Chapter 5 Asthma
Introduction
The National Asthma Council (NAC) defines asthma as a ‘chronic inflammatory disorder of the airways in which many cells and cellular elements play a role, in particular, mast cells, eosinophils, T lymphocytes, macrophages, neutrophils, and epithelial cells’ and a ‘reversible narrowing of the airways in the lungs’.1, 2, 3 Asthma is characterised by allergic inflammation that is the major underlying abnormality affecting the airways.4 This inflammation leads to bronchial hyper-responsiveness to triggers, including infections, allergens and non-specific irritants.
Population-based studies indicate the incidence of asthma is more prevalent in Australia than in Europe or North America.5a Asthma is a significant health problem in Australia, affecting 10% of the population.5b In comparison to international standards, the prevalence of asthma in Australia is high. Studies indicate the prevalence of atopy (a genetic predisposition toward the development of immediate hypersensitivity reactions against common environmental antigens) is increasing worldwide.6
Complementary medicine (CM) use in asthma
With the high prevalence of asthma, it is not surprising that the use of CMs is common, with figures indicating more than 50% use in children and adult asthmatics.7–16 A survey of 48 multicultural parents of children with asthma in the US found up to 81% were using at least 1 form of alternative and complementary treatment for asthma.17 These included prayer, over-the-counter medications, herbal teas, vitamins, and massage. A review of the literature found the use of CM ranged from 4% to 79% in adults and 33% and 89% in children.18 Herbal medicine is commonly used in adults (11%) and children (6%) with asthma.19
An Australian study identified parental dissatisfaction with conventional therapy and concerns about side-effects from steroid use were the most common reason given by parents of children with asthma for using the complementary medicine (CM) and therapy.10
Another study found high CM use amongst children was associated with positive parental beliefs about CM and were significantly associated with greater risks for non-adherence and poorer asthma control.20 Population-based studies of adults found self-treatment with non-prescription products and CMs such as herbal, tea and coffee products is common 21 and associated with increased risk of reported hospitalisation possibly due to delay in utilisation of more efficacious treatments.22
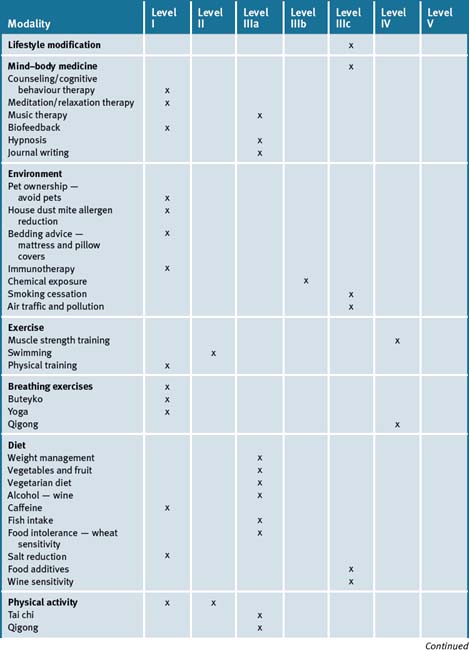
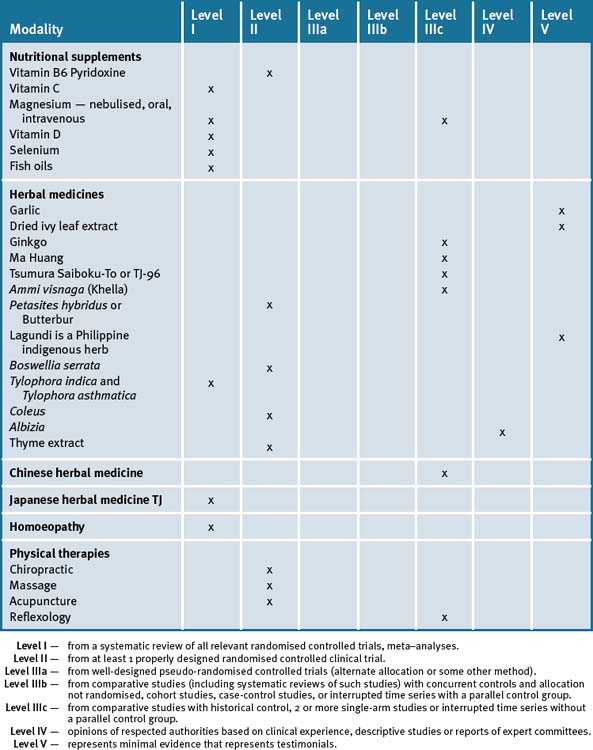
Whilst CM cannot replace drug therapy it may provide a useful adjunct (not alternative), to conventional care by improving quality of life for the asthmatic sufferer. The asthmatic patient still needs monitoring, an action plan, symptomatic relief and should not change their medication dosages without supervision by their doctor. Studies indicate practitioners may help enhance overall wellbeing of the asthma patient by providing advice in improving lifestyle, reducing risk factors and stress levels, appropriate dietary changes, using suitable supplements and changing the environment (see Table 5.1).
adapted from the National Asthma Council 20091
However, systematic reviews of the literature note the methodology of clinical trials with many complementary and alternative medicines are frequently inadequate, with positive results described from limited studies for herbals such as Tylophora indica, music therapy, buteyko, yoga, magnesium and conflicting evidence for homeopathy remedies.23, 24
Risk factors for asthma
Doctor–patient relationship
The doctor–patient relationship can influence communication about asthma management, health outcomes and compliance with medication. A study found that asthma outcomes improved when patient’s physicians encouraged them to participate in decisions about their health care, that contributed to improved quality of life, work disability and need for acute health services.25 Greater participation occurred most commonly with longer consultations and when the patient had seen the same physician for more than 6 months.
Affluent countries and economic development
A global research study evaluating over 54 000 people using standardised questionnaires identified the link between economic development and risk of asthma and atopic sensitisation.26 Children in affluent countries were significantly more likely to have an association with current wheeze, allergy-related asthma, and positive skin prick sensitivity compared with children in less affluent settings. Affluent children with allergic sensitisations were 4.0 times as likely to have asthma compared with non-sensitised children, whereas, children in non-affluent countries were only 2.2 times more likely to have an allergic response and asthma compared with non-sensitised children in the non-affluent countries.
Migration
A large-scale international study of 7794 Chinese adolescents in China and Hong Kong and 2235 Chinese adolescents living in Vancouver, Canada, found the prevalence of asthma was lowest in residents of mainland China compared with those of Hong Kong and those who immigrated to Canada or were born in Canada.27 The incidence for ‘current wheezing’ among boys and girls ranged from 5.9% and 4.3% in Guangzhou (mainland China) respectively, and 11.2% and 9.8% in Canadian-born Chinese adolescents respectively. The prevalence of ever having had asthma ranged from 6.6% (Guangzhou) to 16.6% (Vancouver) for boys and from 2.9% (Gangzhou) to 15.0% (Vancouver) for girls, suggesting asthma symptoms in Chinese adolescents were lowest among residents of mainland China and were greater for those born in Canada. The study also demonstrated that asthma prevalence was higher for Chinese born in Hong Kong and Chinese migrants in Canada. These findings suggest that environmental factors may influence asthma prevalence.
Research involving 211 Australian students who migrated from Asia, the South Pacific, the Middle East, Europe and Africa, demonstrated the prevalence of adolescent migrants living in Australia with asthma was higher compared with similar age groups in their home countries.28 The risk of developing asthma increased by 11% for every year they lived in Australia.
Travel
Travel is a risk factor for asthma — a study of 203 asthmatics visiting 56 countries found frequent use (> or = 3 times weekly) of inhaled bronchodilators before travel and participation in intensive physical exertion during treks significantly increased the risk of asthma attacks.29 Asthmatics should avoid intensive trekking.
Low socioeconomic status (SES)
A US twenty year follow-up study of adults and children demonstrated low socioeconomic status had a negative impact on lung function, after adjusting for smoking status, occupational exposures, and race.30 Researchers reported reductions of forced expiratory volume in 1 second (FEV1) of greater than 300 mL in men and 200 mL in women of low SES compared with high SES.
There are multiple risk factors that may contribute toward the causation and pathogenesis of asthma. Table 5.2 summarises causes and/or triggers of asthma. These are discussed in detail throughout the chapter.
| Atopy |
| Family history |
| Migration |
| Low socioeconomic status |
| Affluent countries |
| Caesarean section |
| Lack of breastfeeding, especially first 6 months of life |
| Serious respiratory infection, especially before age 5 |
| Medical health conditions, for example, viral respiratory infections, gastro-oesophageal reflux disease |
| More than 3 siblings |
General risk factors
A study of Australian rural NSW children aged 3–5 years estimated the prevalence of asthma between 18–22%, and identified clear risk factors for asthma.31 The risk factors which doubled the risk of developing asthma included: atopy; having a parent with a history of asthma; having had a serious respiratory infection in the first 2 years of life; and a high dietary intake of polyunsaturated fats. Protective risk factors include breastfeeding and having 3 or more older siblings.
Other risk factors for asthma include: family history (maternal asthma odds 2.4; paternal 2.1); smoking (1.7 fold); serious respiratory infection before age 5 (2.3 fold); positive skin test to cladosporium (mould), house dust mite (HDM), cat and rye grass pollen; and occupational exposure to allergens.32 Avoidance to these irritants may be of help (see Table 5.3).
Table 5.3 Possible allergen triggers especially in high risk asthmatics33
| Ordinary cow’s milk-based formula in young infants |
| Tobacco smoke |
| Pets e.g. cats, birds, dogs |
| House dust mite (HDM) |
| Cladosporium (mould) |
| Grass pollens — rye |
| Food — baking flour, wheat, milk, nuts, peanut, soy, egg, some fish and shellfish, polyunsaturated fats |
| Chemicals and gases — paint fumes, solvents, latex, synthetic bedding, chlorine |
| Pollution — diesel, wood heater, wood dust |
Caesarean section
Children born by caesarean section are at a higher risk of developing asthma later in life, and the risk was higher if either or both parents were atopic.34
Breastfeeding
Exclusive breastfeeding in the first 3–4 months of life significantly reduces the risk of asthma and atopy. Multiple studies including systematic reviews and meta-analyses have consistently supported this association. 35–39 Also duration of breastfeeding is significant with longer duration greater than 9 months being protective towards risk of asthma and wheeze.40 Furthermore, a prospective birth-cohort study of over 2000 children from antenatal clinics up to 6 years of age, demonstrated children given non-human breast milk in the first 4 months of life were 40% more susceptible to wheeze 3 or more times since 1 year of age.41
The beneficial effect is postulated to be caused by immunomodulatory qualities of breast milk, avoidance of allergens, or a combination of these factors. Interesting, whilst breastfeeding was protective, the association of asthma and breastfeeding was increased in atopic children of asthmatic mothers after 6 years of age.42 Another study failed to demonstrate protective effects of breastfeeding.43 A study of the maternal diet of breastfeeding mothers demonstrated that atopic mothers had a higher intake of total fat and saturated fat and a lower intake of carbohydrate as a percentage of total energy intake compared with non-atopic mothers and was associated with atopic sensitisation of the infant which may explain the higher risk.44 Also higher intake of food allergens may also contribute to this association in atopic mothers.45
Breast milk can contain significant numbers of bifidobacteria with studies demonstrating maternal fecal and breast milk bifidobacterial counts impact on the infants’ fecal Bifidobacterium levels and provide an important source of bacteria in the establishment of infantile intestinal microbiota. A study found allergic mothers had significantly lower amounts of bifidobacteria in breast milk compared with non-allergic mothers and this may impact on the risk of the infant developing allergic disease.46
Of 448 children with a parental history of atopy, children put to bed with a bottle in the first year of life were at higher risk of wheezing at 1–5 years of age.47 The researchers postulated that bronchospasm may be caused by repeated airway irritation due to postprandial reflux.
Antibiotic use and early exposure to respiratory infections
Research indicates that antibiotic use in early infancy (i.e. first 2 years of life) is associated with a 2–3 fold increased risk of developing asthma and hay fever, recognising the role of infections in protecting against asthma.48 The study found that the number of courses of antibiotics during the first year of life was also associated with significant increased risk of asthma with 1–2 courses, but particularly with 3 or more courses when compared with no antibiotic use in the first year of life. In another study involving 1035 children followed up since birth, young children exposed to older children at home or to other children at day care are at increased risk for infections, which in turn may protect against the development of allergic diseases and asthma later in childhood. 49 Another study also found a significant association between antibiotic use and day care in the first year of life and wheezing at 7 years of age.50 Similarly antibiotic use in the first year was significantly associated with greater risk of asthma at age 7 years, particularly with the number of antibiotic courses.51 Compared with children who did not have antibiotics, children who received more than 4 courses of antibiotics were 1.5 times more likely to develop asthma.
Overall, research assessing children who enter day care at an early age, demonstrates exposure to infections in early childhood may prevent and lower the risk of allergy later in life.52, 53 This supports the theory that exposure to infections early in life determines the way the immune system is stimulated, with T-helper cells geared toward fighting infection rather than produce cytokines that promoted allergy.
However, more recent studies found no association of antibiotic use and asthma prevalence suggesting that the reason that some children who’ve been given antibiotics appear to develop asthma is because the symptoms of the chest infection in young children can be confused with the start of asthma.54, 55
Medication
A retrospective study demonstrated that children medicated with paracetamol in the first year of life, are at higher risk of developing asthma; by up to threefold for frequent use.56 Similarly another study found regular paracetamol intake in pregnancy has also been associated with greater risk of developing asthma by 60%, and even up to 85%, in offspring.57 The mechanism behind this finding is not clear. There are many medications known to aggravate asthma such as beta-blockers, non-steroidal anti-inflammatories, aspirin and even the oral contraceptive pill which should be used with caution or avoided in asthmatics.58
The modern diet and obesity
The modern diet low in fresh foods such as fruit, vegetables and fish may be responsible for the rise in asthma. Obesity may be a contributor to allergies. Obese children are at greater risk of asthma, especially girls, according to the UK National Study of Health and Growth in London, which surveyed 15 000 children, aged 4 to 11, independent of ethnicity.59 Obese children are 26% more likely to be more atopic, and sensitivity to milk was at least 50% higher than in normal-weight children.60 Mean total IgE levels were higher among obese and overweight children than normal-weight children. The risk for atopy (any positive specific IgE measurement) was increased in the obese children largely driven by allergic sensitisation to foods.
Also, abdominal adiposity is known to compromise respiratory lung capacity.61, 62, 63
Mattresses
Sleeping on used cot mattress in the first year of life is associated with increased risk of asthma.37 The study assessed 871 New Zealand children of European descent at birth and ages 12 months, 3.5 years and 7 years. The study found that 24% of children suffered from wheezing at 3.5 years and 18% at 7 years when they had a history of sleeping on a used cot mattress. In this study, other than the use of a used cot mattress for sleeping, asthma was also associated with maternal smoking during pregnancy, being in day care, antibiotic use, and the presence of a dog.
Stressful events
Children born to mothers who suffer chronic stress during their early years have a higher risk of asthma rate compared with their peers according to a cohort study of 14 000 children. This finding was independent of income, gender, maternal asthma, urban location or other known asthma risk factors.64 The mechanism for how maternal distress causes asthma is not well understood although depressed mothers were more likely to smoke, less likely to breastfeed and less likely to interact with their infants.
Maternal stress and anxiety in pregnancy during fetal life is a risk factor for asthma during childhood. A longitudinal study of 5810 children recruited during pregnancy found a higher incidence of asthma in children at age 7 with mothers who experienced highest levels of anxiety at 32 weeks gestation compared with mothers with lowest levels of anxiety.65 Poor coping in parenthood is also a known risk factor. Of 150 middle- to upper-class children followed up from birth to 6–8 years of age, one-quarter developed asthma. Both serum IgE levels and parenting difficulties were associated with increased risk of asthma. The researchers concluded that emotional stress may alter both immune and respiratory responses and their results ‘should reinforce the importance of providing support and education to new parents and their children’.65
Acute stressful events and negative life events increase the risk of asthma exacerbations in a study of 90 primary-school Scottish children, particularly in children suffering multiple chronic stressors.66 Asthma was self-monitored twice a week for 3 months. Acute negative events, such as family break-up and death of a grandparent increased the risk of suffering asthma. The risk of asthma attacks increased significantly when the acute stress was added to chronic stress such as poverty, family discord, parental substance abuse or being bullied at school.67
Post–traumatic stress disorder (PTSD)
A study of 3065 male twin pairs (monozygotic and dizygotic twins), who lived together in childhood and served on active military duty during the Vietnam War, found those who suffered greater PTSD symptoms were 2.3 times as likely to have asthma compared with those who suffered from the least PTSD symptoms.69 These findings suggest the emotional association between asthma and PTSD occurred despite twins sharing similar genes. The authors postulated that traumatic stress compromises the immune functioning independent of genes.
Mind–body medicine
General
A review of the literature noted psycho-educational self-management programs, relaxation therapy, biofeedback, and family therapy to be particularly useful in improving asthma outcome and significant beneficial effects were found for relaxation therapy. The researchers also identified biofeedback for ‘respiratory resistance, trachea sounds, and vagal tone’ to show promise.70
A Cochrane review of psychological interventions for adults with asthma identified 15 studies, involving 687 participants, aimed at determining the effect of cognitive behaviour therapy (CBT) on quality of life, and biofeedback and relaxation therapy on pulmonary function, FEV1 and medication use.71 They found conflicting results but overall quality of life improved with CBT and reduced the use of ‘as needed’ medications. Relaxation therapy also reduced the need for ‘as needed medications’ although no significant differences in FEV1 was found. Biofeedback improved asthma and lung functions such as peak expiratory flow rate and FEV1 in 2 studies.
Another Cochrane review assessing psychological interventions for children with asthma found conflicting results amongst the 12 studies of 588 children. Two studies examining the effects of relaxation therapy on peak expiratory flow rate (PEFR) significantly favoured the treatment group (32 L/min, 95% CI 13 to 50 L/min).72
Spiritual healing
An innovative study tried to examine if spiritual healing could assist asthma. Spiritual healing was of no benefit for asthma symptoms when compared with sham healing and control.73
Psychological counselling
Depression and anxiety are more prevalent in severe asthmatics and associated with greater morbidity, mortality and non-compliance, requiring close psychological monitoring and the potential role for effective counselling in young asthmatic patients.74, 75, 76 One study of over 1300 youths aged 11 to 17 years found young people with asthma were twice as likely to suffer depression and anxiety disorders compared with children without asthma.77
A population survey of 3010 participants aged 15 years and over found the prevalence of asthma was 9.9% and identified major depression was significantly higher for those who experienced dyspnoea, wakening at night from asthma, morning symptoms of asthma and reduced quality of life compared with those who did not suffer asthmatic symptoms.78
A case-controlled study of 21 patients from 8 to 18 years of age with severe asthma who were hospitalised and who died of asthma subsequent to discharge identified psychologic risk factors were prominent, such as conflicts between patients’ parents and hospital staff regarding medical management, depressive symptoms and disregard of asthmatic symptoms.79
Psychological approaches such as counselling and biofeedback-assisted relaxation therapy were found to be particularly useful in improving mood and asthma.80, 81, 82
Family therapy
Asthma can be quite stressful for families and can impact parenting and interaction between family members.83, 84, 85 Children of parents who experience major depression or panic attacks are also more likely to develop atopic disorders by 67% and 46% respectively.86 Children with a genetic predisposition to asthma are 3 times more likely to express the illness with domestic stresses and parenting. 87, 88 Research supports the role of family therapy in asthma management.89
Relaxation therapy and meditation
Given that stress may exacerbate asthma, there is a role for stress management in the prevention and management of asthma. A review of the literature of variable quality studies demonstrated that stress can produce bronchoconstriction.90
Relaxation therapy is associated with respiratory muscle relaxation, reduced panic, reduced airway reactivity, improved lung function, promotion of diaphragmatic breathing, reduction in metabolic rate and may have an anti-inflammatory effect. A study found transcendental meditation (TM) can improve respiratory function.91 This 6 month study, with cross-over at 3 months, demonstrated TM can be a useful adjunct to asthma treatment.
However, a UK systematic review of the literature found overall, the methodological quality of the 15 randomised clinical trials (RCTs) on relaxation therapy assessed were poor and better quality studies are required.92
In addition to the abovementioned research, it was found that music therapy can be particularly helpful for relaxation and asthma.93
Biofeedback
Fear and panic are common emotions experienced by asthmatics.94, 95, 96
A review of the literature identified negative emotions such as panic and generalised panic disorder are common in asthmatics. These negative emotions can also affect asthma morbidity.97 They identified self-regulation strategies as useful adjuncts to asthma treatment such as relaxation therapy, electromyographic (EMG) biofeedback, biofeedback to improve sensitivity in perceiving symptoms, and biofeedback training for increasing respiratory sinus arrhythmia. Relaxation-oriented methods were more beneficial amongst asthmatics with panic symptoms.
A number of studies demonstrated that biofeedback is helpful for dealing with asthma symptoms in children and adults.98, 99 A 15 month follow up of asthmatics that included a comprehensive multi-behavioural and desensitisation retraining program using EMG and spirometer feedback to encourage slow diaphragmatic breathing in all situations, demonstrated subjects reported reductions in their asthma symptoms, medication use, emergency room visits, and breathless episodes.100
Electromyographic feedback methods can also be useful by providing feedback on muscle tension and strain.101, 102
Hypnosis
A prospective, randomised, single, blind controlled trial of 39 adults with mild to moderate asthma after a 6-weekly course of hypnotherapy demonstrated a 75% improvement in the degree of bronchial hyper-responsiveness, improved peak expiratory flow rates by 5.5%, and reduction of use of bronchodilators by 26% in the more highly susceptible subjects to hypnosis.103 Daily home recordings of symptoms also improved.
Another study of severe chronic asthmatics inadequately controlled with medication undergoing relaxation induced hypnotherapy reported: improved asthma symptoms; a significant reduction in hospital admissions, from 44 to 13 a year in total; reduced duration of hospitalisation; and reduction of prednisolone use and side-effects experienced from medication.104 Air flow tests remained variable among subjects.
Journal writing
A study of patients with mild-moderate asthma or rheumatoid arthritis demonstrated that writing about emotionally traumatic life experiences over a 4-month period can help reduce chronic disease symptoms.105 With asthma, lung function test forced expiratory volume in 1 second (FEV1) improved from 63.9% at baseline to 76.3% at the 4 month follow-up period of journal writing, with no change in the control group. The rheumatoid arthritis patients also did well with significant reduction of symptoms and disease activity compared with the control group.
In an RCT 137 adult asthmatic patients were randomly assigned to write for 20 minutes, once per week for 3 weeks, about stressful life experiences (n = 41), positive experiences (n = 37), or neutral experiences (n = 36; control group).106 The study found only marginal benefit for FEV1 and for forced vital capacity (FVC) between each group: the stress-writing group demonstrated 4.2% FEV1 and 3.1% FVC improvement, the positive-writing group 1.3% FEV1and 3.6% FVC, and in the control group 3.0% FEV1 and 2.4% FVC.
Laughter
Laughter and excitement can also trigger asthma, such as cough and dyspnoea, with cohort studies suggesting the incidence is common, estimated at 32% in young asthmatics.107 The researchers examined 285 children who had experienced an acute asthma attack and found ‘mirth-triggered’ asthma, especially cough symptom, occurred within 2 minutes of laughing.
Mirth-triggered asthma is an indication of sub-optimal asthma control and treatment.
Pet ownership
Pet keeping in early childhood can impact on allergies and asthma depending on the type of pet, the age and the allergic sensitisation of the individual.108 A review of the literature found recent published studies have produced heterogeneous results.109 Sensitisation to pets is a risk factor for asthma and can occur in children who live in homes with no pets and with low levels of pet. allergen. The authors of the review conclude ‘excluding pets from the home will not necessarily protect children from the development of sensitisation to pets’.
Another systematic review found exposure to pets increases the risk of asthma in children over 6 years of age.110
A large international survey of almost 19 000 adults found the effects of having pets during childhood varied according to the type of pet, the allergic sensitisation of each subject and pet prevalence in the community.111 The survey indicated that keeping a cat doubled the risk of asthma, but only among atopic subjects.
Exposure to a dog in early infancy may protect against atopy due to mediation of cytokine response.106, 112 Further research studying 285 infants demonstrated that children who grow up with a pet dog were less likely to develop allergic sensitisation and atopic dermatitis.113 The rate of allergic sensitisation was 14% lower and the rate of atopic dermatitis 21% lower in children with a dog compared with children with no dog. Similarly, studies suggest early exposure to pets can be protective towards asthma development.114, 115
A recent study of 275 children (3 years of age) at increased risk of developing allergic diseases found exposure to dogs in infancy, and ‘especially around the time of birth, is associated with changes in immune development and reductions in wheezing and atopy’.116
A community sample of 3181 adults aged 26–82 years found keeping a cat or dog in childhood was associated with increased risk of dyspnoea or breathing difficulties.117 Based on current evidence there are mixed findings to pet ownership but it appears dogs may offer some benefit in reducing development of asthma risk in atopic children.
Environment
Thunderstorms
Asthma epidemics may be linked with thunderstorms as pollen counts are high during these times. A deluge of grass pollen during thunderstorms may be the cause of epidemic asthma. It may be wise for asthmatics to stay indoors during thunderstorms. The risk of symptoms doubled if asthma patients were outdoors during thunderstorms, according to an analysis of data of 183 patients following a spring storm.118 Asthmatics sensitive to thunderstorms also tend to suffer hay fever and are allergic to rye grass pollen compared to control groups.119, 120
Farm children
A study surveyed 1333 farmers’ children and a reference group of 566 children aged from 5 to 17 years and found that children with both prenatal and current exposure to farm animals and plants were up to 50% per cent less likely to have allergies such as asthma, eczema and hay fever than the reference group.121
House dust mite (HDM)
House dust mite (HDM) is a major allergen found in house dust. There are various methods available to reduce HDM allergens by chemical or physical means, with the aim to reduce asthma symptoms in those who are sensitive to HDM. There are many studies finding links between HDM and allergic and asthma symptoms. Seasonal fluctuations of HDM numbers determined by humidity in children’s beds may contribute to fluctuations and influence asthma symptoms.122
A large-scale prospective birth-cohort study of 939 newborns, followed up until 7 years of age, cast doubt on the theory of early exposure to HDM allergens as a primary cause of asthma in young children.123 The study did show that children with asthma were more likely to be sensitised to HDM and cat allergens, but those were not the ‘cause’ of asthma.
Bedding and mattress protectors
Higher HDM antigen levels in bedding appear to be a risk factor for persistent bronchial hyper-reactivity in adolescence.124 Exposure to HDM in temperate climates is the strongest environmental risk factor for asthma. A study of 616 pregnant women were randomised to HDM intervention by using impermeable mattress covers and an acaricide washing detergent for bedding. When compared with the control group, these methods were effective in reducing HDM allergens.125
Mattress covers
A European trial involving 636 highly atopic children aged 1.5 to 5 years, with negative skin tests to HDM, were less likely to be sensitised to HDM with a combination of education and a simple preventive measure (mattress encasement) to reduce mite allergen exposure compared with the control group after 1 year.126 Sensitisation to mite allergens was tested by skin-prick test or measured by serum specific immunoglobulin E. Another study also demonstrated allergen-impermeable mattress encasings versus placebo mattress encasings were significantly more effective in reducing HDM allergen levels.127 However, 1 RCT of 47 children found the use of special allergen-occlusive bed covers of little benefit in asthmatic children whose symptoms were triggered by HDM.128
One review of the evidence found that in homes of high-risk atopic infants, the current evidence supports measures to reduce the levels of indoor allergens such as HDM and pets by using mattress and pillow encasings.129 When compared to placebo, semipermeable polyurethane mattress and pillow encasings (allergy control) resulted in a significant perennial reduction of HDM exposure and a significant reduction in the required dose of inhaled steroids by asthmatics.
Synthetic bedding
A number of studies, including cohort studies, demonstrate a clear association between synthetic bedding (e.g. pillow and quilt) and frequent childhood wheeze episodes.130, 131 Children who sleep with synthetic pillows (foam, sponge, polyester, dacron) and quilts are 5 times more likely to have frequent wheezing according to a Tasmanian study of 900 7-year olds.123
A study looking at 758 school children aged 8–10 years demonstrated that those sleeping with synthetic bedding (quilts or pillows) had adverse respiratory outcomes, more asthma and recent wheeze, more than 12 wheezing episodes during the year, and twice the risk of allergic rhino-conjunctivitis if they had a positive skin-prick test than children with a negative test.132
Synthetic bedding (e.g. quilts and pillows) should be avoided in atopic individuals and asthmatics.133
Feathered pillows
It is best to avoid synthetic pillows and use feather pillows as they are associated with a significantly lower rate of wheezing and sensitisation to HDM according to a number of studies.134, 135 Synthetic pillows accumulate more HDM than feathered pillows which may account for the benefit of feather pillows.136–139
Effective pillow encasings can be useful for reducing HDM numbers.
Laundry washing
Washing laundry at 25 degrees Celsius for at least 5 minutes was sufficient to remove most of the cat and mite allergens, according to an Australian study.140 There were no distinct differences between which laundry detergents were used and washing at 60 degrees Celsius reduced more allergens, though the benefits were slight.
Physical and chemical methods
A Cochrane review of 54 trials (3002 patients) of which 36 trials assessed physical methods (26 mattress encasings), 10 chemical methods, and 8 a combination of chemical and physical methods, found most trials were of poor quality and the interventions were statistically of no benefit for health outcomes and asthma symptom score.141 Similarly, a report published in Cochrane PEARLS (Practical Evidence About Real Life Situations) concluded HDM control measures may not reduce asthma symptoms.142
A study found successful attempts to reduce environmental allergens such as avoidance of HDMs, pets, tobacco smoke, promoting longer breastfeeding and avoiding early introduction of foods significantly reduced the risk of asthma by 60% and wheeze by 90% in 2-year olds compared with the control group.143 Table 5.4 provides simple strategies for reducing allergen exposure at home.
Table 5.4 Simple measures for reducing environmental allergens
| Put toys away in cupboards |
| Wipe surfaces weekly with a damp cloth |
| Steam clean carpets bi-annually; use built-in vacuum cleaner |
| Damp mop hard floorboards |
| Hot wash bedding — add a few drops of Eucalyptus oil to wash |
| Place bedding in dryer for 15 minutes weekly |
| Use natural bedding e.g. feathered or cotton pillows and doonas |
| Dust mite covers for bedding |
| Allow sun into house and air house out regularly — daily if possible |
| Avoid smoking inside house |
| Avoid pets in house |
Allergen immunotherapy
Allergen immunotherapy also known as ‘desensitisation’ can occur by injection or sublingual.
The National Asthma Council contains good guidelines on injectable allergen-specific immunotherapy which usually occurs subcutaneously in the skin and should be performed by experienced medical practitioners.144 This is a process that involves gradual increases in quantities of an allergen extract, which modifies the immune response to help reduce airway inflammation and improve asthma control.
A Cochrane review including 75 trials of 3506 participants (3188 with asthma) demonstrated overall immunotherapy significantly reduced asthma symptoms, the use of asthma medications, and improved bronchial hyper-reactivity, with 1 study demonstrating effectiveness equal to inhaled steroids.145 Trials of immunotherapy were tested for HDM allergy, pollen allergy, animal dander, Cladosporium mould allergy, latex and multiple allergens. The authors warned good patient selection was essential as the risk of side-effects included bronchospasm, local reaction, allergic reactions and sometimes anaphylaxis.
A meta-analysis of 9 studies including a total of 441 patients randomised to sublingual immunotherapy (SLIT) or placebo found, overall, there was a significant reduction in both symptoms and medication use following SLIT.146
A study of 253 children suffering grass pollen–induced rhinoconjunctivitis with/without asthma, demonstrated significant improvement with grass tablet Grazax compared with placebo.147 The tablet was generally tolerated, with pruritus the commonest reaction reported by 32% of subjects compared with 2% in the placebo group. Six subjects withdrew due to adverse events.
Damp houses and mould
Dampness in the house is also a risk factor for asthma.
A Munich study of 234 children with asthma, demonstrated that living in damp houses plays a significant risk factor for asthma with increased nocturnal wheeze, shortness of breath and persistence of bronchial hyper-reactivity in adolescence.148 This risk was not attributed or explained by exposure to HDM antigen.
The Skorge et. al. study also found an association of mould exposure with all the respiratory symptoms such as cough and dyspnoea with 3–5% of the frequency of the respiratory symptoms in the study population attributed to exposure to visible moulds.117 Of interest, fitted carpet in the bedroom reduced the risk of respiratory symptoms.
A population-based case–control study that involved researchers visiting and assessing the homes of 121 asthmatic children and 241 controls found visible moisture damage and mould growth in the main living quarters (living room or child’s bedroom) was significantly associated with the development of asthma in early childhood.149
Occupational asthma and chemical exposure
Occupational asthma is caused by reactions to allergens in the workplace.2 The report, Occupational Asthma in Australia, indicates 9–15% of adult-onset asthma cases can be attributed to exposure to causal agents at work such as wood dust, paint fumes, solvents, latex and baking flour.150 The commonest causes of occupational asthma in Australia are wood dust from trees such as the Western red cedar, isocyanates (the raw materials used in polyurethane products), paint fumes, solvents, latex, and flour.
One of the largest population studies of occupational asthma that analysed data on 15 637 randomly selected people aged 20–44 from 26 areas of 12 industrial countries estimated the incidence of occupational asthma in young people at 5–10%.151 A recent population-based study of 13 countries also found that at least 10% of adult onset asthma is related to occupational allergens.152 Those at greatest risk had a history of atopy or parental asthma. High-risk occupations included farmers, painters, plastic workers, cleaners, spray painters and agricultural workers commonly exposed to chemical substances. Asthma risk was also associated with high exposure to dusts, gases and fumes. People who work in commercial greenhouses are also at risk of occupational asthma if they are sensitised to the flowers they grow, according to German researchers.153
Professional and domestic cleaning are also associated with aggravation or inducing asthma due to chemical exposure from cleaning products.154, 155 A 9-year European study of 3503 adults free of asthma at baseline, found 42% of the sample study who used cleaning sprays at least once weekly increased the risk of asthma symptoms by 49% and wheeze by 39%.156 The risk increased with increasing use of cleaning sprays but not liquid products. Glass and furniture cleaning sprays and air-refreshers posed the greatest risk to asthma. The overall prevalence of adult onset asthma related to cleaning sprays was estimated at 15% by researchers.
A study of 4500 Spanish women aged 30–65 years found those who had worked in domestic cleaning had a higher rate of all respiratory symptoms, including asthma compared to those who had never worked in domestic cleaning.157 It was not clear whether exposure to dust mites, cleaning products or other allergens contributed to the increased risk.
Smoking
The association between smoking (passive and active) and asthma is strong and well accepted. The beneficial effects of smoking cessation on lung function are well established. Smoking should be avoided by asthmatics and smoking indoors avoided by other household members (e.g. parental smoking), as even passive smoking may cause or aggravate asthma.158
Traffic and air pollution
Air pollution is associated with impaired health, including reduced lung function in adults and children.159 Air pollution is commonest in major cities and some industrialised areas. Motor vehicles are the main source of air pollution due to particles in suspension, particularly those that use diesel fuel. Air pollution compromises respiratory function. Moving to polluted areas may aggravate lung function in children and adults. A number of studies demonstrate benefit to lung function when children move to cleaner geographic areas.
Epidemiological studies demonstrate an association between the degree of traffic exposure and lung function in asthma. A large-scale study of city school children primarily based in the centre of Oxford (England) demonstrated a statistically significant improvement in peak expiratory flow (PEF) rate and respiratory symptoms among children living where traffic on roads decreased compared to those living where the traffic increased.160
Exposure to traffic pollution, particularly diesel exhaust, is associated with impaired lung function in asthmatics and the rise of atopy.161, 162
A recent prospective birth cohort study based in Germany of children at the age of 4 and 6 found a strong positive association was found between the distance to the nearest main road and asthmatic bronchitis, hay fever, eczema, and sensitisation, especially in those living less than 50 metres from busy roads.163
A study of 460 children in Holland found episodes of wheeze and shortness of breath increased by 140% when exposed to airborne pollutants of small particulate matter and when other pollutants, such as sulphur dioxide and nitrous oxides, were highest.164 The study found children with bronchial hyper-responsiveness and high concentrations of serum total IgE were more susceptible to health problems from air pollution.
Climate change
Climate change gives rise to variations in temperature patterns that impact on plants by varying pollination times and favouring some plant species over others. The presence of high CO2 concentrations and temperatures can increase pollen counts by plants with the subsequent risk of allergic sensitisation in the human population.165 For example, an environmentally controlled greenhouse mimicking climate changes by doubling the atmospheric CO2 concentration stimulated ragweed-pollen production by 61% suggesting significant increases in exposure to allergenic pollen under the scenario of global warming.166
A prospective study of 9651 adults (18 to 60 years of age) randomly selected from population registries found an association between indoor and outdoor pollution (particulate air pollution) and decline in lung function, especially of small airway function.167
Street trees
Children living in areas with more tree-lined streets have lower rates of asthma and a lesser risk of developing asthma, as trees improve air quality or encourage children to play more outdoors.168 Street trees were associated with a lower prevalence of early childhood asthma.
Indoor pollution
Indoor pollution may play a role in the pathogenesis of childhood asthma but not as the cause of asthma in childhood.169 There are potentially multiple indoor allergens that can cause sensitisation such as dust mite, cockroach, pet, rodent allergens, and indoor air pollutants; for example, ozone, particulate matter, nitrogen dioxide, environmental tobacco smoke, sulfur dioxide, and carbon monoxide.170
A study of 409 children in 5 New Zealand communities between the ages of 6 and 12 with diagnosed asthma, were assessed before and after more effective heating was installed in their homes.171 After installing better heating such as heat pumps, flued gas heaters or pellet burners, children demonstrated improved health, less sleep disturbance, reduced asthma symptoms such as wheezing, less coughing at night and overall improved respiratory symptoms. Consequently they had fewer sick days off school and less doctor visits.
A cohort study of children (2–6 years of age) monitored the air in their bedrooms for 3 days to assess the level of indoor pollution specifically for particulate matter, nitrogen dioxide, and ozone.172 They found the level of bedroom air pollutant concentrations did not differ significantly between asthmatic and non-asthmatic subjects. Whilst these substances may aggravate asthma, the study did not support the causative role of these factors for developing asthma.
Overall a systematic review of the literature supports a link between housing improvement, such as rehousing, refurbishment, and energy efficiency measures and health gains after the intervention.173
Of interest, exposure to airborne inhaled allergens released during cooking can provoke asthma in atopic children allergic to foods.174 The study identified inhaling allergens from foods such as fish, chickpeas, and buckwheat during cooking, and even opening packets of peanuts in a confined space, can provoke an asthma attack. Asthmatics with food allergies need dietary advice and need to be aware of environmental measures that may be required to limit exposure to aerosolised food.
Winter air pollution — wood smoke
High wood smoke for home heating causing indoor air pollution, as measured by particulate matter in smoke, can affect the health of boys with asthma by reducing lung function.175 The boys who didn’t have respiratory problems such as asthma were not affected, although all the students (with and without asthma) coughed slightly more on high pollution nights from wood chips.
Installing non-polluting heating in the homes of children with asthma can significantly reduce symptoms of asthma, days off school, visits to health care workers and chemists without significantly improving lung function.176
Swimming pools — chlorine
Swimming offers an excellent way to build up fitness and can improve lung function. However, a number of studies demonstrate exposure to indoor and outdoor chlorinated swimming pools can be detrimental to the airways of swimmers and pool attendants and can be associated with higher risks of asthma, airway inflammation and some respiratory allergies.177–181
In addition, case reports describe occupational asthma in swimming pool attendants, due to poor air quality above indoor chlorinated swimming pools.182 It would appear swimming in non-chlorinated pools such as ozone-treated pools or the beach is preferred for asthmatics.
Exercise
Lack of exercise in Western society, including lack of outdoor activity by children, may contribute to rising incidence of asthma.183 Exercise improves cardiovascular fitness and quality of life in asthmatics.
Trekking
Young adults with mild to moderate asthma are at risk of aggravating their symptoms with high-altitude trekking due to cold, dry air and exposure to new allergens. Of the 88 high-altitude trekkers surveyed, 45% noted their asthma worsened, 37% experienced the worst attack in their life and 11% experienced a life-threatening attack.184
Swimming
While some asthmatics suffer exercise-induced bronchoconstriction, a number of trials have demonstrated the benefits of exercise, such as swimming, that improves aerobic capacity, in the management of asthma particularly.185 Review of available evidence suggests that swimming induces less severe bronchoconstriction than other sports, due to the high humidity of inspired air at water level. Based on the findings of chlorine induced asthma, it is preferable to swim in non-chlorinated pools, such as ozone-treated pools.
Yoga
A double-blind, controlled trial of 56 adult asthmatics on maximum doses of inhaled steroids for poorly controlled asthma were randomised to sessions of Sahaja yoga, a traditional form of ‘yoga meditation’ or a control group for 2 hours on a weekly basis for 4 months. Yoga provided significant benefits in improving asthma hyper-responsiveness at the end of each treatment.186
University students who practised yoga techniques 3 times a week for 16 weeks reported a significant degree of relaxation, positive attitude, better yoga exercise tolerance and lesser usage of beta adrenergic inhalers compared with control groups, but no significant difference in pulmonary function measured with spirometry between the 2 groups.187
A study of 53 asthmatic patients compared with 53 control patients practising 65 minutes of daily yoga over 2 weeks resulted in significantly fewer attacks per week, less use of medication and improved PEFR.188
Similar findings were demonstrated in a study of 570 asthmatic patients, with those undergoing yoga therapy of 2–4 weeks and followed up at 3 and 54 months experiencing significant improvement in PEFR and at least 66% reduction in asthma medication, especially in those who practised consistently on a daily basis for longer periods of time.189
Yoga breathing exercises (pranayama) statistically significantly reduced the dose of histamine needed to provoke a 20% reduction in FEV1 in patients with mild asthma compared with the placebo device.190
Another study demonstrated that a yoga therapy program on 46 indoor patients with chronic bronchial asthma improved exercise tolerance and pulmonary functions, reduced symptom scores and reduced medication requirements, even at 1 year follow-up.191
One trial did not find yoga any different to benefits derived from breath-work and stretching alone. This randomised, controlled, double-masked clinical trial of 62 asthmatics compared the active control involving breath-work and stretching with yoga intervention over a 4-week period.192 Both groups demonstrated significant improvement in post-bronchodilator forced expiratory volume in 1 second and morning symptom scores at 4 and 16 weeks, but no differences were found between the 2 groups.
Qigong
Qigong, part of traditional Chinese medicine (TCM), combines movement, meditation and breathing techniques. It may assist asthmatics according to a pilot study of 30 patients who demonstrated improved lung function tests (peak flow), reduced emergency hospital visits and sick leave.193
Alexander technique
Alexander technique, a physical therapy involving a series of movements designed to correct posture and bring the body into natural alignment, may play a role in management of chronic asthma although robust, well-designed RCTs are required in order to test claims by practitioners.194
Breathing exercises
Dysfunctional breathing
There is a growing body of research that demonstrates a high prevalence of dysfunctional breathing occurs in asthmatics.195, 196 Asthma symptoms can be confused with breathing dysfunction and it is important to identify this difference to tailor appropriate treatment.197 The prevalence of dysfunctional breathing may be as high as one-third of women and one-fifth of men.198
A suitably designed questionnaire may help identify dysfunctional breathing patterns even in children.199, 200 This is an important distinction that should be made as breathing dysfunction can be treated with appropriate breathing exercises.
A cross-sectional study of 219 general practice patients demonstrated that one-third of them, especially young women, diagnosed with asthma have dysfunctional breathing or a combination of both. Treatment for dysfunctional breathing includes relaxation therapy, breathing retraining exercises and reassurance.201
An accompanying editorial concludes ‘asthma and anxiety with dysfunctional breathing are both common conditions and they often coexist’.202 This highlights why it is vital to differentiate dysfunctional breathing associated with anxiety from asthma.
Breathing exercises
There is now ample evidence to demonstrate that breathing exercises can help asthma patients. There are different methods of breathing exercises that may be of help. Some breathing exercises may not improve lung function scores but they appear to play a role in managing symptoms of asthma, improving quality of life and reducing the need for medication.
One study aimed to assess 1 breathing exercise focusing on shallow nasal breathing with those of non-specific upper-body exercises and found little benefit favouring 1 technique over the other.203 Both groups of exercises led to a dramatic reduction in use of reliever medication by 86% and inhaled corticosteroid dose reduced by 50%. The authors recommended breathing exercises be practised twice daily, as a first-line symptom treatment to help reinforce the message of relaxation and self-efficacy. The breathing exercise can be viewed for free online at:
In a prospective parallel-group single-blinded trial of 183 asthmatics, patients were randomised to breathing training or asthma education.204 At 6 months following intervention, there was significant improvement in asthma-specific health status, mood scores (anxiety and depression) and quality of life in asthmatics undergoing breathing training compared with the patient education group, but they did not differ for airway physiology, inflammation or hyper-responsiveness.
In another trial, 85 patients were randomised to a control group or to an intervention group of treatment by the Papworth method, an integrated breathing and relaxation technique used by physiotherapists since the 1960s.205 Both groups received usual medical care. Following 12 months of treatment, there was significant improvement in asthma scores, respiratory function and adverse mood, such as anxiety, for the Papworth group compared with the control group.
A Cochrane review identified 7 studies in total demonstrating that breathing retraining and interventions overall significantly reduced use in rescue bronchodilator, acute exacerbations of asthma and improved quality of life measures.206 However 5 studies compared breathing retraining with no active control and 2 with asthma education control groups illustrating how difficult it is to draw firm conclusions with trials being considerably different. Nevertheless, the authors conclude, in view of improved quality of life with breathing interventions, more trials are warranted.
Inspiratory muscle training
Inspiratory muscle training to improve inspiratory muscle strength training and endurance, also improved asthma symptoms, reduced hospitalisations for asthma, emergency department visits, absence from school or work, and use of medication in patients with asthma.207, 208 These exercises may also benefit patients with cystic fibrosis.209
Buteyko
The buteyko method shows potential but there is still debate until more definitive trials are completed as to whether these benefits are physiological or purely subjective. The high incidence of dysfunctional breathing amongst asthmatics may explain or account for the therapeutic effect of breathing retraining exercises and buteyko method of treating asthma.195, 210
Those practising the buteyko method reduced hyperventilation and their use of beta 2-agonists, with daily inhaled steroid dosage reduced by 49% and observed better quality of life, despite no change in FEV1 levels.211 In another study, buteyko significantly improved quality of life and reduced inhaled bronchodilator use.212 A study of 69 patients found the buteyko technique improved symptoms and reduced the use of bronchodilator use compared with the pranayama breathing exercises (a yoga breathing technique).213
A blinded RCT comparing buteyko breathing with control in 38 people with asthma (18–70 years of age) over 6 months found no significant change in FEV1 but a significant reduction in inhaled steroid use of 50% and beta2-agonist use of 85% at 6 months from baseline compared with the control group.214 The control group remained unchanged with steroid use and there was an observed reduction of beta2-agonist by 37%.
A 2-year Scottish study of 600 asthma patients (aged 18–69 years) randomised to receive buteyko breathing therapy, standard asthma management by physiotherapists, or continued standard asthma management with medication, found buteyko considerably improved asthma symptoms.215 Overall, the buteyko group reduced asthma symptoms by 98%, the need for reliever medications by 98%, preventer medications by 92%, oral preparations by 100%, oral preventers by 96%, and reduced the incidence of colds or viral infections by 20%. This compared with ‘no significant change’ in the other 2 groups.
Dietary changes
Diet can play a major role in the management of asthma. Traditional advice in many cultures (e.g. Chinese and Indian) includes ensuring ‘warm, cooked, spicy foods’ for asthmatics as opposed to cold foods from the fridge. A cup of warm chamomile tea with honey may be beneficial for its calmative effect for coughs, although some allergic asthmatics need to avoid chamomile.
Pregnancy
An assessment of 1253 pregnant women’s diet during pregnancy demonstrated low intake of foods containing vitamin E (not supplements) was associated with increased risk of infants developing asthma symptoms and wheeze.216 Mothers with the lowest vitamin E intake were 5 times more likely to have children with asthma compared with mothers with high intake. Foods rich in vitamin E include vegetable oils, nuts, sunflower seeds and green leafy vegetables.
Mediterranean diet
Fruit and vegetables
Data extracted from a large Europe-wide asthma study, the European Community Respiratory Health Survey, using random samples of 20–44 year old subjects, found an inverse association (i.e. a protective effect) with intake of fruit, vitamins A and C, and riboflavin from dietary intake.217 Research supports the role of high dietary intake of fruits and vegetables as being protective towards asthma.
Adherence to the Mediterranean diet — high in fresh fruit, vegetables, nuts and fish — during childhood appears to be protective towards asthma and rhinitis, reducing the risk by up to 64% compared with low adherence to the diet.218 Wheeze and rhinitis are rare in Crete and the traditional Mediterranean diet appears to play a role. A cross-sectional survey of 690 children (aged 7–18 years) demonstrated 80% of children ate fresh fruit, namely grapes, oranges, apples, and fresh tomatoes, and 68% ate vegetables at least twice a day. Consumption of nuts was also significantly inversely associated with wheezing. Margarine more than doubled the risk of both wheeze and allergic rhinitis.
Similarly a study of over 18 000 children’s diets in Italy found those eating fruit rich in vitamin C up to 5–7 times per week was associated with a reduced incidence of nocturnal cough, chronic cough, non-coryzal rhinitis, shortness of breath and wheeze.219 In children with a history of asthma, those eating fresh fruit at least once a week experienced a lower 1-year occurrence of wheeze (29.3%) than those eating fruit less than once per week (47.1%).
A major study conducted in 10 English and Welsh towns of 2650 children aged 8–11 demonstrated that eating fresh fruit daily was associated with improved lung function by 4.3% (FEV1 of 79ml) in asthmatics suffering wheeze, not related to vitamin C levels.220
High antioxidants, such as ascorbate, may have a protective effect. In a cross-sectional analysis of 4104 children, after controlling for several confounders (sex, study area, paternal education, household density, maternal smoking, paternal smoking, bedroom dampness or mould, parental asthma), intake of citrus fruit or kiwi fruit was found to be a highly significant protective factor for wheeze, shortness of breath, nocturnal cough, rhinitis and chronic cough in the last 12 months, even among children whose intake of fruit was as little as only 1–2 times per week.221
Similar findings were demonstrated in a prospective cohort study of 2512 men aged 45–59 who demonstrated lung function to be 138ml higher in men eating 5 or more apples per week and seemed to be independent of total vitamin E and C intakes.222 Quercetin found in apples (and onions) may contribute to the observed benefits. Traditionally, onions are thought to be beneficial for the prevention and treatment of colds and respiratory problems.
A study of over 63 000 middle-aged men and women found those who smoked, and ate more fruit and soy, were associated with reduced risk of developing cough and chronic respiratory symptoms.223
A diet rich in fish and containing more than 40g a day of ‘fruity vegetables’ — namely tomatoes, eggplants, cucumber, green beans and zucchini — reduces the risk of asthma and allergies according to a 7-year study of 460 Spanish children.224 Children who consumed more than 60g of fish a day and mothers who ate fish during pregnancy were also associated with significantly reduced risks of childhood allergies.
Fish intake
Population studies such as in Eskimo communities demonstrate diets high in fish also have low rates of asthma, whereas diets high in polyunsaturated fats are associated with increased risk of asthma. Ecological and temporal data from population and migration studies suggest that dietary factors may play a role in increased prevalence of asthma. The typical Western diet has 20- to 25-fold more omega n-6 polyunsaturated fatty acids (PUFA) than n-3 PUFA, contributing to a more ‘pro-inflammatory diet’ promoting the release of pro-inflammatory arachidonic acid metabolites (leukotrienes and prostanoids).225
School children who eat fish more than once a week have a significantly reduced risk of developing asthma than those who don’t eat fish.226 After adjusting for other risk factors such as ethnicity, country of birth, atopy, parental smoking and family history, fresh, oily fish (>2% fat) was protective against asthma in childhood. Children with a diet rich in omega-3 fatty acids (e.g. fish and flaxseed oil) had less asthma, as these foods are known to inhibit inflammation. Reduced asthma symptoms with n-3 fatty acid ingestion in the asthma patient who responded appear to be related to 5-series leukotriene production, although the mechanism is still not clear. Those who did not benefit from fish oils displayed a different leukotriene picture: ‘5-series leukotriene excretion with high n-3 PUFA ingestion was significantly greater for responders than for non-responders’.227
A large population-based study, the Respiratory Health in Northern Europe (RHINE) study, of 16 187 subjects aged 23–54 years found that, after excluding other confounders such as smoking, a minimum weekly fish intake reduced the incidence of asthma in adults.228 They found people in Iceland and Norway eat more fish both in childhood and adulthood compared with Sweden, Estonia and Denmark. Fish intake less than once weekly in adults was associated with increased risk of asthma symptoms, while more frequent fish intake did not appear to decrease the risk further as the association was not dose–response related. Those who never ate fish in childhood had the highest risk for asthma and earlier asthma onset. Of interest, daily or no cod oil consumption was associated with asthma, with lowest risk in those taking cod oil some days during the week.
Fast foods and polyunsaturated fat intake
High dietary polyunsaturated fats — for example, margarine and frying in polyunsaturated fats — doubled the risk of developing asthma in preschool children.31
Frequent consumption of hamburgers increased the risk of asthma and bronchial hyper-responsiveness in children even after excluding other risk factors.229 This effect was dose-dependent.
Vegan diets
Vegetarian diets may be protective towards asthma. A study of 35 patients demonstrated significantly reduced asthma symptoms, with 71% reporting improvement at 4 months and 92% at 1 year, and reduction of asthma relieving medication on a vegan diet.230 Part explanation for this observation may also be the increase of intake of fruits and vegetables that are known to help asthma. Patients were also drinking up to 1.5 litres of spring water instead of tap, and there is documentation of the benefits of increasing fluids.
Food allergies versus food intolerances
About 20% of people suffer food-related symptoms but true allergy occurs in about 1–3%, particularly children.231 These symptoms may manifest in different organ systems of the body; for example, the gut, respiratory system, neurological system etc.
Skin prick tests usually detect the presence of food-specific IgE bound to mast cells in the skin causing inflammation, wheal and flare. Serum blood levels for food-specific IgE are measured using RAST tests.232 These tests are frequently associated with false positives.
Skin prick testing is useful to identify IgE mediated food allergies.
A trial exclusion diet carefully formulated to ensure no nutritional deficiencies are occurring may also be of help for some asthmatics to identify any possible food intolerances.233, 234
A study of 322 children (less than 1 year of age) with respiratory allergy placed on a 6-week hypoallergenic restrictive diet consisting of meat base formula, beef, carrots, broccoli and apricots demonstrated significant improvement in respiratory symptom scores in 91% of infants. Food challenge later reproduced symptoms in only 51% of the children. Most importantly the children were followed up for 5 years and only 6% of the children studied showed any evidence of food sensitivity suggesting ‘food allergy tends to be “outgrown” ’. The data suggest young infants with respiratory allergy may benefit from a hypoallergenic diet.235
Delayed introduction of solid foods past 4–6 months of age did not offer benefit or protection from asthma, eczema or allergic rhinitis.236
A randomised, cross-over, double-blind, placebo-controlled trial of 20 subjects with asthma found no statistical correlation between dairy products and bronchoconstriction and no improvement in asthma after exclusion of dairy products.237 However, most children with known beef allergy are also likely to be allergic to cow’s milk and should avoid the consumption of dairy products.238
However, a randomised, cross-over, double-blind, placebo-controlled trial of 20 subjects with asthma found no statistical correlation between dairy products and bronchoconstriction and no improvement in asthma after exclusion of dairy products. There are conflicting studies in this area that require guidance from a well-trained dietician and/or allergy specialist to help identify any food sensitivities that may contribute to the development or aggravation of asthma symptoms. 239
Wheat hypersensitivity
A review of the literature notes ingestion of wheat may contribute to food allergies demonstrated in children and is recognised as a cause for food-dependent exercise-induced anaphylaxis.240 Inhalation of wheat flour can also cause sensitisation known as baker’s asthma, an occupational respiratory allergy.
There are numerous proteins and the insoluble gliadins within wheat that have been implicated in the IgE mediated allergy reaction to ingested wheat. Diagnosis is usually based on history and skin prick testing in general is not useful.241 Wheat allergy may manifest as a myriad of symptoms such as exercise-induced anaphylaxis, occupational asthma and rhinitis or contact urticaria.
If an infant displays respiratory symptoms or asthma during breastfeeding, it is worthwhile for the mother to trial an elimination diet as sensitisation to wheat may occur through maternal milk.242
Weight reduction
Obesity and high body mass index is an independent risk factor for atopy, wheeze, and cough, including in children.243
Weight loss advice for the obese is important. A study of 19 obese asthmatic patients who undertook a weight-loss program over 8 weeks, found that weight loss significantly improved lung function (FEV1 and FVC), asthma symptoms, morbidity and health status.244
Reduction of body mass index in adult obese patients on average from 37.2 to 32.1 kg/m(2) led to a significant increase mean pulmonary function tests such as FEV1 and FVC after weight loss.245 Also functional residual capacity and expiratory reserve volume were significantly higher after weight loss.
A Cochrane review identified 1 trial of 38 patients suffering from chronic asthma and found significant increases in FEV1 and FVC in the active treatment group of dietary calories restriction compared with the control group.246 However, the reviewers concluded more studies are required before any firm conclusions can be drawn from this 1 study.
Salt reduction
High salt intake in the Western diet appears to be correlated with asthma incidence and may exacerbate pulmonary function tests in individuals with exercise-induced asthma. A study based on regional mortality data for England and Wales demonstrated a relationship between asthma mortality and regional per-person purchases of table salt for men but not for women.247
Increasing fluid intake and reducing salt intake may play an important role for exercise-induced asthmatics. Sodium may aggravate asthma symptoms as there appears to be a strong association between sodium dietary intake and bronchial reactivity as demonstrated with histamine challenge tests.248, 249 There was a significant increase in bronchial-reactivity to histamine in 90% of asthmatic patients when their salt intake was increased.247 Also, bronchial reactivity strongly correlated with 24-hour excretion of sodium and was seen as an independent risk factor even after excluding confounders for asthma such as age, family history, atopy and cigarette smoking.
A Cochrane review of the literature found low salt intake is associated with improved pulmonary function but based on the current evidence, including 6 randomised control trials (RCTs), no firm conclusions can be drawn whether salt reduction or exclusion has a place for asthma management. A low sodium diet was associated with significantly lower urine sodium excretion, and less use of reliever bronchodilator than asthmatics on normal or high salt diets.250
Caffeine and green tea
Caffeine and green tea intake may be useful for asthma due to their content of methylxanthine (like theophylline) a natural bronchodilator, and antioxidant properties.251 A Cochrane review of 6 cross-over high-quality trials involving a total of 55 people found, when compared with placebo, caffeine consumption improved lung function for up to 4 hours and FEV1 for up to 2 hours.252 They concluded caffeine appears to ‘improve airways function modestly in people with asthma for up to 4 hours’.
Honey for cough
One study aimed to test the benefits of buckwheat honey prior to bed for nocturnal cough in 105 children with upper respiratory tract infection for up to 7 days and found significant differences in symptom improvement, with honey consistently scoring best when compared with Dextromethorphan or no treatment for any outcome including better quality sleep.253, 254 This may be clinically significant for asthmatics who commonly suffer nocturnal cough, particularly asthma following upper respiratory infections and there is a need to clinically evaluate use of honey in asthmatics.
Food additives
Symptoms related to food additives in atopic individuals appear to involve non-IgE-mediate mechanisms and are usually less severe than food intolerances.255 Asthmatics should be advised to exclude food additives from the diet as they have the potential to trigger asthma, anaphylactic type reactions, rhinitis, urticaria and angioedema.256 Diagnosis is usually based on a history of suspected food containing additives and confirmed by specific challenge. Examples of additives to avoid include tatrazines (jams, butters, candies, cakes), metabisulfites recognised as food additive numbers 220 to 228 (white wine, beer, dried fruit, cordial), benzoates and monosodium glutamate MSG (Chinese Restaurant Syndrome).257, 258
Nutritional medicine
Nutrients may play a complementary, therapeutic role in the management of asthma.
Vitamins
Vitamin B6 Pyridoxine
Pyridoxine is the nutrient best researched for asthma, is often low in asthmatics and may benefit asthma.265 Double-blind studies suggest vitamin B6 (50–200mgs daily) supplementation may reduce the frequency and severity of asthma attacks, including in patients with steroid-dependent asthma,266, 267 and lead to reduction in use of bronchodilators and cortisone.268 However, these doses over 50mg and up to 200mg of vitamin B6 daily is quite concerning as B6 toxicity can cause peripheral neuropathy even in levels up to 50mg daily and with prolonged use in adults.269, 270 It would be advisable to use vitamin B6 for short periods of time and combined in a multi-B supplement.
Nicotinic acid may be of benefit although further trials are warranted.271, 272
Vitamin C Ascorbic acid
There is debate about the role for vitamin C supplementation and its effect on asthma. What is more certain is that a diet low in vitamin C is a risk factor for asthma and diets high in vitamin C are associated with better pulmonary function (e.g. FEV1 and FVC, smoking-related respiratory symptoms, reduced cough and wheeze).273, 274, 275
In a review of 11 studies on vitamin C, 7 studies showed significant improvement in respiratory function following supplementation with 1–2g of oral vitamin C.276 Vitamin C is often low in smokers and is replenished with supplementation.277
A number of studies have demonstrated vitamin C to be useful for asthma and attenuates exercise-induced bronchoconstriction in patients with asthma.278–281
One trial did not show any benefit with vitamin C for asthma.282
A Cochrane review identified 9 studies, randomising a total of 330 participants, and noted most studies were generally poor quality with only 1 small study showing a significant reduction in FEV1 post-exercise.283
A meta-analyses of 7 studies, comprising 13 653 subjects, demonstrated a higher dietary intake of antioxidants (vitamin C and beta-carotene) was not associated with a lower risk of having asthma.284
Vitamin D
A cross-sectional survey of the 14 091 people (20 years of age or over) in the US, after adjusting for other co-factors, found on average the mean FEV1 was 126 mL and the mean FVC was 172 mL greater for those with the highest quintile of serum 25-hydroxy vitamin D level (> or = 85.7 nmol/L) compared with the lowest quintile.285 This suggests there is a strong relationship between serum concentrations of 25-hydroxy vitamin D, FEV1, and FVC and further studies are warranted to determine if vitamin D supplementation is of any benefit in patients with respiratory disease.
Sunshine is the best source of vitamin D (see Chapter 30 on osteoporosis).
Minerals
Magnesium
Oral magnesium supplements
A double-blind placebo-controlled trial of 37 patients (aged 7–19 years) were randomised to either magnesium (300mg/day) or placebo for 2 months.286 Both patient groups received asthma inhaled fluticasone and salbutamol as needed. After 2 months of treatment, compared with the placebo group, the magnesium group demonstrated better lung function, fewer asthma attacks and used less reliever medication. The skin prick tests for recognised allergens also decreased in the magnesium group.
Intravenous (IV) magnesium therapy
Slow IV magnesium is now used in some hospital emergency departments for severe, acute asthma attacks and status asthmaticus. A number of studies demonstrate IV magnesium can help improve lung function in the acute severe asthmatic state.287–290
The mechanism of action for magnesium treatment in asthmatics is due to its smooth relaxant and bronchodilating properties and may also be via an anti-inflammatory effect.291
Serum magnesium levels need to be carefully monitored during IV infusion.
Thirty-eight patients suffering from acute exacerbations of moderate to severe asthma were randomised to either an intravenous infusion of saline placebo or 1.2 g of magnesium sulfate.285
The benefits of IV magnesium sulfate appear to be more effective in the severe asthmatic. A Cochrane review of 7 trials of 665 patients found patients receiving magnesium sulfate demonstrated significant improvements in PEFRs only in those with severe acute asthma.292 It concluded that whilst IV magnesium appears safe and well tolerated, the current evidence does not support the routine use of intravenous magnesium sulfate in all patients with acute asthma.
Another Cochrane review and systematic review of the literature concluded there is a definite role for nebulised inhaled magnesium sulfate in addition to beta2-agonist in the treatment of an acute asthma exacerbation, by improving pulmonary function in patients with severe asthma.293, 294
Selenium
In a double-blinded, controlled study, 24 patients with asthma were randomised to receive either placebo or 100 micrograms of sodium selenite.295 Whilst there were no changes to the placebo group, the asthmatics in the selenium-supplemented group improved significantly.
A recent study of 197 adult asthmatic patients receiving either a high-selenium yeast preparation of selenium (100μg daily) or placebo (yeast only) for 24 weeks demonstrated whilst there was an increase in plasma selenium in the active treatment group, there was no clinical benefit for asthma symptoms except quality of life score compared with the placebo group.296
A Cochrane review concluded there is ‘some indication that selenium supplementation may be a useful adjunct to medication for patients with chronic asthma’.297
Other supplements
Omega-3 fatty acids; Fish oils
A review of the literature found supporting evidence for the beneficial effects of omega-3 fatty acid supplementation for asthma- and exercise-induced bronchoconstriction due to its anti-inflammatory properties reducing airway narrowing, improving asthma symptoms and reducing medication use. Fish oil capsules containing 3.2g eicosapentaenoic acid and 2.2g docohexaenoic acid were compared with placebo capsules containing olive oil taken daily for 3 weeks.225 The authors conclude fish oil supplements have a markedly protective effect in suppressing exercise-induced bronchoconstriction in elite athletes, attributed to their anti-inflammatory properties.
A recent study of fish oil capsules containing 3.2g of eicosapentaenoic acid and 2.0g of docohexaenoic acid or placebo capsules taken daily for 3 weeks also demonstrated marked improvement in pulmonary function, concurrent reduction in bronchodilator use, significant reduction in leukotriene B4 and increase in leukotriene B5 generation from activated polymorphonuclear leukocytes and reduced exercise-induced bronchoconstriction in asthma patients.298 A similar study clearly demonstrated fish oils have a protective effect towards exercise-induced bronchoconstriction, particularly in elite athletes.299
A double-blind, controlled trial of 39 asthmatic children (aged 8–12 years) was randomised for participants to receive fish oil capsules plus canola oil and margarine (omega-3 group) or safflower oil capsules plus sunflower oil and margarine (omega-6 group) over a 6 month period.300 Dietary enrichment of omega-3 fatty acids over 6 months increased plasma levels of nutritional markers such as fatty acids and reduced inflammatory mediators such as stimulated tumour necrosis factor (TNF) alpha production, but had no effect on the clinical severity of asthma in these children compared with baseline results.
However, a Cochrane review identified 9 randomised controlled trials, 8 of which compared fish oil supplements with placebo whilst 1 compared high-dose versus low-dose marine n-3 fatty acid supplementation, and 7 of the 9 studies were conducted in adults. The authors concluded there is ‘little evidence to recommend that people with asthma supplement or modify their dietary intake of marine n-3 fatty acids (fish oil) in order to improve their asthma control’.301
Perilla seed oil, rich in the omega-3 fatty acids, significantly improved lung function tests (FVC and FEV) and suppressed levels of leukotriene B4 (LTB4 and LTC4) inflammatory markers generated by leucocytes compared with corn oil (rich in oimega-6 fatty acid) after 4 weeks of dietary supplementation and may be useful for asthma.302
Herbal medicine
Research for herbs is limited and considering their popularity of use, there is an urgent need for more research. A clinical systematic review of herbs for asthma identified 9 of the 17 trials to be reasonable, well-performed trials reporting clinically relevant improvement in lung function and/or symptom scores. Researchers concluded ‘evidence promising in some cases but not yet definitive’ and there is an urgent need for more research.303
A Cochrane review of herbal interventions for chronic asthma in adults and children identified 27 studies of 21 different herbal preparations randomising a total of 1925 participants.304 The authors conclude that due to the diversity of trials, treatments and evidence base for the effects of herbal treatments from the available data they only provide a small insight into the long-term efficacy and harm profiles. This is understandable as it is difficult to compare 1 type of herb with another, with different study outcomes and quality of trials.
Garlic (Allium sativum)
Garlic was used by the ancient Egyptians, Romans and Greeks for its medicinal properties for asthma and other respiratory related problems. Both onions and garlic are well known for their anti-inflammatory action by inhibiting the lipoxygenase enzyme, and anti-infective effects.
Dried ivy leaf extract
Dried ivy leaf extract, commonly used in Europe, is known for its antispasmolytic and mucolytic effect. A systematic review of the literature identified 5 randomised controlled trials investigating the efficacy of ivy leaf extract preparations in chronic bronchitis, 3 of which were conducted in children and 1 compared ivy leaf extract cough drops to placebo, 1 compared suppositories to drops and 1 tested syrup against drops.305 The review indicated that ivy leaf extract preparations have positive effects with respect to an improvement of respiratory functions of children with chronic bronchial asthma.
Ginkgo biloba
Ginkgo biloba, traditionally used by the Chinese for asthma, showed some benefit in a small, placebo-controlled trial of Ginkgo leaf extract.306
Ma Huang
Ma Huang is a herbal product derived from plants of the Ephedra species. It is a herb traditionally used by the Chinese for thousands of years for the treatment of asthma. The alkaloid compound ephedrine was derived and used in prescription medicine in the early 1900s. Ma Huang availability is now limited due to its abuse potential and its reported cases of hepatotoxicity, including a report of fulminant hepatic failure requiring liver transplant.307, 308
Tsumura Saiboku-To
Tsumura Saiboku-To (or TJ-96) is a mixture of Chinese/Japanese herbs (up to 10 herbs such as ginger, licorice and magnolia) traditionally used for asthma treatment and now approved by the Japanese Government as a treatment for atopic asthma. Several well-performed randomised controlled trials have showed benefit for symptomatic control of asthma when compared with placebo but, unfortunately, no lung function tests were performed other than demonstrating reduced blood and sputum eosinophil count.309, 310, 311 Saiboku-To was noted to have ‘steroid like activity’ and ‘inhibiting the IgE production by mite allergens’.309 The dose used in most trials was 2.5g of TJ-96 orally 3 times daily.
Ammi visnaga (Khella)
Ammi visnaga (Khella), traditionally used by the Egyptians for thousands of years for its bronchorelaxant effect, has very little known toxicity. Disodium cromoglygate is a chemical derivative of Ammi visnaga and today is used as a prophylactic inhaler to stabilise mast cells.312, 313
Petasites hybridus
Petasites hybridus, or Butterbur, historically used by Greeks in the treatment of asthma, this herb resulted in significant improvement of FEV1 and bronchial reactivity on methacholine challenge in a study of 70 asthma or chronic bronchitis patients.314
Lagundi
Lagundi is a Philippine indigenous herb, that contains a smooth muscle relaxant and antihistamine, chrysophenol D, claimed to ease bronchospasm in patients with asthma.315
Boswellia serrata
Boswellia serrata, or Boswellia or Frankincense, is a traditional Ayurvedic Indian gum resin, noted to have anti-inflammatory properties, useful for asthma, arthritis and other inflammatory conditions.316, 317
In a double-blind, placebo-controlled study, B. serrata significantly improved asthma symptoms, dyspnoea and wheezing and lung function tests (FEV1, FVC, PEF), compared with the placebo group, in up to 70% of asthma sufferers over a 6-week period.318
Tylophora indica and Tylophora asthmatica
Tylophora indica and Tylophora asthmatica are Ayurvedic Indian indigenous herbs, of which the leaves were used in the treatment of atopic allergy and asthma for their bronchodilation, anti-inflammatory and mucous-reducing effects. T. indica has been most widely researched, including a number of double-blind studies.319–326
Coleus
Coleus, or Forskolin, isolated from the Indian plant Coleus forskohlii, was tested in double-blind and cross-over studies in healthy volunteers 327 and also in subjects with asthma.328 It demonstrated bronchodilating effects equal to the effects of the pharmaceutical fenoterol.
Albizia labeck
Albizia labeck has traditionally been in use by Ayurvedic physicians for bronchial asthma and eczema.329, 330
Adhatoda vasica
Adhatoda vasica, or Vasicine, is an Indian herb traditionally used for respiratory conditions and is the active alkaloid extracted from vasica.331, 332 Its properties were utilised in the development of Bisolvon, as it has bronchodilating properties and may increase ciliary movement.333 Forty asthmatic patients were randomised to receive Bisolvon intravenously which resulted in fewer bronchial aspirations, less fluid secretions and reduction in total mucus. However, several scientific reports attribute the herb to oxytocic and abortifacient effects.334
Thyme extract
Thyme extract is clinically as effective in managing cough as the pharmaceutical Bisolvon. It is an effective expectorant, bronchospasmolytic and has anti-inflammatory actions.335, 336, 337
Common herbs used as expectorants and for their sympathomimetic effects, yet still not adequately clinically trialled, include Lobelia (Indian tobacco), Euphorbia, Grindelia, Senega, Licorice, Ginseng, Mullein, Zizyphi fructusia and Ziziphi jujube, Cinnamon, Beupleurum, Ligusticum wallichii, Schisandra chinesis, Kan-Lin preparation, ginger and Ginkgo biloba.338
Homeopathy
Two trials published in the Lancet found some benefit for homeopathy and asthma.342, 343
British investigators conducted a double-blind, randomised, controlled trial of homeopathic doses of HDM in 242 asthmatic adults with positive skin tests for HDM. After 4 weeks they found improvements within both groups in key outcome measures (FEV1, quality of life and mood), but differences between groups were not statistically significant.344
A recent randomised placebo-controlled trial of 96 asthmatic children over 12 months demonstrated no benefits with individualised homeopathic remedies over placebo.345
Despite a meta-analysis in the Lancet suggesting a positive effect of homeopathy on asthma, a recent Cochrane review identified 6 randomised-placebo-controlled trials of 556 people and found all of variable quality and with conflicting results.346, 347 Overall the authors concluded there is not enough reliable evidence to support the use of homeopathy for asthma. Another meta-analysis of the literature for homeopathy and asthma and rhinitis noted ‘some positive results were described with homeopathy in good-quality trials in rhinitis, but a number of negative studies were also found’.348
A Lancet review of the literature identified 110 homoeopathy trials in total for various conditions and 110 matched conventional-medicine trials were analysed to compare its effectiveness. They noted biases were present in both placebo-controlled trials of both homoeopathy and conventional medicine and ‘there was weak evidence for a specific effect of homoeopathic remedies’.349 Conventional medicine was more effective.
Manual therapies
A Cochrane review identified a number of manual therapy techniques utilised by chiropractors, osteopaths and physiotherapists and concluded there is insufficient scientific evidence for manual therapies in asthma management and there is a need to conduct well-performed trials.350
Acupuncture
A Cochrane review of 12 studies (350 participants) of variable quality concluded there is not enough evidence to make recommendations of acupuncture treatment for chronic asthma.351 One of the main concerns with acupuncture research is comparing real acupuncture with sham acupuncture which can cause a strong placebo response.
A study comparing the real (laser) acupuncture with placebo acupuncture for exercise-induced asthma found no differences in effects between the 2 methods.352 Another study compared real acupuncture with sham acupuncture and a control group (no needle acupuncture) and still found no notable differences between the 3 groups.353
Despite the Cochrane conclusion and negative findings, a well-performed trial demonstrated real acupuncture to be superior over sham acupuncture for exercise-induced asthmatic children resulting in improved lung function tests FEV1, FVC and PEFR following exercise when compared with sham acupuncture and the control group.354 Interesting, sham acupuncture provided some benefit over the control group also, indicating the placebo effect may have a positive response to asthma outcome too.
Massage
Thirty-two asthmatic children of varying ages were randomly assigned to 20 minutes massage therapy by their parents before bed or relaxation therapy for 30 days.355 In the massage therapy group, the younger children experienced immediate reduction in behavioural anxiety and cortisol levels, improved attitude towards asthma and improved respiratory function (forced expiratory flow from 25 to 75%) compared with the relaxation group.
Massage therapy may also benefit children with other respiratory diseases such as cystic fibrosis by exerting a relaxation effect.356
Speleotherapy for asthma
Speleotherapy, the use of subterranean environments such as staying underground (e.g. in caves and mines), is a popular therapy in Central and Eastern European countries used for the treatment of respiratory disorders such as asthma and chronic obstructive airways diseases. A Cochrane review aimed to identify trials with asthma treatment.357 They identified 3 trials of 124 asthmatic children. Two trials reported short-term benefit of lung function with speleotherapy. The authors concluded that there is still not enough evidence to allow a reliable conclusion as to whether speleotherapy is effective for chronic asthma.
Reflexology
A 10-week study comparing active and placebo reflexology found subjective scores describing symptoms and quality of life, and also bronchial sensitivity to histamine improved in both groups. However, no differences were found between active or placebo reflexology groups.358 Peak flow increased in asthmatics in 1 study on reflexology.359
Conclusion
Asthma can be managed successfully by educating the patient about the condition, and combining pharmaceutical medication with attending to environmental, behavioural and lifestyle factors that promote wellbeing. There are a number of safe complementary medicines and therapies that may be of help. While the science may not be strong for some of these, overall they are generally safe and can improve quality of life for the asthmatic patient. The evidence for lifestyle and complementary medicine and therapies are summarised in Table 5.5.
Clinical tips handout for patients — asthma
1 Lifestyle advice
Sunshine
2 Physical activity/exercise
3 Mind–body medicine
Breathing
Rest and stress management
4 Environment
Precautions
Simple measures for reducing triggers/environmental allergens
| Put toys away in cupboards |
| Wipe surfaces with damp cloth weekly |
| Steam clean carpets bi-annually; in-built vacuum cleaner |
| Damp mop hard floorboards |
| Laundry washing of bedding — add a few drops of Eucalyptus oil to wash |
| Place bedding in dryer and tumble dry for 15 minutes weekly |
| Use natural bedding (e.g. feathered or cotton pillows and doonas) |
| Avoid synthetic bedding |
| Avoid old cot mattresses for newborns |
| Dust mite covers for bedding — mattresses and pillow Use mattress covers such as semipermeable polyurethane mattress and pillow encasings (allergy control) |
| Avoid damp houses and mould |
| Allow sun into house and air house regularly — daily if possible |
| Avoid smoking inside house or, better still, do not smoke |
| Avoid pets in the house; dogs may protect against asthma in some children |
| Occupational asthma is not uncommon: avoid household cleaning sprays, wood dust, chemicals, paint fumes, solvents, latex and wheat flour |
Asthma Information Brochures for consumers:
http://www.nationalasthma.org.au/html/management/infopapers/consumer/1002.asp#asthma
5 Dietary changes
7 Supplements
Vitamin Bs, especially B6
Vitamin C
Vitamin D3 (cholecalciferol 1000 international units)
Doctors should check blood levels and suggest supplementation if levels are low.
Vitamin D3 (cholecalciferol)
Selenium (sodium selenite, organic selenium found in yeast)
Magnesium and calcium (best provided together)
Fish oils
1 Asthma Management Handbook. National Asthma Council. Online. Available: http://www.nationalasthma.org.au/cms/index.php?option=com_content&task=view&id=49&Itemid=29 (accessed 7April 2009) Content created 16 November 2006. Last updated 1 June 2007.
2 National Asthma Council. Asthma Information Brochures for Consumers. Online. Available: http://www.nationalasthma.org.au/html/management/infopapers/consumer/1002.asp#asthma (accessed 7 April 2009).
3 Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland, USA: National Heart, Lung, and Blood Institute; 1997.
4 National Asthma Council website. Available: http://www.nationalasthma.org.au/html/management/infopapers/health_professionals/1002.asp#facts (accessed 7 April 2009).
5(a) Determinants of bronchial responsiveness in the European Community Respiratory Health Survey in Italy. evidence of an independent role of atopy, total serum IgE levels, and asthma symptoms. Allergy. 1998 Jul;53(7):673-681. [No authors listed] PMID:9700036
5(b) ACAM 2008. Asthma in Australia 2008. Cat.no. ACM 14.AiHW. Online. Available: www.asthmamonitoring.org. (accessed Jan 2010).
6 Macan J., Varnai V.M., Maloča I., et al. Increasing trend in atopy markers prevalence in a Croatian adult population between 1985 and 1999. Clinical & Experimental Allergy. Dec 2007;37(12):1756-1763.
7 Blanc P.D., Ware G.K., Katz P.P., et al. Use of herbal products, coffee or black tea, and over-the-counter medications as self-treatments among adults with asthma. J. Allergy Clin. Immunol. 1997;100:789-791.
8 Blanc P.D., Trupin L., Earnest G., et al. Alternative Therapies Among Adults with a Reported Diagnosis of Asthma or Rhinosinusitis: Data from a Population-Based Survey. Chest. 2000;120:1461-1467.
9 Andrews L., Lokuge S., Sawyer M., et al. The use of alternative therapies by children with asthma: A brief report. J Paediatrics Child Health. 1998;34(2):131-134.
10 Shenfield G., Lim E., Allen H. Survey of the use of complementary medicines and therapies in children with asthma. J Paediatrics Child Health. 2002;38(3):252-257.
11 Mazur L.J., De Ybarrondo L., Miller J., et al. Use of alternative and complementary therapies for pediatric asthma. Tex Med. 2001;97:64-68.
12 Ernst E. Use of Complementary therapies in childhood asthma. Pediatric Asthma. Allergy and Immunology. 1998;12:29-32.
13 Janson C., Chinn S., Jarvis D., et al. Physician-diagnosed asthma and drug utilization in the European Community Respiratory Health Survey. Eur Respir J. 1997;10:1795-1802.
14 Andrews L., Lokuge S., Sawyer M., et al. The use of alternative therapies by children with asthma: A brief report. J Paediatrics Child Health. 1998;34:131-134.
15 Ernst E. Complementary Therapies for Asthma: What Patients Use. J Asthma. 1998;35:667-671.
16 Pachter L.M., Cloutier M.M., Bernstein B.A. Ethnomedical (folk) remedies for childhood asthma in a mainland Puerto Rican community. Archives of Pediatric and Adolescent Medicine. 1995;149:982-988.
17 Mazur L.J., De-Ybarrondo L., Miller J., et al. Use of alternative and complementary therapies for pediatric asthma. Texas-medicine. 2001 Jun;97(6):64-68.
18 Slader C.A., Reddel H.K., Jenkins C.R., et al. Complementary and alternative medicine use in asthma: who is using what? Respirology. 2006 Jul;11(4):373-387.
19 Ernst E. Complementary therapies in asthma: what patients use. Journal of Asthma. 1999;36:667-671.
20 Adams S.K., Murdock K.K., McQuaid E.L. Complementary and alternative medication (CAM) use and asthma outcomes in children: an urban perspective. J Asthma. 2007 Nov;44(9):775-782.
21 Blanc P.D., Trupin L., Earnest G., et al. Alternative therapies among adults with a reported diagnosis of asthma or rhinosinusitis: data from a population-based survey. Chest. 2001 Nov;120(5):1461-1467.
22 Blanc P.D., Kuschner W.G., Katz P.P., et al. Use of herbal products, coffee or black tea, and over-the-counter medications as self-treatments among adults with asthma. J Allergy Clin Immunol. 1997 Dec;100(6 Pt 1):789-791.
23 Passalacqua G., Bousquet P.J., Carlsen K.-H., et al. ARIA update: I—Systematic review of complementary and alternative medicine for rhinitis and asthma. J Allergy Clin Immunol. 2006;117:1054-1062.
24 Marks G, Cohen M, Kotsirilos V, et al. Asthma and complementary therapies. A guide for health professionals. National Asthma Council Australia. Online. Available: http://www.nationalasthma.org.au/content/view/54/112/ (accessed 8 June 2009).
25 Adams R.J., Smith B.J., Ruffin R.E. Impact of the physician’s participatory style in asthma outcomes and patient satisfaction. Ann Allergy Asthma Immunol. 2001 Mar;86(3):263-271.
26 Weinmayr G., Weiland S.K., Björkstén B., et al. ISAAC Phase Two Study Group. Atopic sensitization and the international variation of asthma symptom prevalence in children. Am J Respir Crit Care Med. 2007 Sep 15;176(6):565-574.
27 Wang H.-Y., Wong G.W.K., Chen Y.-Z., et al. Prevalence of asthma among Chinese adolescents living in Canada and in China. CMAJ. 2008;11:179. doi:10.1503/cmaj.071797
28 Gibson P.G., Henry R.L., Shah S., et al. Migration to a western country increases asthma symptoms but not eosinophilic airway inflammation. Pediatr Pulmonol. 2003 Sep;36(3):209-215.
29 Golan Y., Onn A., Villa Y., et al. Asthma in adventure travelers: a prospective study evaluating the occurrence and risk factors for acute exacerbations. Arch Intern Med. 2002 Nov 25;162(21):2421-2426.
30 Hegewald MJ, Crapo RO. Socioeconomic status and lung function. Chest 2007 Nov;132(5):1608-14. Online. Available: http://www.chestjournal.org/cgi/content/abstract/132/5/1608 (accessed 8 June 2009)
31 Haby M.M., Peat J.K., Marks G.B., et al. Asthma in preschool children: prevalence and risk factors. Thorax. 2001 Aug;56(8):589-595.
32 Abramson M., Kutin J.J., Raven J., et al. Risk factors for asthma among young adults in Melbourne, Australia. Respirology. 1996 Dec;1(4):291-297.
33 Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol. 2004 June;15(16 suppl.):4-5. 9–32
34 Roduit C., Scholtens S., de Jongste J.C., et al. Asthma at 8 years of age in children born by caesarean section. Thorax. 2009 Feb;64(2):107-113.
35 Gdalevich M., Mimouni D., Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. 2001 Aug;139(2):261-266.
36 Peat J.K., Allen J., Oddy W., et al. Breastfeeding and asthma: Appraising the controversy. Pediatr Pulmonol. 2003 May;35(5):331-334.
37 Oddy W.H. Breastfeeding and asthma in children. A prospective cohort study. Adv Exp Med Biol. 2000;478:393-394.
38 Oddy W.H., de Klerk N.H., Sly P.D., et al. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J. 2002 May;19(5):899-905.
39 Oddy W.H. Breastfeeding and asthma in children: findings from a West Australian study. Breastfeed Rev. 2000 Mar;8(1):5-11.
40 Dell S., To T. Breastfeeding and asthma in young children: findings from a population-based study. Arch Pediatr Adolesc Med. 2001 Nov;155(11):1261-1265.
41 Oddy W.H., Holt P.G., Sly P.D., et al. Association between breastfeeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ. 1999 Sep 25;319(7213):815-819.
42 Wright A.L., Holberg C.J., Taussig L.M., et al. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax. 2001 Mar;56(3):192-197.
43 Kramer M.S., Matush L., Vanilovich I., et al. for the Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group. Effect of prolonged and exclusive breastfeeding on risk of allergy and asthma: cluster randomised trial. BMJ. 2007;335:815. 20 October
44 Hoppu U., Kalliomäki M., Isolauri E. Maternal diet rich in saturated fat during breastfeeding is associated with atopic sensitization of the infant. Eur J Clin Nutr. 2000 Sep;54(9):702-705.
45 Goldman A.S. Association of atopic diseases with breast-feeding: Food allergens, fatty acids, and evolution. The Journal of Pediatrics. 1999;134(1):5-7.
46 Grönlund M.M., Gueimonde M., Laitinen K., et al. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clinical & Experimental Allergy. 2007;37(12):1764-1772.
47 Celedón J.C., Litonjua A.A., Ryan L., et al. Bottle feeding in the bed or crib before sleep time and wheezing in early childhood. Pediatrics. 2002 Dec;110(6):e77.
48 Wickens K., Pearce N., Crane J., et al. Antibiotic use in early childhood and the development of asthma. Clinical & Experimental Allergy. 1999;29(6):766-771.
49 Ball T.M., Castro-Rodriguez J.A., Griffith K.A., et al. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000 Aug 24;343(8):538-543.
50 Mitchell E.A., Robinson E., Black P.N., et al. Risk factors for asthma at 3.5 and 7 years of age. Clinical & Experimental Allergy. 2007;37(12):1747-1755.
51 Kozyrskyj A.L., Ernst P., Becker A.B. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007 Jun;131(6):1753-1759.
52 Haby M.M., Marks G.B., Peat J.K., et al. Day-care attendance before the age of two protects against atopy in preschool age children. Pediatr Pulmonol. 2000 Nov;30(5):377-384. PMID:11064428
53 Krämer U., Heinrich J., Wjst M., et al. Age of entry to day nursery and allergy in later childhood. Lancet. 1999 Feb 6;353(9151):450-454.
54 Kusel M.M.H., de Klerk N., Holt P.G., et al. Clinical & Experimental Allergy, 2008;(38):1921-1928.
55 Wickens K., Ingham T., Epton M., et al. The association of early life exposure to antibiotics and the development of asthma, eczema and atopy in a birth cohort: confounding or causality? Clin Exp Allergy. 2008;38:1318-1324.
56 Beasley R., Clayton T., Crane J., et al. ISAAC Phase Three Study Group. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from Phase Three of the ISAAC program. Lancet. 2008 Sep 20;372(9643):1039-1048.
57 Shaheen S.O., Newson R.B., Henderson A.J., et al. ALSPAC Study Team. Prenatal paracetamol exposure and risk of asthma and elevated immunoglobulin E in childhood. Clin Exp Allergy. 2005;35(1):18-25.
58 Macsali F., Real F.G., Omenaas E.R., et al. Oral contraception, body mass index, and asthma: a cross-sectional Nordic-Baltic population survey. J Allergy Clin Immunol. 2009 Feb;123(2):391-397.
59 Figueroa-Muñoz J.I., Chinn S., Rona R.J. Association between obesity and asthma in 4–11 year old children in the UK. Thorax. 2001 Feb;56(2):133-137.
60 Visness C.M., London S.J., Daniels J.L., et al. Association of obesity with IgE levels and allergy symptoms in children and adolescents: Results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy and Clin Immunol. 2009 Feb 20.
61 Ochs-Balcom H.M., Grant B.J., Muti P., et al. Pulmonary function and abdominal adiposity in the general population. Chest. 2006 Apr;129(4):853-862.
62 Verhulst S.L., Schrauwen N., Haentjens D., et al. Sleep-disordered breathing: a new risk factor of suspected fatty liver disease in overweight children and adolescents? European Respiratory Review. 2008;17:99-100.
63 Oddy W.H., Sherriff J.L., de Klerk N.H., et al. The relation of breastfeeding and body mass index to asthma and atopy in children: a prospective cohort study to age 6 years. Am J Public Health. 2004 Sep;94(9):1531-1537.
64 Kozyrskyj A.L., Mai X.M., McGrath P., et al. Continued exposure to maternal distress in early life is associated with an increased risk of childhood asthma. Am J Respir Crit Care Med. 2008 Jan 15;177(2):142-147.
65 Cookson H., Granell R., Joinson C., et al. Mothers’ anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009 Apr;123(4):847-853. e11
66 Sandberg S., Paton J.Y., Ahola S., et al. The role of acute and chronic stress in asthma attacks in children. Lancet 2000 Dec. 1932;2(9245):356.
67 Sandberg S., Järvenpää S., Penttinen A., et al. Asthma exacerbations in children immediately following stressful life events: a Cox’s hierarchical regression. Thorax. 2004 Dec;59(12):1046-1051. Erratum in: Thorax 2005 Mar;60(3):261. PMID:15563703
68 Sandberg S., McCann D.C., Ahola S., et al. Positive experiences and the relationship between stress and asthma in children. Acta Paediatr. 2002;91(2):152-158. PMID:11952001
69 Goodwin R.D., Fischer F.E. Goldberg JA Twin study of post–traumatic stress disorder symptoms and asthma. Am J Respir Crit Care Med. 2007 Nov 15;176(10):983-987.
70 Lehrer P.M., Sargunaraj D., Hochron S. Psychological approaches to the treatment of asthma. J Consult Clin Psychol. 1992 Aug;60(4):639-643.
71 Yorke J., Fleming S.L., Shuldham C.M. Psychological interventions for adults with asthma. Cochrane Database of Systematic Reviews. (Issue 1):2006. Art No:CD002982. doi:10.1002/14651858.CD002982.pub3
72 Yorke J., Fleming S., Shuldham C. Psychological interventions for children with asthma. Cochrane Database of Systematic Reviews. (Issue 4):2005. Art No:CD003272. doi:10.1002/14651858.CD003272.pub2
73 Cleland J.A., Price D.B., Lee A.J., et al. A pragmatic, three-arm randomised controlled trial of spiritual healing for asthma in primary care. Br J Gen Pract. 2006 Jun;56(527):444-449.
74 Mrazek D.A. Psychiatric complications of pediatric asthma. Ann Allergy. 1992 Oct;69(4):285-290.
75 Ettinger A., Reed M., Cramer J. Epilepsy Impact Project Group. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004 Sep 28;63(6):1008-1014. PubMed PMID:15452291
76 Mrazek D.A., Schuman W.B., Klinnert M. Early asthma onset: risk of emotional and behavioral difficulties. J Child Psychol Psychiatry. 1998 Feb;39(2):247-254. PMID:9669237
77 Katon W., Lozano P., Russo J., et al. The prevalence of DSM-IV anxiety and depressive disorders in youth with asthma compared with controls. J Adolesc Health. 2007 Nov;41(5):455-463.
78 Goldney R.D., Ruffin R., Fisher L.J., et al. Asthma symptoms associated with depression and lower quality of life: a population survey. Med J Aust. 2003 May 5;178(9):437-441.
79 Strunk R.C., Mrazek D.A., Fuhrmann G.S., et al. Physiologic and psychological characteristics associated with deaths due to asthma in childhood. A case-controlled study. JAMA. 1985 Sep 6;254(9):1193-1198.
80 Kern-Buell C.L., McGrady A.V., Conran P.B., et al. Asthma severity, psychophysiological indicators of arousal, and immune function in asthma patients undergoing biofeedback-assisted relaxation. Appl Psychophysiol Biofeedback. 2000 Jun;25(2):79-91.
81 Meuret A.E., Wilhelm F.H., Roth W.T. Respiratory biofeedback-assisted therapy in panic disorder. Behav Modif. 2001 Sep;25(4):584-605. PMID:11530717
82 Noeker M., von Rüden U., Staab D., et al. Processes of body perception and their therapeutic use in pediatrics. From nonspecific relaxation therapy to training to recognize disease-specific symptoms. Klin Padiatr. 2000 Sep–Oct;212;5:260-265. Review. German. PMID:11048285
83 Klinnert M.D., Kaugars A.S., Strand M., et al. Family psychological factors in relation to children’s asthma status and behavioral adjustment at age 4. Fam Process. 2008 Mar;47(1):41-61. PMID:18411829
84 Miller B.D., Wood B.L. Emotions and family factors in childhood asthma: psychobiologic mechanisms and pathways of effect. Adv Psychosom Med. 2003;24:131-160. Review. PMID:14584352
85 Klinnert M.D., Nelson H.S., Price M.R., et al. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001 Oct;108(4):E69. PMID:11581477
86 Mojtabai R. Parental psychopathology and childhood atopic disorders in the community. Psychosom Med. 2005 May–Jun;67(3):448-453.
87 Mrazek D.A., Klinnert M.D., Mrazek P., et al. Early asthma onset: consideration of parenting issues. J Am Acad Child Adolesc Psychiatry. 1991 Mar;30(2):277-282.
88 Mrazek D. Psychiatric complications of paediatric asthma. Annals of Allergy. 1992;69:285-290.
89 Klinnert M.D., Mrazek P.J., Mrazek D.A. Early asthma onset: the interaction between family stressors and adaptive parenting. Psychiatry. 1994 Feb;57(1):51-61.
90 Rietveld S., Everaerd W., Creer T.L. Stress-induced asthma: a review of research and potential mechanisms. Clin Exp Allergy. 2000 Aug;30(8):1058-1066. Review PMID:10931112
91 Wilson A.F., Honsberger R., Chiu J.T., et al. Transcendental meditation and asthma. Respiration. 1975;32(1):74-80.
92 Huntley A., White A.R., Ernst E. Relaxation therapies for asthma: a systematic review. Thorax. 2002 Feb;57(2):127-131.
93 Lehrer P.M., Hochron S.M., Mayne T., et al. Relaxation and music therapies for asthma among patients prestabilized on asthma medication. Journal of Behavioral Medicine. 1994;17(1):1-24.
94 Carr RE. Panic disorder and asthma: causes, effects and research implications. J Psychosom Res 1998 Jan;44(1):43–52. Review Panic disorder and asthma: causes, effects and research implications. PMID:9483463.
95 Creer T.L. Emotions and asthma. J Asthma. 1993;30(1):1-3.
96 Lehrer P.M., Isenberg S., Hochron S.M. Asthma and emotion: a review. J Asthma. 1993;30(1):5-21. Review. PMID:8428858
97 Lehrer P.M. Emotionally triggered asthma: a review of research literature and some hypotheses for self-regulation therapies. Appl Psychophysiol Biofeedback. 1998 Mar;23(1):13-41.
98 Kern-Buell C.L., McGrady A.V., Conran P.B., et al. Asthma severity, psychophysiological indicators of arousal, and immune function in asthma patients undergoing biofeedback-assisted relaxation. Appl Psychophysiol Biofeedback. 2000 Jun;25(2):79-91. PMID:10932333
99 Kotses H, Glaus KD. Applications of biofeedback to the treatment of asthma: a critical review. Biofeedback Self Regul 1981 Dec;6(4):573–93. Applications of biofeedback to the treatment of asthma: a critical review. PMID:7034795.
100 Peper E., Tibbetts V. Fifteen-month follow-up with asthmatics utilizing EMG/incentive inspirometer feedback. Biofeedback Self Regul. 1992 Jun;17(2):143-151.
101 Jahanshahi M., Sartory G., Marsden C.D. EMG biofeedback treatment of torticollis: a controlled outcome study. Biofeedback Self Regul. 1991 Dec;16(4):413-448. PMID:1760462
102 Gallego J., Perez de la Søta A., Vardon G., et al. Electromyographic feedback for learning to activate thoracic inspiratory muscles. Am J Phys Med Rehabil. 1991 Aug;70(4):186-190. PMID:1878176
103 Ewer T.C., Stewart D.E.. Br Med J., Clin Res. Improvement in bronchial hyper-responsiveness in patients with moderate asthma after treatment with a hypnotic technique: a randomised controlled trial, 293. 1986 Nov 1, 1129-1132. 6555
104 Morrison J.B. Chronic asthma and improvement with relaxation induced by hypnotherapy. J R Soc Med. 1988 December;81(12):701-704.
105 Smyth J., Stone A.A., Hurewitz A., et al. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: a randomised trial. JAMA. 1999;281(14):1304-1309.
106 Harris A.H., Thoresen C.E., Humphreys K., et al. Does writing affect asthma? A randomized trial. Psychosom Med. 2005 Jan–Feb;67(1):130-136.
107 Liangas G., Morton J.R., Henry R.L. Mirth-triggered asthma: is laughter really the best medicine? Pediatr Pulmonol. 2003 Aug;36(2):107-112.
108 Svanes C., Heinrich J., Jarvis D., et al. Pet-keeping in childhood and adult asthma and hay fever: European community respiratory health survey. J Allergy Clin Immunol. 2003 Aug;112(2):289-300.
109 Simpson A., Custovic A. Early pet exposure: friend or foe? Current Opinion in Allergy and Clinical Immunology. 2003;3:7-14.
110 Apelberg B.J., Aoki Y., Jaakkola J.J.K. Systematic review: exposure to pets and risk of asthma and asthma-like symptoms. J Allergy Clin Immunol. 2001;107:455-460.
111 Svanes C., Heinrich J., Jarvis D., et al. Pet-keeping in childhood and adult asthma and hay fever: European community respiratory health survey. J Allergy Clin Immunol. 2003 Aug;112(2):289-300.
112 Lau S., Wahn U. Pets—good or bad for individuals with atopic predisposition? J Allergy Clin Immunol. 2003 Aug;112(2):263-264.
113 Gern J.E., Reardon C.L., Hoffjan S., et al. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol. 2004 Feb;113(2):307-314.
114 Marks G.B. What should we tell allergic families about pets? J Allergy Clin Immunol. 2001;108:500-502.
115 Apter A.J. Early exposure to allergen: Is this the cat’s meow or are we barking up the wrong tree. J Allergy Clin Immunol. 2003;111:938-946.
116 Bufford J.D., Reardon C.L., Li Z., et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008 Oct;38(10):1635-1643.
117 Duelien Skorge T., Eagan T.M.L., Eide G.E., et al. Indoor exposures and respiratory symptoms in a Norwegian community sample. Thorax. 2005;60:937-942.
118 Girgis S.T., Marks G.B., Downs S.H., et al. Thunderstorm-associated asthma in an inland town in south-eastern Australia. Who is at risk? Eur Respir J. 2000 Jul;16(1):3-8.
119 Wark P.A., Simpson J., Hensley M.J., et al. Airway inflammation in thunderstorm asthma. Clin Exp Allergy. 2002 Dec;32(12):1750-1756.
120 D’Amato G., Liccardi G., Frenguelli G. Thunderstorm-asthma and pollen allergy. Allergy. 2007 Jan;62(1):11-16.
121 Douwes J., Cheng S., Travier N., et al. Eur Respir J. 2008 Sep;32(3):603-611.
122 Crisafulli D., Almqvist C., Marks G., et al. Seasonal trends in house dust mite allergen in children’s beds over a 7–year period Allergy. 2007 Dec;62(12):1394-1400.
123 Lau S., Illi S., Sommerfeld C., et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000 Oct 21;356(9239):1392-1397.
124 Nicolai T., Illi S., von Mutius E. Effect of dampness at home in childhood on bronchial hyperreactivity in adolescence. Thorax. 1998 Dec;53(12):1035-1040.
125 Mihrshahi S., Marks G.B., Criss S., et al. CAPS Team. Effectiveness of an intervention to reduce house dust mite allergen levels in children’s beds. Allergy. 2003 Aug;58(8):784-789.
126 Tsitoura S., Nestoridou K., Botis P., et al. Randomized trial to prevent sensitization to mite allergens in toddlers and preschoolers by allergen reduction and education: one-year results. Arch Pediatr Adolesc Med. 2002 Oct;156(10):1021-1027.
127 van Strien R.T., Koopman L.P., Kerkhof M., et al. Prevention and Incidence of Asthma and Mite Allergy Study Mattress encasings and mite allergen levels in the Prevention and Incidence of Asthma and Mite Allergy study. Clin Exp Allergy. 2003 Apr;33(4):490-495.
128 Lau S., Illi S., Sommerfeld C., et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000 Oct 21;356(9239):1392-1397. PubMed PMID:11052581
129 Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol. 2004 Jun;15(suppl.)(16):4-5. 9–32
130 Ponsonby A.L., Dwyer T., Kemp A., et al. Synthetic bedding and wheeze in childhood. Epidemiology. 2003 Jan;14(1):37-44.
131 Synthetic pillows and wheezing in childhood. Child Health Alert. 2003 Feb;21:2. (no authors listed)
132 Ponsonby A.L., Gatenby P., Glasgow N., et al. The association between synthetic bedding and adverse respiratory outcomes among skin-prick test positive and skin-prick test negative children. Allergy. 2002 Mar;57(3):247-253.
133 Ponsonby A.L., Dwyer T., Trevillian L., et al. The bedding environment, sleep position, and frequent wheeze in childhood. Pediatrics. 2004 May;113(5):1216-1222.
134 Ponsonby A.L., Kemp A., Dwyer T., et al. Feather bedding and house dust mite sensitization and airway disease in childhood. J Clin Epidemiol. 2002 Jun;55(6):556-562.
135 Strachan D., Carey I.M. Reduced risk of wheezing in children using feather pillows is confirmed. BMJ. 1997 Feb 15;314(7079):518.
136 Rains N., Siebers R., Crane J., et al. House dust mite allergen (Der p 1) accumulation on new synthetic and feather pillows. Clin Exp Allergy. 1999 Feb;29(2):182-185.
137 Crane J., Kemp T., Siebers R., et al. Increased house dust mite allergen in synthetic pillows may explain increased wheezing. BMJ. 1997 Jun 14;314(7096):1763-1764.
138 Mills S., Siebers R., Wickens K., et al. House dust mite allergen levels in individual bedding components in New Zealand. NZ Med J. 2002 Apr 12;115(1151):151-153.
139 Kemp T.J., Siebers R.W., Fishwick D., et al. House dust mite allergen in pillows. BMJ. 1996 Oct 12;313(7062):916.
140 Tovey E.R., Taylor D.J., Mitakakis T.Z., et al. Effectiveness of laundry washing agents and conditions in the removal of cat and dust mite allergen from bedding dust. J Allergy Clin Immunol. 2001 Sep;108(3):369-374.
141 Gøtzsche P.C., Johansen H.K. House dust mite control measures for asthma. Cochrane Database of Systematic Reviews. (Issue 2):2008. Art. No: CD001187. doi: 10.1002/14651858.CD001187.pub3
142 McAvoy B.R.. Cochrane PEARLS (Practical Evidence About Real Life Situations), No. 80, August 2008.
143 Becker A., Watson W., Ferguson A., et al. The Canadian asthma primary prevention study: outcomes at 2 years of age. Allergy Clin Immunol. 2004 Apr;113(4):650-656.
144 National Asthma Council website. Available: http://www.nationalasthma.org.au/html/management/infopapers/health_professionals/1010.asp (accessed 7 April 2009).
145 Abramson M.J., Puy R.M., Weiner J.M. Allergen immunotherapy for asthma. Cochrane Database of Systematic Reviews. (Issue 4):2003. Art No: CD001186. doi: 10.1002/14651858.CD001186
146 Penagos M., Passalacqua G., Compalati E., et al. Metaanalysis of the Efficacy of Sublingual Immunotherapy in the Treatment of Allergic Asthma in Pediatric Patients, 3 to 18 Years of Age. Chest. 2008;133:599-609.
147 Bufe A., Eberle P., Franke-Beckmann E., et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. Journal of Allergy and Clinical Immunology. 2009;123(1):167-173. e7
148 Nicolai T., Illi S., von Mutius E. Effect of dampness at home in childhood on bronchial hyperreactivity in adolescence. Thorax. 1998 Dec;53(12):1035-1040.
149 Pekkanen J., Hyvärinen A., Haverinen-Shaughnessy U., et al. Moisture damage and childhood asthma: a population-based incident case–control study. Eur Respir J. March 2007;29:509-515. doi:10.1183/09031936.00040806
150 Australian Government. Australian Institute of Health and Welfare (AIHW). Bulletin 59; April 2008 Occupational Asthma in Australia http://www.aihw.gov.au/publications/index.cfm/title/10328 (accessed Jan 2010)
151 Kogevinas M., Antó J.M., Sunyer J., et al. Occupational asthma in Europe and other industrialised areas: a population-based study. European Community Respiratory Health Survey Study Group. Lancet. 1999 May 22;353(9166):1750-1754.
152 Kogevinas M., Zock J.P., Jarvis D., et al. Exposure to substances in the workplace and new-onset asthma: an international prospective population-based study (ECRHS-II). Lancet. 2007 Jul 28;370(9584):336-341.
153 Monsó E., Magarolas R., Badorrey I., et al. Occupational asthma in greenhouse flower and ornamental plant growers. Am J Respir Crit Care Med. 2002 Apr 1;165(7):954-960.
154 Medina-Ramón M., Zock J.P., Kogevinas M., et al. Asthma symptoms in women employed in domestic cleaning: a community based study. Thorax. 2003 Nov;58(11):950-954.
155 Jaakkola J.J., Jaakkola M.S. Professional cleaning and asthma. Curr Opin Allergy Clin Immunol. 2006 Apr;6(2):85-90.
156 Zock J.P., Plana E., Jarvis D., et al. The use of household cleaning sprays and adult asthma: an international longitudinal study. Am J Respir Crit Care Med. 2007 Oct 15;176(8):735-741.
157 Medina-Ramón M., Zock J.P., Kogevinas M., et al. Asthma symptoms in women employed in domestic cleaning: a community based study. Thorax. 2003 Nov;58(11):950-954.
158 Belousova E.G., Toelle B.G., Xuan W., et al. The effect of parental smoking on presence of wheez or airway hyper-responsiveness in New South Wales school children. Aust N Z J Med. 1999 Dec;29(6):794-800.
159 Chen E., Schreier H.M., Strunk R.C., et al. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ Health Perspect. 2008 Jul;116(7):970-975. PMID:18629323
160 MacNeill S.J., Goddard F., Pitman R., et al. The Oxford Transport Strategy: impact of a traffic intervention on PEF and wheeze among children. European Respiratory Review June 1. 2008;17:88-89.
161 McCreanor J., Cullinan P., Nieuwenhuijsen M.J., et al. Respiratory Effects of Exposure to Diesel Traffic in Persons with Asthma. N Engl J Med. 2007 December 6;357:2348-2358.
162 Diaz-Sanchez D., Proietti L., Polosa R. Diesel fumes and the rising prevalence of atopy: an urban legend? Curr Allergy Asthma Rep. 2003 Mar;3(2):146-152.
163 Morgenstern V., Zutavern A., Cyrys J., et. al. for the GINI Study Group and the LISA Study Group. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J of Respiratory and Critical Care Medicine. 2008;177:1331-1337.
164 Boezen H.M., van der Zee S.C., Postma D.S., et al. Effects of ambient air pollution on upper and lower respiratory symptoms and peak expiratory flow in children. Lancet. 1999 Mar 13;353(9156):874-878.
165 Bartra J., Mullol J., del Cuvillo A., et al. Air pollution and allergens. J Investig Allergol Clin Immunol. 2007;17(suppl.)(2):3-8.
166 Wayne P., Foster S., Connolly J., et al. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2–enriched atmospheres. Ann Allergy Asthma Immunol. 2002 Mar;88(3):279-282.
167 Downs S.H., Schindler C., Liu S., et al. Reduced Exposure to PM10 and Attenuated Age-Related Decline in Lung Function. NEJM. 2007;357:2338-2347.
168 Lovasi G.S., Quinn J.W., Neckerman K.M., et al. Children living in areas with more street trees have lower prevalence of asthma. J Epidemiol Community Health. 2008 Jul;62(7):647-649.
169 Lau S., Nickel R., Niggemann B., et alMAS Group. The development of childhood asthma: lessons from the German Multicentre Allergy Study (MAS). Paediatr Respir Rev. 2002 Sep;3(3):265-272.
170 Sharma H.P., Hansel N.N., Matsui E., et al. Indoor environmental influences on children’s asthma. Pediatr Clin North Am. 2007 Feb;54(1):103-120. ix
171 Howden-Chapman P., Pierse N., Nicholls S., et al. Effects of improved home heating on asthma in community dwelling children: randomised controlled trial. BMJ. 2008;337:a1411.
172 Diette G.B., Hansel N.N., Buckley T.J., et al. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect. 2007 Nov;115(11):1665-1669.
173 Thomson H., Petticrew M., Morrison D. Health effects of housing improvement: systematic review of intervention studies. BMJ. 2008;337:a1411.
174 Roberts G., Golder N., Lack G. Bronchial challenges with aerosolized food in asthmatic, food-allergic children. Allergy. 2002 Aug;57(8):713-717.
175 Epton M.J., Dawson R.D., Brooks W.M., et al. The effect of ambient air pollution on respiratory health of school children: a panel study. Environmental Health. 2008;7:16.
176 Howden-Chapman P., Pierse N., Nicholls S., et al. Effects of improved home heating on asthma in community dwelling children: randomised controlled trial. BMJ. 2008;337:a1411.
177 Bernard A., Nickmilder M., Voisin C. Outdoor swimming pools and the risks of asthma and allergies during adolescence. Eur Respir J. 2008;32:979-988.
178 Thickett K.M., McCoach J.S., Gerber J.M., et al. Occupational asthma caused by chloramines in indoor swimming-pool air. Eur Respir J. 2002 May;19(5):827-832. PMID:12030720
179 Eggleston PA. Chlorinated pools and the risk of asthma. Environ Health Perspect 2007 May;115(5):A240; author reply A240–1. Chlorinated pools and the risk of asthma. PMID:17520032.
180 Carraro S., Pasquale M.F., Da Frè M., et al. Swimming pool attendance and exhaled nitric oxide in children. J Allergy Clin Immunol. 2006 Oct;118(4):958-960.
181 Bernard A. Chlorination products: emerging links with allergic diseases. Curr Med Chem 2007;14(16):1771–82. Review. Chlorination products: emerging links with allergic diseases. PMID:17627515.
182 Nemery B., Hoet P.H., Nowak D. Indoor swimming pools, water chlorination and respiratory health. Eur Respir J. 2002 May;19(5):790-793.
183 Lucas SR, Platts-Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol 2005 May;115(5):928–34. Review. Erratum in: J Allergy Clin Immunol 2005 Aug;116(2):298.
184 Golan Y., Onn A., Villa Y., et al. Asthma in adventure travellers: a prospective study evaluating the occurrence and risk factors for acute exacerbations. Arch Intern Med. 2002 Nov 25;162(21):2421-2426.
185 Matsumoto I., Araki H., Tsuda K., et al. Effects of swimming training on aerobic capacity and exercise-induced bronchoconstriction in children with bronchial asthma. Thorax. 1999 Mar;54(3):196-201.
186 Manocha R., Marks G.B., Kenchington P., et al. Sahaja yoga in the management of moderate to severe asthma: a randomised controlled trial. Thorax. 2002 Feb;57(2):110-115.
187 Vedanthan P.K., Kesavalu L.N., Murthy K.C., et al. Clinical study of yoga techniques in university students with asthma: a controlled study. Allergy Asthma Proc. 1998 Jan-Feb;19(1):3-9.
188 Nagarathna R., Nagendra H.R. Yoga for bronchial asthma: a controlled study. Br Med J (Clin Res Ed). 1985 Oct 19;291(6502):1077-1079.
189 Nagendra H.R., Nagarathna R. An integrated approach of yoga therapy for bronchial asthma: a 3–54–month prospective study. J Asthma. 1986;23(3):123-137.
190 Singh V., Wisniewski A., Britton J., Tattersfield A. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet. 1990 Jun 9;335(8702):1381-1383.
191 Jain S.C., Talukdar B. Evaluation of yoga therapy program for patients of bronchial asthma. Singapore Med J. 1993 Aug;34(4):306-308.
192 Sabina A.B., Williams A.L., Wall H.K., et al. Yoga intervention for adults with mild-to-moderate asthma: a pilot study. Ann Allergy Asthma Immunol. 2005 May;94(5):543-548.
193 Reuther I. Aldridge D. Qigong Yangsheng as a complementary therapy in the management of asthma: a single-case appraisal. Journal of Alternative & Complementary Medicine. 998;4(2):173-83
194 Dennis J., Cates C.J. Alexander technique for chronic asthma. Cochrane Database of Systematic Reviews. (Issue 2):2000. Art No: CD000995. doi: 10.1002/14651858.CD000995
195 Thomas M., McKinley R.K., Freeman E., et al. The prevalence of dysfunctional breathing in adults in the community with and without asthma. Prim Care Respir J. 2005 Apr;14(2):78-82.
196 Stanton A.E., Vaughn P., Carter R., et al. An observational investigation of dysfunctional breathing and breathing control therapy in a problem asthma clinic. J Asthma. 2008 Nov;45(9):758-765.
197 Keeley D., Osman L. Dysfunctional breathing and asthma. It is important to tell the difference. BMJ. 2001 May 5;322(7294):1075-1076.
198 Thomas M., McKinley R.K., Freeman E., et al. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ. 2001 May 5;322(7294):1098-1100.
199 Thomas M., McKinley R.K., Freeman E., et al. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ. 2001 May 5;322(7294):1098-1100.
200 Bidat E., Sznajder M., Fermanian C., et al. A diagnostic questionnaire for the hyperventilation syndrome in children. Rev Mal Respir. 2008 Sep;25(7):829-838. French
201 Thomas M., McKinley R.K., Freeman E., et al. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ. 2001 May 5;322(7294):1098-1100.
202 Keeley D., Osman L. Dysfunctional breathing and asthma. It is important to tell the difference. BMJ. 2001 May 5;322(7294):1075-1076.
203 Slader C.A., Reddel H.K., Spencer L.M., et al. Double-blind randomised controlled trial of two different breathing techniques in the management of asthma. Thorax. 2006;61:651-656.
204 Thomas M., McKinley R.K., Mellor S., et al. Breathing exercises for asthma: a randomised controlled trial. Thorax. 2009 Jan;64(1):55-61.
205 Holloway E.A., West R.J. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax. Dec 2007;2007(62):1039-1042.
206 Holloway E., Ram F.S.F. Breathing exercises for asthma. Cochrane Database of Systematic Reviews. (Issue 1):2004. Art No: CD001277. doi: 10.1002/14651858.CD001277.pub2
207 Weiner P., Azgad Y., Ganam R., Weiner M. Inspiratory muscle training in patients with bronchial asthma. Chest. 1992;102(5):1357-1361.
208 Weiner P., Azgad Y., Ganam R. Inspiratory muscle training for bronchial asthma. Harefuah. 1992 Feb 2;122(3):155-159. Hebrew. PMID:1563665
209 de Jong W., van Aalderen W.M., Kraan J., et al. Inspiratory muscle training in patients with cystic fibrosis. Respir Med. 2001 Jan;95(1):31-36. PMID:11207014
210 Thomas M., McKinley R.K., Freeman E., et al. Breathing retraining for dysfunctional breathing in asthma: a randomised controlled trial. Thorax. 2003 Feb;58(2):110-115.
211 Bowler S.D., Green A., Mitchell C.A. buteyko breathing techniques in asthma: a blinded randomised controlled trial. Med J Aust. 1998 Dec 7–21;169(11–12):575-578.
212 Opat A.J., Cohen M.M., Bailey M.J., et al. A clinical trial of the buteyko Breathing Technique in asthma as taught by a video. J Asthma. 2000;37(7):557-564.
213 Cooper S., Oborne J., Newton S., et al. Effect of two breathing exercises (buteyko and pranayama) in asthma: a randomised controlled trial. Thorax. 2003 Aug;58(8):674-679.
214 McHugh P., Aitcheson F., Duncan B., et al. buteyko Breathing Technique for asthma: an effective intervention. N Z Med J. 2003 Dec 12;116(1187):U710.
215 McGowan J. Health Education in Asthma Management. Does ‘The buteyko Institute of Method’ make a difference? Thorax Medical Journal. 2003:58. III
216 Devereux G., Turner S.W., Craig L.C., et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5–year-old children.Am J Respir Crit Care Med. 2006 Sep 1;174(5):499-507.
217 Heinrich J., Hölscher B., Bolte G., et al. Allergic sensitization and diet: ecological analysis in selected European cities. Eur Respir J. 2001 Mar;17(3):395-402.
218 Chatzi L., Apostolaki G., Bibakis I., et al. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax. 2007;62:677-683.
219 Forastiere F., Pistelli R., Sestini P., et al. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment. Thorax. 2000 Apr;55(4):283-288.
220 Cook D.G., Carey I.M., Whincup P.H., et al. Effect of fresh fruit consumption on lung function and wheeze in children. Thorax. 1997 Jul;52(7):628-633.
221 Forastiere F., Pistelli R., Sestini P., et al. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment). Thorax. 2000 Apr;55(4):283-288.
222 Butland B.K., Fehily A.M., Elwood P.C. Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax. 2000 Feb;55(2):102-108.
223 Butler L.M., Koh W.P., Lee H.P., et al. Dietary fiber and reduced cough with phlegm: a cohort study in Singapore. Am J Respir Crit Care Med. 2004 Aug 1;170(3):279-287.
224 Chatzi L., Torrent M., Romieu I., et al. Diet, wheeze, and atopy in school children in Menorca, Spain. Pediatric Allergy Immunol. 2007;18:480-485.
225 Mickleborough T.D., Rundell K.W. Dietary polyunsaturated fatty acids in asthma- and exercise-induced bronchoconstriction. Eur J Clin Nutr. 2005 Dec;59(12):1335-1346.
226 Hodge L., Salome C.M., Peat J.K., et al. Consumption of oily fish and childhood asthma risk. Medical Journal of Australia. 1996;164(3):137-140.
227 Broughton K.S., Johnson C.S., Pace B.K., et al. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5–series leukotriene production. Am J Clin Nutr. 1997 Apr;65(4):1011-1017.
228 Laerum B.N., Wentzel-Larsen T., Gulsvik A., et al. Relationship of fish and cod oil intake with adult asthma. Clinical & Experimental Allergy. 2007 Nov;37(11):1616-1623. doi: 10.1111/j.1365–2222.2007.02821.x
229 Wickens K., Barry D., Friezema A., et al. Fast foods — are they a risk factor for asthma? Allergy. 2005 Dec;60(12):1537-1541.
230 Lindahl O., Lindwall L., Spångberg A., et al. Vegan regimen with reduced medication in the treatment of bronchial asthma. J Asthma. 1985;22(1):45-55.
231 Rona R.J., Keil T., Summers C., et al. The prevalence of food allergy: a metaanalysis. J Allergy Clin Immunol. 2007;120:638-646.
232 Ostblom E., Lilja G., Ahlstedt S., et al. Patterns of quantitative food-specific IgE-antibodies and reported food hypersensitivity in 4–year-old children. Allergy. 2008 Apr;63(4):418-424.
233 Bock S.A. Food-related asthma and basic nutrition. J Asthma. 1983;20(5):377-381.
234 Oehling A. Importance of food allergy in childhood asthma. Allergol Immunopathol (Madr). 1981;9:71-73. (suppl.)
235 Ogle K.A., Bullock J.D. Children with allergic rhinitis and/or bronchial asthma treated with elimination diet: a five-year follow-up. Ann Allergy. 1980 May;44(5):273.
236 Zutavern A., Brockow I., Schaaf B., et al. LISA Study Group. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. 2008 Jan;121(1):e44-e52.
237 Woods R.K., Weiner J.M., Abramson M., et al. Do dairy products induce bronchoconstriction in adults with asthma? J Allergy Clin Immunol. 1998 Jan;101(1 Pt 1):45-50.
238 Martelli A., De Chiara A., Corvo M., et al. Beef allergy in children with cow’s milk allergy; cow’s milk allergy in children with beef allergy. Ann Allergy Asthma Immunol. 2002 Dec;89(6):38-43. suppl.1
239 Perry C.A., Dwyer J., Gelfand J.A., et al. Health effects of salicylates in foods and drugs. Nutr Rev. 1996;54(8):225-240.
240 Palosuo K. Current Opinion in Allergy and Clinical Immunology. 2003;3:205-209.
241 Majamaa H., Moisio P., Holm K., et al. Wheat allergy: diagnostic accuracy of skin prick and patch tests and specific IgE. Allergy. 1999;54:851-856.
242 Linna O. Specific IgE antibodies to uningested cereals. Allergy. 1996;51:849-850.
243 Schachter L.M., Peat J.K., Salome C.M. Asthma and atopy in overweight children. Thorax. 2003 Dec;58(12):1031-1035.
244 Stenius-Aarniala B., Poussa T., Kvarnström J., et al. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000 Mar 25;320(7238):827-832.
245 Hakala K., Stenius-Aarniala B., Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000 Nov;118(5):1315-1321.
246 Cheng J., Pan T. Calorie controlled diet for chronic asthma. Cochrane Database of Systematic Reviews. (Issue 2):2003. Art No: CD004674. doi: 10.1002/14651858.CD004674.pub2
247 Burney P.G., Neild J.E., Twort C.H., et al. Effect of changing dietary sodium on the airway response to histamine. Thorax. 1989 Jan;44(1):36-41.
248 Burney P.G., Britton J.R., Chinn S., et al. Response to inhaled histamine and 24 hour sodium excretion. Br Med J (Clin Res Ed). 1986 Jun 7;292(6534):1483-1486.
249 Javaid A., Cushley M.J., Bone M.F. Effect of dietary salt on bronchial reactivity to histamine in asthma. BMJ. 1988 August 13;297:454.
250 Ardern K. Dietary salt reduction or exclusion for allergic asthma. Cochrane Database of Systematic Reviews. (Issue 2):2004. Art No: CD000436. doi: 10.1002/14651858.CD000436.pub2
251 Blanc P.D., Ware G.K., Katz P.P., et al. Use of herbal products, coffee or black tea, and over-the-counter medications as self-treatments among adults with asthma. J Allergy Clin Immunol. 1997;100:789-791.
252 Bara A., Barley E. Caffeine for asthma. Cochrane Database of Systematic Reviews. (Issue 4):2001. Art No: CD001112. doi: 10.1002/14651858.CD001112
253 Paul I.M., Beiler J., McMonagle A., et al. Effect of Honey, Dextromethorphan, and No Treatment on Nocturnal Cough and Sleep Quality for Coughing Children and Their Parents. Arch Pediatr Adolesc Med. 2007;161(12):1140-1146.
254 Warren M.D., Pont S.J., Barkin S.L., et al. The effect of honey on nocturnal cough and sleep quality for children and their parents. Arch Pediatr Adolesc Med. 2007 Dec;161(12):1149-1153.
255 Cardinale F., Mangini F., Berardi M., et al. Intolerance to food additives: an update [Article in Italian]. Minerva Pediatr. 2008 Dec;60(6):1401-1409.
256 Tarlo S.M., Sussman G.L. Asthma and anaphylactoid reactions to food additives. Can Fam Physician. 1993 May;39:1119-1123.
257 Freedman B.J. A dietary free from additives in the management of allergic disease. Clin Allergy. 1977 Sep;7(5):417-421.
258 Stevenson D.D., Simon R.A. Sensitivity to ingested metabisulfites in asthmatic subjects. J Allergy Clin Immunol. 1981 Jul;68(1):26-32.
259 Vally H., Thompson P.J. Allergic and asthmatic reactions to alcoholic drinks. Addict Biol. 2003 Mar;8(1):3-11.
260 Vally H., Thompson P.J. Role of sulfite additives in wine induced asthma: single dose and cumulative dose studies. Thorax. 2001 Oct;56(10):763-769.
261 Vally H., de Klerk N., Thompson P.J. Alcoholic drinks: important triggers for asthma. J Allergy Clin Immunol. 2000 Mar;105(3):462-467.
262 Subiza J., Subiza J.L., Valdivieso R., et al. Toothpaste flavor-induced asthma. J Allergy Clin Immunol. 1992 Dec;90(6 Pt 1):1004-1006.
263 Spurlock B.W., Dailey T.M. Shortness of (fresh) breath–toothpaste-induced bronchospasm. N Engl J Med. 1990 Dec 27;323(26):1845-1846.
264 Baer P.N. Toothpaste allergies. J Clin Pediatr Dent. 1992;Spring;16(3):230-231.
265 Reynolds R.D., Natta C.L. Depressed plasma pyridoxal phosphate concentrations in adult asthmatics. Am J Clin Nutr. 1985 Apr;41(4):684-688.
266 Kaslow J.E. Double-blind trial of pyridoxine (vitamin B6) in the treatment of steroid-dependent asthma. Ann Allergy. 1993 Nov;71(5):492. PubMed PMID:8250357
267 Sur S., Camara M., Buchmeier A., et al. Double-blind trial of pyridoxine (vitamin B6) in the treatment of steroid-dependent asthma. Ann Allergy. 1993 Feb;70(2):147-152. PubMed PMID:8430923
268 Collipp P.J., Goldzier S.III, Weiss N., et al. Pyridoxine treatment of childhood bronchial asthma. Ann Allergy. 1975 Aug;35(2):93-97.
269 Renwick A.G. Toxicology of micronutrients: Adverse effects and uncertainty. J. Nutr. 2006;136:493S-501S.
270 Australian Adverse Drug Reactions Bulletin. Therapeutic Goods Administration, 2008 August;(4):27.
271 Maisel F.E., Somkin E. Treatment of asthmatic paroxysm with nicotinic acid. J Allergy. 1942;13:397-403.
272 Melton G. Treatment of asthma by nicotinic acid. BMJ. 1943; May 15:600-601.
273 Omenaas E., Fluge O., Buist A.S., et al. Dietary vitamin C intake is inversely related to cough and wheeze in young smokers. Respir Med. 2003 Feb;97(2):134-142.
274 Grievink L., Smit H.A., Ocké M.C., et al. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax. 1998 Mar;53(3):166-171.
275 Britton J.R., Pavord I.D., Richards K.A., et al. Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med. 1995 May;151(5):1383-1387.
276 HatchAsthma G.E., Inhaled Oxidants, Dietary Antioxidants. American Journal of Clinical Nutrition. 1995 Mar;61(3):625s-630s. suppl.
277 Lykkesfeldt J., Christen S., Wallock L.M., et al. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and non-smokers with matched dietary antioxidant intakes. Am J Clin Nutr. 2000 Feb;71(2):530-536.
278 Bucca C., Rolla G., Oliva A. 13. Effect of vitamin C on histamine bronchial responsiveness of patients with allergic rhinitis. Ann Allergy. 1990;65:311-314.
279 Anah C.O., Jarike L.N., Baig H.A. High dose ascorbic acid in Nigerian asthmatics. Tropical Geograph. Med. 1980;32:132-137.
280 Schachter E.N., Schlesinger A. The attenuation of exercise-induced bronchospasm by ascorbic acid. Ann Allergy. 1982 Sep;49(3):146-151.
281 Tecklenburg S.L., Mickleborough T.D., Fly A.D., et al. Ascorbic acid supplementation attenuates exercise-induced bronchoconstriction in patients with asthma. Respir Med. 2007 Aug;101(8):1770-1778.
282 Malo J.L., Cartier A., Pineau L., et al. Lack of acute effects of ascorbic acid on spirometry and airway responsiveness to histamine in subjects with asthma. J Allergy Clin Immunol. 1986;78(6):11532-11558.
283 Kaur B., Rowe B.H., Arnold E. Vitamin C supplementation for asthma. Cochrane Database of Systematic Reviews. (Issue 1):2009. Art No: CD000993. doi:10.1002/14651858.CD000993.pub3
284 Gao J., Gao X., Li W., et al. Observational studies on the effect of dietary antioxidants on asthma: a meta-analysis. Respirology. 2008 Jun;13(4):528-536.
285 Black P.N., Scragg R. Relationship between serum 25–hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey Chest. 2005 Dec;128(6):3792-3798.
286 Gontijo-Amaral C., Ribeiro M.A., Gontijo L.S., et al. Oral magnesium supplementation in asthmatic children: a double-blind randomized placebo-controlled trial. Eur J Clin Nutr. 2007 Jan;61(1):54-60.
287 Skobeloff E.M., Spivey W.H., McNamara R.M., Greenspon L. Intravenous magnesium sulfate for the treatment of acute asthma in the emergency department. JAMA. 1989 Sep 1;262(9):1210-1213.
288 Brunner E.H., Delabroise A.M., Haddad Z.H. Effect of parenteral magnesium on pulmonary function, plasma cAMP, and histamine in bronchial asthma. J Asthma. 1985;22(1):3-11.
289 Sydow M., Crozier T.A., Zielmann S., et al. High-dose intravenous magnesium sulfate in the management of life-threatening status asthmaticus. Intensive Care Med. 1993;19(8):467-471.
290 Silverman R.A., Osborn H., Runge J., et al. Acute Asthma/Magnesium Study Group. IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest. 2002 Aug;122(2):489-497.
291 Cairns C.B., Kraft M. Magnesium attenuates the neutrophil respiratory burst in adult asthmatic patients. Acad Emerg Med. 1996 Dec;3(12):1093-1097.
292 Rowe B.H., Bretzlaff J.A., Bourdon C., et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. (2):2000. CD001490
293 Blitz M., Blitz S., Beasely R., et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. (4):2005 Oct 19. CD003898
294 Blitz M., Blitz S., Hughes R., et al. Aerosolized magnesium sulfate for acute asthma: a systematic review. Chest. 2005 Jul;128(1):337-344.
295 Hasselmark L., Malmgren R., Zetterström O., et al. Selenium supplementation in intrinsic asthma. Allergy. 1993;48:30-36.
296 Shaheen S.O., Newson R.B., Rayman M.P., et al. Randomised, double-blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax. June 2007;2007(62):483-490.
297 Allam M.F., Lucena R.A. Selenium supplementation for asthma. Cochrane Database of Systematic Reviews. (Issue 2):2004. Art No: CD003538. doi: 10.1002/14651858.CD003538
298 Mickleborough T.D., Lindley M.R., Ionescu A.A., et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006 Jan;129(1):39-49.
299 Mickleborough T.D., Murray R.L., Ionescu A.A., et al. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003 Nov 15;168(10):1181-1189.
300 Hodge L., Salome C.M., Hughes J.M., et al. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Respir J. 1998 Feb;11(2):361-365.
301 Thien F.C.K., De Luca S., Woods R., et al. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database of Systematic Reviews. (Issue 2):2002. Art No: CD001283. doi: 10.1002/14651858.CD001283
302 Okamoto M., Mitsunobu F., Ashida K., et al. Effects of dietary supplementation with n-3 fatty acids compared with n-6 fatty acids on bronchial asthma. Intern Med. 2000 Feb;39(2):107-111.
303 Huntley A., Ernst E. Herbal medicines for asthma: a systematic review. Thorax. 2000 Nov;55(11):925-929.
304 Arnold E., Clark C.E., Lasserson T.J., et al. Herbal interventions for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. (Issue 1):2008. Art No: CD005989. doi: 10.1002/14651858.CD005989.pub2
305 Hofmann D., Hecker M., Völp A. Efficacy of dry extract of ivy leaves in children with bronchial asthma-a review of randomized controlled trials. Phytomedicine. 2003 Mar;10(2–3):213-220.
306 Li M., Yang B., Yu H., Zhang H. Clinical observation of the therapeutic effect of ginkgo leaf concentrated oral liquor on bronchial asthma. CJIM. 1997;3:264-267.
307 Nadir A., Agrawal S., King P.D., et al. Acute hepatitis associated with the use of a Chinese herbal product, ma-huang. Am J Gastroenterol. 1996 Jul;91(7):1436-1438.
308 Skoulidis F., Alexander G.J., Davies S.E. Ma huang associated acute liver failure requiring liver transplantation. Eur J Gastroenterol Hepatol. 2005 May;17(5):581-584.
309 Urata Y., Yoshida S., Irie Y., et al. Treatment of asthma patients with herbal medicine TJ-96: a randomized controlled trial. 82 Respir Med. 2002 Jun;96(6):469-474.
310 Egashira Y., Nagano H. A multicenter clinical trial of TJ-96 in patients with steroid-dependent bronchial asthma. A comparison of groups allocated by the envelope method. Ann N Y Acad Sci. 1993 Jun 23;685:580-583.
311 Nakajima S., Tohda Y., Ohkawa K., et al. Effect of saiboku-to (TJ-96) on bronchial asthma. Induction of glucocorticoid receptor, beta-adrenaline receptor, IgE-Fc epsilon receptor expression and its effect on experimental immediate and late asthmatic reaction. Ann N Y Acad Sci. 1993 Jun 23;685:549-560.
312 Palecek I. Amni-visnaga. Munchener Medizinische Wochenschrift. 1970, Jan. 30;112(5):199-202.
313 Schindl R. Treatment of asthma with germakellin. Body plethysmographic investigations [Article in German]. Munch Med Wochenschr. 1971 Oct 29;113(44):1471-1474.
314 Zioglo G., Samochoweic L. Study on Clinical Properties and Mechanisms of Action of Petasites in Bronchial Asthma and Chronic Obstructive Bronchitis. Pharm Acta Helv. 1998;72:378-380.
315 Dept Zara P. Science & technology. Phillipine Council for Health Research. 2002.
316 Ammon H.P. Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory diseases [Article in German]. Wien Med Wochenschr. 2002;152(15–16):373-378.
317 Ammon H.P., Safayhi H., Mack T., et al. Mechanisms of Anti-Inflammatory Actions of Curcumine and Boswellic Acids. Journal of Ethno-Pharmacology. 1993;38:113-119.
318 Gupta I., Gupta V., Parihar A., et al. Effects of Boswellia serata Gum Resin in patients with Bronchial Asthma: Results of a double-blind, placebo-controlled, 6 week clinical study. Eur J Med Res. 1998;3(11):511-514.
319 Gupta S., George P., Gupta V., et al. Tylophora indica in bronchial asthma–a double-blind study. Indian J Med Res. 1979 Jun;69:981-989.
320 Mathew K.K., Shivpuri D.N. Treatment of asthma with alkaloids of Tylophora indica: a double-blind study. Aspects Allergy Appl. Immunol. 1974;7:166-179.
321 Shivpuri D.N., Menon M.P.S., Parkash D. A crossover double-blind study on Tylophora indica in the treatment of asthma and allergic rhinitis. J Allergy. 1969;43(3):145-150.
322 Shivpuri D.N., Singhal S.C., Parkash D. Treatment of asthma with an alcoholic extract of tylophora indica: a cross-over, double-blind study. Annals of Allergy. 1972;30(7):407-412.
323 Shivpuri D.N., Agarwal M.K. Effect of Tylophora indica on bronchial tolerance to inhalation challenge with specific allergens. Ann Allergy. 1973;31(2):87-94.
324 Thiruvengadam K.V., Haranath K., Sudarsan S., et al. Tylophora indica in bronchial asthma. J Indian Med Assoc. 1978;71:172-177.
325 Shivpuri D.N., Menon M.P., Parkash D. Preliminary studies in Tylophora indica in the treatment of asthma and allergic rhinitis. J Assoc Physicians India. 1968 Jan;16(1):9-15.
326 Gore K.V., Rao A.K., Guruswamy M.N. Physiological studies with Tylophora asthmatica in bronchial asthma. Indian J Med Res. 1980 Jan;71:144-148.
327 Kaik K.G., Witte P.U. Protective effect of forskolin in acetylcholine provocation in healthy probands. Comparison doses with fenoterol and placebo. (German, English abstract). Wien Med Wochenschr. 1986;136(23–24):637-641.
328 Bauer K., Dietersdorfer F., Sertl K., et al. Pharmacodynamic effects of inhaled dry powder formulations of fenoterol and colforsin in asthma. Clin Pharmacol Ther. 1993 Jan;53(1):76-83.
329 Tripathi R.M., Sen P.C., Das P.K., et al. Studies on the mechanism of action of Albizia Labbek, an Indian indigenous drug used in the treatment of atopic allergy. J. Ethnopharmacol. 1979;1(4):397-400.
330 Tripathi R.M., Sen P.C., Das P.K. Further studies on the mechanism of the anti-anaphylactic action of Albizzia lebbeck, an Indian indigenous drug. J Ethnopharmacol. 1979 Dec;1(4):397-400.
331 Cambridge G.W., Jansen A.B., Jarman D.A. Bronchodilating action of vascinone and related compounds. Nature. 1962;196:1217.
332 Gupta O.P., Sharma M.L., Ghatak B.J., et al. Pharmacological investigations of vasicine and vasicinone- the alkaloids of Adhatoda vasica. Indian J Med. Res. 1977;66(4):680-691.
333 Racle J.P., Girard M., Delage J., et al. Clinical and anatomopathological effect of Bisolvon in respiratory resuscitation. Ann Anesthesiol Fr. 1976;17(1):51-58.
334 Claeson U.P., Malmfors T., Wikman G., et al. Adhatoda vasica: a critical review of ethnopharmacological and toxicological data. J Ethnopharmacol. 2000 Sep;72(1–2):1-20.
335 Meza R.A., Bridges-Webb C., Sayer G.P., et al. The management of acute bronchitis in general practice: results from the Australian Morbidity and Treatment Survey, 1990–1991. Aust Fam Physician. 1994 Aug;23;8:1550-1553..
336 Ernst E., Marz R., Sieder Ch. A controlled multicentre study of herbal versus synthetic secretolytic drugs for acute bronchitis. Phytomedicine. 1997;4:287-293.
337 Blumenthal M, Goldberg A, Brinckmann J. Herbal Medicine. Expanded Commission E monographs. Author Affiliation: American Botanical Council, PO Box 144345, Austin, TX 78714–4345, USA. Editors: Blumenthal M, Goldberg A, Brinckmann J. Herbal medicine. Expanded Commission E monographs, IMC, 2000.
338 Bielory L., Lupoli K. Review article. Herbal Interventions in Asthma and Allergy. Journal of Asthma. 1999;36(1):1-65.
339 Benito M., Jorro G., Morales C., et al. Labiatae allergy: systemic reactions due to ingestion of oregano and thyme. Ann Allergy Asthma Immunol. 1996 May;76(5):416-418.
340 Lemiere C., Cartier A., Lehrer S.B., et al. Occupational asthma caused by aromatic herbs. Allergy. 1996 Sep;51(9):647-649.
341 Hausen B.M., Ketels-Harken H., Schulz K.H. Berufsbedingte Inhalationsallergie durch Pollen von Euphorbia fulgens Karw [Occupational allergy due to inhalation of pollen from Euphorbia fulgens Karw (author’s transl)]. Dtsch Med Wochenschr. 1976 Apr 9;101(15):567-570.
342 Reilly D.T., Taylor M.A., McSharry C., et al. Is homeopathy a placebo response? Controlled trial of homoeopathic potency with pollen in hayfever as a model. Lancet. 1986;2:881-886.
343 Reilly D., Taylor M.A., Beattie N.G.M., et al. Is evidence for homeopathy reproducible? Lancet. 1994;344:1601-1606.
344 Lewith G.T., Watkins A.D., Hyland M.E., et al. Use of ultramolecular potencies of allergen to treat asthmatic people allergic to house dust mite: double-blind randomised controlled clinical trial. BMJ. 2002 Mar 2;324(7336):520. PubMed PMID:11872551; PubMed Central PMCID: PMC67767
345 White A., Slade P., Hunt C., et al. Individualised homeopathy as an adjunct in the treatment of childhood asthma: a randomised placebo-controlled trial. Thorax. 2003 Apr;58(4):317-321.
346 Linde K., Clausius N., Ramirez G., et al. Are the clinical effects of homoeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet. 1997;350:834-843.
347 McCarney R.W., Linde K., Lasserson T.J. Homeopathy for chronic asthma. Cochrane Database of Systematic Reviews. (Issue 1):2004. Art No: CD000353. doi: 10.1002/14651858.CD000353.pub2
348 Passalacqua G., Bousquet P.J., Carlsen K.H., et al. ARIA update: I-Systematic review of complementary and alternative medicine for rhinitis and asthma. J Allergy Clin Immunol. 2006 May;117(5):1054-1062.
349 Shang A., Huwiler-Muntener K., Nartey L., et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet. 2005 Aug 27–2 Sep;366(9487):726-732.
350 Hondras M.A., Linde K., Jones A.P. Manual therapy for asthma. Cochrane Database of Systematic Reviews. (Issue 2):2005. Art No: CD001002. doi: 10.1002/14651858.CD001002.pub2
351 McCarney R.W., Brinkhaus B., Lasserson T.J., et al. Acupuncture for chronic asthma. The Cochrane Database of Systematic Reviews. (Issue 3):2003. Art No: CD000008. doi: 10.1002/14651858.CD000008.pub2
352 Gruber W., Eber E., Malle-Scheid D., et al. Laser acupuncture in children and adolescents with exercise-induced asthma. Thorax. 2002 Mar;57(3):222-225.
353 Medici T.C., Grebski E., Wu J., et al. Acupuncture and bronchial asthma: a long-term randomized study of the effects of real versus sham acupuncture compared to controls in patients with bronchial asthma. J Altern Complement Med. 2002 Dec;8(6):737-750. discussion 751–4
354 Fung K.P., Chow O.K., So S.Y. Attenuation of exercise-induced asthma by acupuncture. Lancet. 1986;Dec 20–27;2(8521–22):1419-1422.
355 Field T., Henteleff T., Hernandez-Reif M., et al. Children with asthma have improved pulmonary functions after massage therapy. J Pediatr. 1998 May;132(5):854-858.
356 Hernandez-Reif M., Field T., Krasnegor J., et al. Children with cystic fibrosis benefit from massage therapy. J Pediatr Psychol. 1999 Apr;24(2):175-181. PMID:10361400
357 Beamon S., Falkenbach A., Fainburg G., et al. Speleotherapy for asthma. Cochrane Database of Systematic Reviews. (Issue 2):2001. Art No: CD001741. doi: 10.1002/14651858.CD001741
358 Brygge T., Heinig J.H., Collins P., et al. No evidence was found that reflexology has a specific effect on asthma beyond placebo influence. Respiratory Medicine. 2001 Mar.
359 Petersen L.N., Faurschou P., Olsen O.T., et al. Footzone therapy and bronchial asthma — a clinically controlled investigation. Ugeskr Laeger. 1992;154:2065-2068.
360 National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: Commonwealth of Australia; 2000.