Chapter 7 Asthma
OVERVIEW AND AETIOLOGY
Asthma is a chronic inflammatory disorder of the airways and may be classed as atopic (extrinsic) or intrinsic.1 It is marked by recurrent attacks of paroxysmal dyspnoea with wheeze, due to spasmodic contraction of the bronchi.1,2 Key indicative symptoms include wheeze, cough, shortness of breath, chest tightness and sputum production.3 The signs and symptoms of asthma may be subtle, and some children present with atypical features such as recurrent respiratory tract infections, seasonal asthma and night-time cough. Often symptoms will present or worsen in relation to certain triggers.3
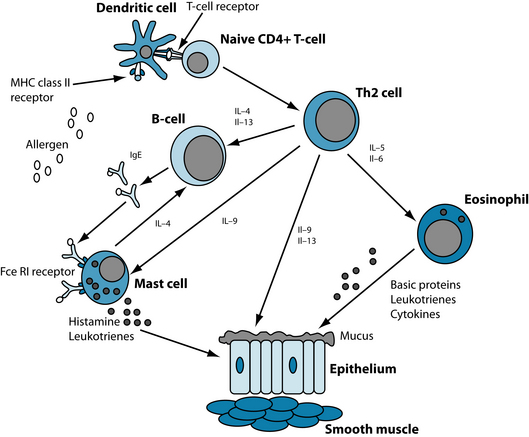
The most common theory of asthma development proposes that the condition is the result of multicellular inflammation driven by airway hyperresponsiveness, with airway remodelling and potentially permanent bronchial obstruction.4,5 This inflammation is due to the recruitment and activation of mast cells, macrophages, dendritic cells, neutrophils and eosinophils with resultant cellular infiltration and airway inflammation.4,5 With the activation of such cells, preformed and generated cytokines and growth factors are released, resulting in the remodelling of the airways with amplified goblet cell
production, smooth muscle hypertrophy and deposition of extracellular proteins.6 The inflammatory mediators also induce changes in the noradrenergic and parasympathetic nervous systems that may lead to bronchial hyperresponsiveness.5
It is postulated that allergen exposure in genetically predisposed individuals leads to T helper type 2 (Th2) proliferation. Th2 cells stimulate B-lymphocytes to produce specific IgE antibodies, which then activate an inflammatory cascade upon subsequent exposure to the allergen (see Figure 7.1).7
In general, infants are born with a disposition towards pro-allergic and pro-inflammatory Th2 immune responses, but early childhood exposure to infections and endotoxins shifts the body towards a predominance of Th1 responses, which suppress Th2 cells and induce tolerance.8 The hygiene hypothesis suggests that in developed countries, the trend towards smaller families,9 cleaner environments10 and early use of vaccinations and antibiotics may deprive children of these Th2-suppressing, tolerance-inducing exposures, partly explaining the continuous increase in asthma prevalence in developed countries.8 It should be noted that, in contrast to this hypothesis, certain studies have identified a pathogenic role for viral respiratory infection in the development of asthma in atopic infants.11
In exercise-induced (intrinsic) asthma, bronchoconstriction seems to be stimulated by moisture loss from the respiratory tract and increased airway cooling due to an increase in ventilation.12 However, despite the lack of identifiable allergenic triggers, immune dysregulation, in the form of inappropriate IgE production and activated T-cells, still seems to be a feature.13
RISK FACTORS
Familial, genetic and environmental factors
Having first-degree relatives with a history of asthma, or a personal or family history of atopy, is a risk factor for the condition.1 Atopic responsiveness is inherited, and genes have been identified that influence bronchial hyperresponsiveness, even in the absence of allergies.14,15
The environment also affects asthma development, particularly during early childhood. Breastfeeding during the neonatal period seems to prevent the development of atopy, perhaps as a desensitisation response to continual oral intake of the allergen.16,17 However, antigen exposure later in infancy seems to promote atopic responses.14 This may partially explain why the early introduction of formula seems to lead to an increase in child body mass index (BMI) and early asthma and atopy.18 Childhood exposure to air pollution has been found to be associated with the development of persistent wheeze and atopy in children living in urban environments.19 Paracetamol use in infancy has also been associated with an increased risk of current wheeze in young children.20
Digestive and dietary factors
Gastrointestinal symptoms appear to be common in children with asthma;21 and an increased prevalence of increased intestinal permeability and cytokines in patients with asthma may support naturopathic theory that leaky gut is associated with the condition.22,23
Gastro-oesophageal reflux has been recognised as a common trigger of asthma,24 via oesophageal acid-induced reflex bronchoconstriction, or microaspiration of acid. A connection has also been made between hypochlorhydria and bronchial asthma.25 This correlation may be the result of inadequate protein digestion,26 increasing atopic allergic reactions. It may also cause poorer nutrient absorption in general,26 affecting the development of atopy and/or bronchial hyperresponsiveness through various deficiencies.
Dietary intake also plays a role in atopic asthma. Nutrient deficiencies, in particular of vitamins D and E, magnesium, potassium, vitamin C and fatty acids, are associated with asthma.27–29 Poor maternal diet has been correlated with a rise in asthma at the ages of 2 and 5 years.28 Asthma is also known to have a number of dietary triggers such as specific ‘allergenic’ foods or food additives.30,31 Hen’s eggs, cow’s milk, soy, wheat, tree nuts, peanuts, fish and shellfish, monosodium glutamate (MSG), tartrazine and sulfites have all been implicated.29
Antibiotic use in the first year of life is associated with an increased risk of wheeze in New Zealand children,20 suggesting that dysbiosis may be a risk factor. This theory is supported by evidence showing that atopic infants have higher levels of i-caproic acid (a marker of Clostridium difficile) and lower populations of lactobacilli, bifidobacteria and Bacteroides than non-atopic children.32
Stress
Stress is a well-known asthma trigger, but it may also play a role in pathogenesis.2,3,33 Parental divorce or separation, exposure to violence and severe disease of a family member all increase the risk of developing atopic conditions.34 Lower socioeconomic status is associated with elevated levels of stress and threat perception, as well as heightened production of IL-5 and IL-13 and higher eosinophil counts in children with asthma.35 Children from this background are more likely to develop asthma, and exhibit poorer health outcomes.36,37 Anxiety disorders and asthma are also common comorbidities, but the exact relationship is unclear.38
Metabolic syndrome
Abdominal obesity and hypertension are both components of metabolic syndrome that increase the risk of asthma-like symptoms.39 Epidemiological studies show that, generally, people with asthma tend to be heavier than those without—a relationship that is more consistent in adults than children.32 Additionally, the risk of asthma seems to increase as body mass index (BMI) rises.40 As both obesity and asthma begin in early life, some researchers have proposed common predisposing factors including genetics, early life weight gain, low physical activity, prenatal diet and nutrition, altered intestinal microflora and adipocytokines.32
CONVENTIONAL TREATMENT
There is a lack of international consensus on conventional diagnosis and treatment, with a significant number of children continuing to suffer symptoms despite current treatment.41 Patient attitudes towards medical professionals and asthma treatment have been found to predict the degree of asthma control a patient is likely to achieve with conventional therapies.42
The National Asthma Education and Prevention Program (NAEPP) proposes a stepwise approach to the management of asthma, based upon severity.3 Medications fall into two categories: short-acting agents designed to relieve airway obstruction in an acute exacerbation, and longer-term prevention therapy:40
| STAGE | ASTHMA SYMPTOMS | RECOMMENDATION |
|---|---|---|
| 1 | Mild, intermittent |
KEY TREATMENT PROTOCOLS
A symptom diary and elimination diet are useful tools to identify trigger factors (see Chapter 5 on food intolerance and allergy). Once known, these should be avoided wherever possible. With time, and naturopathic treatment (including digestive repair and immune support), the patient may be well enough that certain factors cease to be problematic. As the underlying mechanism of airway obstruction is inflammation, a key priority is to manage the inflammatory response with anti-inflammatory, antiallergic and immune-modulating substances, and reduce airway hypersensitivity. On a symptomatic level, respiration will be most efficient and uncomplicated when the bronchioles are clear. Bronchodilation and expectoration are central actions to open the airways and promote symptom-free ventilation. Given the sizeable role of digestive dysfunction in the aetiogenesis of asthma, once symptoms are stabilised, better control and prevention may then be established by redressing intestinal permeability and dysbiosis.
Dampen the inflammatory cascade
Reducing pulmonary inflammation in asthma will improve symptoms and assist in moderating disease progression. A number of herbal agents demonstrate anti-inflammatory actions specific to asthma. Boswellia serrata inhibits 5-lipoxygenase,43 a key cytokine implicated in asthmatic inflammation. Seventy per cent of patients treated with B. serrata showed improvement in their asthma symptoms, as opposed to 27% of controls.44 The anti-inflammatory, antioxidant and antiviral activity of curcumin in Curcuma longa has been effective in treating airway hyperresponsiveness in allergic inflammatory diseases.45,46 The oil of this botanical is also significantly active in removing sputum, relieving cough and preventing asthma,47 and phytochemicals derived from C. longa may interrupt the action of NF-κB (which induces inflammation) and diminish Th2 responses, with a concurrent reduction in asthmatic symptoms.48 Zingiber officinale may also inhibit the release of prostaglandins,49 suppress Th2-mediated immune responses50 and inhibit airway contraction, possibly via blockade of plasma membrane Ca2+ channels.51
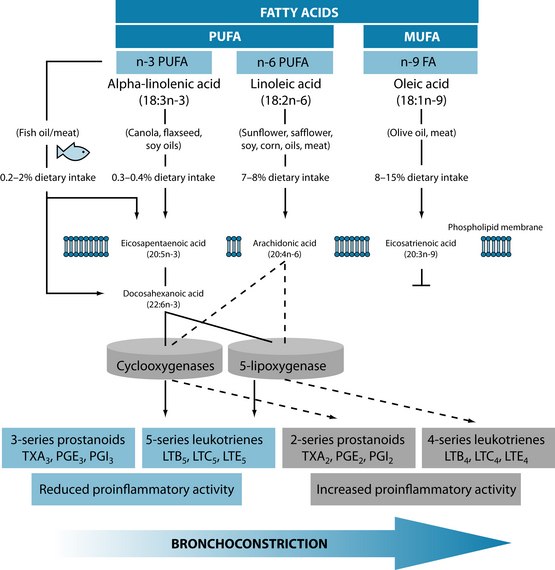
Dietary supplementation with omega-3 fatty acids, zinc and vitamin C significantly improved asthma control, pulmonary function tests and pulmonary inflammatory markers in children with moderately persistent bronchial asthma.52 Benefits from essential fatty acids may be derived as far back as fetal development, with research showing that adequate maternal intake corresponds with lower rates of asthma in offspring (see Figure 7.2).53 The ingestion of 2.7 g of omega-3 polyunsaturated fatty acids for the last 10 weeks of pregnancy was shown to have a significant protective effect against the development of asthma in offspring by the age of 16 years.54
Omega-3 fatty acids may also be beneficial in direct treatment, as 3.2 g EPA and 2.2 g DHA daily for 3 to 10 weeks reduces inflammatory markers, pulmonary compromise (fall in FEV1) by nearly 80% and bronchodilator use by up to 20% in exercise-induced asthma.55,56
In the airways of asthmatics, inflammation is often associated with increased generation of reactive oxygen species and free radical damage.57 Thus the antioxidants vitamins A, C and E may be useful. By reducing the effect of the reactive oxygen species produced in the inflammatory process, these modulate the development of asthma and the impairment of pulmonary function.57 Vitamin C supplementation demonstrates a protective effect against exercise-induced airway narrowing in asthmatic subjects.58 Serum levels of the antioxidants alpha and beta carotene have also been positively correlated to lung function and FEV1 and FVC in epidemiological studies.59,60
Quercetin has the ability to inhibit inflammatory cytokine and chemokine production in acute and chronic inflammatory conditions.61,62 High levels of this flavonoid are found in onions, apples, blueberries, curly kale, hot peppers, green and black tea and broccoli.63,64
In asthma, platelet activating factor (PAF) is released in response to exposure to allergens and induces an inflammatory airway response.65 It is a potent bronchoconstrictor,66 and elicits pulmonary and bronchial oedema, leading to airway obstruction and difficulty breathing.67 While pharmaceutical PAF antagonists have not displayed absolute therapeutical efficacy alone, they may be a useful part of a combined treatment strategy.68
Ginkgo biloba is best known for its well-demonstrated neurological effects.69,70 However, it has also shown activity as a PAF antagonist, exerting an anti-inflammatory action and reducing airway hyperresponsiveness and bronchospasm.71 The most active constituents appear to be quercetin and the ginkgolides, in particular ginkgolide B.72 Some researchers also suggest that G. biloba may modulate lymphocyte activation in asthma,73 and in murine models of asthma the herb appears to impede the disease progress by alleviating all established chronic histological changes of lung except smooth muscle thickness.74 Glycyrrhizae from Glycyrrhiza glabra has also demonstrated ability to inhibit PAF production by human neutrophils in a dose-dependent manner.75 Allium cepa exerts antiasthmatic and anti-PAF effects through its thiosulfinate content. In one study, allergen-induced asthma attacks were almost completely inhibited by an A. cepa extract.76 Thus, there may be potential for garlic, onions and shallots to be included in dietary management of asthma.
Immune regulation
Immune dysregulation is a key feature of atopic asthma (and perhaps intrinsic asthma – see above). Strengthening immune resistance generally, rebalancing T-cell levels and restoring immune homeostasis in the lung may decrease sensitisation to allergens and triggers.7 In addition, viral infections are known to worsen asthma symptoms,3 and immune enhancement will help to prevent these.
Herbal immune modulators such as Echinacea angustifolia may be beneficial in supporting the body’s natural resistance to infection and is particularly efficacious in prophylaxis/treatment of upper respiratory tract infections.77 In addition to its significant immune enhancing properties,78 Andrographis paniculata may also be anti-inflammatory via inhibition of the NF-κB pathway.79 Astragalus membranaceus has good traditional evidence as an immune-enhancing herb, and may potentially play a specific role in treating allergic asthma.80 Also useful is Picorrhiza kurroa, which helps to prevent allergen- and PAF-induced bronchial obstruction.76 General immune supportive nutrients are vitamin C, vitamin A, zinc and selenium (for further immune modulators, refer to Chapter 6 on infectious respiratory disease).
Allergy management
Albizzia lebbeck stabilises mast cell membranes in murine models, suggesting that it may inhibit histamine release in allergenic asthma.81 This herb appears to deliver best results in asthma of less than 2 years’ duration.82 Scutellaria baicalensis contains various flavonoids that suppress eotaxin—a chemokine associated with recruitment of eosinophils to sites of allergic inflammation—indicating a theoretical mechanism for its traditional use in asthma.83 The flavonoid baicalin was associated with significant reductions in inflammatory mediators in patients with bronchial asthma and was five to 10 times more potent than the antiallergic drug azalestine.84
In severe cases, the practitioner may consider immunosuppressive herbs, such as Tylophora indica or Hemidesmus indicus, but caution is urged with regard to dosage, and the patient should be monitored closely. (For protocol on using these herbs, see Chapter 28 on autoimmunity.) Four small, early studies using T. indica for short periods indicate beneficial effects (such as increased peak expiratory flow rate and ventilatory capacity).85–87
In addition to an anti-inflammatory role, quercetin is useful for its anti-allergic qualities. In animal models it reduced the production of IL-4 (a Th2 cytokine) and increased the production of IFN-γ (a Th1 cytokine), potentially indicating a T-cell regulatory effect.88 Dietary intake of antioxidant nutrients such as vitamin A, C, E and selenium may also help to regulate this balance. Supplementation of vitamin E and selenium are reported to promote Th1 differentiation.89–93
Enhance brochodilation
Adhatoda vasica is considered a specific for asthma, and is generally thought to be safe for long-term treatment.94 The alkaloids in A. vasica have been compared to theophylline for their bronchodilator and antiasthmatic actions,95 as they exhibit pronounced protection against allergen-induced bronchial obstruction.76 Euphorbia spp. are also considered a reliable antiasthmatic, particularly in spasmodic forms of the condition. These promote expectoration, allay cough96 and have antiproliferative properties.97
Forskolin from Coleus forskohlii has been shown to increase the levels of cAMP in cells, making it a natural bronchodilator.98 In a model using guinea pig trachea, it showed efficacy in reducing antigen-induced constriction;99 and in early trials it improved forced expiratory volume and decreased airway resistance in male asthmatics.100 Other useful bronchospasmolytics and bronchodilators with traditional evidence are Grindelia camporum and Glycyrrhiza glabra.101,102
Magnesium is also a renowned bronchodilator. It antagonises the movement of calcium across cell membranes, decreasing the uptake and release of the mineral in bronchial smooth muscle, and leading to relaxation and dilation of the airways.103 In acute situations, magnesium sulfate administered intravenously or via a nebuliser appears to be effective in improving the pulmonary function of asthmatics.104,105 In a short-term trial, 400 mg of magnesium was added to a low-magnesium diet for 3 weeks, producing an improvement in symptom scores.106 In asthmatic children, 300 mg/day for 2 months produced reduced bronchial and skin hyperreactivity to known antigens, in addition to fewer asthma exacerbations and less medication use.107
Tea, yerba maté and coffee108 are all agents that may be useful in bronchodilation. The pharmaceutical agent theophylline was derived from tea, and the caffeine that these agents contain may improve lung function for up to 4 hours in people with asthma.109
Digestive connection
The development of asthma has been variously linked to increased digestive permeability,23 oesophageal reflux110 and dysbiosis,111,112 making these the key areas to address.
Inflammation may be attenuated, and the healing of the mucosal lining facilitated, by interventions including vitamin A,113 glutamine114,115 and Aloe vera.116 Improving overall digestive capacity involves the use of herbal bitters such as Gentiana lutea or Peumus boldus and warming digestives such as Zingiber officinale or Cinnamomum cassia in association with enzyme therapy.96,117–119
With dysbiosis, the aim is to reduce overgrowth of detrimental strains of microflora and enhance beneficial strains, following a protocol known colloquially as a ‘weed, seed and feed’. In various animal models, probiotic strains significantly reduce IgE production, airway hyperresponsiveness and/or inflammatory infiltration of the lungs (see the box below).120
For complete protocol and treatment suggestions, refer to the section on the digestive system.
Diet and lifestyle modification
Dietary modification
An elimination diet may be the most successful method of identifying allergens in asthmatic patients (see Figures 5.1 for the elimination diet protocol).122 Egg, shellfish, tree nuts and peanuts are the foods most associated with immediate onset, while those most commonly associated with delayed onset are milk, chocolate, wheat, citrus and food colourings.123
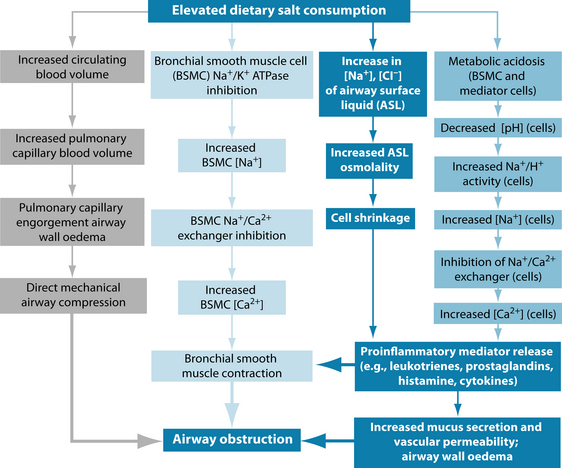
A diet high in sodium may be associated with more severe asthma symptoms in some patients (see Figure 7.3),124 and fast food intake is directly correlated with asthma risk.125
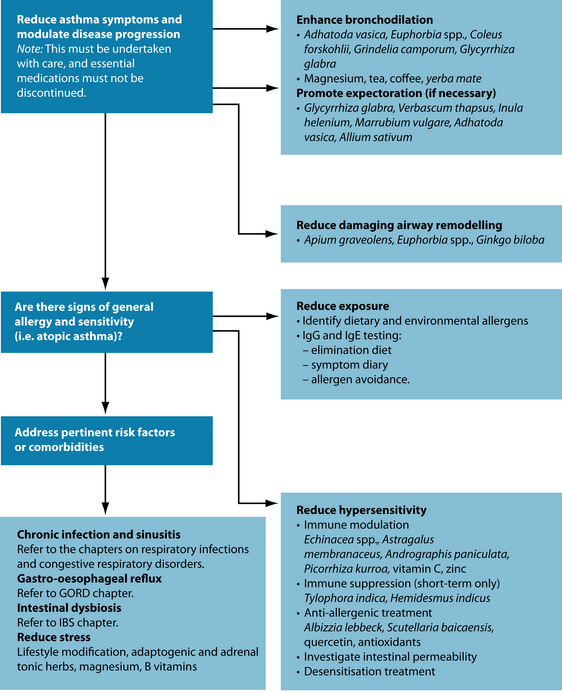
Figure 7.3 The mechanisms by which salt may contribute to airway obstruction and worsening of asthma symptoms108
It has been suggested that oxidative stress may contribute to respiratory pathologies, particularly asthma.126–128 A reduced intake of antioxidant-rich foods induces a worsening of asthma symptoms, and antioxidants are often lower in patients with asthmatic or respiratory distress.129,130 Antioxidant-rich diets are associated with reduced asthma prevalence121 and improved respiratory function.131
Antioxidant supplementation with 50 mg vitamin E and 250 mg vitamin C daily has been found to modulate the effects of pollution on children with asthma.132 Improved beta-carotene levels are associated with a lower incidence of asthma,133 and improved FEV1.59 Lycopene supplementation has been linked with reduced airway neutrophil influx.130 These relationships do not automatically imply a causal relationship and may instead be used as a marker of healthy diets more broadly. This is confirmed by epidemiological evidence that suggests that eating a Mediterranean-style diet, with high levels of fresh fruit, vegetables, omega-3 fatty acid and nuts, may reduce asthma risk in children by up to 80%.134,135 The inclusion of omega-3 fatty acids should be a key priority.108 An avoidance of heavy meals at night has also been proposed in order to manage asthma.136
Lifestyle advice
The association between asthma symptom severity and stress is strong. It is thought that stress exacerbates the immune reactions responsible for airway inflammation in asthma,137 indicating that relaxation therapies may be of particular use. Meditation,138 tai chi139 and yoga140–143 have shown efficacy in this area. The benefits of these therapies may be partly related to their focus on breathing exercises. The yogic science of pranayama is designed to promote deep breathing, expand the lungs and reduce stress. These exercises may help reduce histamine response to allergens, produce decreases in FEV1, peak expiratory flow rate, symptom score and inhaler use, and improve quality of life indexes.144–147 The interventions have little or no effect on lung physiology and are presumed to have a secondary or indirect effect on the condition due in part due to their relaxation effects.
Journalling about stressful experiences not previously disclosed to others has been associated with a 13% improvement in lung function,148 and may be a useful adjunct to treatment.
Regular exercise increases overall quality of life for asthmatics,149 an approach which seems particularly relevant in children.150–152 Maintenance of a healthy weight is important, as asthma prevalence has a positive association with obesity153 and weight loss in overweight patients results in the improvement of asthma symptoms.154
INTEGRATIVE MEDICAL CONSIDERATIONS
Buteyko
The Buteyko breathing technique—which involves making breathing shallow and slow – has demonstrated an ability to reduce medication use and produce improvements in quality of life scores in patients with asthma.155–158
Acupuncture
Acupuncture is useful for the asthmatic patient, and has been used for thousands of years to treat lung dysfunction.159 Approaches generally address excessive or deficient Qi in the respiratory system.159 Reports suggest that it can decrease symptom severity and improve lung function (at least in the short term),159,160 with an immediate bronchodilatory effect, and improvements in FEV after even one treatment.161 A Cochrane review of the English-language evidence determined that more research is needed to make a definitive conclusion.162
Homoeopathy
Homoeopathic treatments for asthma may include Arsenicum Album, Kali Carbonicum, Natrum Sulphuricum and Aconite.33 It is difficult to judge the effectiveness of treatment from a clinical evidence base, as the nature of homoeopathy is that it prescribes so specifically for individuals, and trials are usually conducted with a singular intervention. A Cochrane review found that there was insufficient evidence to reliably assess the role of homoeopathy in asthma treatment.162 However, some individual trials (including the use of allergen isopathy) have yielded promising results on lung function and subjective symptoms.147
Example treatment
Herbal and nutritional prescription
Adhatoda vasica and Euphorbia spp. exhibit bronchodilatory properties.
Astragalus membranaceus is an immune-enhancing agent and tonic, and may be used in this case as there is no apparent acute infection. As an adaptogen it will assist in modulation of the patient’s stress response.
Herbal treatment
| Albizzia lebbeck | 1:2 | 25 mL |
| Adhatoda vasica | 1:2 | 15 mL |
| Euphorbia spp. | 1:2 | 10 mL |
| Astragalus membranaceus | 1:2 | 30 mL |
| Glycyrrhiza glabra | 1:1 | 15 mL |
| Gentiana lutea | 1:2 | 5 mL |
| 100 mL | ||
| Boswellia serrata | 2.0 g | |
| Curcuma longa | 2.0 g | |
| Zingiber officinale | 300 mg | |
| Combined in tablet form: 2 tablets t.d.s Orotalel citrate combination 400 mg b.d. 3:2 gEPA/day 2:2 gDHA/day | ||
Omega-3 fatty acids are included due to marked anti-inflammatory effects.
Expected outcomes and follow-up protocols
The above prescription should produce significant improvements within days. It is suggested that this treatment be maintained for a number of weeks. Once the patient appears stable, treatment can move towards addressing the underlying causes of the condition. Naturopathically, these may be considered to include chronic immune dysregulation as a result of long-term gastrointestinal dysbiosis, and, potentially, increased gut permeability. (See the section on the digestive system.) In addition to the formula Hydrastis canadensis may be added to the formula to enhance repair of the GIT and support the action of the prebiotic fibre supplement. Avena sativa (green) may be included as nervous system support once G. glabra and A. lebbeck are removed from the formula. Substitution is necessary due to concerns with long-term use and hypertension (G. glabra). The patient should continue with the Boswellia combination tablet, decreased to b.d. rather than t.d.s., with the magnesium combination reduced to 400 mg per day, and continue the omega-3 supplementation at the initial dose, while taking a prebiotic supplement to promote digestive function.
| INTERVENTION | KEY LITERATURE | SUMMARY OF RESULTS |
|---|---|---|
| Vitamin C | Meta-analysis found evidence from trials insufficient due to the fact that trials were small, designs varied and reporting was poor.163 | |
| Reviews concluded variously that:164,165,31 |
Note: RCT = randomised controlled trial
Borchers A.T., et al. Probiotics and immunity. J Gastroenterol. 2009;44(1):26-46.
Mickleborough T.D. A nutritional approach to managing exercise-induced asthma. Exerc Sport Sci Rev. 2008;36(3):135-144.
von Mutius E., et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30:1230-1234.
1. Barnes P. Asthma. In: Fauci A.S., et al, editors. Harrison’s principles of internal medicine. New York: McGraw-Hill Companies, Inc., 2008.
2. McCunney R.J. Asthma, genes, and air pollution. J Occup Environ Med. 2005;47(12):1285-1291.
3. National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma. In: In: National Asthma Education and Prevention Program Expert Panel Report 3. Washington: National Institutes of Health; 2007.
4. Anderson G.P. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372(9643):1107-1119.
5. Linzer J.F.S. Review of asthma: pathophysiology and current treatment options. Clin Pediatr Emer Med. 2007;8(2):87-95.
6. Holgate S.T., et al. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105(2 Pt 1):193-204.
7. Ryanna K., et al. Regulatory T cells in bronchial asthma. Allergy. 2009;64(3):335-347.
8. Beers M.H., et al. Merck manual of diagnosis and therapy, 18th edn. West Point: Merck & Co., Inc.; 2006.
9. Strachan D. Hay fever, hygiene, and household size. BMJ. 1989;299:1259-1260.
10. Braun-Fahrländer C., et al. Exposure to farming environment during the first year of life protects against the development of asthma and allergy. Am J Respir Crit Care Med. 2001;163:A157.
11. Kusel M.M., et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105-1110.
12. Carlsen K.H., Carlsen K.C. Exercise-induced asthma. Paediatr Respir Rev. 2002;3(2):154-160.
13. Jayaratnam A., et al. The continuing enigma of non-atopic asthma. Clin Exp Allergy. 2005;35(7):835-837.
14. Kay A.B. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344(1):30-37.
15. Holgate S.T., et al. The genetics of asthma: ADAM33 as an example of a susceptibility gene. Proc Am Thorac Soc. 2006;3(5):440-443.
16. Friedman N.J., Zeiger R.S. The role of breast-feeding in the development of allergies and asthma. J Allergy Clin Immunol. 2005;115(6):1238-1248.
17. Verhasselt V., et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14(2):170-175.
18. Oddy W.H., Sherriff J.L. Breastfeeding, body mass index, asthma and atopy in children. Asia Pac J Public Health. 2003;15(Suppl):S15-S17.
19. Salvi S. Health effects of ambient air pollution in children. Paediatr Respir Rev. 2007;8(4):275-280.
20. Mitchell E.A., et al. Cross-sectional survey of risk factors for asthma in 6–7-year-old children in New Zealand: International Study of Asthma and Allergy in Childhood Phase Three. J Paediatr Child Health. 2009;45(6):375-383.
21. Caffarelli C., et al. Gastrointestinal symptoms in patients with asthma. Arch Dis Child. 2000;82(2):131-135.
22. Hijazi Z., et al. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004;89(3):227-229.
23. Benard A., et al. Increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol. 1996;97:1173-1178.
24. Peterson K.A., et al. The role of gastroesophageal reflux in exercise-triggered asthma: a randomized controlled trial. Dig Dis Sci. 2009;54(3):564-571.
25. Gonzalez H., Ahmed T. Suppression of gastric H2-receptor mediated function in patients with bronchial asthma and ragweed allergy. Chest. 1986;89(4):491-496.
26. Kelly G. Hydrochloric acid: physiological functions and clinical implications. Altern Med Rev. 1997;2(2):116-127.
27. Litonjua A.A., Weiss S.T. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120(5):1031-1035.
28. Seaton A. From nurture to nature – the story of the Aberdeen asthma dietary hypothesis. QJM. 2008;101(3):237-239.
29. Roberts G., Lack G. Food allergy and asthma – what is the link? Paediatr Respir Rev. 2003;4(3):205-212.
30. Ozol D., Mete E. Asthma and food allergy. Curr Opin Pulm Med. 2008;14(1):9-12.
31. Monteleone C., Sherman A.R. Nutrition and asthma. Arch Intern Med. 1997;157(1):23-34.
32. Litonjua A.A., Gold D.R. Asthma and obesity: common early-life influences in the inception of disease. J Allergy Clin Immunol. 2008;121(5):1075-1084. quiz 1085–1086
33. Dupler D. Asthma. In: Krapp K.L., editor. The Gale encyclopedia of alternative medicine. Farmington Hills: Gale Group; 2001:126-132.
34. Williams D.R., et al. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(Suppl 3):S174-S184.
35. Chen E., et al. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117(5):1014-1020.
36. Claudio L., et al. Socioeconomic factors and asthma hospitalization rates in New York City. J Asthma. 1999;36(4):343-350.
37. Ernst P., et al. Socioeconomic status and indicators of asthma in children. Am J Respir Crit Care Med. 1995;152(2):570-575.
38. Goodwin R.D. Asthma and anxiety disorders. Adv Psychosom Med. 2003;24:51-71.
39. Lee E.J., et al. Asthma-like symptoms are increased in the metabolic syndrome. J Asthma. 2009;46(4):339-342.
40. Beuther D.A., Sutherland E.R. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661-666.
41. Bisgaard H., Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. 2007;42(8):723-728.
42. Jones C.A., et al. Predicting asthma control using patient attitudes toward medical care: the REACT score. Ann Allergy Asthma Immunol. 2009;102(5):385-392.
43. Ammon H.P., et al. Mechanism of antiinflammatory actions of curcumine and boswellic acids. J Ethnopharmacol. 1993;38(2–3):113-119.
44. Gupta I., et al. Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. Eur J Med Res. 1998;3(11):511-514.
45. Ram A., et al. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull. 2003;26(7):1021-1024.
46. Kobayashi T., et al. Curcumin inhibition of Dermatophagoides farinea-induced interleukin-5 (IL-5) and granulocyte macrophage-colony stimulating factor (GM-CSF) production by lymphocytes from bronchial asthmatics. Biochem Pharmacol. 1997;54(7):819-824.
47. Li C., et al. Effect of turmeric volatile oil on the respiratory tract. Zhongguo Zhong Yao Za Zhi. 1998;23(10):624-625.
48. Kurup V.P., et al. Immune response modulation by curcumin in a latex allergy model. Clin Mol Allergy. 2007;25(5):1.
49. Mascolo N., et al. Ethnopharmacologic investigation of ginger (I). J Ethnopharmacol. 1989;27(1–2):129-140.
50. Berthe Ahui M.L., et al. Ginger prevents Th2-mediated immune responses in a mouse model of airway inflammation. Int Immunopharmacol. 2008;8(12):1626-1632.
51. Ghayur M.N., et al. Ginger attenuates acetylcholine-induced contraction and Ca2+ signaling in murine airway smooth muscle cells. Can J Physiol Pharmacol. 2008;86(5):264-271.
52. Biltagi M.A., et al. Omega-3 fatty acids, vitamin C and Zn supplementation in asthmatic children: a randomized self-controlled study. Acta Paediatr. 2009;98(4):737-742.
53. Salam M., et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
54. Olsen S.F., et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1):167-175.
55. Mickleborough T.D., et al. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003;168(10):1181-1189.
56. Arm J.P., et al. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax. 1988;43(2):84-92.
57. Riccioni G., et al. Antioxidant vitamin supplementation in asthma. Ann Clin Lab Sci. 2007;37(1):96-101.
58. Tecklenburg S.L., et al. Ascorbic acid supplementation attenuates exercise-induced bronchoconstriction in patients with asthma. Respir Med. 2007;101(8):1770-1778.
59. Grievink L., et al. Serum carotenoids, alpha-tocopherol, and lung function among Dutch elderly. Am J Respir Crit Care Med. 2000;161(3 Pt 1):790-795.
60. Guizhou H.u., Cassano P.A. Antioxidant nutrients and pulmonary function: the Third National Health and Nutrition Examination Survey (NHANES III). Am J Epidemiol. 2000;151(10):975-981.
61. Geraets L., et al. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J Nutr. 2007;137(10):2190-2195.
62. Lim M., et al. Topical antimicrobials in the management of chronic rhinosinusitis: a systematic review. Am J Rhinol. 2008;22(4):381-389.
63. Erdman Jnr JW, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31–June 1, 2005, Washington, DC. J Nutr 2007;137(3 Suppl 1):718S–737S.
64. Manach C., et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S-242S.
65. Kaplan M., et al. Use of herbal preparations in the treatment of oxidant-mediated inflammatory disorders. Complement Ther Med. 2007;15(3):207-216.
66. Hsieh K.H. Effects of PAF antagonist, BN52021, on the PAF-, methacholine-, and allergen-induced bronchoconstriction in asthmatic children. Chest. 1991;99:877-882.
67. Page C.P. The role of platelet-activating factor in asthma. J Allergy Clin Immunol. 1988;81(1):144-152.
68. Kasperska-Zajac A., et al. Platelet-activating factor (PAF): a review of its role in asthma and clinical efficacy of PAF antagonists in the disease therapy. Recent Pat Inflamm Allergy Drug Discov. 2008;2(1):72-76.
69. Mix J.A., Crews W.D. A double-blind, placebo-controlled, randomized trial of Ginkgo biloba extract EGb 761 in a sample of cognitively intact older adults: neuropsychological findings. Hum Psychopharmacol. 2002;17:267-277.
70. Kennedy D.O., et al. The dose-dependent cognitive effects of acute administration of Ginkgo biloba to healthy young volunteers. Psychopharmacol. 2000;151:416-423.
71. Li M., et al. Effects of Ginkgo leaf concentrated oral liquor in treating asthma. Chung Kuo Ching Hsi I Chieh Ho Tsa Chih. 1997;17(4):216-218.
72. Shi C., et al. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against beta-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem Biol Interact. 2009;181(1):115-123.
73. Mahmoud F., et al. In vitro effects of ginkgolide B on lymphocyte activation in atopic asthma: comparison with cyclosporin A. Jap J Pharmacol. 2000;83(3):241-245.
74. Babayigit A., et al. Effects of Ginkgo biloba on airway histology in a mouse model of chronic asthma. Allergy Asthma Proc. 2009;30(2):186-191.
75. Nakamura T., et al. Effects of saiboku-to (TJ-96) on the production of platelet-activating factor in human neutrophils. Ann N Y Acad Sci. 1993;685:572-579.
76. Dorsch W., Wagner H. New antiasthmatic drugs from traditional medicine? Int Arch Allergy Appl Immunol. 1991;94(1–4):262-265.
77. Barrett B. Medicinal properties of echinacea: a critical review. Phytomedicine. 2003;10(1):66-68.
78. Poolsup N., et al. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials. J Clin Pharma Ther. 2004;29(1):37-45.
79. Bao Z., et al. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am J Res Cri Care Med. 2009;179(8):657-665.
80. Shen H.H., et al. Astragalus membranaceus prevents airway hyperreactivity in mice related to Th2 response inhibition. J Ethnopharmacol. 2008;116(2):363-369.
81. Johri R.K., et al. Effect of quercetin and Albizzia saponins on rat mast cell. Indian J Physiol Pharmacol. 1985;29(1):43-46.
82. Bone K.M. A clinical guide to blending liquid herbs. Philadelphia: Churchill Livingstone; 2003.
83. Nakajima T., et al. Inhibitory effect of baicalein, a flavonoid in scutellaria root, on eotaxin production by human dermal fibroblasts. Planta Med. 2001;67(2):132-135.
84. Niitsuma T., et al. Effects of absorbed components of saiboku-to on the release of leukotrienes from polymorphonuclear leukocytes of patients with bronchial asthma. Methods Find Exp Clin Pharmacol. 2001;23(2):99-104.
85. Shivpuri D.N., et al. A crossover double-blind study on Tylophora indica in the treatment of asthma and allergic rhinitis. J Allergy. 1969;43(3):145-150.
86. Shivpuri D.N., et al. Treatment of asthma with an alcoholic extract of Tylophora indica: a cross-over, double-blind study. Ann Allergy. 1972;30(7):407-412.
87. Thiruvengadam K.V., et al. Tylophora indica in bronchial asthma (a controlled comparison with a standard anti-asthmatic drug). J Indian Med Assoc. 1978;71(7):172-176.
88. Park H.J., et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int Immunopharmacol. 2009;9(3):261-267.
89. Broome C.S., et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80(1):154-162.
90. Zheng K., et al. Effect of dietary vitamin E supplementation on murine nasal allergy. Am J Med Sci. 1999;318(1):49-54.
91. Han S.N., et al. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100(4):487-493.
92. Malmberg K.J., et al. A short-term dietary supplementation of high doses of vitamin E increases T helper 1 cytokine production in patients with advanced colorectal cancer. Clin Cancer Res. 2002;8(6):1772-1778.
93. Jeong D.W., et al. Protection of mice from allergen-induced asthma by selenite: prevention of eosinophil infiltration by inhibition of NF-kappa B activation. J Biol Chem. 2002;277(20):17871-17876.
94. Claeson U., et al. Adhatoda vasica: a critical review of ethnopharmacological and toxicological data. J Ethnopharmacol. 2000;72(1–2):1-20.
95. Williamson E. Major herbs of Ayurveda. London: Churchill Livingstone; 2002.
96. Felter HW, Lloyd JU. King’s American dispensatory. Online. Available: http://www.henriettesherbal.com/eclectic/kings/extracta.
97. Chaabi M., et al. Anti-proliferative effect of Euphorbia stenoclada in human airway smooth muscle cells in culture. J Ethnopharmacol. 2007;109(1):134-139.
98. Laurenza A., et al. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci. 1989;10(11):442-447.
99. Burka J.F., Paterson N.A. A comparison of antigen-induced and calcium ionophore A23187 induced contraction of isolated guinea pig trachea. Can J Physiol Pharmacol. 1981;59(10):1031-1038.
100. Lichey I., et al. Effect of forskolin on methacholine-induced bronchoconstriction in extrinsic asthmatics. Lancet. 1984;2(8395):167.
101. Scientific Committee of the British Herbal Medicine Association. British herbal pharmacopoeia. Bournemouth: British Herbal Medicine Association; 1983.
102. Liu B., et al. Isoliquiritigenin, a flavonoid from licorice, relaxes guinea-pig tracheal smooth muscle in vitro and in vivo: role of cGMP/PKG pathway. Eur J Pharmacol. 2008;587(1–3):257-266.
103. Jaber R. Respiratory and allergic diseases: from upper respiratory tract infections to asthma. Prim Care. 2002;29(2):231-261.
104. Rowe B., et al. Magnesium sulfate is effective for severe acute asthma treated in the emergency department. West J Med. 2000;172(2):96.
105. Blitz M., et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. (4):2005. CD003898
106. Hill J., et al. Investigation of the effect of short-term change in dietary magnesium intake in asthma. Eur Respir J. 1997;10(10):2225-2229.
107. Gontijo-Amaral C., et al. Oral magnesium supplementation in asthmatic children: a double-blind randomized placebo-controlled trial. Eur J Clin Nutr. 2007;61(1):54-60.
108. Mickleborough T.D. A nutritional approach to managing exercise-induced asthma. Exerc Sport Sci Rev. 2008;36(3):135-144.
109. Bara A.I., Barley E.A. Caffeine for asthma. Cochrane Database Syst Rev. (4):2001;. CD001112
110. Harding S.M. Gastroesophageal reflux and asthma: insight into the association. J Allergy Clin Immunol. 1999;104(2 Pt 1):251-259.
111. Fukuda S., et al. Allergic symptoms and microflora in schoolchildren. J Adolesc Health. 2004;35(2):156-158.
112. Noverr M.C., Huffnagle G.B. The microflora hypothesis of allergic diseases. Clin Exp Allergy. 2005;35(12):1511-1520.
113. McCullough F.S., et al. The effect of vitamin A on epithelial integrity. Proc Nutr Soc. 1999;58(2):289-293.
114. Scheppach W., et al. Effect of free glutamine and alanyl-glutamine dipeptide on mucosal proliferation of the human ileum and colon. Gastroenterology. 1994;107(2):429-434.
115. Ban K., Kozar R.A. Enteral glutamine: a novel mediator of PPARgamma in the postischemic gut. J Leukoc Biol. 2008;84(3):595-599.
116. Hamman J.H. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13(8):1599-1616.
117. Speisky H., Cassels B.K. Boldo and boldine: an emerging case of natural drug development. Pharmacol Res. 1994;29(1):1-12.
118. Ali B.H., et al. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46(2):409-420.
119. Low Dog T. A reason to season: the therapeutic benefits of spices and culinary herbs. Explore (NY). 2006;2(5):446-449.
120. Borchers A.T., et al. Probiotics and immunity. J Gastroenterol. 2009;44(1):26-46.
121. Hodge L., et al. Assessment of food chemical intolerance in adult asthmatic patients. Thorax. 1996;51:805-809.
122. Ogle K., Bullocks J. Children with allergic rhinitis and/or bronchial asthma treated with elimination diet: a five year follow-up. Ann Allergy. 1980;44:273-278.
123. Carey O., et al. Effect of alterations of dietary sodium on the severity of asthma in men. Thorax. 1993;48(7):714-718.
124. Wickens K., et al. Fast foods—are they a risk factor for asthma? Allergy. 2005;60(12):1537-1541.
125. Rahman I., et al. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533(1–3):222-239.
126. Wood L., et al. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids. 2000;35(9):967-974.
127. Wood L.G., et al. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J. 2003;21(1):177-186.
128. Misso N., et al. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur Respir J. 2005;26:257-264.
129. Wood L.G., et al. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res. 2008;42(1):94-102.
130. Patel B., et al. Dietary antioxidants and asthma in adults. Thorax. 2006;61:388-393.
131. Schünemann H., et al. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. 2001;163(5):1246-1255.
132. Romieu I., et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166(5):703-709.
133. Rubin R., et al. Relationship of serum antioxidants to asthma prevalence in youth. Am J Respir Crit Care Med. 2004;169(3):393-398.
134. Chatzi L., et al. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax. 2007;62(8):677-683.
135. Castro-Rodriguez J.A., et al. Mediterranean diet as a protective factor for wheezing in preschool children. J Pediatr. 2008;152(6):823-828.
136. Singh V., et al. Barriers in the management of asthma and attitudes towards complementary medicine. Respiratory Med. 2002;96(10):835-840.
137. Chen E., Miller G. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21(8):993-999.
138. Wilson A., et al. Transcendental meditation and asthma. Respiration. 1975;32(1):74-80.
139. Chang Y., et al. Tai chi chuan training improves the pulmonary function of asthmatic children. J Microbiol Immunol Infect. 2008;41(1):88-95.
140. Vedanthan P., et al. Clinical study of yoga techniques in university students with asthma: a controlled study. Allergy Asthma Proc. 1998;19(1):3-9.
141. Manocha R., et al. Sahaja yoga in the management of moderate to severe asthma: a randomised controlled trial. Thorax. 2002;57(2):110-115.
142. Nagarathna R., Nagendra H. Yoga for bronchial asthma: a controlled study. Br Med J. 1985;291(6502):1077-1079.
143. Galantino M., et al. Therapeutic effects of yoga for children: a systematic review of the literature. Pediatr Phys Ther. 2008;20(1):66-80.
144. Thomas M., et al. Breathing exercises for asthma: a randomised controlled trial. Thorax. 2009;64(1):55-61.
145. Holloway E., West R. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax. 2007;62(12):1039-1042.
146. von Steinaecker K., et al. Pilot study of breathing therapy in groups for patients with bronchial asthma [in German]. Forsch Komplementmed. 2007;14(2):86-91.
147. Singh V., et al. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet. 1990;335(8702):1381-1383.
148. Smyth J., et al. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: a randomized trial. JAMA. 1999;281(14):1304-1309.
149. Lucas S.R., Platts-Mills T.A. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol. 2005;115(5):928-934.
150. Bonsignore M., et al. Effects of exercise training and montelukast in children with mild asthma. Med Sci Sports Exerc. 2008;40(3):405-412.
151. Fanelli A., et al. Exercise training on disease control and quality of life in asthmatic children. Med Sci Sport Exerc. 2007;39(9):1474-1480.
152. Welsh L., et al. Effects of physical conditioning on children and adolescents with asthma. Sport Med. 2005;35(2):127-141.
153. Beuther D., Sutherland E. Overweight, obesity, and incident asthma: a metaanalysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661-666.
154. Eneli I., et al. Weight loss and asthma: a systematic review. Thorax. 2008;63(8):671-678.
155. Cooper S., et al. Effect of two breathing exercises (Buteyko and pranayama) in asthma: a randomised controlled trial. Thorax. 2003;58(8):674-679.
156. Opat A., et al. A clinical trial of the Buteyko breathing technique in asthma as taught by a video. J Asthma. 2000;37(7):557-564.
157. Cowie R., et al. A randomised controlled trial of the Buteyko method as an adjunct to conventional management of asthma. Respir Med. 2008;102(5):726-732.
158. Bowler S., et al. Buteyko breathing techniques in asthma: a blinded randomised controlled trial. Med J Aust. 1998;169(11–12):575-578.
159. Ngai S., et al. A short review of acupuncture and bronchial asthma – western and traditional Chinese medicine concepts. Hong Kong Physiotherapy J. 2006;24:28-38.
160. Leake R.B., Broderick J. Treatment efficacy of acupuncture: a review of the research literature. Integr Med. 1999;1(3):107-115.
161. Chu K.A., et al. Acupuncture therapy results in immediate bronchodilating effect in asthma patients. J Chin Med Assoc. 2007;70(7):265-268.
162. McCarney R.W., et al. An overview of two Cochrane systematic reviews of complementary treatments for chronic asthma: acupuncture and homoeopathy. Respir Med. 2004;98(8):687-696.
163. Kaur B., et al. Vitamin C supplementation for asthma. Cochrane Database Syst Rev. (1):2009;. CD000993
164. Bielory L., Gandhi R. Asthma and vitamin C. Ann Allergy. 1994;73(2):89-96. quiz 96–100
165. Hatch G.E. Asthma, inhaled oxidants, and dietary antioxidants. Am J Clin Nutr. 1995;61(3 Suppl):625S-630S.
166. Fogarty A., et al. Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respir Med. 2006;100(1):174-179.
167. Schwartz J., Weiss S.T. Relationship between dietary vitamin C intake and pulmonary function in the First National Health and Nutrition Examination Survey (NHANES I). Am J Clin Nutr. 1994;59(1):110-114.
168. Britton J.R., et al. Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med. 1995;151(5):1383-1387.
169. Grievink L., et al. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax. 1998;53(3):166-171.
170. McKeever T.M., et al. Prospective study of diet and decline in lung function in a general population. Am J Respir Crit Care Med. 2002;165(9):1299-1303.
171. Gilliland F.D., et al. Children’s lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol. 2003;158(6):576-584.
172. Ammon H.P., et al. Inhibition of leukotriene B4 formation in rat peritoneal neutrophils by an ethanolic extract of the gum resin exudate of Boswellia serrata. Planta Med. 1991;57(3):203-207.
173. Singh G.B., Atal C.K. Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986;18(3–4):407-412.
174. Vogensen S.B., et al. Preparation of 7-substituted ginkgolide derivatives: potent platelet activating factor (PAF) receptor antagonists. J Med Chem. 2003;46(4):601-608.
175 Tang Y., et al. The effect of Ginkgo biloba extract on the expression of PKCalpha in the inflammatory cells and the level of IL-5 in induced sputum of asthmatic patients. J Huazhong Univ Sci Technolog Med Sci. 2007;27(4):375-380.
176. McHugh P., et al. Buteyko breathing technique for asthma: an effective intervention. N Z Med J. 2003;116(1187):U710.
177. Nagendra H.R., Nagarathna R. An integrated approach of yoga therapy for bronchial asthma: a 3–54-month prospective study. J Asthma. 1986;23(3):123-137.
178. McCarney R.W., et al. Acupuncture for chronic asthma. Cochrane Database Syst Rev. (1):2004;. CD000008
179. Biernacki W., Peake M.D. Acupuncture in treatment of stable asthma. Respir Med. 1998;92(9):1143-1145.