Chapter 34
Atrial Fibrillation
1. How common is atrial fibrillation (AF)?
2. What cardiovascular diseases are likely to coexist in patients with AF?
3. What arrhythmias are related to AF?
4. What are the main anatomic and physiologic substrates for the initiation of AF?
5. Which agents are effective in slowing the ventricular response in acute AF?
6. How do you decide which antithrombotic therapy is most appropriate?
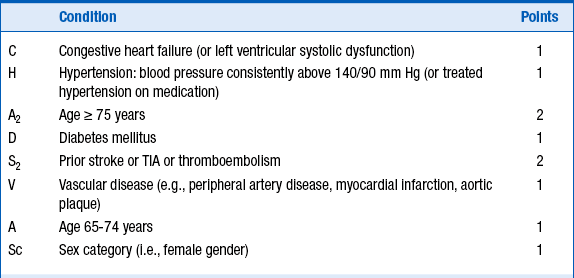
Several schemes for stratification of stroke risk can identify patients who benefit most and least from anticoagulation. The most commonly used tool as outlined in the current guidelines is the CHADS2 score for AF stroke risk scheme (Fig. 34-1), in which patients are stratified for risk according to the presence of moderate risk factors (1 point) and high risk factors (2 points). The use of appropriate anticoagulation is then decided according to the sum of points, as outlined in Table 34-1. A recently updated and modified CHA2DS2-VASc scoring system (Table 34-2) has been validated and used in patients who fall in the intermediate risk category. CHA2DS2-VASc assigns points for risk factors not included in the CHADS2 score, such as female gender, age 65 to 75, and vascular disease.
TABLE 34-1
AMERICAN COLLEGE OF CARDIOLOGY/AMERICAN HEART ASSOCIATION/EUROPEAN SOCIETY OF CARDIOLOGY 2006 GUIDELINES: RECOMMENDED THERAPIES ACCORDING TO CHADS2 STROKE RISK
| Risk Category | Recommended Therapy |
| No risk factors | Aspirin, 81-325 mg daily |
| One moderate risk factor | Aspirin, 81-325 mg daily, or warfarin (INR 2-3, target 2.5) (depending on patient preference) |
| Any high risk factor or >1 moderate risk factor | Warfarin (INR 2-3, target 2.5) |
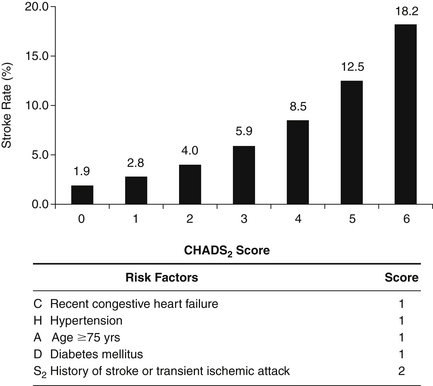
Figure 34-1 CHADS2 stroke risk scheme. The more risk factors, the higher the risk of stroke. (Modified from Rockson SG, Albers GW: Comparing the guidelines: anticoagulation therapy to optimize stroke prevention in patients with atrial fibrillation. J Am Coll Cardiol 43:929-935, 2004.)
7. Why is antithrombotic therapy so important in AF?
AF is an independent risk factor for stroke. The rate of ischemic stroke in patients with nonvalvular AF is about two to seven times that of people without AF, and the risk increases dramatically as patients grow older. Paroxysmal and chronic AF are associated with a similar risk of thromboembolism. Anticoagulation therapy is essential in patients with AF to reduce the risk of embolic stroke. Aspirin (81 or 325 mg/day) reduces the risk of thromboembolism by approximately 35%. Warfarin reduces the risk by 65%. Full-dose warfarin is also superior to low-dose (international normalized ratio [INR] 1.2-1.5) warfarin plus aspirin. The risk of a serious bleeding complication while on warfarin therapy (with an INR goal of 2-3) is 1.3% to 2.5% per year. Although the elderly may have a greater risk of bleeding, they also carry a greater risk of stroke and therefore benefit the most from warfarin therapy.
8. What are some alternatives to warfarin for anticoagulation?
9. When should cardioversion be considered to convert a patient from AF to sinus rhythm?
Cardioversion (CV) may be achieved by means of drugs or direct-current electrical shocks when the patient has new-onset AF that is less than 48 hours in duration. If the duration is more than 48 hours or unknown, the patient should be treated with anticoagulation therapy for 3 weeks before and 4 weeks after CV to prevent thromboembolism. Alternatively, a patient can undergo transesophageal echocardiography (TEE) to rule out left atrial thrombus and, if negative, proceed with CV while receiving appropriate anticoagulation. In cases of early relapse of AF after CV, repeated direct-current cardioversion attempts may be made after administration of antiarrhythmic medication. Administration of flecainide, dofetilide, propafenone, or ibutilide is recommended for pharmacologic cardioversion of AF.
10. How do you approach rate versus rhythm control issues?
11. Which antiarrhythmic drugs are most commonly used to maintain long-term sinus rhythm and prevent AF recurrence?
12. When is catheter ablation of AF indicated, and who is the ideal candidate?
13. What are potential complications of PVI?
14. What is the role of AV nodal ablation followed by permanent pacing in treating AF?
The guidelines specifically state that AV node ablation followed by permanent pacing should be used only as a fallback treatment rather than as a primary strategy, because of the risk of long-term right ventricular (RV) pacing. In general, patients most likely to benefit from this strategy are those with symptoms or tachycardia-mediated cardiomyopathy related to rapid ventricular rate during AF that cannot be controlled adequately with AADs or negative chronotropic medications. Biventricular pacing is often used with or without a defibrillator in patients that are candidates for AV nodal ablation and have a history of cardiomyopathy. Although the symptomatic benefits of AV nodal ablation are clear, limitations include the persistent need for anticoagulation, loss of AV synchrony, and lifelong pacemaker dependency.
15. What is the role of left atrial appendage (LAA) occluders in the prevention of stroke in AF patients?
Bibliography, Suggested Readings, and Websites
1. Gage, B.F., Waterman, A.D., et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870.
2. Heart Rhythm Society, HRA/ECAS expert concensus statement on catheter and surgical ablation of atrial fibrillation (AFib) 2012 Available at http://www.hrsonline.org/Practice-Guidance/Clinical-Guidelines-Documents/Expert-Consensus-Statement-on-Catheter-and-Surgical-Ablation-of-Atrial-Fibrillation-AFib/2012-Catheter-and-Surgical-Ablation-of-AFib Accessed February 11, 2013
3. Hsu, L.-F., Jaïs, P., Sanders, P., et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383.
4. Kalman, J., Kim, Y.-H., Klein, G., et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105.
5. Oral, H., Pappone, C., Morady, F., et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941.
6. Schirmer, S.H., Baumhäkel, M., Neuberger, H.R., et al. Novel anticoagulants for stroke prevention in atrial fibrillation: current clinical evidence and future developments. J Am Coll Cardiol. 2010;56:2067–2076.
7. StopAfib.org. Atrial fibrillation: For patients by patients. Available at http://www.stopafib.org. Accessed February 11, 2013
8. Van Gelder, I.C., Hagens, V.E., Bosker, H.A., et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840.
9. Wolf, P.A., Abbott, R.D., Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988.
10. Wyse, D.G., Waldo, A.L., DiMarco, J.P., et al. The atrial fibrillation follow-up investigation of rhythm management (AFFIRM Investigators). A comparison of rate control and rhythm with atrial fibrillation. N Engl J Med. 2002;347:1825–1833.