Chapter 38 Asthma
Epidemiology, Pathophysiology, and Risk Factors
Epidemiology
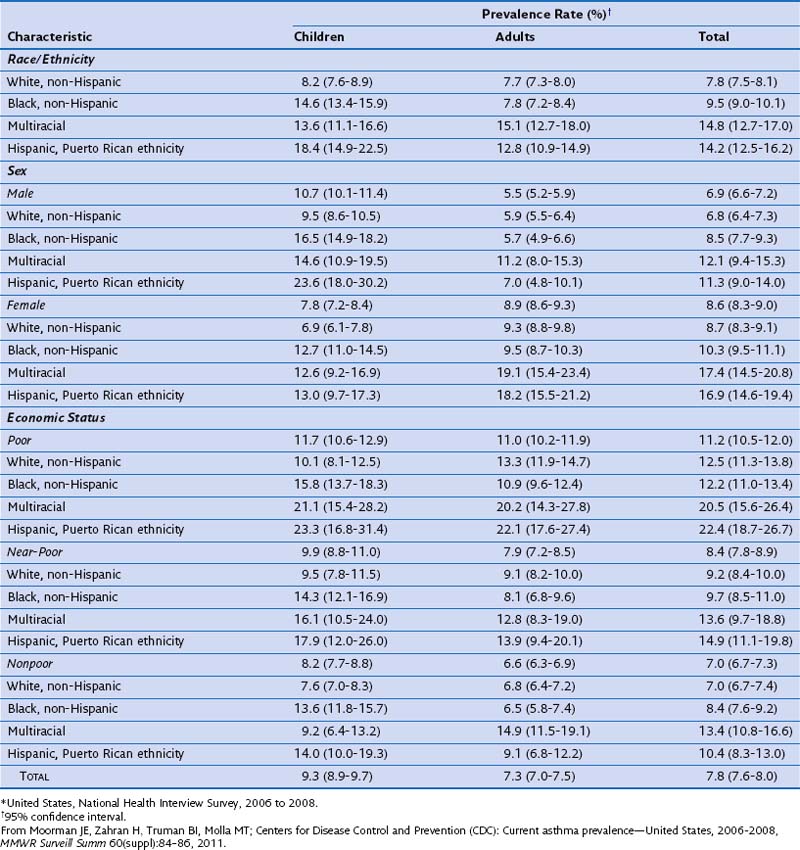
In a morbidity and mortality report from the CDC for the period 2006 to 2008, asthma prevalence was estimated as 7.8% for the U.S. population (Table 38-1). Current asthma prevalence was higher among the multiracial (14.8%), Puerto Rican Hispanics (14.2%), and non-Hispanic blacks (9.5%) than among non-Hispanic whites (7.8%). Current asthma prevalence also was higher among children (9.3%) than among adults (7.3%), among females (8.6%) than among males (6.9%), and among the poor (11.2%) than among the near-poor (8.4%) and nonpoor (7.0%).
Table 38-1 Prevalence of Current Asthma Among Children and Adults by Sex, Race/Ethnicity, and Economic Status–Poverty Level*
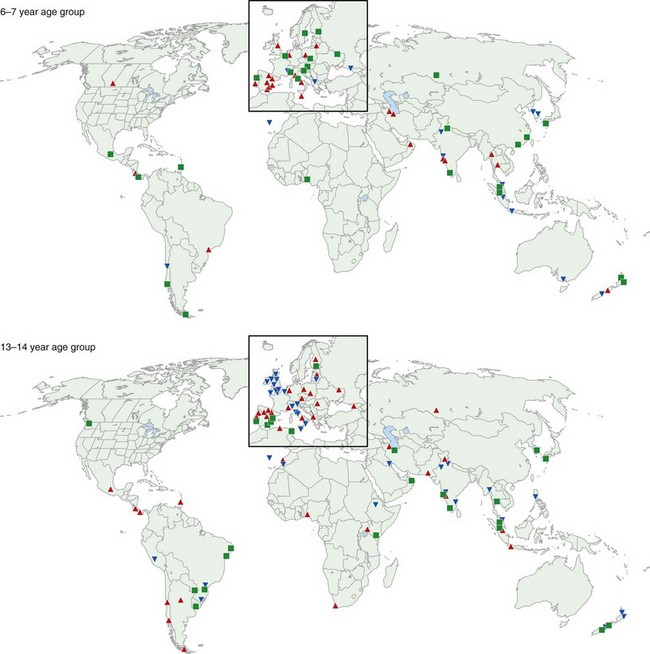
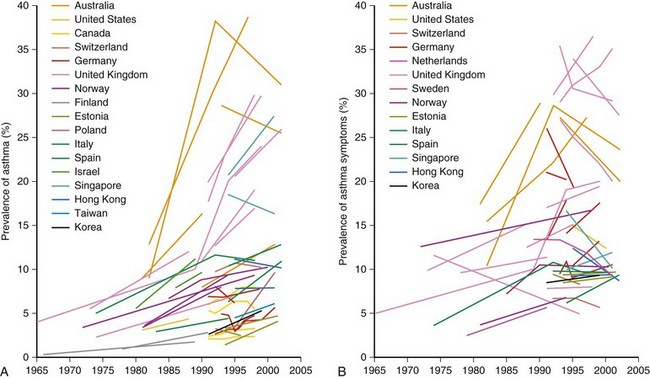
One of the main objectives of the European Community Respiratory Health Survey (ECRHS) was to estimate the variation in prevalence of asthma, asthma-like symptoms, atopic sensitization, and bronchial hyperreactivity, predominantly in Western Europe and many other countries (Figure 38-1). The highest rates in Europe were in the United Kingdom (15.2%), and the lowest were in Georgia (0.28%). Another important ongoing multicenter study, the International Study of Asthma and Allergies in Children (ISAAC), had as its main aim to determine the asthma prevalence in children. A comparative survey conducted in Canadian children between 2 and 7 years of age showed an asthma prevalence of 9.8% overall, with a 4.6% higher rate in boys than in girls (Figure 38-2). GINA embraced the results of both of these valuable studies in the Global Burden of Asthma report and estimated that by 2025, 400 million people around the world will have a diagnosis of asthma. In the same way, this increase in prevalence is directly related to the augmented rates of rhinitis, eczema, and other atopic disorders.
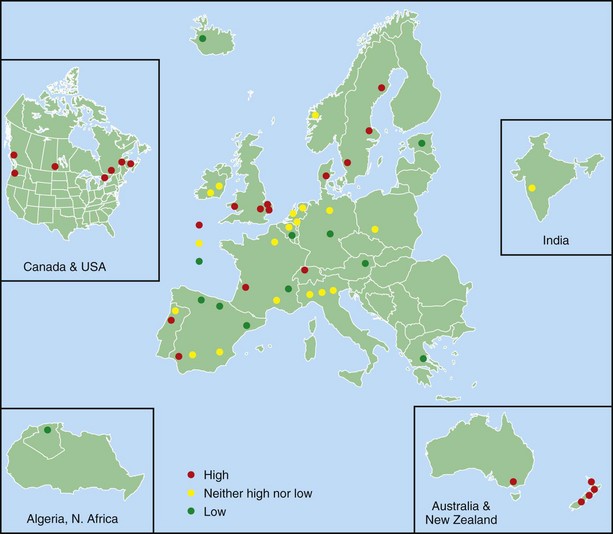
Figure 38-1 Prevalence of asthma in Europe.
(Courtesy European Community Respiratory Health Survey I Centers. Available at: http://www.ecrhs.org/ECRHS%20I.htm.)
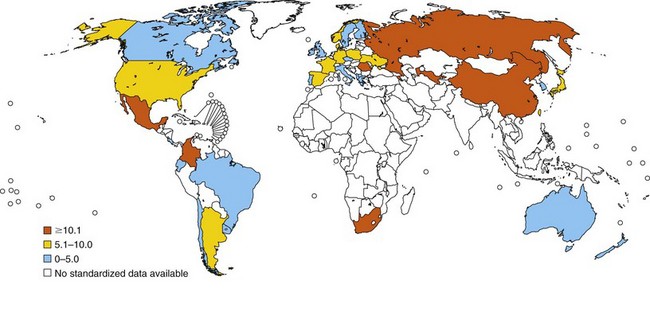
In relation to mortality, asthma accounts for an estimated 250,000 annual deaths worldwide. Statistical data show large differences between countries, and asthma death rates do not parallel prevalence rates (Figure 38-3). Mortality seems to be high in countries where access to essential drugs is low. According to the WHO, many asthma-related deaths are preventable, being a result of suboptimal long-term medical care and delay in obtaining help during the final, fatal attack. In many areas of the world, people with asthma do not have access to basic asthma medications and health care. Nations with the highest death rates are those in which controller medications are not available. In many countries, deaths due to asthma have declined recently as a result of better asthma management. Considerable evidence points to an overall trend of decreasing mortality. For instance, mortality rates in Australia registered by the Australian Institute of Health and Welfare decreased by approximately 70% between 1989 and 2006. Persons older than 65 years of age and people of lower socioeconomic status were at higher risk for dying from asthma, mainly secondary to respiratory infections during winter. Overall, asthma-related mortality in Australia remains uncommon, accounting for 402 (0.30%) in the year 2006 of all deaths (Australian Centre for Asthma Monitoring: Asthma in Australia 2008, AIHW Asthma Series no. 3, Cat. no. ACM 14, Canberra, Australian Institute of Health and Welfare, 2008) (Figure 38-4).
Pathogenesis
A genetic predisposition to develop specific immunoglobulin E (IgE) antibodies directed against common environmental allergens, or atopy, is the strongest identifiable risk factor for the development of asthma. Intrinsic abnormalities in airway smooth muscle and airway remodeling in response to injury and inflammation add to the effects of airway inflammation in creating the clinical presentation of asthma (Figure 38-5).
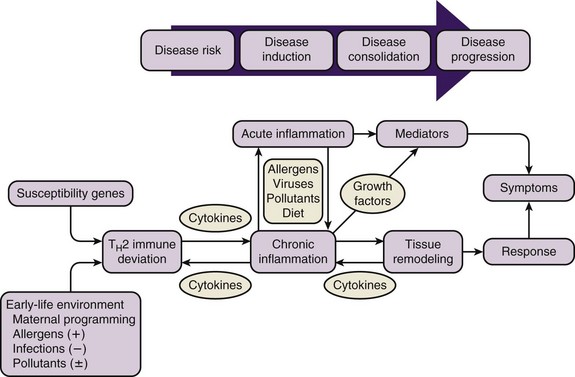
Figure 38-5 Natural history of asthma.
(From Szefler SJ: The natural history of asthma and early intervention, J Allergy Clin Immunol 109:S550, 2002. Adapted from Holgate ST: The cellular and mediator basis of asthma in relation to natural history, Lancet 350[Suppl 2]:5–9, 1997.)
Pathophysiology
Airway narrowing is the final common pathway leading to symptoms and physiologic changes in asthma. As discussed next, several factors contribute to the development of airway narrowing in asthma (Figure 38-6).
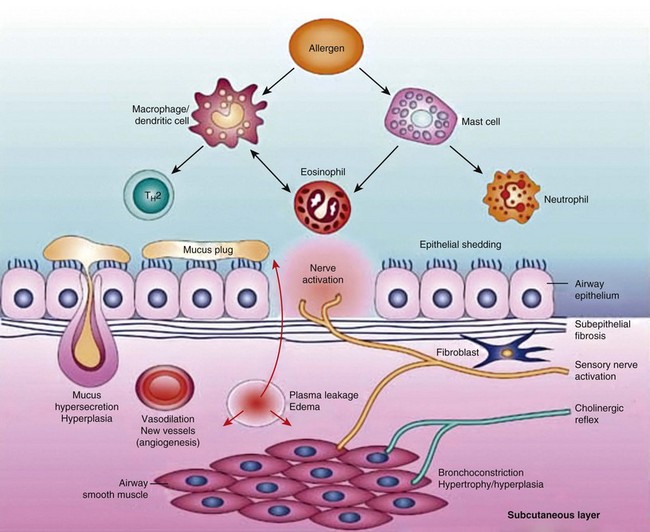
Figure 38-6 Pathobiology of asthma.
(From Barnes PJ: New drugs for asthma, Nat Rev Drug Discov 3:831–844, 2004.)
Airway Remodeling
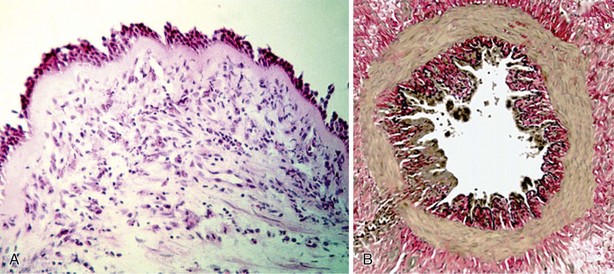
All components of the airway wall have been reported to be thickened in asthma (Figure 38-7). Elements involved in this response are increase in airway smooth muscle, increased myocyte muscle mass, edema, inflammatory cell infiltration, glandular hypertrophy and hyperplasia, and connective tissue deposition. The recently popularized “perturbed equilibrium hypothesis” also suggests that wall thickening can destabilize the dynamic forces that control airway caliber, leading to airway collapse.
Risk Factors
Asthma is a condition characterized by lung airway inflammation initiated and perpetuated by an inappropriate immune response, increased airway responsiveness, and variable airflow obstruction. These results come from complex interactions between multiple genetics and environmental influences. Although a clear cause-and-effect association is not always possible to demonstrate, numerous risk factors have been identified (Box 38-1).
Environmental Factors
Risk Factors in Childhood
Adult-Onset Asthma
Occupational Exposure
Occupational asthma is found predominantly in adults, and workplace sensitization is responsible for 1 in 10 cases of asthma among adults of working age (see Chapter 40). High-risk occupations for the development of asthma include farming and agricultural work, painting, plastic manufacturing, and cleaning. IgE- and cell-mediated allergic reactions are involved in its pathobiology.
Trigger Factors
Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Rep. 2011;32:1–14.
Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743.
Bara I, Ozier A, Tunon de Lara JM, et al. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J. 2010;36:1174–1184.
Bisgaard H, Bønnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126:187–197.
Breysse PN, Diette GB, Matsui EC, et al. Indoor air pollution and asthma in children. Proc Am Thorac Soc. 2010;7:102–106.
Busse WW, Lemanske RFJr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834.
D’Amato G, Cecchi L, D’Amato M, Liccardi G. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J Investig Allergol Clin Immunol. 2010;20:95–102.
Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235.
Global Initiative for Asthma. Global strategy for asthma management and prevention. HHLBI/WHO Workshop report, updated 2011 (article online). http://www.ginasthma.org/. accessed February 2, 2012
Grainge CL, Lau LC, Ward JA, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–2015.
Anderson HR, Gupta R, Strachan DP, Limb ES. 50 years of asthma: UK trends from 1955 to 2004. Thorax. 2007;62:85–90.
Lemanske RFJr, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S95–S102.
Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16:22–27.
Murphy DM, O’Byrne PM. Recent advances in the pathophysiology of asthma. Chest. 2010;137:1417–1426.
Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. 2009;181:E181–E190.
Xepapadaki P, Papadopoulos NG. Childhood asthma and infection: virus-induced exacerbations as determinants and modifiers. Eur Respir J. 2010;36:438–445.