CHAPTER 51 Atherosclerotic Coronary Artery Disease
Imaging of coronary atherosclerosis has depended on coronary angiography as the gold standard since Sones and Shirey developed the technique at the Cleveland Clinic in the 1960s.1 As pathologic studies have described the stages of atherosclerosis and identified underlying plaque components in areas of thrombosis, the limitations of angiography, which outlines the vessel lumen, have emerged. Currently, we are on the brink of new methods of coronary imaging. Noninvasive transthoracic imaging now rivals angiography for the detection of luminal stenosis, and new catheter-based techniques such as intravascular ultrasound (IVUS) promise to identify the lipid component of plaques. Although the in vivo demonstration of vulnerable plaques, allowing for prevention of plaque progression, is yet elusive, it may be only a matter of time before coronary angiography is replaced by noninvasive techniques as the primary tool for coronary atherosclerotic imaging.
PREVALENCE AND EPIDEMIOLOGY
Coronary atherosclerosis is prevalent in developed countries, with obstructive lesions occurring frequently in individuals older than 50 years. The prevalence is affected by age, gender, genetic predisposition, and acquired risk factors. Although there is a downward trend in the prevalence of heart disease (largely caused by coronary atherosclerosis), it remains the leading cause of death in the United States. The prevalence of coronary heart disease from 1999 to 2004 in the U.S. population, as estimated by the National Center for Health Statistics and National Heart, Lung and Blood Institute, was 22.8% for men and 15.4% for women 60 to 79 years old. Approximately 10% of men 50 to 70 years old who die of noncardiac causes have obstructive coronary lesions at autopsy.2
ETIOLOGY AND PATHOPHYSIOLOGY
Atheromatous Plaque Progression
The classic stages of plaque progression, as defined by the American Heart Association (AHA), involve the formation of the necrotic core. A more recent modification of this staging schema3 outlines development of atheroma. The normal human intima contains a small cushion of smooth muscle cells and matrix, which is present from birth and accentuated at branch points; this lesion has been termed adaptive or diffuse intimal thickening. Later in life, with intimal injury, there is influx of lipids, much of which is oxidized; at this stage (pathologic intimal thickening), there is typically influx of small numbers of foamy macrophages, generally luminal in location to the lipid pools. The progression of the pathologic intimal thickening to fibroatheroma (AHA class II-III) is key in the development of lipid-rich plaques that result in symptoms owing to tendency to rupture and thrombose. The features of fibroatheroma include a core of necrotic material (Fig. 51-1), which is composed of apoptotic cell debris–derived smooth muscle cell membranes, macrophages, and red blood cells. Much current imaging technology is focused on the identification of necrotic material because this is the precursor lesion of the most common substrate for coronary atherothrombosis.
Thin Cap Atheroma and the Vulnerable Plaque
The expansion of the atheroma, or necrotic core of the lipid-rich atherosclerotic plaque, results in thinning of the overlying fibrous cap (see Fig. 51-1). An arbitrary fibrous cap thickness of 65 µm has been designated as the criterion for the “vulnerable” plaque or thin cap fibroatheroma.4 The characteristics of thin cap atheroma that render it prone to rupture are unknown, but likely involve physical factors, such as size of the necrotic core, and biologic factors, such as proteolytic enzyme activity within the thinned cap. The goal of imaging to detect thin cap fibroatheroma and distinguish it from fibroatheroma with thicker caps remains elusive, but may eventually enable the prophylactic treatment of lesions prone to rupture with the aim of preventing atherothrombosis.
Nonatheromatous Plaque Progression
In autopsy studies, approximately 15% of patients who die of coronary artery disease (CAD) lack lipid-rich atheromatous lesions. Nevertheless, these patients develop significant luminal narrowing, and luminal thrombus may occur by mechanisms other than rupture of the thin fibrous cap. The major alternative mechanism of atherothrombosis (Fig. 51-2), termed plaque erosion, is of unclear etiology.5 Erosion may result from endothelial injury that results directly in thrombosis, either via coronary spasm or via interaction with matrix receptors. Because plaque erosion thrombi may be small and nonocclusive, they result in layering of smooth muscle cell–rich plaque that expands without a requisite necrotic core, often with little outward expansion (positive remodeling). Although the goal of imaging remains the detection of lipid-rich plaques, a subset of potentially lethal lesions are not detected by modalities that target lipid.
Life Cycling of the Atherosclerotic Plaque
Because coronary imaging seeks to identify lipid-rich plaques, especially plaques with large necrotic cores, mention of the life cycle of lipid-rich lesions and the effect of successive ruptures on plaque composition is relevant. One outcome of plaque rupture, after slowly enlarging core and thinning of the fibrous cap, is occlusive thrombus that organizes as a total occlusion and results in acute coronary syndrome. Much more common after acute rupture is a subocclusive thrombus with healing. The outcome of subocclusive plaque rupture is often plaque enlargement and outward expansion (remodeling) of the arterial wall. The thickening of the fibrous cap that occurs after healed rupture is roughly represented by the AHA class V plaque. Autopsy studies have shown multiple old rupture sites at sites of acute rupture, resulting in compartmentalization of the plaque into areas of lipid core and fibrous healing.6 The site of future rupture may not have a single large lipid compartment, but a heterogeneous makeup that includes fibrous tissue. The consequences for imaging are unclear at this time.
Calcification and Coronary Atherosclerosis: Implications for Imaging
Calcification is a reliable marker for the presence of atherosclerotic plaque because there are no other processes in the coronary arteries that result in calcium deposition. Calcium scoring is a reliable marker for atherosclerotic plaque burden, although in younger patients severe obstructive lesions can occur in the absence of any calcification (see Chapter 32). Calcification is not a marker of plaque instability, however. Solid calcification can be the result of diffuse deposition in fibrous tissue in stable plaques, whereas more stippled calcification, reflective of necrotic core calcification in more heterogeneous plaques, may be more suggestive of an unstable plaque.7
MANIFESTATIONS OF DISEASE
Clinical Presentation
The major clinical presentations of coronary atherosclerosis are stable angina, acute coronary syndromes (ST segment elevation MI and non–ST segment elevation MI), congestive heart failure, and sudden death. Acute coronary syndromes are discussed Chapter 52. Coronary lesions in sudden death are quite heterogeneous because the mechanisms of ventricular arrhythmias vary. In stable angina, there is generally stable plaque with significant obstruction, whereas unstable syndromes are characterized by intraluminal thrombus. In unstable angina or non–ST segment elevation MI, typically nonocclusive thrombi are present, whereas ST segment elevation MI is associated with larger, often occlusive thrombi. Coronary artery lesions in ischemic cardiomyopathy are typically diffuse and multiple, and there is significant myocardial damage generally with transmural healed MI.
Imaging Indications and Algorithm
Currently, angiography is the gold standard for imaging atherosclerotic coronary disease, and other imaging techniques are investigational. CT scanning for coronary calcium is used as identification for coronary risk (see Chapter 32), and IVUS is indicated for the evaluation of graft vascular disease in heart transplant recipients.
The indication for coronary angiography varies by clinical setting. The AHA has set forth algorithms for coronary angiography that are stratified by specific patient groups.8 Patients in whom coronary arteries should be evaluated by angiography include patients with symptoms of stable angina, nonspecific chest pain, unstable angina, recurrence of ischemia after revascularization, and acute MI, and patients undergoing perioperative risk assessment for noncardiac surgery. Patients with valvular heart disease in whom surgery is contemplated, patients undergoing cardiac surgery for other indications including congenital heart disease, and patients with unexplained congestive heart failure may also be candidates for coronary angiography. There are no currently accepted indications for coronary artery imaging other than angiography, although noninvasive modalities, especially multislice CT, are on the brink of being established as a viable option for the identification of coronary artery stenoses (see later).
Imaging Technique and Findings
Angiography
Catheter-based invasive coronary angiography is the gold standard for diagnosing significant (≥50% diameter stenosis) CAD. Despite various, less invasive tests to detect CAD, including stress imaging studies, 35% of patients referred for catheter-based angiography have no significant stenoses, stressing the need for better noninvasive tests. The rate of false-negative results of coronary angiography is not well appreciated, however; one of the few anatomic correlations showed that angiography significantly underestimates lesions in 44% of cases.9 Degree of stenosis as measured by coronary CT angiography shows little correlation between functional severity as measured by fractional flow reserve.10 These findings suggest that angiography is not ideal for identifying coronary stenosis, and that the mere detection of luminal narrowing by even more sophisticated methods may not be as important as functional studies or imaging techniques that show lesion characteristics.
The need to identify functional characteristics of coronary lesions is borne out by follow-up studies. Although the incidence of progression of thin cap atheroma is unknown, at least 6% of patients undergoing percutaneous coronary intervention develop progression of disease requiring intervention for a different target lesion within 1 year.11 The major predictors of plaque progression are multivessel disease, prior percutaneous coronary intervention, and age younger than 65 years. It has also been shown that 17% of acute MI patients with multiple complex angiographic lesions undergo percutaneous coronary intervention of nonculprit lesions within 1 year.12 These data underscore the need for identifying plaques in high-risk patients who would benefit from prophylactic intervention.
Ultrasonography
Conventional IVUS has limited use in de novo coronary atherosclerosis because its resolution of 150 to 300 µm is inadequate for the visualization of plaque components, although the current consensus document on IVUS describes the classification of atherosclerotic plaque based on the echogenicity.13 “Soft plaques” have a low echogenicity and represent high lipid content with or without a necrotic core, whereas “fibrous plaques” have an intermediate echogenicity between soft (echolucent) and high echogenic calcific plaques, which is attributable to a fibrous tissue. Vessel calcification appears as high echogenicity, which causes distal shadowing and obscures visualization of the intima, especially if the calcification is superficial. IVUS is considered more accurate than angiography in the detection of overall coronary plaque burden, a capability that renders it useful to assess progression of disease, an end point used in clinical trials.14
There are several methods of potentially improving the imaging resolution of IVUS to detect lipid-rich plaques and possibly even thin cap fibroatheroma. The use of radiofrequency back-scattered ultrasound signals (IVUS-IB) allows for the detection of lipid-rich plaque, which has been shown to decrease after short-term aggressive statin therapy.15 Similarly, virtual histology IVUS (IVUS-VH) refers to spectral analysis of ultrasound back-scatter radiofrequency signals.16 After the spectral signature of four tissue types (fibrous, fibrofatty, necrotic core, and calcium) is determined (Fig. 51-3), the data are programmed into software. The technique has been validated ex vivo and in vivo, using directional coronary atherectomy specimens. IVUS-VH image acquisition requires ECG gating and motorized catheter pullback, ideally at 0.5 mm/s.
Currently, IVUS-VH cannot distinguish thin cap fibroatheromas based on measurements of cap thickness, although IVUS-derived thin cap fibroatheromas have been described as focal, necrotic core–rich plaques in contact with the lumen and a percent atheroma volume of 40% or greater.16 IVUS-VH defines plaque rupture as “a ruptured capsule with an underlying cavity or plaque excavation by atheromatous extrusion with no visible capsule.”17 Based on these criteria, the numbers and distribution of thin cap fibroatheromas and acute plaque ruptures have been estimated in patients with acute coronary syndromes and stable angina pectoris.17
Computed Tomography
Electron-beam CT was established in the 1980s as a method to detect coronary calcium (see Chapter 32). Current techniques of multidetector CT coupled with angiography (coronary CT angiography) allow the imaging of arteries to detect luminal plaque (Fig. 51-4).
In studies of high-risk patients, multidetector CT has been compared with angiography in the detection of significant coronary stenoses (Fig. 51-5). Using the 17-coronary segment model of the AHA, the number of evaluable segments was greater than 95%, with a sensitivity of 90%, specificity of 95%, positive predictive value of 80%, and negative predictive value of almost 99%.18 Plaque quality, quantity, and morphology such as remodeling have also been assessed by multidetector CT compared with IVUS data.19,20 Because of the improvement in multidetector CT technology, plaque characterization is becoming possible. At this point, multidetector CT to assess plaque composition, specifically thin cap atheroma, is still experimental, however.
Magnetic Resonance Imaging
Challenges to MRI of coronary arteries are similar to the challenges of multidetector CT, and include small caliber of the vessels, cardiac and respiratory motion artifact, vessel tortuosity, and signal differential from fat and myocardium. Coronary MRI is currently limited to evaluation of proximal and mid vessel segments, which are the sites of more than 95% of unstable coronary plaques, as found in autopsy studies.3 Motion artifact affecting signal resolution is almost twice as great for the right compared with the left coronary artery and is minimal immediately before atrial contraction. In contrast to multidetector CT, there is no limitation because of gantry rotation, and gating can be tailored to the individual patient; signal duration is 80 ms for 60 to 70 beats/min. Respiratory motion can be limited by breath-holding, averaging for free breathing and navigator gating. The spatial resolution is theoretically similar to angiography at approximately 200 to 500 µm, but is limited by signal-to-noise ratio and duration of signal acquisition.
There is wide variation in reports of coronary MRI compared with angiography. In a multicenter trial using navigator-gated three-dimensional MR angiography, Kim and colleagues21 reported an overall accuracy of 72% for diagnosing CAD. They reported 100% sensitivity for detection of left main or three-vessel coronary disease, and 100% negative predictive value for diagnosing left main and three-vessel coronary disease. Most segmental data suggest, however, that the predictive values for coronary MR angiography are not as high as with multidetector CT, and there are insufficient data to support clinical coronary MR angiography for the routine identification of coronary disease for chest pain or screening high-risk patients. One exception may be in patients with presumed nonischemic cardiomyopathy, in whom obstructive coronary lesions are excluded using cardiac MR angiography in conjunction with absence of segmental abnormalities on perfusion and viability studies.
Cardiac MR angiography shows promise in the detection of coronary artery bypass graft obstruction, which is excluded by two contiguous transverse images that are patent along the expected course (signal void for spin-echo and bright signal for gradient-echo). There is some evidence that there is less calcification-induced artifact in the detection of significant stenoses compared with multidetector CT.22 Intracoronary stents result in image-degrading signal voids, which depend on the stent material and MRI sequence; stainless steel stents have prominent artifacts, in contrast to tantalum stents, which are not yet approved for coronary use. Assessment of blood flow and direction proximal and distal to the stent provides indirect evidence of stent patency.
Nuclear Medicine
PET/CT is typically used for tumor imaging by tagged metabolites such as 18FDG (18FDG-PET). 11C radiopharmaceuticals can also be used, but because of the short half life of 11C (20 minutes), it is mostly restricted to research settings. The standard applications are oncologic, in the imaging detection of metabolically active tumors and metastases, and neurologic, in the diagnosis of dementias and seizure foci. 18FDG-PET for oncologic use has shown uptake in the aorta and carotid plaques in areas of atherosclerotic inflammation and in vascular grafts.23 There are limitations for the use of 18FDG-PET or hybrid 18FDG-PET/CT in coronary imaging, however, because of a lack of resolution for smaller vessels and the high myocardial uptake of 18FDG. Other positron-emitting tracers, such as radiolabeled ligands that recognize macrophage receptors, including the benzodiazepine receptor ligand [3H]PK 11195, also show promise in imaging atherosclerotic plaques.
Investigational Catheter-Based Imaging Techniques
Emerging technologies with the greatest resolution for coronary imaging are catheter-based. In addition to IVUS-VH, optical coherence tomography (OCT) offers resolution of 10 µm. OCT measures back-reflection of infrared light, analogous to IVUS, which measures reflected sound. OCT provides an image with high resolution, and is highly sensitive to the presence of lipid, including lipid within foam cell macrophages. Because of the high resolution, thin cap fibroatheroma would be expected to be detected by this modality. Acquisition rates are high, and there are no transducers within the catheters. Attenuation by blood and surface foam cells and lack of penetration of deeper regions of plaque remain obstacles to the current use of OCT as a diagnostic tool. Scattering of the signal because of blood necessitates displacing blood during imaging.24
Near-infrared spectroscopy (NIRS) (Fig. 51-6) is a technique that can be adapted to a catheter-based system. In contrast to OCT, these wavelengths are able to penetrate blood, can scan the circumference of the artery, and are performed at a sufficient speed to overcome interference owing to cardiac motion. The resulting spectra can be processed by an algorithm to map out lipid-rich segments of the plaque. NIRS catheter-based systems for the detection of lipid-rich plaque are currently investigational.
TREATMENT OPTIONS
Medical
Medical therapy for CAD includes risk factor modification, lipid lowering with statin drugs, and treatment for comorbid conditions such as hypertension and diabetes. Patients with ischemic cardiomyopathy are treated for congestive heart failure. Acute MI is treated with thrombolytic therapy (see Chapter 52).
Surgical/Interventional
Invasive treatment for coronary atherosclerosis includes thrombolytic therapy with stenting for acute MI and bypass surgery (see Chapter 52). For patients with stable disease, percutaneous intervention with stenting is typically the first-line treatment, especially in patients with focal stenosis and absence of left main disease. There is a current debate between use of bare metal stents and drug-eluting stents; the latter are approved for limited indications, and used on an off-label basis for acute coronary syndromes, distal vessel stenoses, and left main disease. There is no question that drug-eluting stents result in decreased rates of restenosis; however, there may be a slight increase in late stent thrombosis after 1 year compared with bare metal stents.
It remains to be seen if novel plaque imaging technologies allow for stratification of patients who would benefit best from one type of stent versus another—drug-eluting stent versus bare metal stent. Pathologic studies have shown that restenosis and late stent thrombosis rates may be affected by underlying plaque morphology, especially the degree of inflammation and necrotic core within the intima.25,26
Coronary artery bypass graft surgery has been a standard surgical treatment for CAD, especially with multivessel and left main disease. Newer technologies that minimize morbidity include off-pump methods and minimally invasive surgery. There are similar survival rates between percutaneous intervention and bypass surgery in patients with three-vessel disease.27,28
KEY POINTS
 Although angiography remains the gold standard for identifying stenosis in coronary arteries, CT angiography provides an alternative noninvasive approach in selected patients with similar sensitivity and specificity.
Although angiography remains the gold standard for identifying stenosis in coronary arteries, CT angiography provides an alternative noninvasive approach in selected patients with similar sensitivity and specificity. Identification of stenoses in itself does not predict future unstable events (i.e., thrombosis). The goal of coronary artery imaging is to identify the “vulnerable plaque.”
Identification of stenoses in itself does not predict future unstable events (i.e., thrombosis). The goal of coronary artery imaging is to identify the “vulnerable plaque.” Nuclear medicine technologies including PET and SPECT detect functional deficit caused by CAD, but they do not provide anatomic details of coronary stenoses.
Nuclear medicine technologies including PET and SPECT detect functional deficit caused by CAD, but they do not provide anatomic details of coronary stenoses.Ben-Haim S, Israel O. PET/CT for atherosclerotic plaque imaging. Q J Nucl Med Mol Imaging. 2006;50:53-60.
Burke AP, Joner M, Virmani R. IVUS-VH: a predictor of plaque morphology? Eur Heart J. 2006;27:1889-1890.
Burke AP, Virmani R, Galis Z, et al. 34th Bethesda Conference: Task force #2—what is the pathologic basis for new atherosclerosis imaging techniques? J Am Coll Cardiol. 2003;41:1874-1886.
Chien C, Feng YF, Arora RR. Advances in computed tomography-based evaluation of coronary arteries: a review of coronary artery imaging with multidetector spiral computed tomography. Rev Cardiovasc Med. 2007;8:53-60.
Choi SH, Chae A, Chen CH, et al. Emerging approaches for imaging vulnerable plaques in patients. Curr Opin Biotechnol. 2007;18:73-82.
Davies JR, Rudd JH, Weissberg PL. Molecular and metabolic imaging of atherosclerosis. J Nucl Med. 2004;45:1898-1907.
De Feyter PJ, Meijboom WB, Weustink A, et al. Spiral multislice computed tomography coronary angiography: a current status report. Clin Cardiol. 2007;30:437-442.
de Roos A, Kroft LJ, Bax JJ, et al. Applications of multislice computed tomography in coronary artery disease. J Magn Reson Imaging. 2007;26:14-22.
Kolodgie FD, Burke AP, Farb A, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16:285-292.
Manning WJ, Nezafat R, Appelbaum E, et al. Coronary magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2007;15:609-637.
Meijs MF, Meijboom WB, Cramer MJ, et al. Computed tomography of the coronary arteries: an alternative? Scand Cardiovasc J. 2007;41:277-286.
Schuijf JD, Jukema JW, van der Wall EE, et al. The current status of multislice computed tomography in the diagnosis and prognosis of coronary artery disease. J Nucl Cardiol. 2007;14:604-612.
1 Proudfit WL, Shirey EK, Sones FMJr. Selective cine coronary arteriography: correlation with clinical findings in 1,000 patients. Circulation. 1966;33:901-910.
2 Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25-e146.
3 Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262-1275.
4 Burke AP, Farb A, Malcom GT, et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276-1282.
5 Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core: a frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354-1363.
6 Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934-940.
7 Burke AP, Taylor A, Farb A, et al. Coronary calcification: insights from sudden coronary death victims. Z Kardiol. 2000;89(Suppl 2):49-53.
8 Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation. 1999;99:2345-2357.
9 Staiger J, Adler CP, Dieckmann H, et al. Postmortem angiographic and pathologic-anatomic findings in coronary heart disease: a comparative study using planimetry. Cardiovasc Intervent Radiol. 1980;3:139-143.
10 Wijpkema JS, Dorgelo J, Willems TP, et al. Discordance between anatomical and functional coronary stenosis severity. Neth Heart J. 2007;15:5-11.
11 Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111:143-149.
12 Goldstein JA, Demetriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915-922.
13 Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478-1492.
14 Bose D, von Birgelen C, Erbel R. Intravascular ultrasound for the evaluation of therapies targeting coronary atherosclerosis. J Am Coll Cardiol. 2007;49:925-932.
15 Kawsasaki M, Sano K, Okubo M, et al. Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound. J Am Coll Cardiol. 2005;45:1846-1953.
16 Rodriguez-Granillo GA, Garcia-Garcia HM, McFadden EP, et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005;46:2038-2042.
17 Hong MK, Mintz GS, Lee CW, et al. A three-vessel virtual histology intravascular ultrasound analysis of frequency and distribution of thin-cap fibroatheromas in patients with acute coronary syndrome or stable angina pectoris. Am J Cardiol. 2008;101:568-572.
18 Rubinshtein R, Halon DA, Gaspar T, et al. Usefulness of 64-slice multidetector computed tomography in diagnostic triage of patients with chest pain and negative or nondiagnostic exercise treadmill test result. Am J Cardiol. 2007;99:925-929.
19 Leber AW, Becker A, Knez A, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672-677.
20 Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and non-calcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14-17.
21 Kim WY, Danias PG, Stuber M, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863-1869.
22 Liu X, Zhao X, Huang J, et al. Comparison of 3D free-breathing coronary MR angiography and 64-MDCT angiography for detection of coronary stenosis in patients with high calcium scores. AJR Am J Roentgenol. 2007;189:1326-1332.
23 Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818-1824.
24 Manfrini O, Mont E, Leone O, et al. Sources of error and interpretation of plaque morphology by optical coherence tomography. Am J Cardiol. 2006;98:156-159.
25 Farb A, Weber DK, Kolodgie FD, et al. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105:2974-2980.
26 Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435-2441.
27 Hannan EL, Wu C, Walford G, et al. Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331-341.
28 Yang JH, Gwon HC, Cho SJ, et al. Comparison of coronary artery bypass grafting with drug-eluting stent implantation for the treatment of multivessel coronary artery disease. Ann Thorac Surg. 2008;85:65-70.

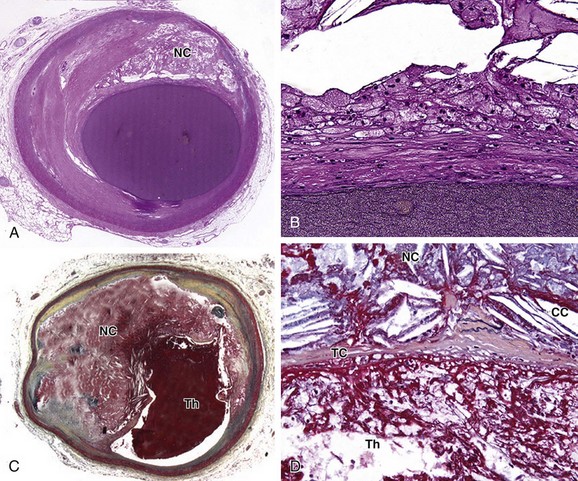
 FIGURE 51-1
FIGURE 51-1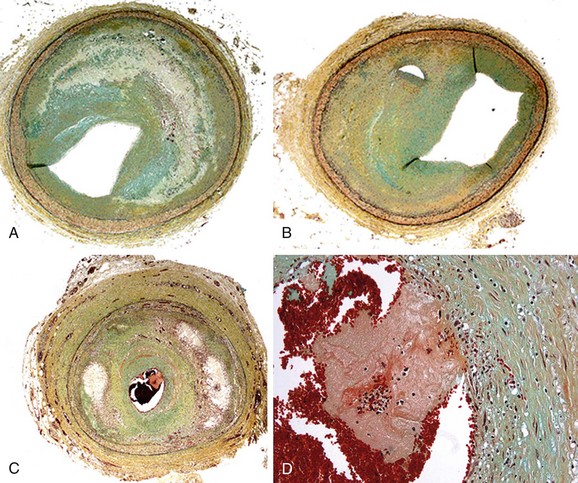
 FIGURE 51-2
FIGURE 51-2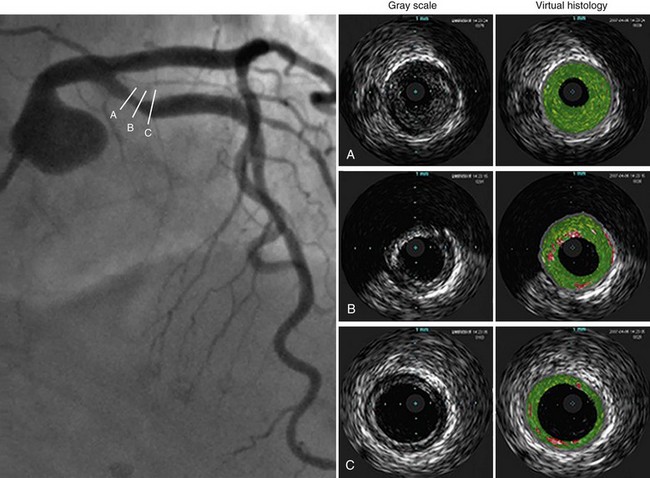
 FIGURE 51-3
FIGURE 51-3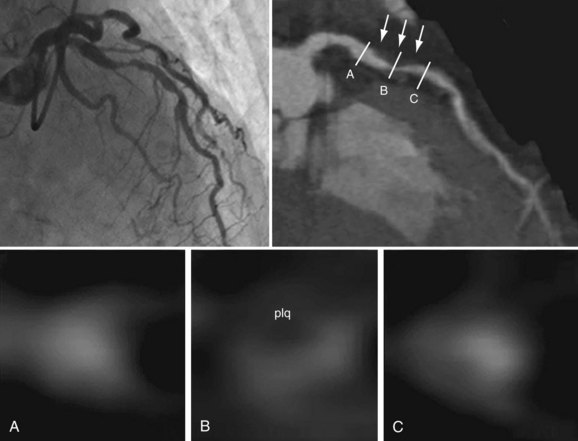
 FIGURE 51-4
FIGURE 51-4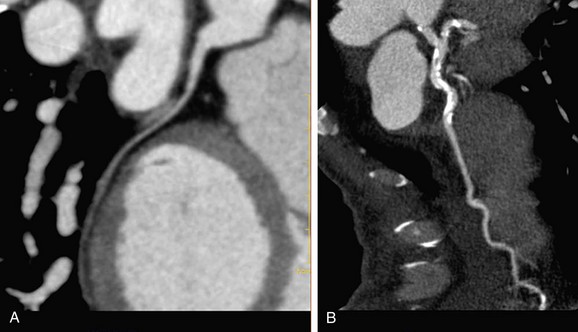
 FIGURE 51-5
FIGURE 51-5
 FIGURE 51-6
FIGURE 51-6

