Chapter 28
Atherosclerotic Risk Factors
Diabetes
Matthew G. Nayor, Joshua A. Beckman
Based on a chapter in the seventh edition by Peter Sheehan
Diabetes is characterized by chronic hyperglycemia resulting either from a lack of insulin production (type 1) or from insulin resistance (type 2). In the last several decades, an alarming rise in the global prevalence of diabetes has been seen. The cost to the health care system is enormous because the medical expenditures of people with diabetes are two to three times higher than those of the rest of the population.1
The harmful consequences of diabetes are primarily vascular and are routinely divided into microvascular and macrovascular categories. The most important microvascular complications are retinopathy and nephropathy; people with diabetes have a 20-fold increased relative risk of blindness and a 25-fold higher relative risk of end-stage renal disease compared with people without diabetes. Macrovascular disease is characterized by atherosclerosis.2 Diabetes is an important risk factor for the development and severity of all forms of atherosclerosis, including peripheral artery disease (PAD), coronary artery disease (CAD), and cerebrovascular disease (CVD). Most of the 230,000 diabetes-related deaths in the United States every year are due to cardiovascular disease.1 Diabetes also increases the risk of ischemic stroke two- to threefold and accounts for 60% of nontraumatic lower-limb amputations.3–7 These financial and physical costs are expected to increase in the next decades as the prevalence of diabetes continues to rise worldwide.
Epidemiology
Over the last several decades, the global prevalence of diabetes has steadily increased, with an estimated 366 million people worldwide currently diagnosed with the condition. This number is predicted to reach 500 million by 2030, which equates to an annual increase in diabetes prevalence that is 1.7 times faster than the annual growth of the world’s population. In the United States, 8.3% of the population, or 25.8 million people, have diabetes and 79 million people have prediabetes (characterized by insulin resistance).2 Although the prevalence of diabetes is shifting to a younger demographic as the overall population becomes more obese, the risk of diabetes continues to increase with age, and as many as 26.9% of all Americans over the age of 65 have diabetes.1 The risk is higher for non-Hispanic blacks and Hispanic Americans than for whites or Asian Americans.1
Lifestyle changes related to increasing industrialization and economic development appear largely responsible for the dramatic rise in diabetes prevalence. Chief among these are the increasing rates of obesity, which have been attributed to a sedentary lifestyle and a Western diet rich in high-calorie food. The prevalence of obesity in the United States is increasing at an alarming rate and directly corresponds to the number of new diagnoses of diabetes. In fact, from 2000 to 2005, the rate of obesity increased by 24%. During that same time period, the prevalence of diabetes in the United States increased from 12% to 16.3%.1 Recent data, however, point to the specific role of sugar consumption, independent of obesity and other types of food intake.8 Indeed, no other food type was associated with diabetes, whereas a direct relationship was found between the amount of sugar intake and diabetes risk.
Average medical expenditures for patients with diabetes are 2.3 times higher than for those without diabetes. In 2007, the total cost of diabetes in the United States was estimated to be $218 billion. Perhaps most profound is the relationship between diabetes and cardiovascular death, which accounts for the majority of diabetes-related deaths in the United States annually.1
Classification of Diabetes
Type 1
Type 1 diabetes is characterized by an absolute deficiency in insulin secretion and accounts for 5% to 10% of diabetes diagnoses.9 It results from cellular-mediated autoimmune destruction of the pancreatic β cells and requires both genetic and environmental factors to cause the disease state. Markers of immune destruction of the β cell are present in 70% to 90% of patients and can aid in the diagnosis. These include islet cell autoantibodies, autoantibodies to insulin, anti−glutamic acid decarboxylase antibodies, and autoantibodies to tyrosine phosphatase IA-2 and IA-2β.10
Typically, type 1 diabetes presents with acute hyperglycemia or ketoacidosis as the first disease manifestation. Type 1 diabetes (previously known as “juvenile-onset” diabetes) often presents in children and adolescents but can present at any age. It also frequently develops in patients who have other autoimmune diseases such as lupus, rheumatoid arthritis, and Hashimoto’s thyroiditis.9 As a result of the absolute deficiency in insulin secretion, patients with type 1 diabetes are reliant on insulin replacement therapy.
Type 2
Type 2 diabetes results from a combination of insulin resistance and inadequate compensatory insulin secretion. It accounts for 90% to 95% of diabetes cases.9 Although type 2 was previously referred to as either “adult-onset” or “non–insulin-dependent” diabetes, these terms are less accurate because many patients eventually require insulin treatment and because type 2 diabetes can develop at practically any age. The pathogenesis of type 2 diabetes is heterogeneous, with both environmental and genetic causes. Obesity is strongly related to insulin resistance and is the most important environmental factor. Although insulin resistance is clearly necessary for the development of type 2 diabetes, incomplete compensatory rise in insulin secretion (relative deficiency) must also be present for hyperglycemia to result. This concept was illustrated by DeFronzo et al, who demonstrated that plasma insulin response to ingested glucose increases progressively until the fasting glucose concentration reaches 120 mg/dL. Thereafter, increases in fasting glucose are associated with progressive decline in insulin secretion.11 The genetic predisposition to type 2 diabetes is also well defined. The lifetime risk of developing type 2 diabetes is 40% in individuals with one parent affected and 70% if both parents are affected.12 In type 2 diabetes, hyperglycemia tends to develop slowly, and the symptoms are therefore more subtle. These include polyuria, polydipsia, weight loss, and polyphagia. People with type 2 diabetes have varying levels of insulin resistance and deficiencies in insulin secretion, and titration of different medications is often necessary to achieve appropriate glycemic control.
Diabetes and Vascular Disease
Vascular disease is the most significant cause of morbidity and mortality in people with diabetes.3 Microvascular diseases, such as retinopathy, nephropathy, and neuropathy, are associated with a direct relationship between level of hyperglycemia and disease severity; thus these diseases are more prominent in type 1 diabetes, with its long duration of hyperglycemia exposure.3 On the other hand, macrovascular complications such as CAD, PAD, and CVD, although responsible for the majority of deaths in patients with diabetes, have a modest relationship to glycemia.13
Coronary Artery Disease
Diabetes is associated with a significantly increased risk of developing CAD, and patients with diabetes and CAD have been shown to have worse outcomes. People with diabetes account for 30% of patients presenting with acute coronary syndromes, although they make up only 8% of the general population.14 Furthermore, 75% of people with diabetes die of complications related to CAD.15 People with diabetes tend to present with CAD at a younger age than patients without diabetes. It is estimated that diabetes leads to clinically evident CAD as much as 15 years earlier than otherwise expected.16 Once diagnosed with CAD, persons with diabetes have a higher risk of cardiovascular death, recurrent myocardial infarction (MI), stroke, and coronary stent thrombosis.17 The risk is further increased in patients on insulin therapy, which likely serves as a marker of disease severity. In addition, following MI, the 1-month mortality rate is 58% higher in people with diabetes.18
Despite the increased rates of cardiovascular morbidity and mortality, asymptomatic diabetic patients do not require stress testing to determine whether there is silent coronary heart disease. In the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study, 22% of patients had silent ischemia.19 The strongest predictor for silent ischemia was diabetes duration. Despite the higher rate of silent ischemia, the DIAD study made clear that conducting stress testing in asymptomatic patients with diabetes to find coronary heart disease does not reduce cardiovascular outcomes beyond standard risk factor modification.20 The results were similar in the Do You Need to Assess Myocardial Ischemia in Type-2 diabetes (DYNAMIT) trial.21 Therefore evaluation of patients with diabetes undergoing noncardiac surgery does not differ from other patient populations and should follow the 2007 American College of Cardiology/American Heart Association (ACC/AHA) guidelines, which emphasize assessment of cardiovascular symptoms and functional capacity.22
Peripheral Artery Disease
The prevalence of PAD varies significantly based on the age of the population studied. In the National Health and Nutrition Examination Survey (NHANES), the prevalence of PAD in Americans older than 40 years was 4.3%.23 However, it was 19.8% in men 65 years and older in the German Epidemiological Trial on Ankle Brachial Index (GETABI).24 Targeted screening can more clearly identify a population at risk. In the PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) trial, nearly 7000 subjects were screened in primary care practices, provided they met one of the following criteria: age 70 years or greater or ages 50 to 69 years with a history of diabetes and/or smoking.25 Using these criteria, 29% of subjects were found to have PAD. In patients with diabetes, risk of PAD is increased by older age, duration of diabetes, and presence of peripheral neuropathy. In the Edinburgh Artery Study, the prevalence of PAD was 12.5% in patients with normal glucose tolerance test, 19.9% in patients with impaired glucose tolerance testing, and 22.4% in patients with diabetes.26 Using the ABI for diagnosis, another survey found the prevalence of PAD to be 20% in people 40 years and older with diabetes, nearly 5 times that expected in patients without PAD.7 The risk of PAD is also known to be higher in African Americans and Hispanic Americans with diabetes.5
Diabetes significantly increases both the incidence and severity of limb ischemia because of several associated factors.27 The distribution of the PAD is different in patients with diabetes compared with those without it. Patients with diabetes and PAD tend to have involvement of the more distal arteries, particularly the popliteal and tibial arteries, making limb-salvage revascularization more challenging.5,6 The neuropathy that often develops in people with diabetes presents several additional challenges. First, sensory neuropathy reduces the ability to avoid injury by decreasing normal sensation and withdrawal to pain. In addition, symptoms common to advanced disease may be less appreciated and may lead to delay in diagnosis.6 Diabetic peripheral neuropathy also leads to limited joint mobility (due to motor neuropathy), decreased proprioception and pain sensation (due to sensory neuropathy), and decreased sweating (due to autonomic neuropathy). The motor neuropathy fosters the formation of a swan-neck foot deformity, which greatly increases the pressure to the ball of the foot making ulceration more likely.28,29
As a result, diabetes is the most common cause of nontraumatic lower extremity amputation in the United States, accounting for 55% of amputation-related hospitalizations.30 For people 65 to 74 years old, the risk of amputation is increased more than 20-fold compared with those without PAD and diabetes.31 The combination of PAD and diabetes is of additional clinical importance given its association with cardiovascular events. Patients with both diabetes and PAD are at extremely high risk of adverse cardiovascular events. In the Heart Protection Study (HPS), the risk of cardiovascular event over a 5-year period was 10% for people with diabetes, 20% for those with PAD, and 30% for patients with both.32
Vascular Evaluation of Patients with Diabetes
The vascular evaluation of patients with diabetes differs little from evaluation of patients without diabetes and is well reviewed elsewhere in this text. One important diagnostic consideration, however, is the increased likelihood of noncompressible pedal vessels and subsequent falsely elevated ABI results in patients with diabetes. Diabetes does provide further challenges and requires additional evaluation for a comprehensive assessment, particularly regarding risk factor assessment and the evaluation of neuropathy.
The presence of neuropathy is an important risk multiplier not seen with other risk factors. Diabetic peripheral neuropathy is characterized by a symmetric sensorimotor polyneuropathy.33 It starts distally, moves proximally, and results in a typical “glove and stocking” distribution.34 Motor deficits are rare in the early stages of diabetic peripheral neuropaty. Burning, tingling, and shooting pains are frequently described and are typically worse at night.34 Of note, the degree of pain and subjective symptoms are not reliable indicators of sensory nerve damage. Therefore careful examination and monitoring of patients with diabetes are necessary because sensory loss can exacerbate foot ulceration and lead to unintentional injuries. Careful peripheral neurologic examination is recommended annually in patients with diabetes.35 Electrophysiologic testing is rarely necessary. The examination should focus on inspection of the extremities and feet for signs of skin change, hair loss, ulceration, or increased dryness. Full sensory and motor exam should then be performed with the addition of monofilament testing plus vibration sensation (using 128-Hz tuning fork), pinprick sensation, or ankle reflexes.35
Pathophysiology of Vascular Disease in Diabetes
Diabetes leads to increased atherosclerotic vascular disease by a number of mechanisms, including metabolic derangements, hypercoagulability, inflammation, vascular dysfunction, and neuropathy. These alterations result in a phenotypic change in the blood vessel from one of homeostasis to an atherogenic phenotype characterized by endothelial cell dysfunction, oxidative stress mediated by increased production of free radicals, and vascular smooth muscle dysfunction.
Dysmetabolism and Vascular Dysfunction
The cardinal metabolic derangements in diabetes are each associated with a wide variety of insults that attenuate the vasculature’s ability to maintain equilibrium and foster an environment permissive for the development of atherosclerosis. The two fundamental derangements include hyperglycemia and insulin resistance.
Both hyperglycemia and insulin resistance are associated with atherosclerosis. Increases in the rate of atherosclerotic events begin with modest increases in fasting glucose levels in the normal range. Insulin resistance, independent of hyperglycemia, is associated with atherosclerosis and predicts cardiovascular events.36 Indeed, a majority of patients with coronary heart disease have insulin resistance or frank diabetes. In the Euro Heart Survey performed in 110 medical centers in 25 nations, 4961 subjects with coronary artery disease but no known diabetes were enrolled, and a majority of these patients were subsequently found to have diabetes, impaired glucose tolerance, or impaired fasting glucose.37 The results have been replicated in non-European populations as well.38
The impact of diabetes on the vascular endothelium represents an important link between the dysmetabolism of diabetes and the atherosclerosis that causes the majority of morbidity and mortality. The vascular endothelium plays a fundamental role in vascular homeostasis, regulating vascular tone, platelet activity, leukocyte adhesion and diapedesis, and vascular smooth muscle cell migration and proliferation.39 The endothelium regulates vascular homeostasis through the elaboration of autocrines and paracrines that modulate the structure and function of vascular cells. The endothelium-derived vasodilator, nitric oxide (NO), is constitutively produced in healthy endothelial cells by endothelial nitric oxide synthase (eNOS). The production of NO is closely adjusted by a wide variety of chemical and biomechanical stimuli. In addition to its potent vasodilatory properties, NO reduces production of proinflammatory chemokines and cytokines through inhibition of inflammatory transcription factors, which subsequently limits platelet activation. In contrast, decreased bioavailability of NO enhances an environment of vascular injury and atherogenesis.40 NO bioavailability is reduced in basic investigations, animal models, and humans with insulin resistance and frank diabetes mellitus.41–44 Endothelial dysfunction, found in both hyperglycemia and impaired endothelial insulin signaling, may link insulin resistance to its heightened risk of atherosclerosis, MI, and death. Thus endothelial dysfunction participates in the development and progression of atherosclerosis and may facilitate its adverse sequelae.
Hyperglycemia impairs vascular function through an increase in the production of reactive oxygen species (ROS), oxidative stress, and consequent impairment in endothelial function.45 Hyperglycemia-induced ROS inactivates endothelium-derived NO.45 Reduced NO bioavailability fosters atherogenesis and predicts a heightened risk of cardiovascular outcomes.46,47 Through a variety of mechanisms, hyperglycemia increases ROS production and impairs endothelial function. Hyperglycemia increases mitochondrial generation of superoxide anion, leading to cellular mitogenic pathway activation including polyol and hexosamine flux, advanced glycation end products (AGEs), protein kinase C (PKC) activation, and nuclear factor κB (NF-κB)–mediated vascular inflammation.48,49 Indeed, ROS lead to upregulation and nuclear translocation of NF-κB subunit p65 and transcription of proinflammatory genes encoding for monocyte chemoattractant protein-1 (MCP-1), selectins, vascular cell adhesion molecule-1 (VCAM-1), and intracellular adhesion molecule-1 (ICAM-1). These events facilitate adhesion of monocytes to the vascular wall and translocation into the subendothelium with subsequent formation of foam cells (Fig. 28-1).
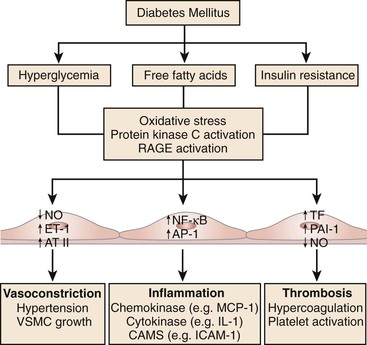
Figure 28-1 The metabolic abnormalities that characterize diabetes—particularly hyperglycemia, free fatty acids, and insulin resistance—provoke molecular mechanisms that alter the function and structure of blood vessels.
The second cardinal marker of dysmetabolism in diabetes is insulin resistance. Insulin resistance likely precedes the onset of hyperglycemia by many years. In diabetes, insulin resistance affects many tissues, including skeletal muscle, liver, adipose, and blood vessels. One possible mechanism by which insulin resistance can impair vascular function is the byproduct of the resistance on adipose tissue. Adipose is an important source of inflammatory mediators and free fatty acids (FFA).50 Obese patients with type 2 diabetes have increased plasma levels of free fatty acids and inflammatory markers.51 Impaired endothelial cell insulin signaling may also be salient. In mice, a loss of insulin signaling in the vascular endothelium leads to diminished endothelial nitric oxide synthase levels, endothelial dysfunction, expression of adhesion molecules, and atherosclerotic lesions.52 Another study confirmed the importance of endothelial insulin signaling by showing that genetic disruption of endothelial insulin receptor substrate 2 (IRS-2) reduces glucose uptake by skeletal muscle,53 whereas restoration of insulin-induced eNOS phosphorylation restored capillary recruitment as well as insulin delivery.53 These novel findings strengthen the central role of endothelium in obesity-induced insulin resistance, suggesting that blockade of vascular inflammation and oxidative stress may be a promising approach to prevent metabolic disorders. Notably, pharmacologic improvement of insulin sensitivity in patients with type 2 diabetes and metabolic syndrome is associated with restoration of flow-mediated vasodilation.54–56
The atherogenic effects of insulin resistance are also due to changes in lipid profile such as high triglycerides, low HDL cholesterol, increased remnant lipoproteins, elevated apolipoprotein B (ApoB), and small and dense LDL.57 Once circulating free fatty acids reach the liver, VLDL are assembled and made soluble by increased synthesis of apolipoprotein B. VLDL are processed by cholesteryl ester transfer protein (CETP), allowing transfer of triglycerides to LDL, which become small and dense and, hence, more atherogenic. Atherogenic dyslipidemia is a reliable predictor of cardiovascular risk, and its pharmacologic modulation may reduce vascular events in subjects with type 2 diabetes and metabolic syndrome.58–60
Platelet Dysfunction and Coagulation Cascade
Platelet dysfunction has also been shown to play a role in thrombosis, complicating atherosclerotic plaque rupture in diabetes. Glycoprotein Ib and IIb/IIIa expression is upregulated in diabetes, which leads to increased amounts of von Willebrand factor and platelet-fibrin interaction.61 Hyperglycemia also impairs calcium homeostasis, which alters calcium-dependent platelet aggregation and activation.62 Procoagulant factors (factor VIII, thrombin, and tissue factor) are increased and endogenous anticoagulants and fibrin inhibitors (thrombomodulin, protein C, plasminogen activator inhibitor 1) are decreased in a chronic hyperglycemic state.63–66 Diabetes therefore leads to increased platelet aggregation and a shift in favor of procoagulant factors of the thrombotic cascade. These alterations contribute to the propensity not only for atherosclerosis, but for pathologic plaque rupture resulting in acute coronary syndrome, ischemic stroke, and acute limb ischemia, which are known to be more common in people with diabetes.
Treatment of Patients with Diabetes And PAD
The two most important goals in the treatment of patients with PAD and diabetes are improving limb outcomes (i.e., improving claudication symptoms and preventing progression to critical limb ischemia) and decreasing morbidity and mortality from cardiovascular disease and stroke. An aggressive approach to risk factor modification and medical treatment is necessary to achieve both goals. Target-driven medical intervention can reduce the risk of cardiovascular by ≈50% in patients with type 2 diabetes.67 A sample treatment algorithm for patients with PAD and diabetes is available in Box 28-1.
Preventive Foot Care
Peripheral neuropathy, ischemia, and infection form the etiologic triad of diabetic foot complications.68 Commonly, diabetic foot ulcers and infections begin as small wounds that are not recognized and treated in the early stages because of symptoms may be masked by sensory neuropathy. Therefore careful screening and early intervention are important in preventing diabetic foot complications. The American Diabetes Association (ADA) recommends annual foot examination to identify high-risk conditions before complications develop.69 Proper foot care and hygiene are the hallmarks of preventive therapy. Areas of bony deformity or rubor should be treated with offloading and inserts as well as special footwear to prevent progression.68
Glycemic Control
The study of glycemic control and the impact on macrovascular events has occurred in two phases. The first phase was the United Kingdom Prospective Diabetes Study (UKPDS). In this study, a strategy of intensive glycemic control (hemoglobin A1c goal of 7.0% versus 7.9%) led to a 25% reduction in microvascular endpoints.13 Neither cardiovascular death nor stroke was impacted by tight control, but MI trended toward improvement. The possibility of glucose control improving cardiovascular outcomes was further supported by the Epidemiology of Diabetes Interventions and Complications study in patients with type 1 diabetes.70 Based on this work, three large trials were performed to determine whether tight glucose control (hemoglobin A1c <6.5%) was better than standard control (hemoglobin A1c of 7%). In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, a strategy of targeting a hemoglobin A1c of 6% versus 7% to 7.9% in order to reduce adverse cardiovascular events was evaluated in 10,251 patients.71 The study was stopped early because the intensive glucose-lowering strategy was found to increase cardiovascular mortality without reducing other major cardiovascular events. The Action in Diabetes and Vascular Disease (ADVANCE) study and the Veterans Affairs Diabetes Trial (VADT) both also failed to demonstrate a reduction in cardiovascular outcomes in patients with improved glycemic control.72,73 Based on the lack of success in these large trials, the ADA recommended targeting a hemoglobin A1c of 7.0%.74 Although it has not been proven to improve PAD symptoms or amputation rates, the ACC/AHA consensus guidelines on PAD treatment also support a hemoglobin A1c goal of 7.0%.75
In addition to lowering glucose, insulin-sensitizing agents have been studied in relation to cardiovascular events. Metformin, a biguanide, has demonstrated improvements in cardiovascular outcomes. In UKPDS, among obese subjects, metformin reduced diabetes-related death and all-cause mortality by 42% and 36%, respectively.3 In the 10-year follow-up of UKPDS, metformin reduced MI by 33% and mortality by 27% compared with subjects treated with sulfonylureas, despite a lack of difference in achieved glycemia after the clinical trial ended.76 In a Danish study, patients treated with insulin secretagogues had an approximate 20% increase in all-cause mortality compared with patients treated with metformin.77 Thus metformin is the recommended first-line hypoglycemic agent to be used in patients with type 2 diabetes.
However, not all insulin-sensitizing agents are beneficial.78 For example, the thiazolidinediones have a mixed cardiovascular outcomes record. In the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive), pioglitazone missed its primary cardiovascular endpoint when compared with placebo but showed benefit in its composite secondary endpoint (all-cause mortality, nonfatal MI, and stroke).79 Rosiglitazone, on the other hand, has been linked to an increased risk of MI.80 Table 28-1 briefly reviews the different classes of medications available to treat hyperglycemia.
Table 28-1
Summary of the Most Common Oral Hypoglycemic Agents
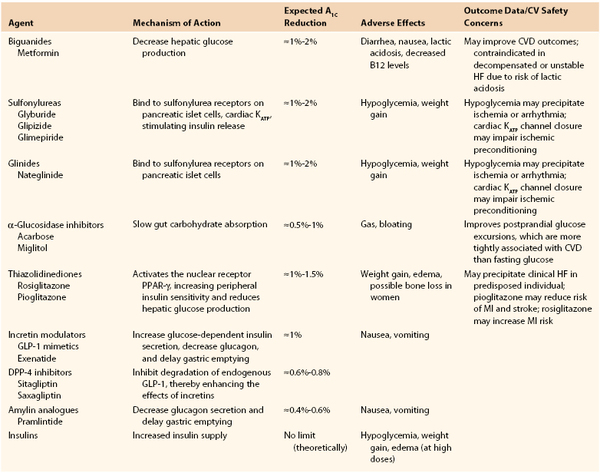
CVD, Cardiovascular disease; GLP-1, glucagon-like peptide 1; GIP-1, glucose-dependent insulinotropic peptide; HF, heart failure; MI, myocardial infarction.
Adapted from Inzucchi SE, et al: New drugs for the treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation 117:574, 2008.
With so many medication classes, the choice of agent for each patient may seem daunting. The ADA published A Consensus Algorithm for the Initiation and Adjustment of Therapy in 2009 to aid in these decisions. According to this algorithm, lifestyle interventions are the first step of treatment, followed by metformin, if still necessary. If lifestyle interventions and maximal tolerated dose of metformin do not achieve a hemoglobin A1c less than 7%, another medication should be added. Either sulfonylurea or insulin is the suggested second medications for this step. If euglycemia is still not achieved, insulin therapy should be started or intensified until goal hemoglobin A1c is achieved. The ADA therefore relegates the other oral and subcutaneous medications to a “second-tier” algorithm, which it states is appropriate in certain individualized circumstances.74
Risk Factor Control
Aggressive risk factor modification is the cornerstone for reductions in atherosclerosis-related events. Much of this therapy is discussed in other chapters in the text; here we provide diabetes-specific data for risk factor reduction.
Dyslipidemia
Numerous large-scale clinical trials have shown a significant benefit with statins in patients with diabetes and elevated LDL or even average LDL.81,82 In the Heart Protection Study (HPS), 3000 subjects with diabetes and without evidence of atherosclerotic disease at entry but with total cholesterol >135 mg/dL were randomized to simvastatin or placebo. A 34% risk reduction was observed in the combined endpoint of coronary heart disease, stroke, and revascularization in simvastatin-treated group.32 The Collaborative Atorvastatin Diabetes Study (CARDS) enrolled participants with diabetes without evidence of vascular disease but with one other cardiovascular risk factor (hypertension, retinopathy, smoking, or micro- or macroalbuminuria) and randomized these subjects to 10 mg/day of atorvastatin versus placebo.83 The mean LDL level in this trial was 117 mg/dL. The results showed a 30% reduction in the composite primary endpoint of major cardiovascular events. The most significant finding of this trial is that the benefit occurred irrespective of baseline LDL levels. Despite numerous trials and recent efforts at targeted drug development, to date, no lipid-lowering medications other than statins have been shown to reduce the incidence of stroke, MI, or cardiovascular mortality. Indeed, niacin, fenofibrate, and novel agents that raise HDL (cholesteryl ester transfer protein inhibitors) have all been shown in large-scale clinical trials to provide no benefit beyond statins in cardiovascular risk reduction.84–86
Hypertension
Control of hypertension has been shown in multiple studies to decrease the risk of macrovascular disease and death in patients with diabetes. Indeed, blood pressure control was the first treatment to show reductions in mortality. In UKPDS, “tight blood pressure control” (a mean blood pressure of 144/82 mm Hg in the treatment arm compared with 154/87 mm Hg in the control arm) was associated with a 32% risk reduction in death related to diabetes and 44% risk reduction in strokes.87 In the Appropriate Blood-Pressure Control in Diabetes (ABCD) trial, normotensive patients with diabetes were randomized to intensive diastolic blood pressure control (with a goal of decreasing the diastolic blood pressure by 10 mm Hg from the baseline value) or moderate blood pressure control.88 The intensive blood pressure control group was treated with either nisoldipine (a calcium-channel blocker) or enalapril (an angiotensin-converting enzyme [ACE] inhibitor). Intensive diastolic blood pressure control resulted in improved cardiovascular outcomes in the ACE inhibitor group and had a beneficial effect on albuminuria, retinopathy, and strokes in patients treated with either medication.89 When patients with PAD were specifically analyzed, a significant and dramatic reduction (38.7% compared with 13.6%) was seen in MI, stroke, and cardiovascular death in the patients in the intensive blood pressure control groups.90 In response to these and other studies showing similar outcomes, the goal blood pressure for patients with diabetes is less than 130/80 mm Hg.91
Further studies have shed light on the importance of the renin-angiotensin axis in patients with diabetes. ACE inhibitors and angiotensin II receptor blockers reduce the risk of progressive nephropathy in both type 1 and type 2 diabetes.92,93 In the Heart Outcomes Prevention Evaluation (HOPE) study, normotensive subjects with diabetes and known vascular disease or a vascular disease risk factor were treated with ramipril or placebo. The ramipril group had a modest drop in blood pressure and a significant decrease in death, myocardial infarction, and stroke.94 In hypertensive patients with diabetes, antagonism of the renin-angiotensin axis is especially important. In the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, patients with diabetes and hypertension were randomized to losartan or atenolol treatment. The blood pressure lowering was equivalent in both groups, but losartan reduced the incidence of cardiovascular death, stroke, and myocardial infarction by 24% and total mortality by 39%.95 The effect of ACE inhibitors and angiotensin II receptor blockers on diabetic foot ulcers and lower extremity amputations in patents with diabetes and PAD was studied by Margolis et al.96 They found significant reduction in diabetic foot ulcers and lower extremity amputations in patients treated with angiotensin II receptor blockers for 1 year compared with those who received ACE inhibitors.96 This finding requires further validation.
Despite being proven inferior to angiotensin receptor blockers in the LIFE trial, β blockers do have a continued role in patients with diabetes and CAD. Their use, historically, has been limited out of concern for masking hypoglycemia symptoms in diabetics receiving glucose-lowering treatment. However, in a retrospective study of 45,000 patients admitted to the hospital after MI, β blockers reduced the rate of death at 1 year by 23% in patients with type 2 diabetes, compared with 13% in patients without diabetes, along with no significant increase in hypoglycemia-related complications.97 Continued use of β blockers in patients with diabetes and CAD remains standard of care given their benefit for CAD-related risk reduction.
Antiplatelet Therapy
Antiplatelet therapy is a key component of secondary prevention measures in patients with diabetes and known atherosclerotic vascular disease. The Antiplatelet Trialists’ Collaboration reviewed 195 trials including >135,000 patients at high risk of vascular disease and found that platelet antagonists (mostly aspirin) reduced the risk of stroke, myocardial infarction, and vascular death.98 Based on this evidence and several older trials, in 2007 the ADA and the AHA jointly recommended aspirin therapy (75-162 mg/d) for primary prevention in patients with diabetes at increased cardiovascular risk. This included people over the age of 40, with a family history of cardiovascular disease, hypertension, smoking, dyslipidemia, or proteinuria.99 However, since that recommendation was published, two randomized controlled trials were published that cast doubt on the benefit of aspirin for primary prevention in diabetes.100,101 In response, in 2010, the ADA, the AHA, and the American College of Cardiology Foundation (ACCF) convened an expert panel to reexamine the evidence and recommendations. They concluded that the effect of aspirin for primary prevention of cardiovascular events in adults with diabetes remains unclear.102 Their recommendations were that low-dose (75-162 mg/d) aspirin use should be tailored to cardiovascular risk and that patients at low risk (men <50 years old, women <60 years old with no major additional risk factors) should not be prescribed aspirin. Aspirin “might be considered for people at intermediate risk” (younger patients with one or more risk factors or older patients with no risk factors) and aspirin use was determined “reasonable” for those adults with diabetes who are at increased CVD risk (men >50 years old, women >60 years old with one or more of the following risk factors: smoking, hypertension, dyslipidemia, family history of premature CVD, or albuminuria).102
The picture is similarly cloudy when considering antiplatelet therapy for CVD risk reduction in patients with known PAD. A subgroup of the Antithrombotic Trialists’ Collaboration meta-analysis included 9214 patients with PAD and found a significant reduction in serious vascular events with aspirin therapy.98 The 2005 ACC/AHA PAD guidelines recommend antiplatelet therapy in individuals with PAD with an “A” level of evidence.75 However, more recent data question the benefits of this therapy. A meta-analysis by Berger et al in 2009 evaluated studies with a total of 5269 participants with PAD and found only a significant but small reduction in nonfatal stroke for patients treated with aspirin, but no significant decrease in cardiovascular event rates.103 In the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes (JPAD) trial, patients with diabetes were randomized to low-dose aspirin (81-100 mg) versus placebo. No reduction was found in the risk of major cardiovascular events.101 The Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial evaluated aspirin efficacy in patients with both diabetes and PAD. The 1276 patients with diabetes and PAD (based on ABI less than 0.99) were randomized to low-dose aspirin plus antioxidant treatment, low-dose aspirin plus placebo, antioxidant plus placebo, or double placebo. No significant difference was seen among the four groups in death from coronary heart disease, nonfatal MI, stroke, above-the-ankle amputation, or critical limb ischemia.100 Although aspirin is still recommended by the ADA and ACCF/AHA PAD guidelines (updated in 2011), recent evidence has cast doubt on the previous conventional wisdom that aspirin reduces cardiovascular events in patients at risk.104 The most relevant data to date (although involving a relatively small number of patients) suggest no benefit of aspirin in prevention of cardiovascular or cerebrovascular events in patients with PAD and diabetes. Nevertheless, the clinical standard will likely be to continue aspirin in these patients pending further studies in a larger number of patients.
Another important question is whether a different antiplatelet agent should be used in PAD patients. In the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial, patients with non–ST-elevation MI, ischemic stroke, or PAD were randomized to treatment with aspirin versus clopidogrel. A subset analysis demonstrated that the 3866 patients with diabetes had a 12.5% reduction in major cardiovascular events with clopidogrel versus aspirin.105 The higher cost and increased bleeding risk with clopidogrel have limited its use for this indication. However, now that clopidogrel is available in generic form, it may find increased use in patients who are considered to be at higher risk.
Medical Treatment for Symptomatic Improvement of PAD
Once a diagnosis of PAD has been made, therapies to preserve exercise tolerance and improve symptoms should be initiated. Exercise therapy is the most beneficial intervention for these goals, and cilostazol may improve exertional capacity and symptoms as well.
Exercise Therapy
Exercise therapy is effective in improving the symptoms of intermittent claudication. Randomized controlled trials of supervised exercise training programs have repeatedly supported this benefit.106,107 The optimal exercise program, based on a meta-analysis by Gardner et al includes walking for at least 30 minutes more than three times per week for 6 months.108 In another meta-analysis of supervised exercise for PAD, Bendermacher et al found an average improvement in maximum walking time of 6.5 minutes with supervised exercise, which is superior to any medication studied to date.109 The Claudication: Exercise versus Endoluminal Revascularization (CLEVER) study randomized patients (approximately 25% of whom had diabetes) with aortoiliac PAD to optimal medical care, optimal medical care plus supervised exercise, or optimal medical care plus stent revascularization.110 The primary endpoint was a graded treadmill test at 6 months compared with baseline. The supervised exercise group had the greatest improvement in walking time but quality of life assessment by questionnaire was most improved in the stenting group. This finding will be further evaluated in the larger SUPERvised Exercise Therapy or Immediate PTA for Intermittent Claudication In Paatients with an Iliac Artery Obstruction (SUPER) Trial, which is ongoing.111 Consistent with these data, the ACC/AHA guidelines on PAD recommend a supervised exercise program as the initial treatment modality for patients with claudication with an “A” level of evidence.75 Indeed, although patients with diabetes typically have more impairment at baseline, exercise therapy increases walking distance in patients with diabetes as well as in claudicants without diabetes.112
Cilostazol
Cilostazol is a phosphodiesterase-3 inhibitor that has vasodilator, antiproliferative, and antiplatelet effects.113 Although it has not been shown to have any effect on mortality, it improves symptoms of claudication in approximately 50% of patients with and without diabetes.114 A Cochrane Review of seven randomized double-blind trials evaluating the use of cilostazol found significantly increased walking times in patients with stable intermittent claudication.115 The ACC/AHA guidelines for treatment of PAD state that cilostazol at a dose of 100 mg twice daily is an effective therapy to alleviate symptoms and improve walking distance in patients with intermittent claudication and they recommend consideration of a therapeutic trial in all patients with lifestyle-limiting symptoms.75 In one study that compared the effects in subjects with and without diabetes, cilostazol increased walking distance similarly in both groups.116 Cilostazol is generally well tolerated, with adverse effects of headaches, nausea, diarrhea, pain, infection, upper respiratory symptoms, palpitations, arrhythmias, and peripheral edema occurring in approximately 5% of people based on a meta-analysis.115 Cilostazol is contraindicated in patients with congestive heart failure or moderate to severe renal or hepatic impairment.
Pentoxifylline
Pentoxifylline is a methylxanthine derivative that acts by inhibiting phosphodiesterase and by potentiating the effects of endogenous prostacyclin.117 Numerous studies have been performed comparing pentoxifylline treatment with both placebo and other therapies. A Cochrane Review of these studies concluded that the data were poor, the results varied widely, and definitive evidence of efficacy was lacking.117 When evaluated head to head, cilostazol significantly improved walking distances compared with placebo, whereas pentoxifylline was no different from placebo.118 The ACC/AHA guidelines state that pentoxifylline “may be considered as a second-line alternative therapy to cilostazol” and agree that the clinical effectiveness of pentoxifylline has not been sufficiently proven.75 Pentoxifylline has fallen out of routine use.
Statins
Beyond their reduction of cardiovascular events, statins have also been shown to improve walking distance and reduce the progression of symptomatic claudication. In one prospective trial of patients with PAD randomized to atorvastatin 10 mg/day, atorvastatin 80 mg/day or placebo, the patients in the high-dose atorvastatin group experienced improvement in pain-free walking distance and subjective physical activity questionnaire scores.119 A retrospective analysis of the Scandinavian Simvastatin Survival Study (4S) found that patients with dyslipidemia and CAD had a 38% risk reduction in developing new or worsening claudication symptoms when treated with simvastatin.120
ACE Inhibitors
In a small randomized, double-blind, placebo-controlled trial, Ahimastos et al found that ramipril improved pain-free and maximum walking time significantly in the 20 patients assigned to the treatment arm. These authors have recently published a follow-up study randomizing 212 claudicants to ramipril or placebo. The ramipril-treated arm enjoyed significant increases in pain-free walking time, total walking time, and ankle-brachial index compared with placebo.121
Referral for Revascularization
When medical therapy fails, revascularization (either surgical or percutaneous) may be necessary. Indications for peripheral revascularization procedures for PAD are the same in patients with and without diabetes. In PAD, these include disabling claudication, critical limb ischemia (rest pain or tissue loss), or nonhealing or infected foot ulcers that are expected to benefit from revascularization. However, patients with diabetes may have foot ulcers of multifactorial etiology.
Diabetic foot ulcers, which occur in 15% of patients with diabetes, represent a difficult diagnostic and therapeutic issue for clinicians.122 The majority of foot ulcers will heal on their own, whereas 10% to 15% will remain active and 5% to 24% will eventually require amputation.123 Diabetic neuropathy is a common factor in 90% of foot ulcers.124 As discussed earlier, in patients with peripheral diabetic neuropathy, ulcer formation is accelerated by loss of sensation, bony foot deformities, decreased sweating, loss of skin integrity, and repeated minor injuries. Crucial to the management of diabetic foot ulcers is the determination of whether the ulcer is neuropathic, neuroischemic, or ischemic in origin. This is accomplished by vascular assessment and physical examination. Diabetic foot ulcers are therefore defined as (1) neuropathic, if neuropathy is present but no evidence of ischemia exists; (2) ischemic, if PAD is present but not neuropathy; and (3) neuroischemic, if neuropathy and ischemia coexist.123 The hallmarks of treatment for neuropathic ulcers are offloading of the effected limb, clean dressings, and antibiotics if signs of infection exist. Debridement also can stimulate healing in the appropriate situation.68 Neuroischemic and ischemic ulcers require revascularization if conservative measures fail.
Summary and Future Directions
Diabetes increases the risk of vascular disease, including cardiovascular, cerebrovascular, peripheral vascular, and microvascular diseases. Coronary artery disease is responsible for the majority of the deaths in patients with diabetes, but stroke, claudication, critical limb ischemia, diabetic foot ulcers, retinopathy, and nephropathy all contribute to the overall health care expenditures and morbidity in patients with diabetes. Numerous metabolic, thrombotic, and vascular derangements occur in diabetes and explain the accelerated atherosclerosis and increased rate of thrombosis characteristic of diabetic vascular disease. Treatment of PAD in patients with diabetes involves therapies to improve claudication symptoms and aggressive risk factor modification aimed at improving cardiovascular outcomes and overall mortality. As the worldwide incidence of diabetes grows, these complications will become more and more important to global health care delivery.
Selected Key References
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2012;35(Suppl 1):S64–S71.
The most recent diagnostic criteria for diabetes.
Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O’Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R. The Prevention of Progression of Arterial Disease and Diabetes (POPADAD) Trial: Factorial Randomised Placebo Controlled Trial of Aspirin and Antioxidants in Patients with Diabetes and Asymptomatic Peripheral Arterial Disease. [Diabetes Registry Group, Royal College of Physicians of Edinburgh] BMJ: British Medical Journal. 2008;337.
One of several large recent randomized trials that put the value of aspirin therapy into question for the patient with peripheral artery disease.
Group UKPDS. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ: British Medical Journal. 1998;703–713.
The first work demonstrating a mortality benefit with risk factor modification in patients with diabetes.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). [American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation] J Am Coll Cardiol. 2006;47(6):e1–e192.
A multispecialty review of the key information and recommendations for patients with peripheral artery disease.
UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
The first work demonstrating a possible link between hypoglycemic therapy and reductions in myocardial infarction.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. US Department of Health and Human Services, Centers for Disease Control and Prevention. 2011.
2. Donnelly R, et al. Vascular complications of diabetes. BMJ. 2000;320(7241):1062–1066.
3. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865.
4. Fowkes GR, et al. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh artery study. Am J Epidemiol. 1992;135(4):331–340.
5. PERIPHERAL IOF. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333.
6. Jude EB, et al. Peripheral arterial disease in diabetic and nondiabetic patients. A comparison of severity and outcome. Diabetes Care. 2001;24(8):1433–1437.
7. Elhadd T, et al. Pilot study of prevalence of asymptomatic peripheral arterial occlusive disease in patients with diabetes attending a hospital clinic. Practical Diabetes International. 1999;16(6):163–166.
8. Basu S, et al. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLos ONE. 2013;8(2):e57873.
9. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2012;35(Suppl 1):S64–S71.
10. Atkinson MA. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harbor Perspectives in Medicine. 2012;2(11).
11. DeFronzo R. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687.
12. Kobberling J, et al. Empirical risk figures for first degree relatives of non-insulin dependent diabetics. The Genetics of Diabetes Mellitus. 1982;201–209.
13. Turner R, et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
14. American Heart Association. Heart disease and stroke statistics—2004 update. American Heart Association: Dallas, Texas; 2004.
15. American Diabetes Association. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes. Diabetes Care. 1998;21(9):1551–1559.
16. Booth GL, et al. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. The Lancet. 2006;368(9529):29–36.
17. Wiviott SD, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with Prasugrel–Thrombolysis in myocardial infarction 38. Circulation. 2008;118(16):1626–1636.
18. Miettinen H, et al. Impact of diabetes on mortality after the first myocardial infarction. Diabetes Care. 1998;21(1):69–75.
19. Wackers FJT, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27(8):1954–1961.
20. Young LH, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes. JAMA: The Journal of the American Medical Association. 2009;301(15):1547–1555.
21. Lièvre MM, et al. Detection of silent myocardial ischemia in asymptomatic patients with diabetes: results of a randomized trial and meta-analysis assessing the effectiveness of systematic screening. Trials. 2011;12:23.
22. Fleisher LA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association. J Am Coll Cardiol. 2007;50(17):e159–e242.
23. Selvin E, et al. Prevalence of and risk factors for peripheral arterial disease in the United States results from the national health and nutrition examination survey, 1999-2000. Circulation. 2004;110(6):738–743.
24. Diehm C, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172(1):95–106.
25. Hirsch AT, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA: The Journal of the American Medical Association. 2001;286(11):1317–1324.
26. Fowkes F, et al. Edinburgh artery study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20(2):384–392.
27. Abbott RD, et al. Epidemiology of some peripheral arterial findings in diabetic men and women: experiences from the Framingham study. Am J Med. 1990;88(4):376–381.
28. Bus SA, et al. Intrinsic muscle atrophy and toe deformity in the diabetic neuropathic foot a magnetic resonance imaging study. Diabetes Care. 2002;25(8):1444–1450.
29. Van Schie CH, et al. Muscle weakness and foot deformities in diabetes relationship to neuropathy and foot ulceration in Caucasian diabetic men. Diabetes Care. 2004;27(7):1668–1673.
30. McDaniel MD, et al. Basic data related to the natural history of intermittent claudication. Ann Vasc Surg. 1989;3(3):273–277.
31. Diabetes-related amputations of lower extremities in the Medicare population–Minnesota, 1993-1995. MMWR Morb Mortal Wkly Rep. 1998;(47):649–652.
32. Yusuf S. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Commentary. Lancet. 2002;360(9326):7–22.
33. Tesfaye S, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293.
34. Tesfaye S, et al. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res. 2012;28:8–14.
35. Executive summary: Standards of medical care in diabetes—2013. Diabetes care. 2013;36(Suppl 1):S4–S10.
36. DeFronzo R. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard lecture 2009. Diabetologia. 2010;53(7):1270–1287.
37. Bartnik M, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe: the Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004;25(21):1880–1890.
38. Tamita K, et al. Newly diagnosed glucose intolerance and prognosis after acute myocardial infarction: comparison of post-challenge versus fasting glucose concentrations. Heart. 2012;98(11):848–854.
39. Verma S, et al. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108(17):2054–2059.
40. Moncada S, et al. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012.
41. Fujii K, et al. Effect of diabetes mellitus on flow-mediated and endothelium-dependent dilatation of the rat basilar artery. Stroke. 1992;23(10):1494–1498.
42. Altan V, et al. The effects of type-1 and type-2 diabetes on endothelium-dependent relaxation in rat aorta. Pharmacology Biochemistry and Behavior. 1989;33(3):519–522.
43. Beckman J, et al. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103:1618–1623.
44. Steinberg HO, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601.
45. Creager MA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108:1527–1532.
47. Lerman A, et al. Endothelial function cardiac events. Circulation. 2005;111(3):363–368.
48. Nishikawa T, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790.
49. Giacco F, et al. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070.
50. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–176.
51. Cavelti-Weder C, et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35(8):1654–1662.
52. Rask-Madsen C, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell metabolism. 2010;11(5):379–389.
53. Kubota T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell metabolism. 2011;13(3):294–307.
54. Vitale C, et al. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med. 2005;258(3):250–256.
55. Naka KK, et al. Rosiglitazone improves endothelial function in patients with type 2 diabetes treated with insulin. Diabetes and Vascular Disease Research. 2011;8(3):195–201.
56. Wang T, et al. Relation of improvement in endothelium-dependent flow-mediated vasodilation after rosiglitazone to changes in asymmetric dimethylarginine, endothelin-1, and C-reactive protein in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2006;98(8):1057.
57. Zhang J, et al. Apolipoprotein AI and B levels, dyslipidemia and metabolic syndrome in south-west Chinese women with PCOS. Human reproduction. 2012;27(8):2484–2493.
58. Lee M, et al. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: a meta-analysis. Atherosclerosis. 2011;217(2):492–498.
59. Fruchart J, et al. The residual risk reduction initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102(10):1K–34K.
60. Arca M, et al. Usefulness of atherogenic dyslipidemia for predicting cardiovascular risk in patients with angiographically defined coronary artery disease. Am J Cardiol. 2007;100(10):1511–1516.
61. Vinik AI, et al. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24(8):1476–1485.
62. Li Y, et al. Platelet hyperactivity and abnormal Ca2 homeostasis in diabetes mellitus. American Journal of Physiology-Heart and Circulatory Physiology. 2001;280(4):H1480–H1489.
63. Ceriello A, et al. Hyperglycemia-induced thrombin formation in diabetes: the possible role of oxidative stress. Diabetes. 1995;44(8):924–928.
64. Hafer-Macko CE, et al. Thrombomodulin deficiency in human diabetic nerve microvasculature. Diabetes. 2002;51(6):1957–1963.
65. Pandolfi A, et al. Plasminogen activator inhibitor type 1 is increased in the arterial wall of type II diabetic subjects. Arterioscler Thromb Vasc Biol. 2001;21(8):1378–1382.
66. Ren S, et al. Impact of diabetes-associated lipoproteins on generation of fibrinolytic regulators from vascular endothelial cells. Journal of Clinical Endocrinology & Metabolism. 2002;87(1):286–291.
67. Gæde P, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393.
68. Kalish J, et al. Management of diabetic foot problems. Journal of Vascular Surgery. 2010;51(2):476–486.
69. Mayfield JA, et al. Preventive foot care in diabetes. [American Diabetes Association] Diabetes Care. 2004;27(Suppl 1):S63–S64.
70. Nathan D, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643.
71. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559.
72. ADVANCE Collaborative Group, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572.
73. Abraira C, et al. Glycaemic separation and risk factor control in the veterans affairs diabetes trial: an interim report. Diabetes, Obesity and Metabolism. 2009;11(2):150–156.
74. Nathan DM, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203.
75. Hirsch AT, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A. J Am Coll Cardiol. 2006;47(6):e1–e192.
76. Holman RR, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589.
77. Schramm TK, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32(15):1900–1908.
78. Inzucchi SE, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379.
79. Dormandy JA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macroVascular events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289.
80. Nissen SE, et al. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471.
81. Goldberg RB, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. Circulation. 1998;98(23):2513–2519.
82. Pyörälä K, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: a subgroup analysis of the Scandinavian simvastatin survival study (4S). Diabetes Care. 1997;20(4):614–620.
83. Colhoun HM, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. The Lancet. 2004;364(9435):685–696.
84. Boden W, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. NEJM. 2011;365(24):2255–2267.
85. Barter PJ, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122.
86. Tonkin AM, et al. Effects of combination lipid therapy in the management of patients with type 2 diabetes mellitus in the action to control cardiovascular risk in diabetes (ACCORD) trial. Circulation. 2010;122(8):850–852.
87. Group UKPDS. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ: British Medical Journal. 1998;703–713.
88. Estacio RO, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338(10):645–652.
89. Schrier RW, et al. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61(3):1086–1097.
90. Mehler PS, et al. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation. 2003;107(5):753–756.
91. American Diabetes Association. Treatment of hypertension in adults with diabetes (position statement). Diabetes Care. 2003;(26):S80–S82.
93. Parving HH, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878.
94. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259.
95. Dahlöf B, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. The Lancet. 2002;359(9311):995–1003.
96. Margolis DJ, et al. The differential effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with respect to foot ulcer and limb amputation in those with diabetes. Wound Repair and Regeneration. 2010;18(5):445–451.
97. Chen J, et al. Beta-blocker therapy for secondary prevention of myocardial infarction in elderly diabetic patients: results from the national cooperative cardiovascular project. J Am Coll Cardiol. 1999;34(5):1388–1394.
98. Trialists’ Collaboration A. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
99. Buse JB, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30(1):162–172.
100. Belch J, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;331:a1840.
101. Ogawa H, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes. JAMA: the Journal of the American Medical Association. 2008;300(18):2134–2141.
102. Pignone M, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. J Am Coll Cardiol. 2010;55(25):2878–2886.
103. Berger JS, et al. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease. JAMA: the Journal of the American Medical Association. 2009;301(18):1909–1919.
104. Rooke TW, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2011;58(19):2020–2045.
105. Bhatt DL, et al. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol. 2002;90(6):625.
106. Leng G, et al. Exercise for intermittent claudication (Cochrane review). The Cochrane Library. 2000;1:12–15.
107. Larsen O, et al. Effect of daily muscular exercise in patients with intermittent claudication. Lancet. 1966;2(7473):1093.
108. Gardner A, et al. Exercise rehabilitation programs for the treatment of claudication pain: a meta-analysis. ACC Current Journal Review. 1996;5(2):31.
109. Bendermacher B, et al. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2006;2(2).
110. Murphy TP, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125(1):130–139.
111. Frans F, et al. SUPERvised exercise therapy or immediate PTA for intermittent claudication in patients with an iliac artery obstruction–a multicentre randomised controlled trial; SUPER study design and rationale. Eur J Vasc Endovasc Surg. 2012;43(4):446–471.
112. Van Pul KM, et al. Effect of supervised exercise therapy for intermittent claudication in patients with diabetes mellitus. Annals of Vascular Surgery. 2012;26(7):957–963.
113. Vodnala D, et al. Medical management of the patient with intermittent claudication. Cardiol Clin. 2011;29(3):363.
114. Pande RL, et al. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vascular Medicine. 2010;15(3):181–188.
115. Robless P, et al. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev. 2008;1.
116. Rendell M, et al. Cilostazol treatment of claudication in diabetic patients. Current Medical Research and Opinion. 2002;18(8):479–487.
117. Salhiyyah K, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2012.
118. Dawson DL, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109(7):523.
119. Mohler ER III, et al. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108(12):1481–1486.
120. Pedersen TR, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). Am J Cardiol. 1998;81(3):333–335.
121. Ahimastos AA, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication. A randomized controlled trial ramipril among patients with PAD and claudication. JAMA. 2013;309(5):453–460.
122. Paulose-Ram R, et al. Lower extremity disease among persons aged > or = 40 years with and without diabetes, United States, 1999-2002. MMWR Morb Mortal Wkly Rep. 2005;54:1158–1160.
123. Alexiadou K, et al. Management of diabetic foot ulcers. Diabetes therapy: research, treatment and education of diabetes and related disorders. 2012;3(1):4.
124. Tesfaye S, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM complications study. Diabetologia. 1996;39(11):1377–1384.