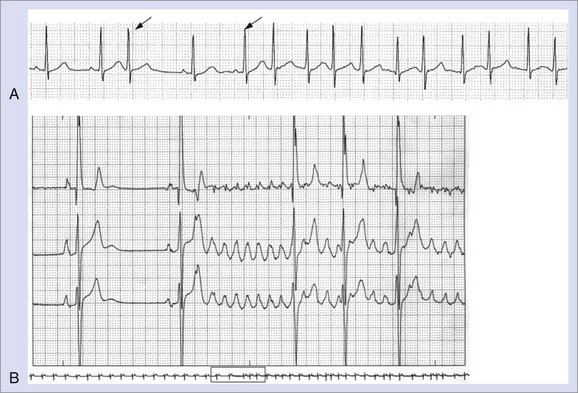
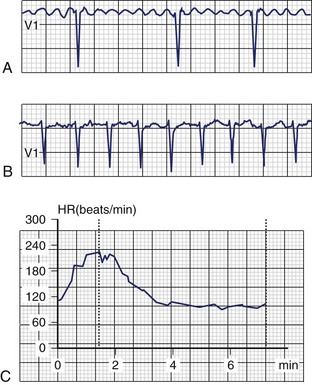
Less commonly, AF with fast, uncontrolled ventricular rates may cause symptomatic left ventricular dysfunction in patients with no or minimal structural heart disease. Such ventricular dysfunction associated with significant heart dilatation and symptoms of heart failure is termed tachycardia-induced cardiomyopathy. It is usually applied to persistent forms of the arrhythmia, and it is generally accepted that sustained ventricular rates of above 120 beats/min may pose a risk but may also occur in individuals with frequent paroxysms, in whom rate control is difficult. Tachycardia-induced cardiomyopathy may reverse completely after sinus rhythm is restored or adequate ventricular rate control is achieved either by pharmacologic or nonpharmacologic means (Figures 42-8 and 42-9).
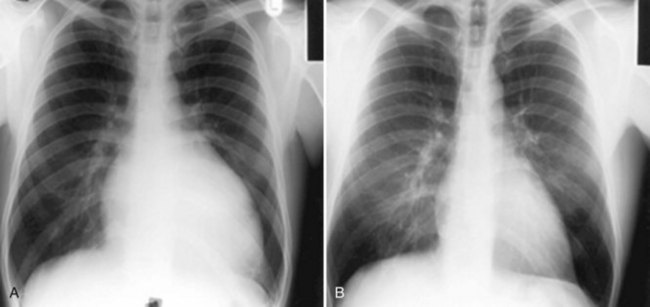
FIGURE 42-8 Reversal of cardiac enlargement on chest radiographs 3 months after restoration of sinus rhythm.
Hospitalizations
According to some surveys, the number of AF-related hospitalizations almost tripled in 2000 compared with the previous 2 decades and continues to grow, probably because of an increasing proportion of the population surviving to very old age with greatly improved health care. Patients with AF can be admitted for treatment of AF (e.g., cardioversion, initiation of antiarrhythmic drug therapy, or ablation) and cardiovascular adverse events (e.g., proarrhythmia, progression of heart failure, stroke, or MI) or noncardiac causes. Analysis from the Atrial Fibrillation Follow up Investigation of Rhythm Management (AFFIRM) trial has linked cardiovascular hospitalization with increased risk of subsequent death.77 The association between cardiovascular hospitalization and adverse outcome has also been reported in the Stockholm Cohort Study of Atrial Fibrillation, which showed that individuals who spent more than 2% of their follow-up period in the hospital with a cardiovascular diagnosis had a significantly higher mortality rate than those who had spent less time in the hospital (36 vs. 8.2 deaths per 100 patient-years).78 Time in-hospital greater than 2% increased the risk of subsequent death by a factor of 2.5, and rehospitalization for cardiovascular reason within 3 months increased the risk of death by a factor of 1.4 in the early cohort and 2.43 in the later cohort, for which a better account of therapy was available.
Quality of Life
Patients with AF generally have impaired quality of life compared with healthy control subjects—matched samples from the general population or patients with stable CAD—and is similar on most scales to health impairment seen in patients with MI or CHF and more significant structural heart disease.79 The degree of reported reductions in quality of life is determined by the form of AF and the presence of other diseases that may affect the individual perception of health. Thus patients with multiple comorbidities are more likely to report poorer quality of life compared with patients with established AF and those with less significant underlying heart disease. Paroxysmal AF is usually symptomatic and is associated with impaired quality of life. Patients with this variety report better quality of life and had significantly higher scores in physical functioning, vitality, mental health, and emotional health when they perceive themselves to be in sinus rhythm.80 Women are more likely to report poor quality of life compared with men, despite comparable severity of underlying pathology. Even patients with asymptomatic AF report a significantly poorer perception of general health and decreased global life satisfaction compared with their healthy counterparts, despite similar scores on all other scales.81
Both antiarrhythmic drug therapy and ablation-based therapies have been shown to render a significant proportion of AF asymptomatic, which may result in improvement in quality of life. Of interest, when quality of life was compared in patients with AF who were randomly assigned to AV node ablation or AV node modification, ablation was shown to result in a greater improvement in quality of life and reduction of symptoms compared with modification, probably because of better control of the rate and regularity of ventricular response after abolishing fibrillatory conduction to the ventricles.82 Recently, several AF-specific quality of life questionnaires have been proposed, but all require validation.83,84
Silent Atrial Fibrillation
AF is typically associated with a variety of symptoms: palpitations, dyspnea, chest discomfort, fatigue, dizziness, and syncope (Figure 42-10).85 The paroxysmal forms are more likely to be symptomatic and frequently present with specific symptoms, whereas the permanent forms are usually associated with less-specific symptoms. However, a substantial proportion of patients may not experience any symptoms or these symptoms may be subtle and may not be reported.
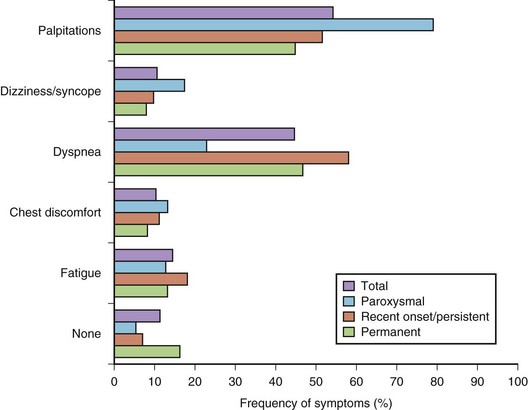
FIGURE 42-10 Distribution of symptoms in different forms of atrial fibrillation.
(Modified from Lévy S, Maarek M, Coumel P, et al, on behalf of the College of French Cardiologists: Characterization of different subsets of atrial fibrillation in general practice in France: The ALFA Study, Circulation 99:3028–3035, 1999.)
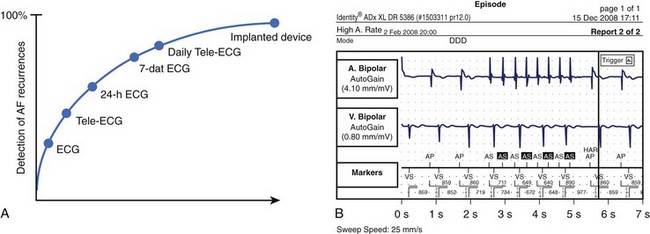
The prevalence of sustained silent AF found incidentally during routine physical examinations, preoperative assessments, occupation assessments, or population surveys is believed to be 25% to 30%, but modern implantable rhythm control devices such as pacemakers and implantable cardioverter defibrillators (ICDs) have revealed that up to 50% to 60% may have unsuspected episodes of the arrhythmia, with almost half of these lasting more than 48 hours.8,86 Patients with unrecognized AF do not receive appropriate preventive therapy and are at greater risk of stroke or, in the case of persistent rapid ventricular rates, tachycardia-induced cardiomyopathy. For example, AF may be found incidentally in about 20% to 25% of admissions for stroke.
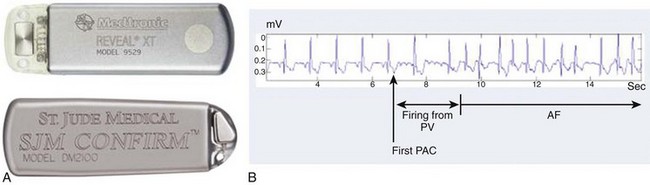
Studies that used regular ECG monitoring have demonstrated that up to 90% of recurrences may go unreported by patients but can only be detected by daily transtelephonic ECG transmission (Figure 42-11).87 The clinical significance of very short paroxysms of AF, which are difficult to detect by conventional methods of rhythm monitoring but can be logged by implantable device diagnostics, has recently been demonstrated in the The Relationship Between Daily Atrial Tachyarrhythmia Burden from Implantable Device Diagnostics and Stroke Risk (TRENDS) study in patients treated with a pacemaker for sinus node dysfunction.88,89 AF burden of 5.5 hours or greater on any of 30 prior days doubled the risk of a thromboembolic event.
Furthermore, an exact definition of “asymptomatic” AF does not exist. A patient may claim to have no symptoms but report feeling much better after cardioversion. Studies have also shown that quality of life is reduced even when a patient is classified as asymptomatic.81 Level of acceptable symptoms may also vary according to age and comorbidities.
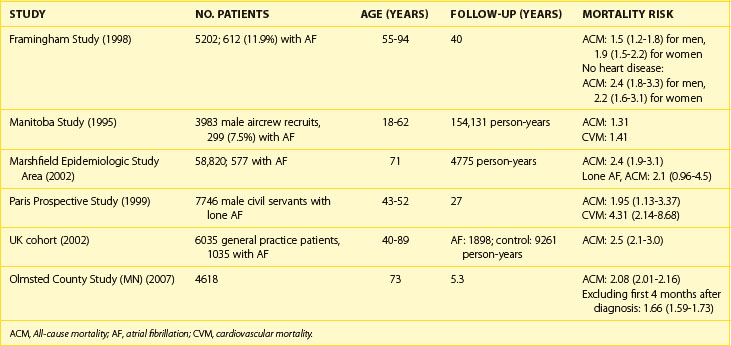
Mortality
Association of AF with death has been demonstrated in epidemiologic studies. Data from the Framingham Study suggest that the risk of death conferred by AF is nearly doubled even in the absence of identifiable structural heart disease.90 The risk of death associated with AF is significantly higher in women than in men (OR, 1.9 vs. 1.5). Data from other epidemiologic studies are convincing (Table 42-3). AF is an independent predictor of death in patients with a range of cardiovascular pathologies: CHF, hypertension, MI with left ventricular dysfunction, and ACS. AF increases cause-specific mortality: SCD, death from heart failure, and fatal strokes. A mechanistic basis for the link between AF and each of the components of total mortality does exist (Figure 42-12). Although the exact mechanism by which AF can increase the risk of SCD in the general AF population (apart from rare cases of SCD in WPW syndrome) has not been well established, proarrhythmia from antiarrhythmic drugs and ischemia-induced VT and VF, particularly in patients with severe underlying disease, are plausible explanations.
Current data do not offer a definitive answer whether AF truly is an independent risk factor for death or whether it is a marker indicating a sicker patient. However, even lone AF has been reported to entail 4.2-fold increase in all-cause mortality and an almost twofold increase in cardiovascular mortality, including SCD, in the Paris Prospective Study.91
Costs
The high lifetime risk for AF and increased longevity underscore the important public health burden imposed by AF across the world. The majority of AF expenditure constitutes hospital charges for frequent and often prolonged admissions for AF and particularly its costly complications such as stroke and heart failure. In addition, the cost of caring for patients with other cardiovascular pathologies is significantly higher in the presence of AF. The cost of caring for patients with AF also continues to be significantly higher during the 2 to 3 years after initial hospitalization. The arrhythmia costs of the health care budget in the United Kingdom and has almost doubled (0.9% to 2.4%) over the past 5 years.92 Direct cost estimates ranged from $2000 to $14,200 per patient-year in the United States and from 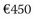 to
to 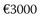 in Europe.92 Inpatient care accounts for 50% to 70% of the annual direct costs.
in Europe.92 Inpatient care accounts for 50% to 70% of the annual direct costs.
Based on data from the retrospective analysis of three federally funded databases in the United States in 2001, total annual costs for treatment of AF were estimated at $6.65 billion, including $2.93 billion (44%) for hospitalizations for AF, $1.95 billion (29%) for the incremental inpatient cost of AF as a comorbid diagnosis, $1.53 billion (23%) for outpatient treatment of AF, and $235 million (4%) for prescription drugs.93
Relationship Between Atrial Fibrillation and Atrial Flutter
Epidemiology
Data on the prevalence and incidence of AFL in the general population are limited. The report from the Marshfield Epidemiologic Study Area (MESA), based on a selected sample of 58,820 residents served by the Marshfield Clinic in Wisconsin, suggests that the incidence of AFL is 0.88 per 1000 person-years, but this figure includes 58% of individuals in whom both AFL and AF have been diagnosed.94 As a sole arrhythmia, the incidence of AFL is relatively low and clearly stands at less than 1%.
Associated Disease and Prognosis
AFL is commonly associated with organic heart disease (see Figure 42-2). In the MESA population, nearly all cases of AFL were linked to comorbid conditions such as CHF, hypertension, and COPD or occurred in association with a specific precipitating event (i.e., major surgery, pneumonia, or acute MI).94 Only 1.7% of cases had no structural cardiac disease or precipitating causes (lone AFL).
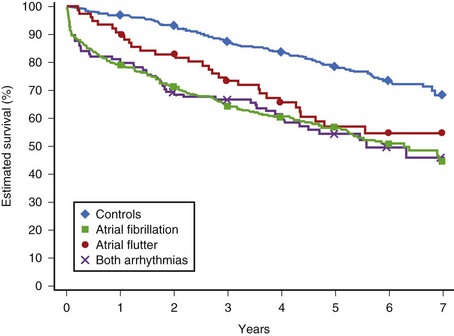
AFL and AF share similar risk factors and have the same risk of CHF and thromboembolism in the presence of risk factors for stroke. Similar to AF, AFL is associated with an increase in mortality rate by a factor of 1.7 as a sole arrhythmia and a factor of 2.5 if it coexists with AF (Figure 42-13).95
Interrelationship
Typical AFL is a classic macro–re-entrant arrhythmia that develops from a functional conduction block at the cavotricuspid isthmus.96 This mechanism of initiation and maintenance is markedly different from AF, and typical AFL can be cured relatively easily by ablation. Furthermore, AFL and AF respond differently to antiarrhythmic drugs. In this respect, patients with AFL should be distinguished from patients with AF.
However, the close clinical interrelationship between AF and AFL should not be disregarded. In many instances, AFL is preceded by a transitional rhythm in the form of AF of variable duration, which facilitates the occurrence of a functional block between the venae cavae necessary for the maintenance of the AFL macro–re-entrant circuit. Conversely, clinical and experimental studies in postoperative AF and in the canine sterile pericarditis model have demonstrated that a very fast AFL may lead to AF.96
The close interrelationship between AF and AFL is reflected in the high prevalence of patients in whom both arrhythmias coexist and the significant proportion of patients (30% to 70%) who develop AF after successful ablation of the cavotricuspid isthmus for AFL, even if AF has never been documented before ablation. In a recent meta-analysis of long-term outcomes of ablation for typical AFL (158 studies, 10,719 patients), the overall incidence of AF was 33.6% during an average follow-up of 15 months; AF occurred in 23.1% of patients with no history of AF and in 52.7% of patients with a history of AF before ablation.97 Such a high proportion of patients developing AF within a relatively short time after ablation suggests that AFL may be an early marker of atrial remodeling that forms a substrate for AF. In contrast, antiarrhythmic drugs with sodium channel–blocking properties, such as class IC agents and amiodarone, which slow conduction in the atria, can convert AF into AFL, probably by facilitating the formation of functional block in the right atrium. Ablation of the cavotricuspid isthmus has been effectively used in such patients to prevent the recurrence of AF.
Anatomy and Pathology
Structurally, the atria are complex structures because of the gaps in their walls for the openings of the connecting veins and for the valvular openings. The conduction of the cardiac impulse from the sinus node to the AV node travels through preferential pathways formed by the remaining myocardium. In discussing the atria, the anatomy of the right and left atria and their venous connections, the sinus and AV nodes, the nodal approaches, and the pathologic changes that may be involved in atrial arrhythmias are all considered. Gross dissections of the atrial walls help reveal the complex arrangement of the myocardial strands that make up the atrial walls.98 Even though the myocardial strands do not precisely replicate the syncytia of myocytes that are visible only on microscopy, they provide a useful guide to the general orientation of the myocytes, allowing inferences to be made on the direction of conduction.99–101 On histology, pathologic changes in the atrial myocardium occur from middle age onward. Myocyte loss occurs to varying extents followed by replacement with fat and fibrous tissue.102 In the normal aging process, some myocytes may be atrophied, some are hypertrophied, and others are in various stages of degeneration. The conduction tissues are also affected by aging-related changes.103 Furthermore, the atrial chambers become enlarged even in normal individuals with sinus rhythm older than 50 years.104 Myocyte hypertrophy, myolysis, apoptosis, and irreversible fibrosis are more pronounced in patients with atrial arrhythmias.
Right Atrium
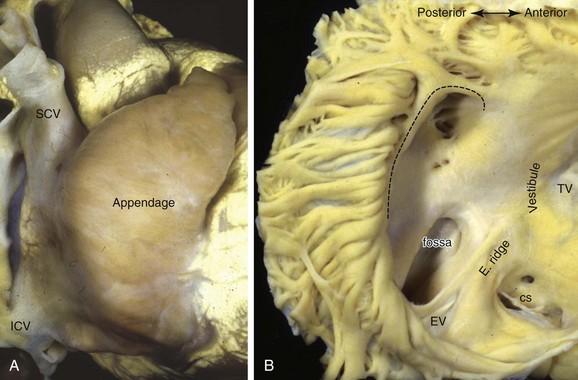
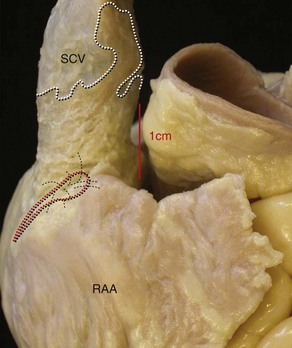
The dominant feature of this chamber is the large, triangular shaped appendage, which is located anterolaterally (Figures 42-14 and 42-15). Usually, a fat-filled groove, corresponding internally to the terminal crest (crista terminalis), can be seen along the lateral wall. The sinus node is located subepicardially in this groove, close to the superior cavoatrial junction (see Figure 42-15). In most individuals, the node is located anterolaterally in a tadpole shape with the head nearest the junction and the tail penetrating into the musculature of the terminal crest.105 In approximately 10%, the node is horseshoe shaped and located mediolaterally in the terminal groove.106 Right atrial musculature often extends superiorly over the wall of the superior vena cava (SVC), unlike inferiorly where atrial muscle fades out or terminates close to the entrance of the inferior vena cava (IVC). Prongs of sinus node tissues often interdigitate with the musculature of the terminal crest and may even extend into the muscular sleeves that surround the SVC (see Figure 42-15).105,107 The tail portion often fragments into clusters of specialized cells toward the middle or even lower portions of the terminal crest. The prongs and the clusters may have some role in focal atrial tachycardia (AT).
The terminal crest demarcates the junction between the rough-walled atrial appendage and the smooth-walled venous (intercaval) appendage on the endocardial surface. Most commonly it is ridgelike and composed of myocardial strands that are aligned longitudinally.108 An extensive array of pectinate muscles arise nearly perpendicularly from the terminal crest to spread throughout the entire wall of the appendage, reaching to the lateral and inferior walls of the atrium (see Figure 42-14) to the atrial vestibule that separates the appendage from the tricuspid annulus. The pectinate muscles branch into fine and interconnecting muscle bundles. In between the bundles, the wall of the appendage is very thin, almost parchment-like in some cases. Potentially, this arrangement may play a role in initiating intra-atrial re-entry.
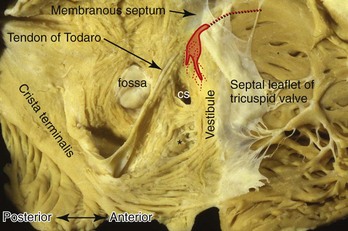
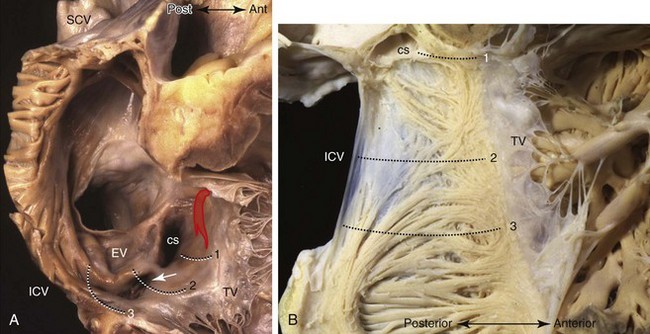
The atrial vestibule is the smooth muscular rim that surrounds the tricuspid orifice (see Figure 42-14, B) and the short extensions of its musculature inserting into the atrial surface of the leaflets. Importantly, the inferior to inferolateral segment of the right atrial vestibule, as viewed in left anterior oblique projection, is the anterior part of the cavotricuspid isthmus, the isthmus that is targeted as the zone of slow conduction in common AFL.109,110 The posterior part of the isthmus contains the terminal ramifications from the terminal crest as it approaches the orifice of the IVC (Figure 42-16).109 While the anterior part of the isthmus is always muscular, being the atrial vestibule, the middle and posterior parts are highly variable in topography and wall thickness.111,112 The posterior part adjoining the eustachian valve tends to be composed of fibrous tissues, but the middle part contains variable extents and thicknesses of criss-crossing muscle bundles separated by thin membranous tissue (Figure 42-17). A pouchlike recess, the sub-eustachian sinus, is common and is 5 mm or more deep in 20% of patients.113 The eustachian valve, which guards the entrance of the IVC, is variably developed. Usually, it is a triangular flap of fibrous or fibromuscular tissue that inserts medially to the eustachian ridge, or the sinus septum, which is the border between the fossa ovalis and the coronary sinus. The free border of the eustachian valve continues as a tendon of Todaro, which runs in the musculature of the sinus septum.114 It is the posterior border of the triangle (of Koch) that delineates the location of the AV node (see Figure 42-16).115,116 The anterior border is marked by the hinge line of the septal leaflet of the tricuspid valve. Superiorly, the central fibrous body is the landmark for the penetrating bundle of His. Thus the inferior border of the triangle is the orifice of the coronary sinus, together with the vestibule immediately anterior to it. This vestibular portion, also known as the septal isthmus or, more accurately, the paraseptal isthmus (see Figure 42-17), is the area often targeted for ablation of the slow pathway in AVNRT. Although the body of the compact node is located toward the apex of Koch’s triangle, it sends two prongs inferiorly toward the mitral and tricuspid orifices. The rightward extensions have been implicated in this arrhythmia.117 This area also contains the zone of transitional cells that provides the inferior and posterior inputs to the compact node. The so-called fast pathway in AVNRT corresponds to the area of musculature close to the apex of the triangle of Koch. The superior zone of transitional cells and myocardial strands from the anterior limbic band of the fossa ovalis sweep into this area overlying the body of the compact node.
Left Atrium
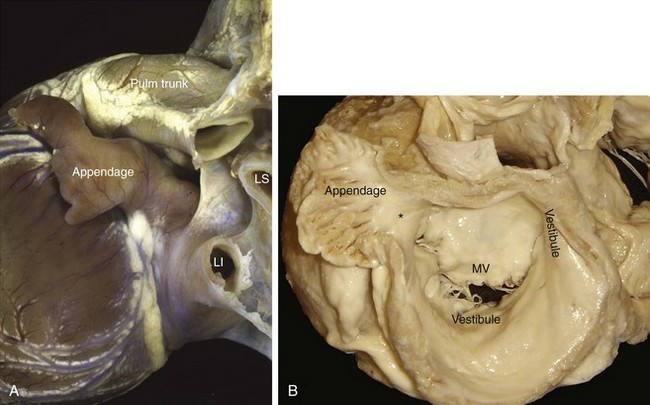
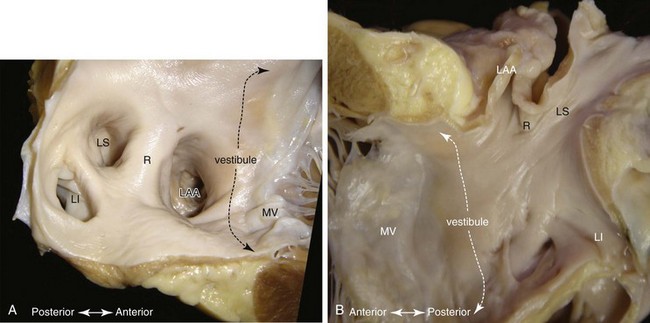
Although the left atrium has similar component parts as the right atrium, its fingerlike appendage is distinctive. It can have a variable number of lobes, bends, or branches, but characteristically, the appendage is a cul-de-sac with a narrow opening to the rest of the chamber (Figure 42-18).118 In contrast to the right atrium, no terminal crest or sinus node is present in the left atrium. The so-called ridge (crest) on the endocardial surface between the left atrial appendage (LAA) and the left superior pulmonary vein is an infolding of the atrial wall (Figure 42-19) that contains the remnant of the vein of Marshall and neural elements on the epicardial side. Nearly all the pectinate muscles in the left atrium are confined within the appendage. They form a complicated network lining the endocardial surface. The major part of the atrium, including the septal component, is smooth walled. The smoothest parts are the walls forming the atrial body, the superior and posterior walls that make up the large pulmonary venous component, and the vestibule that is the atrial outlet. Like the right vestibule, the left atrial vestibule also extends a very short distance over the atrial surface of the mitral leaflets. Although appearing fairly uniform, the atrial walls are composed of three or more overlapping “layers” of differently aligned myocardial strands.98,119,120 The “layers” are not insulated by fibrous tissue sheaths, but abrupt changes in orientations may account for the unusual patterns of conduction.121 Marked regional variations in thickness are also present.122,123 A small area of the anterior wall just behind the aorta is usually thin, whereas the superior wall, or dome, is thicker but tends to thin out near the left pulmonary veins.
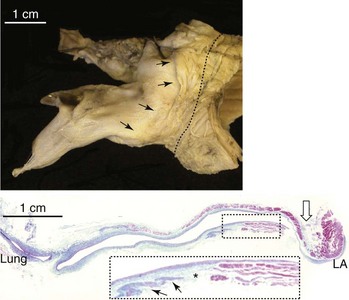
The transition between the atrium and the vein is smooth. The musculature of the atrial wall extends to varying lengths onto the outside of the venous walls, with the longest sleeves along the upper veins (Figure 42-20).119,124,125 The right lower veins tend to have the shortest extensions or none at all. The sleeves closest to the venous insertions are thicker and more complete around the vein. Nerve bundles and ganglionated plexi are abundant at the venoatrial junctions and the adjoining walls.126,127 The margins of the sleeves become thinner and irregular toward the lung hila. Pathologic studies have been inconclusive regarding the role of these myocardial sleeves in triggering AT and AF because they are found in asymptomatic adults as well as in patients with atrial arrhythmias. Like the myocardium of the atrial body, the sleeves also undergo aging changes. Islands of viable myocytes among fibrotic areas are often found (see Figure 42-20).125 Myocyte hypertrophy, fibrosis, and sleeve discontinuity are more frequently seen in tissues from patients with AF.128 Other studies have shown mainly circularly orientated myocardial fibers with interdigitating longitudinally and obliquely orientated fibers in the sleeves.101,124 These may set the scene for micro–re-entry.129
Although nodelike cells have been described in murine models, they have never been found human tissues until in the study by Perez-Lugones and colleagues, but controversy remains.124,125,130–132 Recently, interstitial cells of Cajal, previously identified as capable of providing pacemaker activity in the gut, were described for the first time in human atrial myocardium. They were found in higher density in the myocardial sleeves of pulmonary veins from patients with AF.133–135
Atrial Septum and Inter-atrial Connections
Viewed from the right atrium, the endocardial aspect of the true septum is confined to the valve of the fossa ovalis and the raised muscular rim (limbus) that is immediately around it (see Figure 42-14, B). The rim is an infolding of the right atrial wall harboring on its epicardial surface the fatty tissues of the inter-atrial groove. Although belonging to the right atrium, the leftward part of the muscular fold apposing the valve of the fossa continues into the musculature of the left atrial wall. The left atrial aspect of the septum is the valve itself. In most adult hearts, the fossa valve is thin, ranging from 0.4 mm to 3.4 mm. It usually is composed of two myocardial layers separated by fibrous tissue and sandwiched by fatty tissue beneath the endocardial surfaces.136,137 This arrangement may explain asynchronous and discordant activation of the right and left septal surfaces, as observed by Lemery and colleagues, and may participate in the left septal flutter circuit, as reported by Marrouche and colleagues.138,139
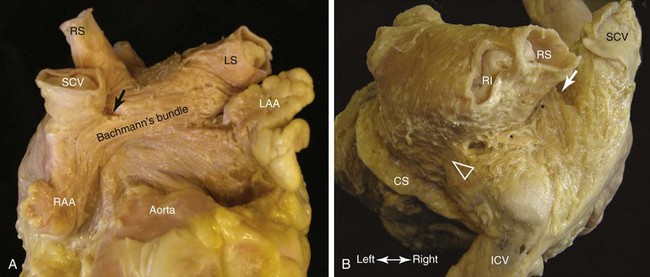
Muscular continuity between the atria peripheral to the septum is frequently found as bridges in the subepicardium.101,102,136 In the majority of hearts, the most prominent inter-atrial bridge is Bachmann’s bundle (Fig. 42-21, A). This is a broad muscular band that runs in the subepicardium connecting the anterior right atrial wall of the superior cavoatrial junction with the anterior wall of the left atrium. The myocardial strands in Bachmann’s bundle, as in the terminal crest, are well aligned. The posterior and inferior bridges joining the left atrium to the intercaval area on the right provide the potential for posterior breakthrough of sinus impulse (see Figure 42-21, B).139 In one third of hearts, the inferior inter-atrial connection is thicker or as thick as Bachmann’s bundle.136 Multiple smaller inter-atrial bridges are frequently present, giving the potential for macro–re-entry. Some connect the muscular sleeves of the right pulmonary veins to the right atrium, and some connect the SVC to the left atrium.101,120 Inferiorly, further muscular bridges from the left atrial wall often overlie and run into the wall of the coronary sinus.120 Fine bridges connecting the remnant of the vein of Marshall to the left atrium have also been demonstrated.140
Pathologic Substrates
In assessing pathologic changes in the myocardium for atrial arrhythmia, consideration must be given to changes associated with normal aging. Further compounding the issue are well-recognized conditions in older adults, such as senile amyloidosis, sick sinus syndrome, CAD, hypertensive heart disease, CHF, which can affect the myocardium as well as conduction tissues. Increased connective tissue, fibro-fatty changes, myocyte degeneration in the myocardium, and fibro-fatty degeneration or fibrosis of the conduction tissues are common. In patients with arrhythmias, some investigators reported increased interstitial fibrosis, myocyte myolysis, and inflammatory infiltrates in AF.141–144 In addition, recent microinfarcts are more often found in prominent muscle bundles such as the terminal crest and Bachmann’s bundle of AF patients, which suggests that atrial MI may be more common than previously thought.145
Clinical Electrocardiography
Atrial Flutter
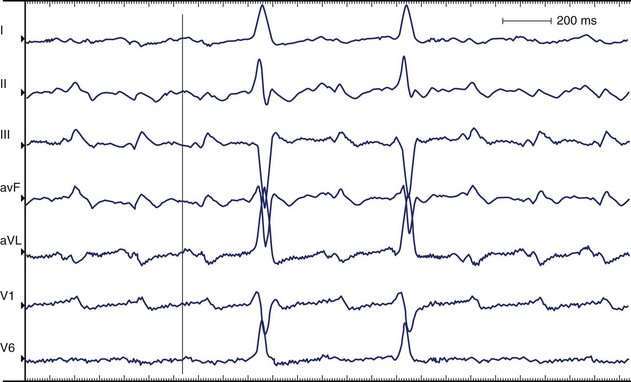
AFL is more organized than AF, features a saw-toothed pattern of regular activation that is particularly apparent in leads II, III, and aVF, and does not have an isoelectric baseline between deflections. The rate is typically 240 to 300 beats/min. Major types of AFL include typical (isthmus dependent, clockwise, and counterclockwise AFL) and atypical (isthmus independent) AFL. In the presence of anatomically predetermined slow conduction in the crista terminalis, atrial stimuli wavefront creates unidirectional block in the isthmus and maintains the AFL re-entry circuit. In typical AFL (Figure 42-22) that rotates counterclockwise in the right atrium, the flutter waves are inverted in leads II, III, aVF, and V6 and upright in lead V1. Biphasic or negative deflections in V1 are less common. Leads I and aVL usually have low amplitude deflections.146,147 When the activation sequence is reversed (clockwise rotation), the flutter waves may be upright in leads II, III, and aVF and inverted in lead V1. Wide negative deflections in lead V1 and a positive flutter wave in V6 are characteristic of clockwise rotation in the right atrium.146,147 The flutter waves are positive in V6. The surface lead characteristics that differentiate clockwise and counterclockwise AFL are summarized in Table 42-4.
Table 42-4 Predominant Flutter Wave Morphology in Typical Clockwise and Counterclockwise Right Atrial Flutter
| ECG LEAD | CLOCKWISE FLUTTER | COUNTERCLOCKWISE FLUTTER |
|---|---|---|
| II, III, aVF | Positive or negative | Negative |
| I | Positive | Biphasic or isoelectric |
| aVL | Biphasic or isoelectric | Positive |
| V1 | Negative or biphasic | Positive |
| V6 | Positive | Negative |
Counterclockwise Atrial Flutter
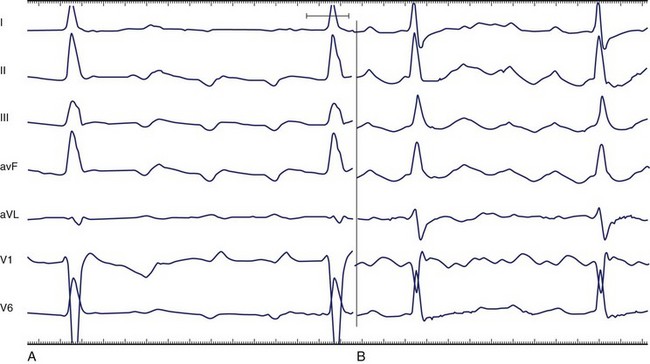
The silent isoelectric zone of the ECG that precedes the negative deflections in the inferior leads corresponds to activation of the low right atrium and isthmus between the tricuspid annulus and the IVC and precedes activation of the left atrium, which begins from the lower septum.146–152 The left atrial activation sequence is the predominant determinant of the flutter wave morphology (Figure 42-23, A).
In typical counterclockwise right AFL, the left atrium is activated from both the inferior septum and the superior septum.148 The posterior wall is activated preferentially from the inferior septum, and the anterior wall is activated from the superior septum. Activation of the lateral wall of the left atrium reflects variable inputs from both regions. Studies showed that left atrial activation coincided with the negative component in leads I, II, III, aVF, and V6 and the first flat or slowly rising component in V1. Activation of the lateral wall of the right atrium coincided with the positive deflections in leads I, V1, and V6 and the upstroke component in the inferior leads. The plateau duration in lead III correlated with the time required for conduction through the isthmus between the tricuspid annulus and the IVC.
In studies using body surface mapping with simultaneous endocardial mapping, the flutter wave cycle length could be divided into three time segments.151 Caudocranial activation of the right atrial septum occurred in conjunction with proximal-to-distal activation along the coronary sinus and corresponded to the initial segment of the flutter wave. Craniocaudal activation of the right atrial free wall occurred during the intermediate portion of the flutter wave, and activation of the lateral sub-eustachian isthmus occurred during the terminal flutter wave.
Clockwise Atrial Flutter
The silent or isoelectric zone of clockwise AFL is shorter compared with counterclockwise AFL.146,147,151 In studies, a saw-tooth pattern with a negative deflection in the inferior leads observed in clockwise AFL was interpreted as being very similar to the pattern observed in counterclockwise AFL.146 A shorter plateau phase was accompanied by widening of the negative component of the flutter wave. A negative flutter wave in V1 was a constant finding, and flutter waves were predominantly positive in V6 (see Figure 42-23, B). Caudal to cranial activation of the lateral wall of the right atrium corresponded to the end of the plateau and the descending part of the negative portion of the flutter wave. The ascending portion of the flutter wave corresponded to the descending activation of the septum and occurred synchronously with proximal-to-distal activation in the coronary sinus.
Activation of the lateral right atrium from caudal to cranial corresponded to an inverted component on the inferior leads of variable amplitude just before the development of upright notched flutter waves.147 In some patients, this period was an electrically silent isoelectric segment. However, in this study, all the patients with clockwise AFL had prominent upright flutter waves in the inferior leads. The upstroke began when the wavefront of activation reached the superior part of the crista terminalis in the vicinity of Bachmann’s bundle. This also corresponded with the onset of the major deflections in the precordial leads. The bulk of the flutter wave was presumably determined by the left atrium.
During clockwise AFL, a dominant breakthrough to the left atrium in the high anteroseptal area and a second breakthrough in the low posterior septal area were observed.148,149 Left atrial activation was coincident with positive components on the surface of ECG leads I, II, II, aVF, and V6 and the first negative component in V1. Activation of the lateral wall of the right atrium coincided with the negative components in lead I, inferior leads, and V6. The body surface maps attributed the initial segment of the flutter wave to craniocaudal excitation of the right atrial septum.151 The intermediate segment corresponded to excitation of the isthmus and proximal-to-distal activation along the coronary sinus. The terminal segment corresponded to caudocranial excitation of the right free wall.
Difficulties with Electrocardiogram Interpretation
The interpretation of AFL morphologies depends on a sufficient degree of AV block to separate the flutter wave from ventricular activation and repolarization. Atypical forms of AFL with diverse flutter wave morphologies that do not have a standard nomenclature complicate ECG assessments. Sometimes, the flutter wave morphology is low in amplitude or may be obscured by ventricular repolarization when the ventricular response is rapid. ECGs of atypical right atrial macro–re-entrant circuits can be difficult to interpret.152,153 Complex forms of left atrial macro–re-entry, which may resemble typical right AFL, tend to have predominantly positive flutter waves in V1.154 Several types of atypical AFL were demonstrated, including AFL in the low right atrial free wall, AFL involving the high right atrium (SVC–atrial septum area), and AFL involving two or four pulmonary vein orifices, mitral annulus isthmus, and the fossa ovalis area.
Figure 42-24 was recorded from a patient who had undergone a Fontan operation to treat transposition of the great vessels. The patient developed AFL, which involved rotation around a scar on the lateral aspect of the right atrium.
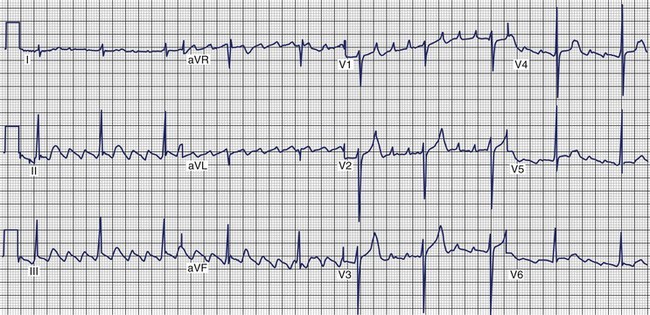
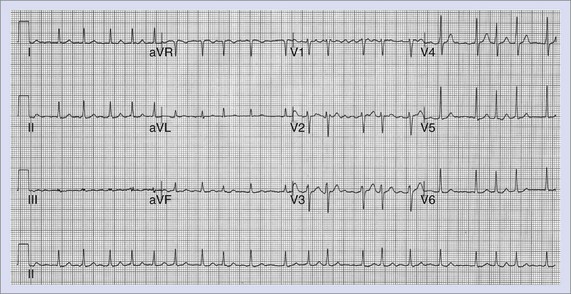
Atrial Fibrillation
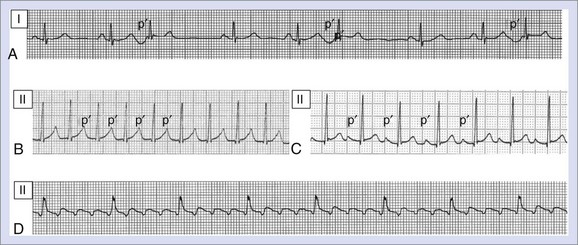
AF consists of rapid oscillations or fibrillatory waves that vary in size, shape, and timing (Figure 42-25). The ventricular response to AF depends on the electrophysiological properties of the AV node, the effects of drugs, and the balance between sympathetic and parasympathetic tones. The R-R intervals are irregular unless the patient has AV block or a paced rhythm. Paroxysmal AF appears to be highly dependent on initiation by atrial ectopy: 93% of spontaneous episodes of paroxysmal AF were triggered by atrial premature depolarizations, and 6.4% were preceded by typical AFL as documented by 12-lead Holter recordings.152 The morphology of the initiating P waves was used to estimate the origin of triggering events: 77.5% arose from the left atrium, 2% were of right atrial origin, and 13.5% were nonspecific. Generally, an increase was observed in the frequency of atrial ectopy in the 30 seconds that preceded the onset of AF. The beats that initiated AF had shorter coupling intervals than those that failed to initiate AF.155 More than half the episodes were also preceded by cycle length variation with short-long sequences. Although there appeared to be qualitative differences in the homogeneity of right atrial activation in paroxysmal AF compared with chronic AF, no consistent ECG criteria have been developed to distinguish the standard 12-lead ECG morphology of chronic and paroxysmal AF.156
Basic Electrophysiology
The basic mechanisms underlying cardiac arrhythmias are discussed in detail in Chapters 3 and 4 and are not repeated here. This section deals in detail with the present state of knowledge about the basic electrophysiology of AF.
Historical Aspects
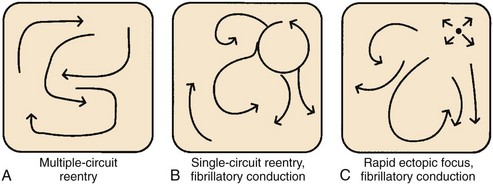
In the late 1800s, AF was shown to be the mechanism underlying “delirium cordis,” in which the heart was noted to beat without any apparent regularity. With the subsequent development of electrocardiography and of methods to study cardiac electrophysiology, three basic theories about the mechanism of AF emerged.157 These mechanisms are illustrated in Figure 42-26.
Mines and Garrey observed the occurrence of regular re-entry and fibrillation in cardiac tissue preparations and considered AF to be caused by continuous irregular re-entrant activity occurring in a dyssynchronous fashion in various atrial regions (“multiple circuit re-entry”; see Figure 42-26, A). Garrey first put forward the idea that fibrillation requires a critical mass of tissue to support a sufficient number of irregular re-entrant wavefronts to maintain the arrhythmia.
Lewis believed that AF is caused by a single rapid macro–re-entry circuit (see Figure 42-26, B), with wavefronts emanating from the primary “driver” circuit breaking against regions of varying and greater refractoriness, producing “fibrillatory conduction” and the irregular global activity characterizing the arrhythmia.158 Others held that AF is caused by very rapid activity, with either a single source giving rise to fibrillatory conduction (see Figure 42-26, C) or multiple ectopic foci producing fibrillation by virtue of dyssynchronous activity and colliding wavefronts. In the subsequent years, various lines of evidence pointed to the relevance of multiple circuit re-entry to clinical AF, and from then until relatively recently, multiple circuit re-entry (see Figure 42-26, A) was widely assumed to be the dominant mechanism underlying clinical AF.
Moe refined the concept of multiple circuit re-entry by suggesting that activity during AF need not involve complete re-entry circuits beginning and ending at the same location but, rather, simultaneous wavelets that either extinguish (not contributing to arrhythmia maintenance) or succeed in continuously encountering excitable tissue and maintaining the arrhythmia.159 Moe viewed the maintenance of AF as a probabilistic function of tissue properties and size, with a minimum temporal density of re-entrant wavelets needed to sustain the arrhythmia.
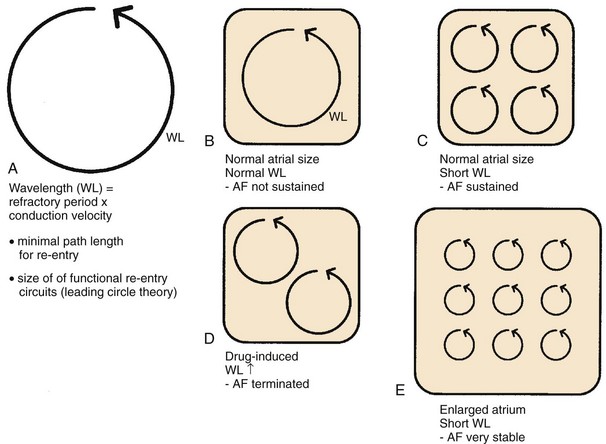
Allessie subsequently added a quantitative element to Moe’s reasoning by emphasizing the importance of the wavelength (Figure 42-27). The wavelength (see Figure 42-27, A and B) is the distance traveled by a cardiac impulse during the refractory period (refractory period ± conduction speed) and indicates the shortest path length that can maintain re-entry. In circuits shorter than the wavelength, the head of the impulse will impinge on a still-refractory tail after one cycle, and the impulse will die out. According to Allessie’s “leading circle” concept of functional re-entry, functional re-entry circuits naturally form around a perimeter equal to the wavelength.160 Allessie postulated that shorter wavelengths favor AF maintenance by increasing the number of simultaneous functional re-entrant circuits that the atria can accommodate (see Figure 42-27, C). A corollary of this notion is that multiple circuit re-entry AF can be terminated or prevented by increasing the refractory period and consequently the wavelength, thereby reducing the number of circuits possible and making AF maintenance less likely (see Figure 42-27, D).161 By contrast, factors that reduce the wavelength (such as short refractory periods and slow conduction) should favor AF.162 Atrial dilatation should also favor re-entry by increasing atrial size and thereby increasing the number of circuits that the atria can accommodate (see Figure 42-27, E).163 Heterogeneity in refractory properties should favor AF by promoting the fractionation of impulses into multiple re-entrant wavefronts.
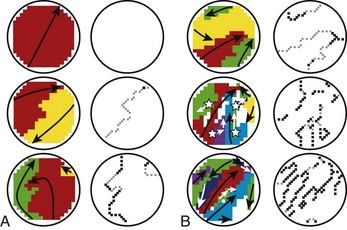
Recently, a significant increase in longitudinal dissociation, consisting of lines of block running parallel to the atrial muscle has been found during intraoperative epicardial mapping in patients with persistent AF as opposed to patients with acutely included AF (Figure 42-28).164 The amount of dissociation was sixfold greater in longstanding persistent AF, suggesting that it may play a role in the self-perpetuation of the arrhythmia.
Electrical Remodeling
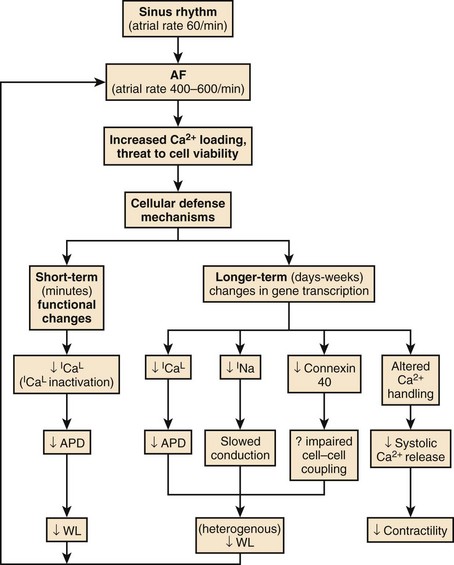
A key concept in understanding AF is that of electrical remodeling.165 It has become obvious over the past few years that atrial electrical properties are altered by sustained AF such that the atria become more susceptible to the initiation and maintenance of the arrhythmia.166 The primary factor driving AF-induced remodeling appears to be AT, and similar changes can be produced by other atrial tachyarrhythmias such as AFL and AT. Electrical remodeling occurs in a time-related fashion following the onset of AF and likely involves a series of adaptations stimulated by increased cellular calcium (Ca2+) loading from the increased rate of activation, with Ca2+ entering the cell with each activation.167 The electrophysiological changes caused by tachycardia-induced atrial electrical remodeling are summarized in Figure 42-29.
Short-term changes involve functional alterations, primarily Ca2+ current (ICa) inactivation, that reduce the action potential duration (APD), the refractory period, and the wavelength and thereby promote AF. Longer-term changes include alterations in the production of ion channel proteins, among which a key alteration appears to be downregulation in the L-type Ca2+ current (ICa.L) that maintains the plateau of the action potential and triggers cardiac contraction.168 ICa.L downregulation reduces APD and attenuates APD adaptation to rate, which is largely caused by tachycardia-dependent ICa.L reduction (that normally reduces APD at fast rates). In addition, sodium (Na+) current also appears to be reduced, decreasing conduction velocity, and the intercellular coupling channel protein, connexin 40, is reduced in a heterogeneous fashion. The resulting heterogeneous reductions in atrial wavelength make AF more likely to sustain itself and increase the vulnerability of the atria to AF re-induction should the arrhythmia be terminated.
Structural Remodeling
Autopsy studies of atrial tissues from patients with AF often show extensive atrial fibrotic changes, particularly in association with conditions such as mitral valve disease, CHF, and senescence. Recent clinical studies suggest that patients with AF have activation of the atrial renin-angiotensin system and related mitogen-activated protein kinases with profibrotic actions. In an animal model of CHF, sustained AF can be induced fairly readily, with an underlying substrate that involves local conduction abnormalities related to intense atrial interstitial fibrosis. AF in this model sometimes has the appearance of macro–re-entry with fibrillatory conduction, consistent with the mechanism illustrated in Figure 42-26, B.169 In addition to causing abnormalities in conduction, structural remodeling is often associated with atrial dilatation, favoring AF, as illustrated in Figure 42-27, E. Cellular electrical properties are also altered in CHF but not in the same way as with atrial tachycardia-induced electrical remodeling.169 APD is not shortened, so the wavelength is not reduced; however, the activity of the Na+-Ca2+ exchange current (NCX) is increased. The NCX exchanges Ca2+ accumulated in the cell during the action potential for extracellular Na+, with one intracellular Ca2+ ion exchanged for three extracellular Na+ ions. Thus, the NCX carries one net positive charge into the cell with each cycle. When NCX activity is enhanced, delayed afterdepolarizations (DADs) and triggered ectopic activity can result. CHF therefore favors AF both by creating a structural substrate for atrial re-entry and by producing a functional basis for atrial ectopic activity that can trigger re-entry.
Synthesis of Physiological Determinants in Atrial Fibrillation
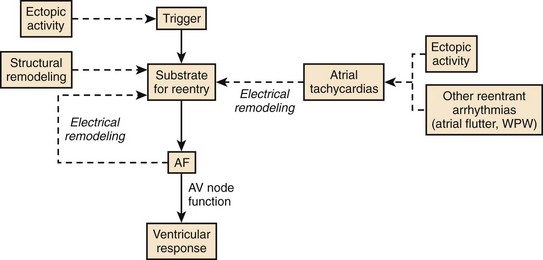
Figure 42-30 presents a synthesis of the physiological determinants of AF described earlier. Ectopic activity plays an important role by triggering re-entry and AF in the presence of a vulnerable substrate such as that created by structural remodeling. In addition, continuous atrial ectopic activity can generate ATs that cause atrial electrical remodeling, which produces a substrate for AF. Thus ectopic activity can result in both the substrate for AF (atrial remodeling) and the trigger that acts on the substrate to cause the arrhythmia. Other atrial tachyarrhythmias (including AFL, AVRT, and AVNRT) can also cause tachycardia-dependent remodeling and produce a substrate for AF, accounting (at least in part) for the occurrence of AF in association with these arrhythmias in some patients. If AF is induced by any mechanism, electrical remodeling will ensue, causing heterogeneous wavelength decreases that promote multiple circuit re-entry. Thus, electrical remodeling tends to make multiple circuit re-entry the final common pathway of AF, irrespective of the initial mechanism of the arrhythmia. As discussed earlier, even though AF may begin as macro–re-entry with fibrillatory conduction (as in some cases of experimental heart failure–related AF) or as a rapidly discharging focus with fibrillatory conduction, by the time patients get medical attention, they may already have multiple circuit AF because of remodeling.
Recent discoveries regarding AF mechanisms cast an interesting light on the theories of AF in the early twentieth century, as illustrated in Figure 42-26. Recent work suggests that all the original ideas regarding AF mechanisms are probably accurate for some subsets of patients. Furthermore, the subsequent dominance of the multiple circuit re-entry concept is also well founded, since electrical remodeling acts to make multiple circuit re-entry the final common pathway of AF in many cases. The dynamic nature of AF mechanisms needs to be considered to understand the mechanisms of the arrhythmia and to optimize therapy in any given patient.
Investigations
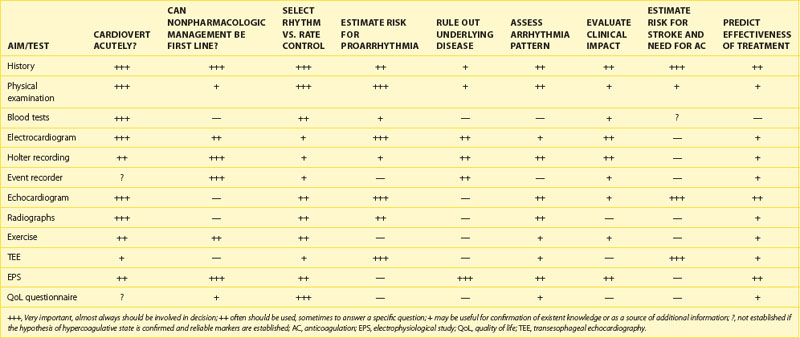
A detailed algorithm for evaluation of patients with newly diagnosed AF has been proposed by the Canadian Cardiovascular Society (CCS) and incorporates medical, family and social histories; physical examination; risk assessment; and essential investigations (Box 42-1).170
Box 42-1 Baseline Evaluation in Patients with Atrial Fibrillation Proposed by the Canadian Cardiovascular Society
From Healey JS, Parkash R, Pollak T, et al, for the CCS Atrial Fibrillation Guidelines Committee: Canadian Cardiovascular Society atrial fibrillation guidelines 2010: Etiology and initial investigations, Can J Cardiol 27(1):31–37, 2011.
History
Etiology and Pattern
Next, the duration and pattern of AF (e.g., first detected, paroxysmal, persistent, or unknown duration), the symptomatic status, and clinical impact should be assessed, and the risk of potential complications and progression should be estimated. This assessment is important for the subsequent selection of appropriate investigations from the extensive range of possible tests. The assessment allows the determination of the principal management strategies, including the restoration and maintenance of sinus rhythm (the rhythm control strategy), control of the ventricular rates, and lifelong anticoagulation (Table 42-5).
Symptoms
The ESC and CCS guidelines introduced score systems to quantify the severity of AF-related symptoms and their impact on quality of life: the European Heart Rhythm Association (EHRA) and the Severity of Atrial Fibrillation (SAF) (Table 42-6) scales.63,170 Both scales are based on a subjective perception of symptoms by the patient and include four and five grades, respectively, ranging from the asymptomatic status to severe symptoms disrupting every day activity and significantly affecting quality of life.
Table 42-6 Assessment of Symptoms Related to Atrial Fibrillation and Impact on Quality of Life by EHRA score and CCS SAF Scale
| EHRA CLASS | SYMPTOMS |
|---|---|
| EHRA I | No symptoms |
| EHRA II | Mild symptoms; normal daily activity not affected |
| EHRA III | Severe symptoms; normal daily activity affected |
| EHRA IV | Disabling symptoms; normal daily activity discontinued |
| SAF SCORE | IMPACT ON QUALITY OF LIFE |
|---|---|
| Class 0 | No symptoms |
| Class 1 | Minimal effect on quality of life |
| Class 2 | Minor effect on quality of life |
| Class 3 | Moderate effect on quality of life |
| Class 4 | Severe effect on quality of life |
CCS SAF, Canadian Cardiovascular Society; Severity of Atrial Fibrillation; EHRA, European Heart Rhythm Association.
From Camm AJ, Kirchhof P, Lip GY, et al: Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC), Europace 12:1360–1420, 2010; Healey JS, Parkash R, Pollak T, et al, for the CCS Atrial Fibrillation Guidelines Committee: Canadian Cardiovascular Society atrial fibrillation guidelines 2010: Etiology and initial investigations, Can J Cardiol 27(1):31–37, 2011.
Many cases of AF are discovered incidentally and are thought to be truly asymptomatic. However, symptoms are often not perceived as physicians tend to focus on palpitations as the most important feature of the arrhythmia, but patients are often more aware of exertional dyspnea, poor exercise tolerance and fatigue, especially if the ventricular rate is slow (see Figure 42-10).85 The true impact of the disease may be missed, and patients with longstanding, unrecognized, and untreated arrhythmia may be exposed to high risk of thromboembolic complications or tachycardia-induced left ventricular dysfunction with stroke and heart failure being the first clinical manifestations. Many patients may have both silent and symptomatic bouts of the arrhythmia, and considerable discrepancy exists between reported symptoms and documented rhythms. For example, patients may complain of episodic palpitations when rapid or highly irregular ventricular rates are present but may not be aware of their arrhythmia during slow ventricular rates (Figure 42-31). Therapeutic intervention may render AF asymptomatic or modify the patient’s perception of the arrhythmia.
Electrocardiography
12-Lead Electrocardiogram
A proper evaluation of the 12-lead ECG is vital for several reasons. Firstly, the diagnosis must be confirmed (see Figure 42-25), and the type of the arrhythmia should be differentiated, as confusion may arise from other causes of irregular pulse, for example, frequent atrial or ventricular premature beats or intermittent heart block (Figure 42-32). Conversely, a regular, relatively slow pulse can be found in patients with AFL or AT if the ventricular rate is pharmacologically slowed down (see Figure 42-32). AF may coexist with complete heart block where atrial fibrillatory activity is visible as the baseline but where the ventricular rhythm is slow and regular. Second, the presence of pre-excitation syndrome on baseline ECG determines further investigation and management. Third, evidence of left atrial enlargement during sinus rhythm, previous MI, ST-segment and T-wave changes, left ventricular hypertrophy, and axis deviation can be useful in the identification of coexistent disease and assessment of risk of recurrence. Finally, monitoring of the P-R and Q-T intervals and the QRS complex width is essential for the prevention of proarrhythmias during antiarrhythmic drug therapy.
Holter Monitoring
Ambulatory 24-hour Holter monitoring usually is useful for the diagnosis of frequent paroxysms of the arrhythmia and for the assessment of the effects of rate-slowing agents if rate control was selected as a principal management strategy (Figure 42-33). In some cases, it may provide information pertinent to the principal management of the arrhythmia, as documentation of frequent atrial premature beats or fast atrial tachycardia preceding AF may be crucial for the selection of nonpharmacologic treatment alternatives (Figure 42-34). Digital ambulatory recorders, which enable standard 12-lead configurations similar to that of the digital diagnostic-quality 12-lead ECG machines, may increase diagnostic accuracy.
Loop Recorders
When paroxysms of the arrhythmia are infrequent or when asymptomatic episodes are suspected, a loop event recorder with transtelephonic or telemetric facilities can offer long-term, continuous ECG monitoring and event-specific recording. ECG loop recorders have a retrospective (loop) memory, which continuously records and deletes the patient’s ECG. They can be both implantable and external devices and have a patient activation function that allows the patient to activate ECG storage as a result of symptoms and an auto-activation feature that allows the automatic capture of arrhythmic events. The probability of capturing the arrhythmic event varies from 65% to 88% (Figure 42-35).171 Implantable loop recorders are superior to Holter monitoring systems in the diagnosis of very infrequent arrhythmic attacks, determination of onset patterns, identification of asymptomatic arrhythmias, assessment of treatment efficacy, and detection of proarrhythmias.172
One of such devices, Reveal XT (Medtronic Inc., Minneapolis, MN) has been tested in patients who underwent pulmonary vein ablation and has been shown to identify AF recurrences with a 92% positive predictive accuracy.173 These devices can be useful in identifying AF as a cause of cryptogenic strokes when no specific risk factors are present.
Exercise Stress Test
Exercise stress test can be used for exclusion of adrenergically mediated or exercise-induced AF and for the assessment of the effectiveness of rate control therapy in patients with permanent AF (Figure 42-36). It is also generally performed in individuals with suspected CAD, especially, if type IC antiarrhythmic drug therapy is planned.
Blood Tests
Essential blood tests include a full blood count and hemoglobin, serum electrolytes, including serum potassium and magnesium levels, and the assessment of thyroid function. A lipid profile is often warranted in patients with risk factors for CAD. Of interest, several reports identified the association between low levels of high-density lipoprotein (HDL) cholesterol and risk of AF.174
Thyrotoxicosis accounts for 1% to 2% of new cases of AF but abnormal thyroid function tests can be found in 4% to 12.5% of AF patients.39 Recognition of a hyperthyroid state is important because cardioversion and long-term maintenance of sinus rhythm are unlikely as long as the underlying condition persists. Thyroid hormones have a direct positive chronotropic effect and may enhance cardiac sensitivity to catecholamines by increasing β-adrenergic receptor density. In some patients, no obvious symptoms of hyperthyroidism are present or only subtle signs of an overactive thyroid are present (apathetic hyperthyroidism), and the arrhythmia can be the only clinical marker of the disease.
Anemia may contribute to dyspnea or may precipitate atrial tachyarrhythmias. The white cell count may suggest the presence of inflammatory mechanisms precipitating the arrhythmia. Measures of the acute phase response, such as the erythrocyte sedimentation rate (ESR) and C-reactive protein, may give further guidance where inflammatory or malignant processes are suspected. Increased levels of C-reactive protein have been associated with the development of AF in the general population and with recurrent AF after cardioversion and may be a marker of the inflammatory mechanism underlying the arrhythmia but currently are only measured for research purposes.174
Imaging
Echocardiography
TTE is essential if active management is contemplated. Valve heart lesions, severe enough to cause AF, are usually evident on clinical examination but may not be discovered. Echocardiographic measurement of left ventricular hypertrophy and left ventricular dysfunction is important for the guidance of prophylactic antiarrhythmic drug therapy in patients with hypertension and CHF. The left atrial volume at the time of mitral valve opening is now routinely measured (Figure 42-37, A). Three-dimensional echocardiography allows assessing left atrial volumes at different phases of the cardiac cycle. TTE can be effectively used to assess left atrial mechanical function, but its sensitivity in diagnosis of an LAA thrombus is low.
Transesophageal echocardiography (TEE) is capable of providing high-quality images of cardiac structure and function and is the most sensitive and specific technique to detect the presence of thrombi in the left atrium and the LAA, which have been found in 5% to 15% of patients with AF prior to cardioversion (see Figure 42-37, B).175 TEE has proved to be useful in the stratification patients with AF and AFL with regard to risk of ischemic stroke and has been suggested for the guidance of elective cardioversion.176
Left Atrial Imaging
New techniques such as three-dimensional echocardiography, color tissue Doppler imaging, speckle tracking, and delayed enhancement cardiac MRI are increasingly being used to determine left atrial function and structure.177 Cardiac CT and MRI are routinely used to ascertain the anatomy of the left atrium and pulmonary veins prior to ablation and as part of image integration with real-time electroanatomic mapping during ablation (see Chapter 17). Left atrial volume and the amount of fibrosis (Utah score) have been linked to the progression of AF (see Figure 42-7) and the risk of stroke and can be used to guide the ablation strategy.68,178 These imaging techniques can potentially be used for the assessment of reversal of atrial remodeling and efficacy of therapy.
Brain Imaging
In patients with a suspected history of TIAs, CT or MRI imaging can reveal previous silent cerebral infarcts and influence the decision in favor of life-long anticoagulation (see Figure 42-37, C and D). Recently, the presence of microbleeds on Baring imaging has been linked to increased risk of subsequent ischemic and hemorrhagic strokes.179 Microbleeds are particularly significant in patients with AF on oral anticoagulation. Of note, a lower risk of hemorrhagic stroke on therapy with new anticoagulants such as dabigatran compared with warfarin can probably be explained by the fact that the latter penetrates the hemato-encephalic barrier and may have a local effect.
Electrophysiological Study
Electrophysiological study (EPS) is indicated for the identification of the specific arrhythmia mechanism, for example, in patients with suspected AVRT or AVNRT, which may trigger AF. EPS can also identify sinus node dysfunction and define the mechanism of wide QRS complex tachycardia. The mechanisms of AF, mapping, and effectiveness of therapy can be judged and are dealt with in more detail in Chapters 28, 91, and 93.
Principles of Practice
The fundamental principles of therapy of AF include (Figure 42-38): (1) risk stratification and prevention of thromboembolic complications and stroke; (2) ventricular rate control, if expedient restoration and maintenance of sinus rhythm is impossible or not contemplated (e.g., accepted AF); (3) electrical or pharmacologic restoration and maintenance of sinus rhythm; (4) selection of an appropriate long-term rhythm control strategy and identification of AF amenable to ablation; (5) identification and correction of risk factors and elimination of precipitating agents; and (6) treatment of the underlying pathology. Therapies currently available for management of AF are summarized in Table 42-7.
Anticoagulation
Pathogenesis of Stroke in Atrial Fibrillation
Absence of organized mechanical contraction of fibrillating atria with a consequent increase in atrial pressure, atrial stretch, and dilation caused by multiple pathophysiological mechanisms compensating for reduced cardiac output, generate conditions for blood stasis and thrombus formation (Figure 42-39).180 Atrial stretch results in the increased production of ANP and decreased secretion of vasopressin, which may cause hemoconcentration within the fibrillating atria. A hypercoagulable state and increased platelet activation have been found in association with AF, adding to the risk of thrombus formation. Endothelial injury and systemic inflammation are also contributory factors. The left atrium andthe LAA, particularly because of its anatomic features (distensibility and trabeculations), are the main source of cardioembolic emboli in AF.181 With TEE, left atrial thrombi can be found in about 14% of patients with AF or AFL, even if they were effectively anticoagulated, and spontaneous echocardiographic contrast can be seen in 52% of patients.176–178,181 The LAA is the source of thrombi in 80% to 90% of patients.182
Risk Stratification
Stroke
A number of models have been devised to predict the risk of stroke and the likelihood of benefit from therapy with either warfarin or aspirin. Patients with valvular AF (mitral valve stenosis or prosthetic valves) are considered high risk, independently of other risk factors, and anticoagulation is mandatory in this patients.183 Major risk factors for stroke in nonvalvular AF were identified on the basis of the pooled analysis of 1593 untreated patients from five primary-prevention trials of warfarin (known as Atrial Fibrillation Investigators’ risk stratification model) and the results from 2012 participants from the aspirin arms of the Stroke Prevention in Atrial Fibrillation I to III (SPAF) studies.184–186 Both reports have shown that hypertension, prior stroke or TIA, diabetes, and advanced age are all associated with increased risk of stroke. The SPAF investigators also introduced left ventricular dysfunction and female gender as risk factors. According to these schemes, patients with no major risk factors have a 0.9% to 1% probability of stroke, those with 1 (or 2) risk factors are at moderate risk, which is estimated at 2.6% to 5.7%, and individuals with more than 2 risk factors have a risk of stroke of 7.1% to 8.1%. All subsequent risk stratification schemes used a combination of these factors.
The most widely accepted CHADS2 scheme is an amalgamation of individual risk factors: congestive heart failure, hypertension, age >75 years, diabetes mellitus, each of which is assigned one point, and prior stroke or TIA, which is given 2 points (hence, the subscript “2”).185–187 The CHADS2 score system was designed to simplify the determination of stroke risk in general practice. Using this system, the stroke rate per 100 patient-years without anti-thrombotic therapy is expected to increase by a factor of 1.5 for each 1-point increase from 1.9 for a score of 0, to 18.2 for the highest score of 6 (Table 42-8).
Table 42-8 CHADS2 Stroke Risk Stratification in Nonvalvular Atrial Fibrillation
| HISTORY | CHADS2 RISK SCORE |
|---|---|
| Prior stroke or TIA | 2 |
| Age >75 years | 1 |
| Hypertension | 1 |
| Diabetes mellitus | 1 |
| Heart failure | 1 |
| CHADS2 SCORE | ADJUSTED STROKE RATE (%/YEAR) (95% CI) |
|---|---|
| 0 | 1.9 (1.2-3.0) |
| 1 | 2.8 (2.0-3.8) |
| 2 | 4.0 (3.1-5.1) |
| 3 | 5.9 (4.6-7.3) |
| 4 | 8.5 (6.3-11.1) |
| 5 | 12.5 (8,2-17.5) |
| 6 | 18.2 (10.5-27.4) |
CHADS2, Cardiac failure, hypertension, age, diabetes, stroke [doubled] risk stratification system; TIA, transient ischemic attack; CI, confidence interval.
From Gage BF, Waterman AD, Shannon W, et al: Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation, JAMA 285:2864–2870, 2001; and Van Walraven WC, Hart RG, Wells GA, et al: A clinical prediction rule to identify patients with atrial fibrillation and a low stroke risk while taking aspirin, Arch Intern Med 163:936–943, 2003.
Although it provides a valuable first step in identifying patients for whom anticoagulation is mandatory, the CHADS2 scheme allows a rather crude estimate of stroke risk as it does not take into consideration less validated risk factors such as gender, age between 65 and 75 years, the presence of obstructive artery disease (coronary, carotid, peripheral), chronic kidney dysfunction and proteinuria, and thyrotoxicosis. Furthermore, it underappreciates the importance of advanced age (>75 years), which is probably a more powerful risk factor than previously thought. Some of these factors have been incorporated in the CHA2DS2VASc system adopted by the ESC guidelines (Table 42-9).187
Table 42-9 CHA2DS2VASc Stroke Risk Stratification in Nonvalvular Atrial Fibrillation
| HISTORY | CHA2DS2VASC RISK SCORE |
|---|---|
| Congestive heart failure or left ventricular dysfunction | 1 |
| Hypertension | 1 |
| Age ≥75 years | 2 |
| Diabetes mellitus | 1 |
| Stroke, TIA, or thromboembolic vascular disease (CAD, CArD, PAD) | 2 |
| Age 65-74 years | 1 |
| Gender (female) | 1 |
CHA2DS2VASc, Cardiac failure, hypertension, age (doubled), diabetes, stroke (doubled); vascular disease: myocardial infarction, aortic plague, peripheral arterial disease; CAD, coronary artery disease; TIA, transient ischemic attack; CArD, complex aortic plaque disease; PAD, pulmonary artery disease.
The advantage of the CHA2DS2VASc system is that is appears to perform best identifying patients at truly low risk of stroke and those who would benefit from anticoagulation but who would be deemed as low risk by other risk stratification systems (Figure 42-40).
Although TEE is not routinely used for this purpose, it can offer further refining of risk stratification based on specific findings such as an enlarged LAA with reduced inflow and outflow velocities and thrombi and complex aortic plaque (thick >4 mm, mobile, pedunculated).188 Assessment of indexes of hypercoagulability and endothelial dysfunction or damage (e.g., von Willebrand factor, P-selectin, fibrin, D-dimer, thrombomodulin) for prediction of stroke in AF remains a research tool.
Bleeding
The efficacy of anti-thrombotic therapy for the prevention of ischemic stroke should be balanced against the risk of major hemorrhagic complications, particularly cerebral hemorrhage, which is usually fatal or significantly disabling. The risk of hemorrhagic events is related to the intensity of anticoagulation, patient age, and fluctuations of previously therapeutic international normalized ratio (INR) values caused by drug or food interactions.189 Overall, randomized trials comparing outcomes of therapy with full-dose warfarin versus placebo did not show unacceptably higher rates of major hemorrhagic complications, including intracranial bleeding, most of which occurred in patients older than 75 years.
An assessment of bleeding risk is part of patient assessment prior to initiating anticoagulation.190 Several risk stratification schemes for bleeding in AF populations have been proposed: HEMORR2HAGES and HAS-BLED (Table 42-10).191,192 The limitation of these schemes is that they share many risk factors that have also been linked to stroke.
Therapy
Aspirin and Clopidogrel
Aspirin acts by irreversibly acetylating platelet cyclooxygenase-1, thereby blocking arachidonic acid metabolism and the formation of thromboxane A2, which potentiates platelet aggregation and the release of granule contents. It is less effective when stasis and enhanced coagulation, rather than platelet activation in response to endothelial injury, are the principal mechanisms of thrombosis as occurs in the fibrillating atria or in the venous system. Consequently, therapy with aspirin reduced the risk of stroke by about 20%, with the variable magnitude of the effect, depending on the study design and dose range.193 In direct comparisons with warfarin in older adults enrolled in the Birmingham Atrial Fibrillation Treatment of the Aged (BAFTA) study, aspirin was associated with a 48% higher risk of stroke compared with adjusted-dose warfarin, whereas no differences in bleeding complications were seen.194 Aspirin alone is not recommended for the prevention of stroke, and alternative therapies (e.g., LAA occlusion devices) should be used for stroke prevention in patients who cannot take warfarin.
Clopidogrel selectively and irreversibly blocks platelet P2Y12 adenosine diphosphate (ADP) receptors and prevents ADP-induced platelet activation. A combination of aspirin with clopidogrel has been tested in the W (“W” stands for “warfarin”) arm of the Atrial fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) in 6706 high-risk patients with AF.195 The study was stopped prematurely because of the clear superiority of warfarin. A 44% increase in a composite primary endpoint of stroke, systemic embolism, MI, and vascular death and a 72% increase in any stroke associated with anti-platelet therapy were seen, whereas the likelihood of ischemic stroke was more than doubled.
However, combination therapy outperformed aspirin alone in the twin ACTIVE A study, which showed a modest, but statistically significant, 11% reduction the primary endpoint and a 28% reduction in the rate of stroke.196 Therefore, the combination therapy may be used in patients with contraindications to warfarin, although a risk of clopidogrel (as well as aspirin) resistance exists.197
Warfarin
Warfarin, a vitamin K antagonist, reduces the synthesis of biologically inactive forms of thrombin and clotting factors VII, IX and X, which requires vitamin K. Anticoagulation with adjusted-dose warfarin (target INR, 2.5; range, 2 to 3) is recommended in all patients at risk but without a contraindication. This strategy is also pertinent for patients with moderate risk on the basis of an individualized approach that balances the risks and benefits of oral anticoagulation or aspirin, but preference should be given to warfarin. Warfarin has consistently reduced the risk of ischemic stroke or systemic embolism by about two thirds compared with no treatment and by 30% to 40% compared with aspirin in high-risk patients with AF (Figure 42-41).193 The number needed to treat (NNT) to prevent one stroke has been estimated to be 100 patient-years for those at low risk (≤2% per year) compared with 25 patient-years for those at high risk (≥6% per year).
International Normalized Ratio Monitoring
Despite it proven efficacy, warfarin is substantially underused (Table 42-11). Warfarin has a narrow therapeutic window, and its management is complicated by multiple drug interactions and variations in dietary intake of vitamin K, prompting regular, and sometimes intense, monitoring of coagulation parameters and dose adjustment, and stringent control of diet, alcohol, and co-medications. Warfarin has a relatively slow onset of action (2 to 7 days), and early thrombogenic risk increases because of the depletion of natural anticoagulation proteins C and S. Intracranial bleeding is the most serious complication with warfarin, as it is associated with high mortality or disability rates.
Table 42-11 Use of Warfarin for Prevention of Atrial Fibrillation–Related Stroke in Epidemiologic Studies
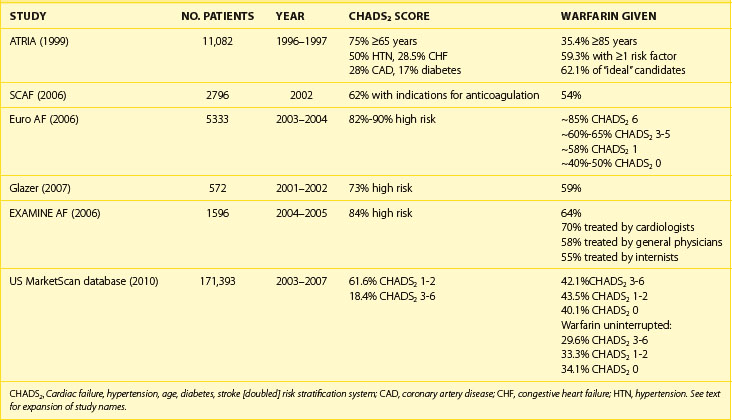
Genetic determination of the response to warfarin also exists. Patients with variant alleles (CYP2C9*2 and CYP2C9*3) of the liver cytochrome P-450 2C9 (CYP2C9) isoenzyme, which is involved in warfarin metabolism, are slow metabolizers of warfarin and can be exposed to higher concentrations of the drug and the associated risk of over-anticoagulation. Conversely, individuals with polymorphisms in the gene encoding for the vitamin K epoxide reductase complex 1 (VKORC1), which is the target protein for warfarin, exhibit increased resistance or a greater sensitivity to warfarin.198 Although it is possible to screen patients for their potential response to warfarin by using genetic testing, it is limited by associated costs and availability.
Strict INR control is essential to ascertain the highest efficacy and safety of anticoagulation with warfarin, but it is difficult to achieve for the reasons mentioned above. The intensity of anticoagulation with an INR in the range between 2 and 3 is considered to achieve maximum efficacy in the prevention of stroke and an acceptable level of risk of bleeding (Figure 42-43).199 A population-average model estimated that INR should be maintained within the therapeutic range for at least 60% of the time on therapy to ensure any benefit from anticoagulation; below this threshold, little benefit of anticoagulation exists.200 Compared with the recommended INR values, INR less than 2 confers a 3.6-fold increased risk of stroke in patients younger than 75 years and a twofold increased risk in those older than 75 years, whereas INR above 4 is associated with an exponential increase in bleeding risk. Self-monitoring of INR has a limited applicability.43
Anticoagulation in Atrial Flutter
AFL is now considered to be associated with the risk of stroke as in AF, in the presence of risk factors. This is further supported by the fact that AF often coexists with AFL. TEE studies have shown that LAA flow velocity is lower in patients with AFL compared with sinus rhythm but higher than during AF. In the Flutter Atriale Società Italiana di Ecografia Cardiovascolare (FLASIEC) study, 2.4% of patients with AFL had left atrial thrombi.201 The same criteria for anticoagulation should therefore be applied in patients with isolated AFL as recommended for AF.
New Anticoagulants
Two main avenues of research into new anticoagulants emerged: (1) oral direct thrombin inhibitors (dabigatran) and (2) factor Xa inhibitors (e.g., rivaroxaban, apixaban, edoxaban) (Table 42-12). A brief summary is provided below, and the reader is referred to Chapter 82 for a detailed treatment of the subject.
Dabigatran
The efficacy and safety of two doses of dabigatran (110 mg twice daily or 150 mg twice daily) compared with open-label warfarin has been demonstrated in the Randomized Evaluation of Long-Term Anticoagulant Therapy (RE-LY) trial with more than 18,000 patients with AF and cardiovascular and thromboembolic risk (average age, 72 years; mean CHADS score, 2.1; and history of MI [17%], stroke [20%], and CHF [32%]).202
Both doses of dabigatran performed better compared with warfarin despite tight INR control (64% of the time within the therapeutic range) (see Figure 42-41). Dabigatran 110 mg twice daily had similar efficacy for the primary endpoint of stroke or systemic embolism (event rates per year, 1.54% vs. 1.71% on warfarin; P < .001 for noninferiority) with fewer major bleeds and fewer hospitalizations, and dabigatran 150 mg twice daily had better efficacy (event rates per year, 1.11% vs. 1.71% on warfarin; P < .001 for superiority), lower mortality rate, and similar major bleeding rates. Compared with warfarin, the risk of major bleeds was 20% lower for dabigatran 110 mg twice daily (3.57% vs. 2.87% per year) but similar for 150 mg twice daily (3.57% vs. 3.32% per year). Importantly, intracranial bleeds were significantly less with both doses of dabigatran than with warfarin. However, high-dose dabigatran was associated with significantly more gastrointestinal bleeds compared with warfarin.
Consequently, the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines recommended dabigatran as a useful alternative to warfarin for the prevention of stroke in AF.203 No evidence of its performance in patients with prosthetic heart valves or hemodynamically significant valve disease exists, and the drug should not be used in these patients as well as in individuals with severe renal failure (creatinine clearance <15 mL/min), or advanced liver disease.
Factor Xa Inhibitors
Rivaroxaban proved to be at least as effective as warfarin in preventing AF-related strokes in 14,264 patients enrolled in a double-blind, double-dummy (unlike the RE-LY study)trial, the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial.204 The primary outcome of stroke or systemic embolism occurred in 1.7% in the rivaroxaban group and 2.2% in the warfarin group (P for noninferiority <.001). In the prespecified on-treatment analysis, rivaroxaban was associated with a 21% reduction in the primary endpoint events compared with warfarin (1.7% vs. 2.15%; P = .015) (see Figure 42-41). Similar to dabigatran, rivaroxaban causes fewer intracranial bleeding compared with warfarin (0.5% vs. 0.67%).
Apixaban at 5 mg twice daily significantly outperformed aspirin in the Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial of 5599 patients, who, for a variety of reasons, were not candidates for warfarin.205 Apixaban reduced the risk of stroke or systemic embolism by 55% compared with aspirin (1.6% vs. 3.7%) without an increase in the risk of major bleeding (1.4% vs. 1.2%; P = .57; Figure 42-41).
Left Atrial Appendage Occluder Devices
A meta-analysis of 23 studies in approximately 5000 subjects with rheumatic or nonrheumatic AF in settings of cardiac surgery, autopsy, or TEE, which revealed that LAA thrombi were found in 57% cases of rheumatic AF and 91% of nonrheumatic AF, has set the stage to investigate the benefit of LAA occlusion.206 LAA excision is often performed adjunct to open-heart surgery in patients with AF. LAA ligation or ectomy via thoracoscopy or a limited sternotomy is not widely used, but new tools are under development. Transcatheter devices are discussed in detail in Chapter 98. Early studies showed the feasibility and safety of a percutaneous LAA transcatheter occluder (PLAATO, ev3 Inc., Plymouth, MN).207 Successful occlusion of LAA was achieved in 90% of patients, with acceptable perioperative morbidity and mortality rates. The incidence of stroke was 2.3% per year compared with the 6.6% per year expected rates of stroke with no warfarin administration warfarin according to the CHADS2. Several other devices (see Fig. 42-43) are currently under investigation (see Chapter 98).
The performance of the Watchman device (Atritech, Plymouth, MN) against dose-adjusted warfarin has been tested in the Protection in Patients with Atrial Fibrillation (PROTECT-AF) study of 800 patients with a CHADS2 score of 1 or less.208 After implantation, warfarin was continued for 45 days to allow endothelialization of the device. Warfarin was discontinued and replaced with aspirin and clopidogrel for 6 months if TEE at 45 days showed either no or minimal flow into the LAA and no thrombus and no clinical events occurred. At 45 days, 85% of patients in the device group were able to discontinue warfarin. The primary composite endpoint of all strokes, systemic thromboembolism, and cardiovascular or unexplained death and systemic thromboembolism occurred at a rate of 3 per 100 patient-years in the device group and 4.4 per 100 patient-years in the warfarin group. However, the primary safety endpoints, which included peri-procedural events (stroke and long-term bleeding or device embolization) were more common in the device group (7.4 vs. 4.4 per 100 patient-years), with pericardial tamponade occurring in 5.5%. This has been explained by a steep learning curve; in the subsequent Continued Access Protocol (CAP) registry, the incidence of pericardial effusion decreased to 1.1%.209 The performance of the device will be further tested in several randomized controlled studies and registries (Table 42-13).