CHAPTER 312 Assessment of the Cervical Spine after Trauma
Acute injuries of the spine and spinal cord are among the most common causes of death and disability resulting from trauma.1–4 Organized trauma systems, improvements in prehospital care, advances in surgical stabilization techniques, and specialized centers for rehabilitation have all contributed to improving outcomes after cervical spinal cord injury. Despite these advances, however, prevention of injuries altogether or, at a minimum, prevention of additional secondary injury will have the greatest impact on overall outcomes.
Epidemiology
Accidents are the fourth leading cause of death in the United States, after heart disease, cancer, and stroke, and account for approximately 50 deaths per 100,000 population annually.5 Early deaths tend to result from exsanguination, whereas later posttraumatic deaths (those occurring after the first hour of hospitalization) tend to result from severe neurological injury.6
The prevalence of spinal cord injury varies for different geographic regions, as well as for different groups according to age, gender, and race.7–9 Spinal cord injuries, like most traumatic injuries, tend to occur in a young, predominantly male population, although a second peak is observed in the elderly.7 Young males are affected 3 to 20 times more often than females.7,10,11 In the elderly, there does not appear to be a difference in the relative number of men versus women affected, although this is in part explained by the much smaller number of men making up an elderly population.7
Estimates of the number of patients living with a spinal cord injury in the United States alone range from 185,000 to 400,000. Approximately 2000 U.S. hospital beds are required each year for the care of these patients.12 According to Kraus and colleagues, of the approximately 14,000 people who sustain spinal cord injuries each year, 4200 die before reaching the hospital and an additional 1500 patients die during the initial hospitalization.13
Although cervical spine fractures account for 20% to 30% of all spine fractures, cervical spinal cord injuries make up more than half of all spinal cord injuries. Fortunately, only 10% to 20% of cervical fractures result in spinal cord injuries.14 Cervical cord injuries result in profound physical disability and have a considerable societal impact. In 1990, the direct cost of spinal cord injury was estimated to be $4 billion, with lost wages being estimated at $3.4 billion.15
Reliable estimates of the prevalence of cervical spine injury after trauma are difficult to make because the inclusion criteria for various studies differ markedly. In addition, the proportion of cervical fractures that are found to be unstable is not clearly known. Most authors suggest that cervical injuries occur in 2% to 6.6% of patients after blunt trauma.16–19
A recent meta-analysis of 65 studies published between 1985 and 2008 attempted to determine the prevalence of cervical spine fracture and also to define the rate of instability.20 These authors found an overall 3.7% incidence of traumatic cervical spine injury. However, there was a significant difference observed according to the level of consciousness. In alert patients, the prevalence of cervical spine injury was 2.8%, whereas patients who could not be evaluated clinically (intoxicated, altered mentation) were found to have a prevalence of 7.7%. In addition, for all detected cervical spine injuries, 41.9% were subsequently determined to be unstable.
The likelihood of a concomitant cervical spine injury in a patient with a head injury has also been well known since the 1920s21 and is reported to range between 4% and 8%.22 The severity of the head injury tends to positively correlate with the likelihood of a spine injury,22–25 as does the mechanism of the head injury. For example, although vehicle-related head injuries may be associated with an approximately 10% rate of concomitant spine fracture, severe penetrating head injuries such as gunshot wounds are rarely accompanied by spine fractures.22 The spine injuries associated with moderate or severe head injury also more frequently involve the upper cervical spine.
Motor vehicle accidents cause between 35% and 45% of all spinal cord injuries.7,26 The cervical region is the segment of the spine most frequently injured in vehicular crashes, especially when shoulder and lap belt restraints are not worn.27,28 The National Crash Severity Study found that in accidents in which the vehicle was damaged severely enough to be towed from the scene, 1 in 300 occupants sustained a severe neck injury.28 A recent study found that for drivers and front seat passengers hospitalized after a crash, 12.5% sustained a spine fracture, the majority of which were cervical.29 This same study found that in these patients severe fractures accounted for only 8% and there appeared to be a protective effect of concomitant seat belt and air bag use. Along similar lines, the overall incidence of severe neck injury increased to 1 in 14 for passengers ejected from the vehicle.28
Falls are the most common cause of cervical spine and spinal cord injuries in the elderly, with more than 70% of injuries in this age group resulting from this mechanism.30,31 In addition, cervical injuries in young children and toddlers are frequently the result of falls.32 For both extremes of the age spectrum, the injuries sustained in this manner more frequently involve the upper cervical spine and are often of lesser severity.
Pediatric spinal trauma occurs at an incidence of 1.8 cases per 100,000 population, with 80% of incidents occurring in patients older than 10 years.33 Pediatric cervical spine injuries occur at different levels according to age group. Although three fourths of injuries in patients younger than 18 years occur below C4, between 70% and 87% of injuries affecting patients younger than 8 years occur at C3 or higher.33 Upper cervical spine injuries are more likely to be fatal, with atlanto-occipital dislocation (AOD) being associated with mortality rates of 70% to 100%.33–36
Occipitocervical injuries as a result of air bag deployment have been identified as a specific risk to small children.37–39 Standard three-point restraints have also been implicated in upper cervical spine injuries in young children.40 Greater public awareness of these dangers, coupled with improved pediatric restraint systems and wider use of pediatric safety seats, should reduce the incidence of these life-threatening injuries.
Children older than 11 years appear to have injury patterns more similar to those in adult patients, with injuries more frequently involving C4 or below. Older children are also less likely than younger children to suffer severe cervical spine injury. Although vehicular trauma is the most common mechanism in all pediatric age groups, in older children, sports-related injuries replace falls as the next most common mechanism.41
Acute Care of Cervical Spine Injuries
Prehospital Management
The potential for an unstable spine must be considered at the scene of the accident even as the initial priorities of airway, breathing, and circulation are addressed and the patient is prepared for extrication and moved. Increased awareness of the potential for instability and implementation of advanced immobilization techniques during extrication and transport have been associated with a decline in complete spinal cord lesions.42 Gillingham43 and Geisler and associates44 both reported on vertebral injuries made worse by well-intentioned but faulty first aid at the accident scene.
Commonly, airway obstruction after trauma is the result of prolapse of the tongue or airway obstruction by blood, secretions, or foreign bodies. Frequently, the airway can be established by using the chin lift or jaw thrust technique to bring the tongue forward. The mouth and oropharynx should be checked for debris and cleared either manually or with suction. In a semiconscious or unconscious patient, an oropharyngeal or nasopharyngeal airway should be inserted gently if indicated. An esophageal obturator airway can be inserted in an unconscious patient who has suffered respiratory arrest, although it is contraindicated in those with an intact gag reflex. The laryngeal mask airway has been used increasingly in this setting with good results.45–47
If a satisfactory airway or adequate ventilation cannot be established or maintained with the previous methods, endotracheal intubation is required. Gentle, manual in-line traction should be performed during this maneuver. Care should be taken to avoid overly vigorous traction because significant spinal distraction carries a risk for neurological injury.48 Although blind nasal intubation has been advocated in this setting, it is contraindicated in those with a basilar skull fracture. The latter is suspected in individuals with raccoon eyes, significant craniofacial trauma, Battle’s sign, or clear fluid in the nose or nasal secretions.
Assessment of the circulation can be difficult in the setting of acute trauma. Although hypotension is usually the result of hypovolemia or cardiac dysfunction, hypotension in a spinal cord–injured patient may be the result of loss of sympathetic tone with decreased peripheral vascular resistance.49,50 This results in venous pooling and decreased cardiac preload, which is exacerbated by the lack of reflex, sympathetically mediated tachycardia. Unlike patients with hypotension as a result of acute blood loss, these patients may appear to be peripherally well perfused with pink warm extremities—so-called warm shock.
Extrication of a patient with a suspected spinal injury requires immobilization of the neck and maintenance of normal axial alignment of the body. Except in the presence of extreme circumstances, such as fire, no patient should be moved before rigorous spinal stabilization is achieved. Soft collars allow complete neck movement in all planes and should not be used.51,52 Instead, if a patient is seated in a vehicle or in a position that makes full access difficult, a rigid collar can be placed for some support. With the patient still in the vehicle, a half backboard can be used for removal of the victim from the vehicle. Placement of the individual in the supine position on a long backboard is accomplished as soon as possible. Immobilization is augmented with sandbags or plastic intravenous bags placed along the head and neck with tape passed from one edge of the backboard to the other across the forehead.53,54 This method allows free movement of the jaw and lower part of the face for easy airway control.
The half backboard and long backboard may not be suitable for extrication of victims in all circumstances. Other alternatives include the Kendrick extrication device, which is a close-fitting jacket with extensions behind and on both sides of the head and neck, and the scoop sledge stretcher.55 Pediatric patients also present unique problems with regard to immobilization. Because of the proportions of a young child, immobilization on a standard backboard may result in neck flexion. The use of specially designed pediatric backboards can minimize this problem.56
A motorcyclist, bicyclist, or athlete may be found at an accident scene with a helmet still in place. In-line traction should be applied for removal, with a second rescuer supporting the head and neck. If the helmet cannot easily be removed, it can be left in place during transport as long as the patient’s airway is not compromised. For sports injuries in particular, it is appreciated that only the facemask of the helmet need be removed to obtain access to the airway; the rest of the helmet can be removed later, thereby preventing any manipulation before evaluation of the injury.57,58
Acute Evaluation and Management in the Emergency Department
A full skeletal x-ray series, including the chest and pelvis, should be performed as indicated (also see the section on imaging). This is especially important in a neurologically impaired patient because 11% of fractures associated with head or spinal cord injury are missed in the initial assessment and an unconscious, confused, or neurologically impaired patient may have abdominal pathology that is obscured by the neurological injury.59 In addition, cervical spine fractures are often associated with fractures elsewhere in the spinal axis.
Disruption of sympathetic nerve function at T8 or above is frequently associated with hypothermia. Euthermia should be restored by external warming, administration of warmed intravenous fluids, and heated inspired air if the patient is intubated. Although theoretical considerations have supported the concept of controlled hypothermia in spinal cord injury, the limited clinical data available to date do not support widespread application of this strategy in clinical practice.60
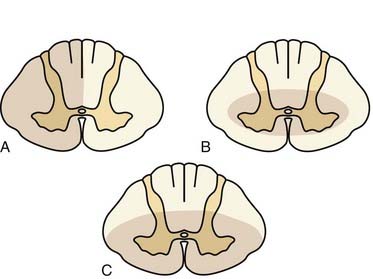
Injury to the spinal cord occurs because of stretching, crushing, vascular compromise, or compression. Certain types of injury mechanisms are more likely to be associated with specific neurological findings on clinical examination (Fig. 312-1). Spinal cord hemisection leading to Brown-Séquard syndrome usually results from penetrating trauma but may also be seen after trauma with epidural cord compression.59 On clinical examination, one finds contralateral dissociated sensory loss (i.e., loss of pain and temperature sensation caudal to the lesion) with preserved light touch because of redundant ipsilateral and contralateral axonal pathways (anterior spinothalamic tract). Ipsilaterally, one finds proprioceptive loss and motor paralysis below the lesion. Among the incomplete spinal cord syndromes, Brown-Séquard syndrome has the best prognosis, with approximately 90% of patients regaining the ability to ambulate independently and control sphincter function.61
After acute hyperextension injury, frequently in patients with preexisting congenital spinal stenosis, central cord syndrome may be evident.62 Patients with this syndrome have greater weakness in the upper extremities than in the lower extremities. Various degrees of sensory disturbance occur below the level of the lesion, and sphincter disturbance is commonly present. Only about half of these patients eventually recover enough neurological function in the lower extremities to ambulate independently.61,63–65 Recovery of upper extremity function is also poor, and fine motor control is usually absent. Bowel and bladder control is frequently recovered.
In patients who experience vertical compression or hyperflexion injuries, an anterior cord syndrome, also known as anterior spinal artery syndrome, may occur.66 Cord infarction in the vascular territory supplied by the anterior spinal artery is the proposed mechanism. These patients exhibit motor and sensory disturbance below the level of the lesion in the presence of intact posterior column function. This leads to a dissociated sensory loss, with loss of pain and temperature sensation caudal to the lesion but preservation of joint position sense and two-point discrimination. Anterior cord syndrome has the poorest prognosis of the incomplete cord syndromes. Only 10% to 20% of patients recover functional motor control and the ability to ambulate.61
Many potential treatment strategies for acute spinal cord injury have been examined experimentally, including hypothermia, hyperbaric oxygen, electromagnetic fields, immobilization, and various pharmacologic agents given shortly after injury, such as intravenous lidocaine, melatonin, steroids (e.g., dexamethasone, methylprednisolone, 21-aminosteroids), and opiate antagonists such as naloxone.67–75 Although several earlier studies suggested that naloxone and glucocorticoids were ineffective in the treatment of acute spinal cord injury, other studies showed beneficial effects.68,76–78 The Second National Acute Spinal Cord Injury Study (NASCIS 2) revealed in a prospective, randomized, double-blind study that high-dose methylprednisolone was associated with improved neurological outcome in spinal cord–injured patients when compared with placebo or naloxone.79 This was followed by NASCIS 3, which compared 24- and 48-hour treatment with methylprednisolone with 24-hour treatment with tirilazad, a 21-aminosteroid antioxidant.80 The results were stratified by interval between injury and initiation of treatment. The study concluded that if treatment could be initiated within 3 hours after injury, a 24-hour period of treatment with methylprednisolone should be instituted. If the treatment could not be started within 8 hours of injury, steroids were of no benefit. Although there continue to be methodologic questions about the study (e.g., there was no placebo control group), it remains the most complete and definitive study on the subject of pharmacologic treatment of spinal cord injury to date.
A number of other agents have shown promise in the treatment of spinal cord injury, including calcium channel blockers such as nimodipine, modulators of excitotoxicity such as phencyclidine or dextrorphan, and blockers of lipid peroxidation and membrane disruption such as 21-aminosteroids and GM1 ganglioside.81–87 Unfortunately at this time, only MPSS has been shown to benefit patients, and even the long-term benefits in patients treated with MPSS remain questionable.88,89
Imaging
Plain Radiography
A complete cervical spine series consists of a lateral cervical projection, an anteroposterior view, an open-mouth view of the odontoid, and oblique films.90,91 A pillar view, a swimmer’s view, and dynamic studies are supplemental and may be considered to more fully evaluate the extent of injury.91,92 The optimal plain film evaluation of the cervical spine depends on the patient’s clinical condition and neurological status and the circumstances and magnitude of the injury.
A cross-table lateral view should be the first film obtained in the cervical spine series, and in patients with multiple trauma, this film should precede all others. This projection is accurate in revealing posttraumatic abnormalities approximately 70% to 83% of the time.90,91,93–98 Completion of the series, however, markedly increases its sensitivity.90,93,94,99,100
The initial cross-table film should be performed without traction because of the potential for atlanto-occipital or atlantoaxial dissociation or other major ligamentous disruption.101 However, this initial film is frequently inadequate to assess the lower cervical spine. Optimal visualization on the lateral projection should include the C7-T1 disk space. Depression of the shoulders by pulling down on the arms will frequently allow visualization when not precluded by other injuries. In some patients, a swimmer’s view may be required to visualize the cervicothoracic junction.
The lateral films should be evaluated for alignment, bony abnormalities, disk space abnormalities, and soft tissue abnormalities. Although careful evaluation of the bony anatomy is of primary importance, close attention should also be paid to soft tissue details. Subtle findings such as prevertebral swelling may be the only radiologic sign of an acute injury on plain radiography.102,103
The pillar view allows direct visualization of the individual lateral masses. In addition, the dens can be assessed if the open-mouth view is difficult or inadequate. Miller and colleagues recommended inclusion of the pillar view in all radiographic assessments of the cervical spine after trauma.104 Because significant head rotation is required for this view, it has largely been supplanted by evaluation with CT.
Flexion-extension films are frequently advocated to assess ligamentous integrity in intact patients after trauma. They are clearly contraindicated in a patient with a neurological deficit, obvious fracture, or instability or in a patient with a history of a transient, resolved deficit after trauma. Acute intoxication, disorientation, and heavy analgesic administration are also contraindications. The confounding aspects of pain or muscle spasm limiting full mobility are also problematic in the acute setting. Because of the aforementioned considerations, a number of authors have found flexion-extension radiographs to be of no benefit acutely.105–108 As MRI is increasingly becoming available, ligamentous integrity is frequently being assessed by this means.
Asymptomatic Patients
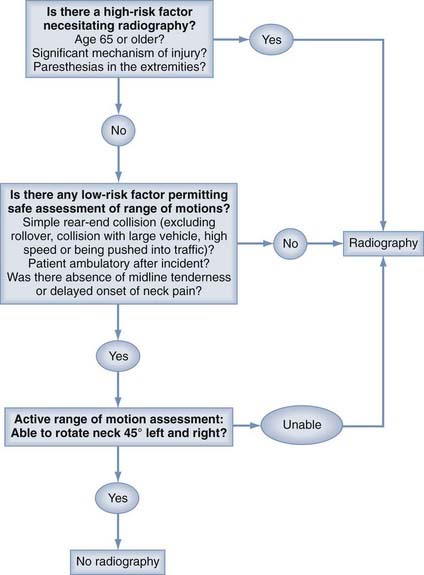
It has long been recommended that all trauma patients undergo radiographic evaluation of the cervical spine. Conversely, a policy of imaging all trauma patients has been challenged because of the issues of unnecessary radiation exposure, overuse of resources, and increased cost.109–113 It is estimated that more than 1 million patients with blunt trauma and some potential for cervical spine injuries are seen in U.S. emergency departments annually.114 Several authors have found, however, that for neurologically intact patients, the incidence of spinal injury or acute fracture is less than 1%.115–117 Consequently, there have been a number of efforts to identify clinical decision rules that would be highly sensitive for the detection of cervical spine injuries in patients who are alert and stable, thus providing both greater consistency and selectivity in the use of radiography.113,118,119 Hoffman and coworkers proposed five criteria that classified patients as having a low probability of injury (Table 312-1). When applied to 34,069 patients undergoing cervical spine radiography, these criteria identified all but 8 of the 818 patients who had cervical spine injuries (sensitivity of 99%).118 Of these 8 patients falsely identified as being unlikely to have an injury, only 2 had what was previously defined by the authors as a “clinically significant” injury. According to the authors, use of the criteria would have avoided imaging in 12.6% of the overall 34,069 patients studied. In another similar effort, the Canadian C-Spine Rule (CCR) investigators developed an algorithm based on three determinations: a high-risk factor necessitating radiography; low-risk factors, one or more of which allow assessment of range of motion; and active assessment of range of motion (Fig. 312-2). In a subsequent study of 8924 patients in which the incidence of cervical spine injury was 1.7%, the CCR was found to be 100% sensitive and 42.5% specific for “significant”injuries.119
From Hoffman JR, Wolfson AB, Todd K, et al. Selective cervical spine radiography in blunt trauma: methodology of the National Emergency X-Radiography Utilization Study (NEXUS). Ann Emerg Med. 1998;32:461-469.
In 2002, recommendations were published in a supplement to Neurosurgery regarding radiographic assessment of asymptomatic trauma patients.120 In the discussion, asymptomatic patients were defined as those who
It has been estimated that approximately a third of patients evaluated in emergency departments will be asymptomatic according to these criteria.118,121,122 With this definition in mind, the literature was reviewed and found to contain nine class I studies and numerous reports of class II and class III evidence. The resulting conclusion of this review is that asymptomatic patients “do not require radiographic assessment of the cervical spine after trauma.” Nonetheless, the incidence of cervical spine injuries in asymptomatic patients from the various study cohorts ranged from 1.9% to 6.2%.
Despite the aforementioned studies, considerable variability remains in practice. Comparison of the National Emergency X-Radiography Utilization Study (NEXUS) and CCR rules suggests that the latter may have greater sensitivity, yet many physicians are reluctant to perform an adequate range-of-motion assessment.123 Both methods, when consistently and accurately applied, would appear to perform better than unstructured physician judgment and have the potential to reduce cervical spine radiographs in nearly a third of patients with blunt injuries.124
Computed Tomography
At the present time, CT is the tomographic method of choice for the evaluation of acute spinal trauma.125 Thin-section CT should be used to further evaluate areas of obvious or suspected cervical spine injury detected on plain radiography.43,125–128 CT is also indicated if the plain film study is inconsistent with the patient’s clinical condition, for injuries resulting in neurological deficit, for fractures involving the posterior arch of the cervical canal, and for every fracture with suspected retropulsion of bone fragments into the canal.129,130 CT should usually precede other supplemental studies, including MRI, dynamic studies, and digital subtraction angiography (DSA).
A potential exception to the rule of proceeding directly to CT after demonstration of an injury on initial cervical spine films may be patients with a fracture-dislocation or other subluxation and an acute neurological deficit. In these patients, early decompression of the cord is paramount, and every effort should be made to reduce the malalignment as rapidly as possible.3,130 Application of Gardner-Wells tongs or a similar device followed by the application of traction in a controlled fashion with frequent monitoring via fluoroscopy or lateral plain films should be considered if feasible. Once alignment has been restored or an inability to effect reduction has been established, CT or in some cases MRI is then performed.
With CT, bone is imaged in exquisite detail, with clear demonstration of even small cortical disruptions, and encroachment of bone on the spinal canal can easily be seen. This is particularly useful for C1 and C2 fractures because the precise fracture subtype is often difficult to discern on plain films.131–133 Combination fractures involving both the atlas and axis are difficult to assess without CT, and axial views are particularly useful for evaluating injuries to the vertebral body and posterior elements.131,133 Reformatted images in the sagittal and coronal planes are now routinely performed at most centers. Images reformatted in oblique planes may also be obtained.102 Three-dimensional reformatted CT images are also occasionally of use in understanding complex fractures, although the processing time and the technical aspect required to produce these images may limit their use in the acute setting.98
When the sensitivity of CT for the detection of cervical spine injuries has been compared with that of plain radiographs, CT is significantly better (e.g., 98% versus 52%), which has caused some to advocate it as the initial screening test for the cervical spine in high-risk patients.134
Magnetic Resonance Imaging
In addition, MRI provides imaging of the ligaments, muscles, and other surrounding soft tissues. Spinal epidural hematomas, intramedullary hematomas, spinal cord contusions, myelomalacia, and spinal cord edema are usually, but not always well visualized with MRI.135–142
MRI can directly demonstrate disruption of some ligamentous structures of the cervical spine that cannot be visualized with other radiologic techniques, such as the transverse ligament at C1-2.137,143,144
Cervical imaging with MRI can also permit delineation of the vertebral arteries and can detect injury, although CT angiography (CTA) and DSA remain the primary means of diagnosing vascular abnormalities. Some centers use two-dimensional time-of-flight MR angiography (MRA) sequences to increase sensitivity for vascular injury. Using this methodology, Friedman and colleagues identified vertebral artery injuries in 24% of patients with severe, nonpenetrating cervical spine trauma.145
Because of the ability to visualize injury to soft tissues such as ligaments, muscles, and joint capsules, there is considerable interest in the use of MRI to “clear” the spine in patients with inconclusive or negative plain films or CT who cannot be evaluated or nonetheless are suspected of having a serious injury. Benzel and associates performed MRI on a series of 174 patients with clinical features worrisome for an occult injury but with normal findings on plain radiography. Soft tissue abnormalities, including disk disruptions and ligamentous injuries, were found in 36%.146
A meta-analysis of five studies investigating the use of MRI for evaluating patients with suspected cervical spine trauma (level I data) found a negative predictive value of 100% and therefore concluded that MRI could be considered a “gold standard” for clearance of the cervical spine; however, the false-positive rate could not be fully determined in this review.147
Myelography
Intrathecal administration of water-soluble contrast medium, followed by plain radiography or CT, provides visualization of the spinal cord silhouette, subarachnoid space, and nerve roots. It demonstrates intramedullary and extramedullary mass lesions, obstruction to flow of cerebrospinal fluid (CSF), root avulsions, dural tears, and with delayed CT images, posttraumatic syringomyelia.148,149 However, very little direct information can be obtained about the intrinsic pathology of the cord in the setting of acute injury, especially when compared with MRI. In the context of trauma, CT after the intrathecal administration of contrast material has largely supplanted the plain film myelography examination because less patient movement is required. If CT myelography is performed in patients with cervical injuries, the contrast agent may be introduced via the lateral C1-2 approach, thereby further minimizing movement of the patient.
Other Imaging Modalities
CTA and, less frequently, MRA have been advocated to detect vertebral artery injuries when cervical fractures or dislocations are thought to be associated with high suspicion for vertebral artery injury.150 Radionuclide studies have been used historically to detect occult fractures of the spine but have little application in acute trauma.
Classification of Cervical Spine Injuries
Universally accepted classifications of acute cervical spine injuries do not exist. Some of the classifications focus on the neurological aspect without analysis of the bony or soft tissue injury.151,152 Others subdivide injuries in terms of the specific bony or soft tissue pathology without considering the biomechanics or mechanism of injury.3,153 Still others focus on the mechanism without addressing the neurological aspects. When the reasons for classifying cervical spine injuries are considered, it seems apparent that the mechanism of injury, pattern of bony or soft tissue disruption, and type and degree of neurological injury are all relevant to such issues as assessment of stability, overall prognosis, selection of treatment, and collection of meaningful outcome data. A number of authors have suggested systems based primarily on injury mechanism.154,155 Recently, the Spine Trauma Study Group proposed a novel classification system termed the Subaxial Injury Classification and Severity Score (SLIC).156 This classification scores three major characteristics of the injury: injury morphology, status of the diskoligamentous complex, and neurological status (Table 312-2).
TABLE 312-2 The Subaxial Injury Classification and Severity Score System
| CHARACTERISTIC | POINTS |
|---|---|
| Morphology | |
| No abnormality | 0 |
| Compression | 1 |
| Burst | 1-2 |
| Distraction (e.g., facet perch, hyperextension) | 3 |
| Rotation/translation (e.g., facet dislocation, unstable teardrop or advanced-staged flexion compression injury) | 4 |
| Diskoligamentous Complex | |
| Intact | 0 |
| Indeterminate (e.g., isolated interspinous widening, MRI signal change only) | 1 |
| Disrupted (e.g., widening of anterior disk space, facet perch or dislocation, kyphotic deformity) | 2 |
| Neurological Status | |
| Intact | 0 |
| Root injury | 1 |
| Complete cord injury | 2 |
| Incomplete cord injury | 3 |
| Ongoing cord compression (in the setting of a neurological deficit) | 1 |
Biomechanical studies and autopsy or cadaver experiments have established the fundamental relationships among injury mechanism, force vectors, and the resulting osseous and ligamentous injuries of the spine.157–163 In such controlled laboratory experiments, pure force vectors such as flexion, extension, vertical compression (axial load), lateral flexion, rotation, or a combination of forces (e.g., simultaneous flexion and rotation) have been shown to produce specific injury patterns. Clinically, however, the causative (vector) force of injury must be inferred from historical, physical, or radiologic evidence because the mechanisms of injury are certainly not controlled. Furthermore, in all probability, the injury is a result of multiple simultaneous forces with one predominant force vector rather than a single pure force. Nonetheless, the similarities observed between the injuries produced in the laboratory with relatively pure force vectors and the patterns seen clinically support the concept that most clinical examples are the result of a predominant injury vector.164
Two- and Three-Column Concepts
Holdsworth’s two-column concept of the spine is an aid to defining stability in the thoracolumbar spine.52,165–167 The anterior column is made up of the anterior longitudinal ligament, vertebral body, intervertebral disk, and posterior longitudinal ligament; the posterior column consists of all the skeletal and ligamentous structures posterior to the posterior longitudinal ligament. This concept had been invaluable in understanding the pathophysiology of injuries occurring as a result of flexion, extension, and other forces on the cervical spine. More recently, Denis’ redefinition of this column concept to include a third, middle spinal column has added substantially to our understanding of the biomechanics of injury and to the definitions of instability after cervical spine trauma.168 The middle column consists of the posterior third of the vertebral body, the anulus fibrosus, and the posterior longitudinal ligament; the posterior column is formed by the posterior neural arch, spinous process, and articular processes and corresponding articular capsules. Although the three-column concept was designed specifically for thoracolumbar fractures, both the two- and three-column models are helpful in further redefining the biomechanics of cervical spine injury as well.
Simultaneous flexion plus rotation involves flexion with the head slightly rotated at the outset. Such forces tend to produce partial disruption of the annulus, posterior ligaments, and capsule of the facet joint and result in unilateral dislocation of the facet on the side opposite the direction of rotation.169 Unilateral facet dislocation may be associated with an impaction fracture of the articular masses on the dislocated facet joint, but such a fracture is not usually a major component of the injury.
Because of the complexity of injury forces, classifications that attempt to infer the primary force vector are subject to interpretation. The SLIC system, alluded to earlier, instead focuses on morphology. In addition, it accounts for ligamentous injury, which factors greatly into management decisions, as well as neurological status, which is important in terms of both acute management and prognosis. A study evaluating clinical use of the classification demonstrated high degrees of interrater reliability.170 Further work to assess the applicability and validity of the SLIC system are ongoing.
Cervical Spine Stability
The principal goals in the management of potential spinal instability are prevention of secondary neurological injury and provision of an optimal environment for recovery of any existing neurological injury along with the restitution of stability to the osteoligamentous structures. Achievement of these goals requires an understanding of bony and ligamentous pathology, the nature of the neural tissue injury, and the capacity for healing that can be expected. In one early review, which largely antedated modern immobilization techniques, new signs or symptoms of cervical spinal cord compression developed in 10% of patients during emergency department evaluation or during the acute phase of hospitalization.171 These findings underscore the importance of recognizing spinal instability and taking appropriate measures to immobilize the spine and protect the spinal cord and nerve roots. If stability cannot be established, it is always safer to assume that the spine is unstable until it can be proved otherwise.
The unique anatomy and the wide range of motion of the occipitoatlantoaxial region differentiate this area from the rest of the cervical spine. Instability of the craniocervical junction is determined by the integrity of the interrelated bony and ligamentous structures. The latter can be subdivided into outer and inner ligament groups. The outer ligaments include the articular capsules, the anterior and posterior atlanto-occipital membranes, and the nuchal ligament.172 The inner ligaments include the paired alar ligaments, the apical ligament, the transverse atlantal ligament, the vertically oriented cruciform ligament, and the tectorial membrane. Werne, in a detailed analysis of the ligamentous stability of this region, concluded that hyperflexion is limited by bony contact between the anterior rim of the foramen magnum and the tip of the dens whereas hyperextension and vertical distraction are primarily limited by the tectorial membrane.173 Lateral bending was thought to be controlled primarily by the alar ligaments. The relative importance of these various structures was determined by sequential sectioning of the ligamentous elements.
Various anatomic structures have been implicated in the stability of the lower cervical spine. Both Bailey and Bedbrook believed that the disk and the anterior and posterior longitudinal ligaments were the most important structures for stability in the lower cervical spine.174–177 Experimental work done by both Roaf and Munro supports these observations.161,178
More recently, work by White and Panjabi provided additional information on the ligaments’ role in maintaining cervical spine stability.52 From observations on cadavers, they concluded that the loss of function of either all of the anterior or all of the posterior bony or ligamentous structures could render the lower cervical spine unstable. Furthermore, they determined that horizontal and angular displacement between cervical vertebrae did not exceed 2.7 mm or 10.7 degrees, respectively, before complete failure of the motion segment occurred. Based on these data and in conjunction with other concepts of stability, they constructed a graded checklist system for determining instability of the lower cervical spine.147 Points are assigned if anterior or posterior column destruction is noted, sagittal angulation is greater than 11 degrees, sagittal plane translation is greater than 3.5 mm, there is spinal cord injury or a positive stretch test, nerve root damage or disk narrowing is present, or it is anticipated that the patient will place great stress on the cervical spine. A patient with more than 5 points is considered to have an unstable lower cervical spine. However, it is possible for patients with lower scores to have spinal instability and for patients with higher scores to be clinically asymptomatic. For this reason there is no substitute for repeat examinations to identify potential spinal instability. These authors also noted that flexion-extension radiographs may be hazardous in patients with occult ligamentous instability and recommended the so-called stretch test as a safer alternative.162
Closed Reduction
Frequently, patients will be seen who have an acute neurological deficit referable to the spinal cord and who have obvious subluxation on imaging with resultant cord compression. Common examples include patients with unilateral or bilateral locked facets, cervical burst fractures, displaced type II odontoid fractures, and the like. Early closed reduction of these injuries with the use of tong or halo traction devices has been advocated for decades.179–182 The rationale for this approach is to achieve rapid cord decompression along with the modicum of stability and immobilization afforded by the traction device. This practice of early closed reduction has been questioned by a number of authors who cite the potential for neurological deterioration as a result of displacement of disrupted disk material into the canal during reduction.183–185 These authors recommend early evaluation with MRI followed by anterior diskectomy and subsequent reduction if a herniated disk is observed. The frequency of disk disruption/herniation on prereduction MRI is relatively high, 42% in the series reported by Rizzolo and coworkers,186 yet the clinical significance of the finding is debated. Advocates of rapid cord decompression cite the delay incurred by screening MRI.
In 1999, Grant and coauthors reported a retrospective review of 82 examinable patients treated by rapid, fluoroscopically controlled, closed reduction of unstable cervical spine fractures. Traumatic disk disruption or herniation was noted in 46% of the patients (usually observed on postreduction MRI), and partial or complete spinal cord injury was found in 56%. Significant improvements were seen in mean American Spinal Injury Association (ASIA) motor scores 24 hours after injury in patients with both complete and incomplete injuries, with only 1 patient exhibiting deterioration (1.2%).187
A recent review that attempted to define the role of closed reduction and the utility of prereduction MRI was unable to find conclusive (class I or II) evidence supporting either practice.188 Nonetheless, on the basis of considerable class III data, the authors concluded that reduction of the cervical spine in awake patients by traction is possible in approximately 80% of patients, with transient neurological deficits occurring in 2% to 4% and permanent deterioration in only approximately 1%. Prereduction MRI was not considered necessary for most patients, although those with altered consciousness impeding accurate neurological assessment could be evaluated with this modality as an option. It would also be advisable to evaluate patients with normal neurological function or minor deficits before closed reduction, assuming that adequate immobilization can be ensured during transport and performance of the scan.
Restoration of Spinal Stability
External Orthoses
Spinal orthotic devices work by limiting physical movement to a greater or lesser degree, depending on device design and stiffness, fit, patient anatomy, level of injury, and the nature of the instability being treated. To reduce motion, the orthotic must contact various firm points of the anatomy above and below the level to be braced, such as the mandible, the chest and upper part of the back, and the occiput. The area that is best stabilized is typically midway between the contact points. This means that upper cervical or cervicothoracic injuries may be ineffectively braced in a cervical collar. A halo apparatus with fixation to the skull will more effectively immobilize the upper cervical spine.189,190 A cervicothoracic brace, which extends contact further down the thorax and especially over the sternum, may be more effective in stabilizing lower cervical injuries than a collar, especially in flexion.
Although many injuries can be managed successfully with external orthoses, there are potential problems that need to be considered. Immobilization will be associated with later decreased mobility, joint stiffness, and frequently, muscular pain that may need aggressive physiotherapy to address. Some orthoses may be associated with skin breakdown, particularly in patients with altered consciousness, obesity, or advanced age. The halo orthosis may also be poorly tolerated by elderly patients and in some with advanced respiratory compromise. In addition, numerous cases of skull perforation by halo pins with subsequent infectious complications, including brain abscess, meningitis, and osteomyelitis, have been reported.191,192
Surgical Stabilization
The timing of surgical intervention in the context of cervical fracture or dislocation remains controversial. Patients exhibiting a complete neurological deficit several hours or more after injury are unlikely to improve. Conversely, anecdotal cases of rapid early reduction/decompression of apparently complete injuries with dramatic recovery of function have been noted. If the patient exhibits progressive neurological decline after an injury, there is little argument about acute decompression. The timing of decompression of patients with stable deficits is less clear.193,194 Arguments in favor of early surgical decompression center on minimization of secondary injuries with improved perfusion, decreased anatomic distortion, and restoration of CSF circulation. In addition, early restoration of spinal stability reduces the risk for further mechanical injury and allows more rapid mobilization. Advocates of delayed surgery note that the vast majority of the cord injury occurs at impact, that the injured cord is more vulnerable to the manipulation and hemodynamic changes that might occur during early surgical intervention, and that concurrent injuries may increase the surgical risk and potential morbidity.195–198 Additionally, it has been noted that closed reduction may achieve rapid decompression without subjecting the patient to the risks associated with early surgery.
In a multicenter study, Marshall and colleagues examined the frequency of worsening in 283 patients with spinal cord injury.198 Overall, they noted neurological deterioration in 4.9% of patients. For the small number of patients in the series with cervical cord injury, worsening was observed only in those undergoing surgical stabilization within 5 days of injury. Patients undergoing surgery 6 or more days after injury had no deterioration. These authors subsequently concluded that early surgery in patients with spinal cord injury should be undertaken only to avoid further deterioration in neurological function.
Specific Lesions and Their Management
Occipital Condyle Fractures
Condylar fractures are increasingly being recognized with the use of CT and multiplanar reconstruction. Although not technically cervical spine fractures, they are mentioned here because they are often detected when evaluating a trauma patient for a spine injury. Most patients with condylar fractures have signs or symptoms of head injury. Approximately 40% of patients with occipital condyle fractures have lower cranial nerve injuries (primarily cranial nerve XII), some of which can develop in delayed fashion.199
Condylar fractures are usually classified into three types.200 Type I fractures result from an axial load with crushing or comminution of the condyle without significant displacement. Although the ipsilateral alar ligament may be incompetent, these injuries are typically stable and treated with a cervical collar. Type II fractures are nondisplaced linear fractures that extend onto the occipital bone. In the absence of occipitocervical subluxation, these fractures are frequently stable and not associated with ligamentous disruption. They may require no specific treatment or a collar for comfort. Displacement or rotation of the occiput in relation to C1 warrants careful investigation for an unstable injury. Type III condylar fractures, which account for about 75% of occipital condyle fractures, involve avulsion of the alar ligament.201 The mechanism is thought to involve lateral bending associated with rotation. These injuries may have concomitant injury to the contralateral alar ligament or the tectorial membrane that renders them unstable. Treatment in these cases consists of craniocervical fusion. MRI may aid in the assessment of ligamentous injury.
Atlanto-occipital Dislocation
These injuries may be missed on radiographic assessment, in part because of a low index of suspicion for these injuries and also because axial studies may not be carried up to the level of the foramen magnum. On lateral plain radiographs, these injuries may be seen as increased distance between the tip of the dens and the clivus or as anterior or posterior displacement of the skull in relation to the upper cervical spine. A number of publications have attempted to provide indices or measurements to aid in the diagnosis of AOD, including those by Wholey, Powers, Pang, and their colleagues.202–205
Traynelis and colleagues classified AOD into three different categories.206 Type I is anterior dislocations, Type II is longitudinal (distraction)-type dislocations, and type III consists of posterior dislocation of the occiput in relation to the atlas.
Cervical traction is relatively contraindicated in patients with these injuries because of the potential for worsening the distraction and exacerbating high cervical or medullary injury. The rate of neurological deterioration in patients with AOD treated by traction may be approximately 10%.207 Emergency surgical stabilization or immediate application of a halo orthosis has been advocated to provide some degree of cervical stabilization while the patient’s other injuries can be addressed. Surgical management typically involves occipitoatlantal or, more commonly, occipitocervical fusion.
Atlas Fractures
Isolated fractures of the atlas account for approximately 5% to 10% of cervical spine injuries.132,133,135,208 The anatomic features of this vertebra and its relationship with the occipital condyles and lateral masses of C2 make it susceptible to axial loading and direct fracture of the posterior arch. Atlas fractures are often seen in combination with fractures of the occipital condyle or the axis.131 Four basic injury patterns tend to occur: posterior arch fracture, lateral mass fracture, Jefferson’s fracture, and transverse fracture of the anterior arch.
Jefferson’s fractures, first described by the neurologist Sir Jeffrey Jefferson in 1920, are the result of an axial load that displaces the lateral masses laterally and causes multiple fractures of the anterior and posterior arches.21 These fractures, although not generally associated with neurological injury, may be unstable, especially if the lateral masses are displaced sufficiently to disrupt the transverse atlantal ligament. Most authors suggest that this may have occurred if the total lateral displacement exceeds 7 mm.209 Jefferson’s fractures deemed unstable are usually managed with a halo orthosis. Isolated fractures of the anterior arch are uncommon and are usually oriented in the axial plane. They are avulsion injuries caused by a hyperextension mechanism with avulsion of the attachment of the longus colli. These fractures will usually heal satisfactorily with a cervical collar.
Axis Fractures
The axis vertebra has unique osseous anatomy, as well as unique ligamentous, muscular, and vascular relationships, which can produce a number of distinct injury patterns. Fractures of C2 are usually classified as odontoid fractures, lateral mass fractures, fractures of the pars (traumatic spondylolisthesis or hangman’s fracture), and miscellaneous combination fractures. Approximately 20% of acute cervical spine fractures affect the axis vertebra,210 with a type II odontoid fracture being the most common.
Odontoid Fractures
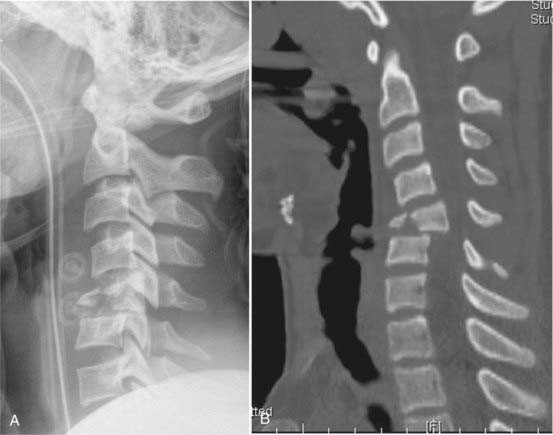
Fractures of the odontoid process, or dens, (Fig. 312-3) account for approximately 7% to 14% of cervical spine fractures and almost 60% of axis fractures.132
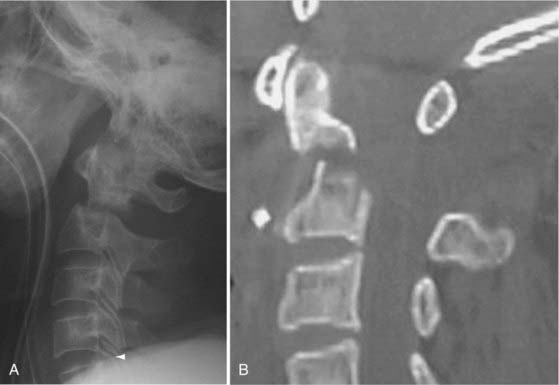
FIGURE 312-3 Type III odontoid fracture. Because reduction was difficult to maintain, screw fixation was necessary.
Odontoid fractures are generally subdivided according to the classification of Anderson and D’Alonzo.210 Type I fractures involve avulsion of the apical aspect of the dens by the apical or alar ligaments, or by both.
Type II fractures, which are the most common, involve an axially or obliquely oriented fracture across the base of the dens without involvement of the C2 body. These fractures are noteworthy because of their high rate of nonunion. Occasionally, there is significant comminution at the fracture line, which further complicates management and predisposes to nonunion. Hadley and colleagues termed these type IIA fractures.211
Treatment with a halo orthosis has frequently been advocated for type II fractures, although rates of nonunion ranging from 25% to 63% have been reported.212 Surgical stabilization with an anterior odontoid screw is thought to offer greater rates of healing while preserving atlantoaxial rotation.213 Such treatment requires an intact transverse atlantal ligament and is problematic in patients with oblique fractures, a barrel chest, and severe osteopenia. Posterior atlantoaxial fusion by a variety of techniques can be considered in such cases.
Clinical factors that correlate with higher rates of nonunion for type II fractures remain controversial.132,211–214 Some factors that have been implicated include increasing age (especially >65 years), greater degrees of dens displacement (especially >6 mm), and posterior displacement of the dens.
Traumatic Spondylolisthesis
Traumatic spondylolisthesis of C2, also termed a hangman’s fracture, involves bilateral fractures through the pars interarticularis or pedicles. In some cases, the fracture may extend onto the posterior axis body. Concomitant ligamentous injury and the associated instability largely dictate the subsequent management. Levine and Edwards proposed a four-level classification.215 Type I fractures demonstrate no angulation or translation of C2 relative to C3 and can be managed with a rigid collar. Type II fractures demonstrate significant angulation (>11 degrees) and translation (>3.5 mm). Type IIA injuries in the Levine and Edwards classification exhibit no translation but high degrees of angulation. Type III injuries are rare; they consist of high degrees of angulation and translation in which there is concomitant unilateral or bilateral facet dislocation at C2-3.
Most cases of traumatic spondylolisthesis are not associated with neurological injury. Type I injuries are typically treated with a rigid collar. Type II, IIA, and III hangman’s fractures are frequently treated with a halo orthosis. Vaccaro and coworkers demonstrated that early immobilization in a halo was a viable treatment option for type II and IIA hangman’s fractures.216 Some type III injuries are reduced with traction before placement in a vest. Some cases of significant angulation of C2 on C3 that cannot be reduced with a halo orthosis can be treated by anterior interbody fusion and plate fixation.217,218
Combined Atlantoaxial Fractures
Concomitant fractures of the atlas and axis are not uncommon and occur in 5% to 53% of patients, depending on the exact fracture patterns.131 Because these combined injuries present unique management challenges, they have been the subject of several publications.219–221 Many authors have noted that patients who have sustained such injuries are at greater risk for neurological injury or death than are patients with atlas or axis fractures alone.131,219,221–223
Treatment of combined C1-2 fractures is predicated on involvement of both vertebrae and the potential for ligamentous instability created by the injury. Surgical stabilization is more frequently used than for isolated C1 or C2 fractures, which is appropriate given the greater degree of bony and soft tissue injury incurred. Treatment options include cervical orthoses (collar, halo, Minerva, SOMI) alone, an odontoid screw combined with an orthosis, transarticular screw fixation, occipitocervical fusion, and posterior C1-2 fusion with screw fixation. Although no firm guidelines exist for this heterogeneous group of fractures, a recent review attempted to categorize the literature in this regard.221 Not surprisingly, there were no class I or class II studies on the subject, with most information coming from retrospective case series or case reports. The authors of this report concluded that the nature of the axis fracture was the primary consideration dictating the treatment approach; however, the competence of the transverse ligament and atlas ring was also significant. Recently, screw fixation into the lateral masses of the atlas has emerged as a common means of dealing with a number of these fractures when the C1 arch is incompetent with or without compromise of the transverse atlantal ligament.
Subaxial Fractures
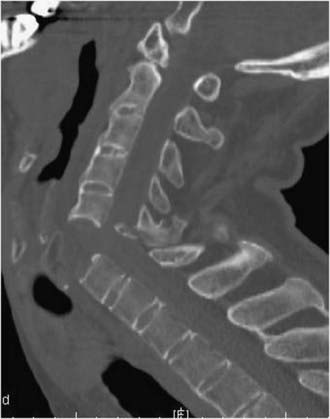
Burst Fractures
Burst fractures in the cervical spine are largely the result of axial compression forces, occasionally with a flexion component. These injuries involve two columns and are associated with retropulsion of bone into the canal (Fig. 312-4).
Hyperextension Injuries
Hyperextension injuries often occur as a result of falls or vehicular injuries. A common clinical scenario is a patient initially seen with a central cord syndrome after a fall or rear-end collision.62 In many of these cases, the patient has no gross spinal instability and no major fracture. There is evidence of preexisting spondylosis and stenosis. MRI will often demonstrate a signal abnormality in the cord.
Higher degrees of angular acceleration may result in disruption of the anterior longitudinal ligament and disk with a “fish mouth” anterior deformity on plain film or sagittal imaging (Fig. 312-5). Fractures of the spinous processes or laminae may also occur with this mechanism. In some cases of unstable hyperextension injury, the spine will have returned to a normal position and the bony anatomy on plain radiographs will appear normal. Prevertebral soft tissue swelling may initially be the only x-ray finding. MRI will demonstrate the ligamentous injury to best advantage.
Spinal Cord Injury without Radiographic Abnormality
As the name implies, SCIWORA is an entity in which there is evidence of spinal cord injury in the absence of radiographic signs of fracture or dislocation. The term, initially coined by Pang and Wilberger, referred to injuries that were most frequently described in children and resulted from the greater mobility of the pediatric osteoligamentous complex.224 The relatively underdeveloped cervical musculature in young children coupled with ligamentous laxity and the disproportionate weight of the head contributes to these injuries. A variety of mechanisms are seen, including hyperextension, flexion, flexion-extension, distraction, and direct crushing. The neurological deficits are frequently severe and result from cord transection or severe partial cord syndromes.224–229 With the advent of MRI, the term SCIWORA is somewhat of a misnomer because ligamentous and cord injury is usually demonstrable.
In adults, such injuries were initially ascribed to hyperflexion-distraction with immediate reduction by muscular action or transient disk prolapse during forced flexion.230,231 Most authors now agree that the predominant mechanism is acute hyperextension in the context of preexisting spondylosis and canal stenosis.62,232–234 The typical clinical picture is that of a central cord syndrome as described earlier.
Concomitant Vascular injury
The association of vertebral or carotid artery injury with cervical spine fractures is well known, although the incidence of such lesions remains controversial. Clearly, the anatomic relationship of the vertebral arteries and the cervical spine places these vessels at risk with fracture-dislocations and the like. Because these vascular injuries are often clinically inapparent, the incidence was formerly thought to be low. The availability of noninvasive screening modalities such as MRA or CTA has provided further information. Several studies have reported rates of vertebral artery occlusive lesions of between 17.2% and 25.5% in patients with cervical spine fractures or dislocations.235–238 Lesser injuries, such as intimal tears or dissections, could be presumed to be even more common.
With the ability to screen patients with MRA or CTA rather than DSA, the true incidence of these injuries and their clinical significance will become better understood.239
The role of antiplatelet or anticoagulant therapy in these cases, although advocated by some groups,235–237 remains controversial and is complicated by the presence of associated injuries.
Benzel EC, Hart BL, Ball PA, et al. Magnetic resonance imaging for the evaluation of patients with occult cervical spine injury. J Neurosurg. 1996;85:824-829.
Benzel EC, Larson SJ. Functional recovery after decompressive spine operation for cervical spine fractures. Neurosurgery. 1987;20:742-746.
Bohlman HH. Acute fractures and dislocations of the cervical spine: an analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119-1142.
Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597-1604.
Brunette DD, Rockswold GL. Neurologic recovery following rapid spinal realignment for complete cervical spinal cord injury. J Trauma. 1987;27:445-447.
Cothren CC, Moore EE, Ray CEJr, et al. Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury. Surgery. 2007;141:76-82.
Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
Dickman CA, Hadley MN, Browner C, et al. Neurosurgical management of acute atlas-axis combination fractures: a review of 25 cases. J Neurosurg. 1989;70:45-49.
Doran SE, Papadopoulos SM, Ducker TB, et al. Magnetic resonance imaging documentation of coexistent traumatic locked facets of the cervical spine and disc herniation. J Neurosurg. 1993;79:341-345.
Ducker TB. Treatment of spinal cord injury. N Engl J Med. 1990;322:1459-1461.
Grant GA, Mirza SK, Chapman JR, et al. Risk of early closed reduction in cervical spine subluxation injuries. J Neurosurg. 1999;90(suppl 1):13-18.
Hadley MN, Dickman CA, Browner C, et al. Acute axis fractures: a review of 229 cases. J Neurosurg. 1989;71:642-647.
Hanson JA, Deliganis AV, Baxter AB, et al. Radiologic and clinical spectrum of occipital condyle fractures: retrospective review of 107 consecutive fractures in 95 patients. AJR Am J Roentgenol. 2002;178:1261-1268.
Holly LT, Kelly DF, Counelis GJ, et al. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors and injury characteristics. J Neurosurg. 2002;96:285-291.
Johnson RM, Hart DL, Simmons EF, et al. Cervical orthoses: a study comparing their effectiveness in restricting cervical motion in normal subjects. J Bone Joint Surg Am. 1977;59:332-339.
Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73:680-691.
Marshall LF, Knowlton S, Garfin SR, et al. Deterioration following spinal cord injury: a multicenter study. J Neurosurg. 1987;66:400-404.
McCall T, Fassett D, Brockmeyer D. Cervical spine trauma in children: a review. Neurosurg Focus. 2006;20(2):E5.
Pang D, Nemzek WR, Zovickian J. Atlanto-ocipital dislocation—part 2: the clinical use of (occipital) condyle-C1 interval, comparison with other diagnostic methods, and the manifestation, management and outcome of atlanto-occipital dislocation in children. Neurosurgery. 2007;61:995-1015.
Pang D, Wilberger J. Spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1982;57:114-129.
, 2002 Radiographic assessment of the cervical spine in asymptomatic trauma patients. Neurosurgery. 2002;50(suppl):S30-S35.
Rekate H, Theodore N, Sonntag VK, et al. Pediatric spine and spinal cord trauma: state of the art for the third millennium. Childs Nerv Syst. 1999;15:743-750.
Stiell IG, Wells GA, Vandeheem K, et al. The Canadian C-Spine rule study for alert and stable trauma patients. JAMA. 2001;286:1841-1848.
Tator C. Epidemiology and general characteristics of the spinal cord injured patient. In: Benzel E, Tator C, editors. Contemporary Management of Spinal Cord Injury. Park Ridge, IL: American Association of Neurological Surgeons; 1995:9-20.
Vaccaro AR, Hurlburt RJ, Patel AA, et al. The subaxial cervical spine injury classification system: a novel approach to recognize the importance of morphology, neurology and integrity of the discoligamentous complex. Spine. 2007;32:2365-2374.
White A, Panjabi M. Clinical Biomechanics of the Spine. Philadelphia: JB Lippincott; 1990.
1 Bohlman H. Complications of treatment of fractures and dislocations of the cervical spine. In: Epps C, editor. Complications of Orthopedic Surgery. Philadelphia: JB Lippincott; 1985:897-918.
2 Bohlman H, Ducker T, Lucas J. Spine and spinal cord injuries. In: Rothman R, Simeone F, editors. The Spine. Philadelphia: WB Saunders; 1982:661-757.
3 Bohlman HH. Acute fractures and dislocations of the cervical spine: an analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119-1142.
4 Bolesta M, Bohlman H. Late complications of cervical fractures and dislocations and their surgical treatment. In: Frymoyer J, editor. The Adult Spine: Principles of Practice. New York: Raven Press; 1991:1107-1126.
5 Yashon D. Spinal Injury. Norwalk, CT: Appleton-Century-Crofts; 1986.
6 Peng R, Chang C, Gilmore D, et al. Epidemiology of immediate and early trauma deaths at an urban level I trauma center. Am Surg. 1998;64:950-954.
7 Fabio A, Weiss H, Forjoh S, et al. Head and Spinal Cord Injuries in Pennsylvania 1995-1998. Pittsburgh Center for Violence and Injury Control, Department of Emergency Medicine, Allegheny University of the Health Sciences, 1998.
8 Devivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411-1419.
9 Nobunaga A, Go B, Karunas R. Recent demographic and injury trends in people served by the model spinal cord injury care systems. Arch Phys Med Rehabil. 1999;80:1372-1382.
10 Green BA, Gabrielsen MA, Hall WJ, et al. Analysis of swimming pool accidents resulting in spinal cord injury. Paraplegia. 1980;18:94-100.
11 Hall J, Buke D. Diving injury resulting in tetraplegia. Med J Aust. 1978;1:171.
12 DeVivo MJ, Fine PR, Maetz HM, et al. Prevalence of spinal cord injury: a reestimation employing life table techniques. Arch Neurol. 1980;37:707-708.
13 Kraus JF, Franti CE, Riggins RS, et al. Incidence of traumatic spinal cord lesions. J Chronic Dis. 1975;28:471-492.
14 Hu R, Mustard CA, Burns C. Epidemiology of incident spinal fracture in a complete population. Spine. 1996;21:492-499.
15 Tator C. Epidemiology and general characteristics of the spinal cord injured patient. In: Benzel E, Tator C, editors. Contemporary Management of Spinal Cord Injury. Park Ridge, IL: American Association of Neurological Surgeons; 1995:9-20.
16 Ajani AE, Cooper DJ, Scheinkestrel CD, et al. Optimal assessment of cervical spine trauma in critically ill patients: a prospective evaluation. Anaesth Intensive Care. 1998;26:487-491.
17 Demetriades D, Charalambides BS, Chahwan S, et al. Non-skeletal cervical spine injuries: epidemiology and diagnostic pitfalls. J Trauma. 2000;48:724-727.
18 Scheinarts PJ, Diaz J, Kaiser C, et al. Prospective comparison of admission computed tomographic scan and plain films of the upper cervical spine in trauma patients with altered mental status. J Trauma. 2001;51:663-669.
19 Pasquale M, Fabian TC. Practice management guidelines for trauma: EAST Ad Hoc Committee on Guideline Development—identifying cervical spine instability after trauma. J Trauma. 1998;44:941-956.
20 Milby AH, Halpern CH, Guo W, et al. Prevalence of cervical spine injury in trauma. Neurosurgical Focus. 2008;25(5):E10.
21 Jefferson G. Fractures of the atlas vertebra. Report of four cases and a review of those previously recorded. Br J Surg. 1920;7:407-422.
22 Holly LT, Kelly DF, Counelis GJ, et al. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors and injury characteristics. J Neurosurg. 2002;96:285-291.
23 Michael DB, Guyot DR, Darmody WR. Coincidence of head and cervical spine injury. J Neurotrauma. 1989;6:177-189.
24 Piatt JH. Detected and overlooked cervical spine injury in comatose victims of trauma: report from the Pennsylvania Trauma Outcomes Study. J Neurosurg Spine. 2006;5:210-216.
25 Cooper DJ, Ackland HM. Clearing the cervical spine in unconscious head injured patients—the evidence. Crit Care Resusc. 2005;7:181-184.
26 NSCIC. Spinal Cord: Facts and Figures at a Glance. Survey of Model Systems Spinal Cord Injury Rehabilitation Centers. Birmingham AL: National Spinal Cord Injury Statistical Center; 2000.
27 Ersmark H, Lowenheilm P. Factors influencing the outcome of cervical spine injuries. J Trauma. 1988;28:407-410.
28 Huelke DF, Mendelsohn RA, States JD, et al. Cervical fractures and fracture-dislocations sustained without head impact. J Trauma. 1978;18:533-538.
29 Wang MC, Pintar F, Yoganandan N, et al. The continued burden of spine fractures after motor vehicle crashes. Clinical article. J Neurosurg Spine. 2009;10:86-92.
30 Fassett DR, Harrop JS, Maltenfort M, et al. Mortality rates in geriatric patients with spinal cord injuries. J Neurosurg Spine. 2007;7:277-281.
31 Lomoschitz FM, Blackmore CC, Mirza SK, et al. Cervical spine injuries in patients 65 years old and older: epidemiologic analysis regarding the effects of age and injury mechanism on distribution, type and stability of injuries. AJR Am J Roentgenol. 2002;178:573-577.
32 McCall T, Fassett D, Brockmeyer D. Cervical spine trauma in children: a review. Neurosurg Focus. 2006;20(2):E5.
33 Niteki S, Moir C. Predictive factors in of the outcome of traumatic cervical spine fracture in children. J Pediatr Surg. 1994;29:1409-1411.
34 Eleraki MA, Theodore N, Adams M, et al. Pediatric cervical spine injuries. Report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
35 Rekate H, Theodore N, Sonntag VK, et al. Pediatric spine and spinal cord trauma: state of the art for the third millennium. Childs Nerv Syst. 1999;15:743-750.
36 Shamoun JM, Riddick L, Powell RW. Atlanto-occipital subluxation/dislocation: a “survivable” injury in children. Am Surg. 1999;65:317-320.
37 Giguere JF, St-Vil D, Turmel A, et al. Airbags and children: a spectrum of C-spine injuries. J Pediatr Surg. 1998;33:811-816.
38 Maxeiner H, Hahn M. Airbag-induced lethal cervical trauma. J Trauma. 1997;42:1148-1151.
39 Arbogast KB, Durbin DR, Kallan MJ, et al. Injury risk to restrained children exposed to deployed first- and second-generation air bags in frontal crashes. Arch Pediatr Adolesc Med. 2005;259:342-346.
40 Deutsch RJ, Badaway MK. Pediatric cervical spine fracture caused by an adult 3-point seatbelt. Pediatr Emerg Care. 2008;24:105-108.
41 Bilston LE, Brown J. Pediatric spinal injury type and severity are age and mechanism dependent. Spine. 2007;32:2339-2347.
42 Gunby I. New focus on spinal cord injury. JAMA. 1981;245:1201-1206.
43 Gillingham J. The problem of head and spinal cord injuries: prevention of the second accident. Med Sci Law. 1970;10:104-109.
44 Geisler W, Wynne-Jones M, Jousse A. Early management of the patient with trauma to the spinal cord. Med Serv J Can. 1966;22:512-523.
45 Moller F, Andres AH, Langenstein H. Intubating laryngeal mask airway (ILMA) seems to be an ideal device for blind intubation in case of immobile spine. Br J Anaesth. 2000;85:493-495.
46 Schuschnig C, Waltl B, Erlacher W, et al. Intubating laryngeal mask and rapid sequence induction in patients with cervical spine injury. Anaesthesia. 1999;54:793-797.
47 Waltl B, Waltl B, Leitgeb J, et al. Tracheal intubation and cervical spine excursion: direct laryngoscopy vs intubating laryngeal mask. Anaesthesia. 2001;56:221-226.
48 Bivins HG, Ford S, Bezmalinovic Z, et al. The effect of axial traction during orotracheal intubation of the trauma victim with an unstable cervical spine. Ann Emerg Med. 1988;17:25-29.
49 Meyer G, Berman I, Doty D, et al. Hemodynamic responses to acute quadriplegia with or without chest trauma. J Neurosurg. 1971;34:168-177.
50 Troll GF, Dohrman GJ. Anaesthesia of the spinal cord-injured patient: cardiovascular problems and their management. Paraplegia. 1975;13:162-171.
51 Johnson RM, Hart DL, Simmons EF, et al. Cervical orthoses: a study comparing their effectiveness in restricting cervical motion in normal subjects. J Bone Joint Surg Am. 1977;59:332-339.
52 White A, Panjabi M. Clinical Biomechanics of the Spine. Philadelphia: JB Lippincott; 1990.
53 Dunford J. Spinal column trauma. In: Baxt W, editor. Trauma: The First Hour. Norwalk, CT: Appleton-Century-Croft; 1984:171-219.
54 Podolsky S, Baraff LJ, Simon RR, et al. Efficacy of cervical spine immobilization methods. J Trauma. 1983;23:461-465.
55 Chesnut R, Marshall L. Early assessment, transport, and management of patients with post-traumatic spinal instability. In: Cooper P, editor. Management of Post-traumatic Spinal Instability. Park Ridge, IL: American Association of Neurolgical Surgeons; 1990:1-17.
56 Boswell H, Dietrich A, Shiels WE, et al. Accuracy of visual determination of neutral position of the immobilized pediatric cervical spine. Pediatr Emerg Care. 2001;17:10-14.
57 Gastel JA, Palumbo MA, Hulstyn MJ, et al. Emergency removal of football equipment: a cadaveric cervical spine injury model. Ann Emerg Med. 1998;32:411-417.
58 Waninger KN. On-field management of potential cervical spine injury in helmeted football players: leave the helmet on!. Clin J Sports Med. 1998;8:124-129.
59 Rumana C, Baskin D. Brown-Sequard syndrome produced by cervical disc herniation: case report and literature review. Surg Neurol. 1996;45:359-361.
60 Dietrich WD3rd. Therapeutic hypothermia for spinal cord injury. Crit Care Med. 2009;37(suppl):S238-S242.
61 Greenberg M. Incomplete spinal cord injuries. In: Greenberg M, editor. Handbook of Neurosurgery. 2nd ed. Lakeland, FL: Greenberg Graphics; 1991:495-496.
62 Hughes J, Brownell B. Spinal cord damage from hyperextension injury in cervical spondylosis. Lancet. 1963;1:687-690.
63 Dai L, Jia L. Central cord injury complicating acute cervical disc herniation in trauma. Spine. 2000;25:331-335. discussion 336
64 Schneider R, Cherry G, Patek H. The syndrome of acute central cervical spinal cord injury: with special reference to the mechanisms involved in hyperextension injuries of the cervical spine. J Neurosurg. 1954;11:546-577.
65 Schneider R, Thompson J, Bebin J. The syndrome of acute central cervical spinal cord injury. J Neurol Neurosurg Psychiatry. 1958;21:216-227.
66 Lifeso RM, Colucci MA. Anterior fusion for rotationally unstable cervical spine fractures. Spine. 2000;25:2028-2034.
67 Black P, Markowitz R. Experimental spinal cord injury in monkeys: comparison of steroids and local hypothermia. Surg Forum. 1971;22:409-411.
68 Ducker TB, Hamit HF. Experimental treatments of acute spinal cord injury. J Neurosurg. 1969;30:693-697.
69 Faden AI, Jacobs TP, Holaday JW. Opiate antagonist improves neurologic recovery after spinal injury. Science. 1981;211:493-494.
70 Faden AI, Jacobs TP, Mougey E, et al. Endorphins in experimental spinal cord injury: therapeutic effect of naloxone. Ann Neurol. 1992;10:326-332.
71 Fujimoto T, Nakamura T, Ikeda T, et al. Potent protective effects of melatonin on experimental spinal cord injury. Spine. 2000;25:769-775.
72 Kaptanoglu E, Tuncel M, Palaoglu S, et al. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J Neurosurg. 2000;93(1 suppl):77-84.
73 Taskiran D, Tanyalcin T, Sozemen EY, et al. The effects of melatonin on the antioxidant system in experimental spinal injury. Int J Neurosci. 2000;104:63-73.
74 Ducker TB, Salcman M, Daniell HB. Experimental spinal cord trauma. III. Therapeutic effect of immobilization and pharmacologic agents. Surg Neurol. 1978;10:71-76.
75 Ducker TB. Treatment of spinal cord injury. N Engl J Med. 1990;322:1459-1461.
76 Brodkey JS, Richards DE, Blasingame JP, et al. Reversible spinal cord trauma in cats: additive effects of direct pressure and ischemia. J Neurosurg. 1972;37:591-593.
77 Smith AJ, McCreery DB, Bloedel JR, et al. Hyperemia, CO2 responsiveness and autoregulation in the white matter following experimental spinal cord injury. J Neurosurg. 1978;48:239-251.
78 Wallace MC, Tator CH. Failure of naloxone to improve spinal cord blood flow and cardiac output after spinal cord injury. Neurosurgery. 1986;18:428-432.
79 Bracken MB, Shepard MJ, Collins WF, et al. A randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405-1411.
80 Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597-1604.
81 Anderson DK, Braughler JM, Hall ED, et al. Effects of treatment with U-74006F on neurological outcome following experimental spinal cord injury. J Neurosurg. 1988;69:562-567.
82 Bose B, Osterholm JL, Kalia M. Ganglioside-induced regeneration and reestablishment of axonal continuity in spinal cord transected rats. Neurosci Lett. 1986;63:165-169.
83 Faden AL, Simon RP. A potential role for excitotoxins in the pathophysiology of spinal cord injury. Ann Neurol. 1988;23:623-626.
84 Fehlings MG, Tator CH, Linden RD. The effect of nimodipine and dextran on axonal function and blood flow following experimental spinal cord injury. J Neurosurg. 1989;71:403-416.
85 Lehmann J, Sills M, Tsai C, et al. Dextromethorphan modulates the NMDA-type receptor–associated ion channel by binding to its closed state. In: Cavalheiro E, editor. Frontiers in Excitatory Amino Acid Research. New York: Allan R Liss; 1988:571-578.
86 Martinez-Arizala A, Rigamonti DD, Long JB, et al. Effects of NMDA receptor antagonists following spinal ischemia in the rabbit. Exp Neurol. 1990;108:232-240.
87 Steinberg GK, Saleh J, Kunis D. Delayed treatment with dextromethorphan and dextrorphan reduces cerebral damage after transient focal ischemia. Neurosci Lett. 1988;89:193-197.
88 Hurlburt RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93(1 suppl):1-7.
89 Short DJ, El Masry WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury—a systematic review from a clinical perspective. Spinal Cord. 2000;38:273-286.
90 Galli R, Spaite D, Simon R. The spine. In: Galli R, Spaite D, Simon R, editors. Emergency Orthopedics. Norwalk, CT: Appleton & Lange; 1989:96-104.
91 Harris JHJr, Edeiken-Monroe B, Kopaniky DR. A practical classification of acute cervical spine injuries. Orthop Clin North Am. 1986;17:15-30.
92 Gehweiler JA, Osborne RLJr, Becker RF. The Radiology of Vertebral Trauma. Philadelphia: WB Saunders; 1980.
93 Blahd WHJr, Iserson KV, Bjelland JC. Efficacy of the posttraumatic cross table lateral view of the cervical spine. J Emerg Med. 1985;2:243-249.
94 Gerrelts BD, Petersen EU, Mabry J, et al. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31:1622-1626.
95 Mace SE. Emergency evaluation of cervical spine injuries: CT versus plain radiographs. Ann Emerg Med. 1985;14:973-975.
96 Shaffer MA, Doris PE. Limitation of the cross table lateral view in detecting cervical spine injuries: a retrospective analysis. Ann Emerg Med. 1981;10:508-513.
97 Streitwieser DR, Knopp R, Wales LR, et al. Accuracy of standard radiographic views in detecting cervical spine fractures. Ann Emerg Med. 1983;12:538-542.
98 Wojcik WG, Edeiken-Monroe BS, Harris JHJr. Three-dimensional computed tomography in acute cervical spine trauma: a preliminary report. Skeletal Radiol. 1987;16:261-269.
99 Doris PE, Wilson RA. The next logical step in the emergency radiographic evaluation of cervical spine trauma: the five-view trauma series. J Emerg Med. 1985;3:371-385.
100 Ross S, Schwab CW, David ET, et al. Clearing the cervical spine: initial radiographic evaluation. J Trauma. 1985;27:371-385.
101 Heiden JS, Weiss MH, Rosenberg AW, et al. Management of cervical spinal cord trauma in southern California. J Neurosurg. 1975;43:732-736.
102 Eideken-Monroe B, Wagner LK, Harris JHJr. Hyperextension dislocation of the cervical spine. AJR Am J Roentgenol. 1986;146:803-808.
103 Taylor A, Blackwood W. Paraplegia in cervical injuries with normal radiographic appearance. J Bone Joint Surg Br. 1948;30:245-248.
104 Miller JD, Gehweiler JA, Martinez S, et al. Significant new observations on cervical spine trauma. AJR Am J Roentgenol. 1978;130:659-663.
105 Brady WJ, Moghtader J, Crutcher D, et al. ED use of flexion-extension cervical spine radiography in the evaluation of blunt trauma. Am J Emerg Med. 1999;17:504-508.
106 Dwek JR, Chung CB. Radiography of cervical spine injury in children: are flexion-extension radiographs useful for acute trauma? AJR Am J Roentgenol. 2000;174:1617-1619.
107 Pollack CJ, Hendley G, Martin D, et al. Use of flexion-extension radiographs of the cervical spine in blunt trauma. Ann Emerg Med. 2001;38:8-11.
108 Wang JC, Hatch JD, Sandhu HS, et al. Cervical flexion and extension radiographs in acutely injured patients. Clin Orthop Relat Res. 1999;365:111-116.
109 Velmahos GC, Theodorou D, Tatevossian R, et al. Radiographic cervical spine evaluation in the alert asymptomatic blunt trauma victim: much ado about nothing? J Trauma. 1996;40:768-774.
110 Bayless P, Ray VG. Incidence of cervical spine injuries in association with blunt head trauma. Am J Emerg Med. 1989;7:139-142.
111 Hoffman JR, Mower WR, Wolfson AB, et al. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma: National Emergency X-Radiography Utilization Group. N Engl J Med. 2000;343:94-99.
112 Hoffman JR, Shriger DL, Mower W, et al. Low risk criteria for cervical spine radiography in blunt trauma: a prospective study. Ann Emerg Med. 1992;21:1454-1460.
113 Hoffman JR, Wolfson AB, Todd K, et al. Selective cervical spine radiography in blunt trauma: methodology of the National Emergency X-Radiography Utilization Study (NEXUS). Ann Emerg Med. 1998;32:461-469.
114 McCaig LF. National Hospital Ambulatory Medical Care Survey: 1992 emergency department summary. Adv Data. 1994;245:1-12.
115 Diliberti T, Lindsey RW. Evaluation of the cervical spine in the emergency setting: who does not need an x-ray? Orthopedics. 1992;15:179-183.
116 Bachulis BL, Long WB, Hynes GD, et al. Clinical indications for cervical spine radiographs in the traumatized patient. Am J Surg. 1987;153:473-478.
117 Reid DC, Henderson R, Saboe L, et al. Etiology and clinical course of missed spine fractures. J Trauma. 1987;27:980-986.
118 Hoffman JR, Mower WR, Wofson AB, et al. Validity of a set of criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med. 2000;343:94-99.
119 Stiell IG, Wells GA, Vandeheem K, et al. The Canadian C-Spine rule study for alert and stable trauma patients. JAMA. 2001;286:1841-1848.
120 Radiographic assessment of the cervical spine in asymptomatic trauma patients. Neurosurgery. 2002;50(suppl):S30-S35.
121 Bayless P, Ray VG. Incidence of cervical spine injuries in association with blunt head trauma. Am J Emerg Med. 1989;7:139-142.
122 Kriepke DL, Gillespie KR, McCarthy MC, et al. Reliability of indications for cervical spine films in trauma patients. J Trauma. 1989;20:1438-1439.
123 Eyre A. Overview and comparison of NEXUS and Canadian C-Spine Rules. Am J Clin Med. 2006;3:12-15.
124 Bandiera G, Stiell IG, Wells GA, et al. The Canadian C-Spine Rule performs better than unstructured physician judgment. Ann Emerg Med. 2003;42:395-402.
125 Keene JS, Goletz TH, Lilleas F, et al. Diagnosis of vertebral fractures: a comparison of conventional radiography, conventional tomography and computed axial tomography. J Bone Joint Surg Am. 1982;64:586-594.
126 Cacayorin ED, Kieffer SA. Applications and limitations of computed tomography of the spine. Radiol Clin North Am. 1982;20:185-206.
127 Ghoshhajra K, Rao KC. CT in spinal trauma. J Comput Tomogr. 1980;4:309-318.
128 Maravilla KR, Cooper PR, Sklar FH. The influence of thin section tomography on the treatment of cervical spine injuries. Radiology. 1978;127:131-139.
129 Brant-Zawadzki M, Miller EM, Federle MP. CT in the evaluation of spine trauma. AJR Am J Roentgenol. 1981;136:369-375.
130 Sonntag VK, Hadley MN. Nonoperative management of cervical spine injuries. Clin Neurosurg. 1988;34:630-649.
131 Dickman CA, Hadley MN, Browner C, et al. Neurosurgical management of acute atlas-axis combination fractures: a review of 25 cases. J Neurosurg. 1989;70:45-49.
132 Hadley MN, Dickman CA, Browner C, et al. Acute axis fractures: a review of 229 cases. J Neurosurg. 1989;71:642-647.
133 Hadley MN, Dickman CA, Browner C, et al. Acute traumatic axis fractures: management and long term outcome. Neurosurgery. 1988;23:31-35.
134 Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injuries: a metaanalysis. J Trauma. 2005;58:902-905.
135 Betz RR, Gelman AJ, De Felipp GJ, et al. Magnetic resonance imaging (MRI) in the evaluation of spinal cord injured children and adolescents. Paraplegia. 1987;25:92-99.
136 Chakeres DW, Flickinger F, Bresnahan JC, et al. MR imaging of acute spinal cord trauma. AJNR Am J Neuroradiol. 1987;8:5-10.
137 Goldberg AL, Rothfuss WE, Deeb ZL, et al. The impact of magnetic resonance on the diagnostic evaluation of acute cervicothoracic spinal trauma. Skeletal Radiol. 1988;17:89-95.
138 Hackney DB, Asato R, Joseph PM, et al. Hemorrhage and edema in acute spinal cord compression: demonstration by MR imaging. Radiology. 1986;161:387-390.
139 Kadoya S, Nakamura T, Kobayashi S, et al. Magnetic resonance imaging of acute spinal cord injury: report of three cases. Neuroradiology. 1987;29:252-255.
140 Kalfas I, Wilberger J, Goldberg A, et al. Magnetic resonance imaging in acute spinal cord trauma. Neurosurgery. 1988;23:295-299.
141 Pan G, Kulkarni M, MacDougall D, et al. Traumatic epidural hematoma of the cervical spine: diagnosis with magnetic resonance imaging. J Neurosurg. 1988;68:798-801.
142 White P, Seymour R, Powell N. MRI assessment of the prevertebral soft tissues in acute cervical spine trauma. Br J Radiol. 1999;72:818-823.
143 Schaefer D, Flanders A, Northrup B, et al. Magnetic resonance imaging of acute cervical spine trauma. Spine. 1989;14:1090-1095.
144 McArdle CB, Crofford MJ, Mirfakhraee M, et al. Surface coil MR of spinal trauma: preliminary experience. AJNR Am J Neuroradiol. 1986;7:885-893.
145 Friedman D, Flanders A, Thomas C, et al. Vertebral artery injury after acute cervical spine trauma: rate of occurrence as detected by MR angiography and assessment of clinical consequences. AJR Am J Roentgenol. 1995;164:443-447.
146 Benzel EC, Hart BL, Ball PA, et al. Magnetic resonance imaging for the evaluation of patients with occult cervical spine injury. J Neurosurg. 1996;85:824-829.
147 Muchow RD, Resnick DK, Abdel MP, et al. Magnetic resonance imaging (MRI) in the clearance of cervical spine blunt trauma: a metaanalysis. J Trauma. 2008;64:179-189.
148 Allen RL, Perot PLJr, Gudeman SK. Evaluation of acute nonpenetrating cervical spinal cord injuries with CT metrizamide myelography. J Neurosurg. 1985;63:510-520.
149 Cooper PR, Cohen A, Rosiello A, et al. Posterior stabilization of cervical spine fractures and subluxations using plates and screws. Neurosurgery. 1988;23:300-306.
150 Willis BK, Greiner F, Orrison WW, et al. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery. 1994;34:435-441.
151 Marar BC. Hyperextension injuries of the cervical spine: the pathogenesis of damage to the spinal cord. J Bone Joint Surg Am. 1974;56:1655-1662.
152 Norrell H. Fractures and dislocations of the spine. In: Rothman R, Simeone F, editors. The Spine. Philadelphia: WB Saunders; 1975:529-566.
153 Norton W. Fractures and dislocations of the cervical spine. J Bone Joint Surg Am. 1962;44:115-139.
154 Allen BLJr, Ferguson RL, Lehmann TR, et al. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine. 1982;7:1-27.
155 Harris JH, Edeiken-Monroe B, Kopansiky DR. A practical classification of acute cervical spine injuries. Orthop Clin North Am. 1986;17:15-30.
156 Vaccaro AR, Hurlburt RJ, Patel AA, et al. The subaxial cervical spine injury classification system: a novel approach to recognize the importance of morphology, neurology and integrity of the discoligamentous complex. Spine. 2007;32:2365-2374.
157 Bauze RJ, Ardran GM. Experimental production of forward dislocation in the human cervical spine. J Bone Joint Surg Br. 1978;60:239-245.
158 Gosch HH, Gooding E, Schneider RC. An experimental study of cervical spine and cord injuries. J Trauma. 1972;12:570-576.
159 Maiman DJ, Yoganandan N. Biomechanics of cervical spine trauma: clinical neurosurgery. In: Proceedings of the Congress of Neurological Surgeons. Baltimore: Williams & Wilkins; 1989.
160 Marar BC. The pattern of neurological damage as an aid to the diagnosis of the mechanism in cervical-spine injuries. J Bone Joint Surg Am. 1974;56:1648-1654.
161 Roaf R. A study of the mechanics of spinal injuries. J Bone Joint Surg Br. 1960;42:810-823.
162 White AAIII, Johnson RM, Panjabi MM, et al. Biomechanical analysis of clinical stability of the cervical spine. Clin Orthop Relat Res. 1975;109:85-96.
163 Yoganandan N, Sances A, Maiman DJ, et al. Experimental spinal cord injuries with vertical impact. Spine. 1986;11:855-860.
164 Sypert G. Management of lower cervical spinal instability. In: Wilkins R, editor. Neurosurgery Update II: Vascular, Spinal, Pediatric, and Functional Neurosurgery. New York: McGraw-Hill; 1991:234-244.
165 Holdsworth F. Fractures, dislocations and fracture-dislocations of the cervical spine. J Bone Joint Surg Am. 1970;52:1534-1551.
166 Lloyd S. Fracture dislocation of the spine. Med Rec. 1907;71:465-470.
167 Panjabi M, White A, Johnson R. Cervical spine mechanics as a function of transection of components. J Biomech. 1975;8:327-336.
168 Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
169 Braakman R, Vinken PJ. Unilateral facet interlocking in the lower cervical spine. J Bone Joint Surg Br. 1967;49:249-257.
170 Patel AA, Dailey A, Brodke DS, et al. Subaxial cervical spine trauma classification: the Subaxial Injury Classification system and case examples. Neurosurg Focus. 2008;25(5):E8.
171 Rogers W. Fractures and dislocations of the cervical spine: an end result study. J Bone Joint Surg Am. 1957;39:3341-3376.
172 Fielding J, Burstein A, Frankel V. The nuchal ligament. Spine. 1976;1:3-14.
173 Werne S. Studies in spontaneous atlas dislocation. Acta Orthop Scand Suppl. 1957;23:1-50.
174 Bailey RW. Observation of cervical intervertebral disc lesions in fractures and dislocations. J Bone Joint Surg Am. 1963;45:461-470.
175 Bedbrook G. Are cervical spine fractures ever unstable? J West Pac Orthop Assoc. 1969;6:729.
176 Bedbrook G. Closed injuries of the cervical spine and spinal cord extension-rotation injuries. Proc Veterans Admin Spinal Cord Injury Conf. 1973;19:58-59.
177 Bedbrook G. Compression, flexion and extension injuries of the cervical spine with tetraplegia. In: Proceedings of the 19th Veterans Administration Spinal Cord Injury Conference. Washington, DC: Veterans Administration; 1977:6-28.
178 Munro D. The factors that govern the stability of the spine. Paraplegia. 1966;3:219-228.
179 Alexander EJr, Davis CHJr, Forsyth HF. Reduction and fusion of fracture dislocation of the cervical spine. J Neurosurg. 1967;27:588-591.
180 Brunette DD, Rockswold GL. Neurologic recovery following rapid spinal realignment for complete cervical spinal cord injury. J Trauma. 1987;27:445-447.
181 Crutchfield W. Skeletal traction in treatment of injuries to the cervical spine. JAMA. 1954;155:29-32.
182 Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179-192.
183 Eismont FJ, Arena MJ, Green BA. Extrusion of an intervertebral disc associated with traumatic subluxation or dislocation of cervical facets. J Bone Joint Surg Am. 1991;73:1555-1560.
184 Doran SE, Papadopoulos SM, Ducker TB, et al. Magnetic resonance imaging documentation of coexistent traumatic locked facets of the cervical spine and disc herniation. J Neurosurg. 1993;79:341-345.
185 Maiman DJ, Barolat G, Larson SJ. Management of bilateral locked facets of the cervical spine. Neurosurgery. 1986;18:542-547.
186 Rizzolo SJ, Piazza MR, Cotler JM, et al. Intervertebral disc injury complicating cervical spine trauma. Spine. 1991;16(6 suppl):S187-S189.
187 Grant GA, Mirza SK, Chapman JR, et al. Risk of early closed reduction in cervical spine subluxation injuries. J Neurosurg. 1999;90(suppl 1):13-18.
188 Initial closed reduction of cervical spine fracture-dislocation injuries. Neurosurgery. 2002;50(suppl):S44-S50.
189 Wolf J, Johnson R. Cervical orthoses. In: In The Cervical Spine Research Society (ed): The Cervical Spine. Philadelphia: JB Lippincott; 1983:54-61.
190 Hartman JT, Palumbo F, Hill BJ. Cineradiography of the braced normal cervical spine: a comparative study of five commonly used cervical orthoses. Clin Orthop Relat Res. 1975;109:97-102.
191 Garfin SR, Botte MJ, Waters RL, et al. Complications in the use of the halo fixation device. J Bone Joint Surg Am. 1986;68:320-325.
192 Saeed MU, Dacuycuy MA, Kennedy DJ. Halo pin insertion–associated brain abscess: case report and review of the literature. Spine. 2007;32:E271-E274.
193 Benzel EC, Larson SJ. Recovery of nerve root function after complete quadriplegia from cervical spine fractures. Neurosurgery. 1986;19:809-812.
194 Benzel EC, Larson SJ. Functional recovery after decompressive spine operation for cervical spine fractures. Neurosurgery. 1987;20:742-746.
195 Braakman R, Penning G. Injuries of the cervical spine. In: Vinken P, Bruyn G, Braakman R, editors. Handbook of Clinical Neurology: Injuries of Spine and Spinal Cord. Amsterdam: North Holland; 1976:227-376.
196 Cheshire DJ. The stability of the cervical spine following the conservative treatment of fractures and fracture-dislocations. Paraplegia. 1969;7:193-203.
197 Guttmann L. The conservative management of closed injuries of the vertebral column resulting in damage to the spinal cord and spinal roots. In: Vinken R, Bruyn G, Braakman R, editors. Handbook of Clinical Neurology: Injuries of Spine and Spinal Cord. Amsterdam: North Holland; 1975:285-306.
198 Marshall LF, Knowlton S, Garfin SR, et al. Deterioration following spinal cord injury: a multicenter study. J Neurosurg. 1987;66:400-404.
199 Tuli S, Tator CH, Fehlings MG, et al. Occipital condyle fractures. Neurosurgery. 1997;41:368-377.
200 Anderson PA, Montesano PX. Morphology and treatment of occipital condyle fractures. Spine. 1988;13:731-736.
201 Hanson JA, Deliganis AV, Baxter AB, et al. Radiologic and clinical spectrum of occipital condyle fractures: retrospective review of 107 consecutive fractures in 95 patients. AJR Am J Roentgenol. 2002;178:1261-1268.
202 Wholey MH, Bruwer AJ, Baker HL. The lateral roentgenogram of the neck (with comments on the atlanto-odontoid-basion relationship). Radiology. 1958;71:350-356.
203 Powers B, Miller MD, Kramer RS, et al. Traumatic anterior atlanto-occipital dislocation. Neurosurgery. 1979;4:12-17.
204 Dublin AB, Marks WM, Weinstock D, et al. Traumatic dislocation of the atlanto-occipital articulation (AOA) with short-term survival: with a radiographic method of measuring the AOA. J Neurosurg. 1980;52:541-546.
205 Pang D, Nemzek WR, Zovickian J. Atlanto-ocipital dislocation—part 2: the clinical use of (occipital) condyle-C1 interval, comparison with other diagnostic methods, and the manifestation, management and outcome of atlanto-occipital dislocation in children. Neurosurgery. 2007;61:995-1015.
206 Traynelis VC, Marano GD, Dunker RO, et al. Traumatic atlanto-occipital dislocation: case report. J Neurosurg. 1986;65:863-870.
207 Diagnosis and management of traumatic atlanto-occipital dislocation injuries. Neurosurgery. 2002;50(suppl):S105-S111.
208 Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73:680-691.
209 Fielding JW, Cochran GV, Lawsing JFIII, et al. Tears of the transverse ligament of the atlas: a clinical and biomechanical study. J Bone Joint Surg Am. 1974;56:1683-1691.
210 Anderson LD, D’Alonzo RT. Fractures of the odontoid process of the axis. J Bone Joint Surg Am. 1974;56:1663-1674.
211 Hadley MN, Browner C, Liu SS, et al. New subtype of acute odontoid fractures (type IIA). Neurosurgery. 1988;22:67-71.
212 Ekong CE, Schwartz ML, Tator CH, et al. Odontoid fracture: management with early mobilization using the halo device. Neurosurgery. 1981;9:631-637.
213 Subach BR, Monroe MA, Haid RW, et al. Management of acute odontoid fractures with single-screw anterior fixation. Neurosurgery. 1999;45:812-819. discussion 819-820
214 Dunn ME, Seljeskog EL. Experience in the management of odontoid process injuries: an analysis of 128 cases. Neurosurgery. 1986;18:306-310.
215 Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67:217-226.
216 Vaccaro AR, Madigan L, Bauerle WB, et al. Early halo immobilization of displaced traumatic spondylolisthesis of the axis. Spine. 2002;27:2229-2233.
217 Caspar W, Barbier DD, Klara PM. Anterior cervical fusion and Caspar plate stabilization for cervical trauma. Neurosurgery. 1989;25:491-502.
218 Tuite G, Papadopoulos SM, Sonntag VK. Caspar plate fixation for the treatment of complex hangman’s fractures. Neurosurgery. 1992;30:761-764. discussion 764-765
219 Fowler JL, Sandhu A, Fraser RD. A review of fractures of the atlas vertebra. J Spinal Disord. 1990;3:19-24.
220 Gleizes V, Jacquot FP, Signoret F, et al. Combined injuries in the upper cervical spine: clinical and epidemiological data over a 14 year period. Eur Spine J. 2000;9:386-392.
221 Management of combination fractures of the atlas and axis in adults. Neurosurgery. 2002;50(suppl):S140-S147.
222 Hanigan WC, Powell FC, Elwood PW, et al. Odontoid fractures in elderly patients. J Neurosurg. 1993;78:32-35.
223 Fujimura Y, Nishi Y, Kobayashi K. Prognosis of neurological deficits associated with upper cervical spine injuries. Paraplegia. 1995;33:195-202.
224 Pang D, Wilberger J. Spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1982;57:114-129.
225 Burke DC. Traumatic spinal paralysis in children. Paraplegia. 1974;11:268-276.
226 Cheshire DJ. The paediatric syndrome of traumatic myelopathy without demonstrable vertebral injury. Paraplegia. 1977;15:74-85.
227 Dunlap J, Morris M, Thompson R. Cervical spine injuries in children. J Bone Joint Surg Am. 1958;40:681-686.
228 Glasauer FE, Cares HL. Biomechanical features of traumatic paraplegia in infancy. J Trauma. 1943;13:166-170.
229 Papavasiliou V. Traumatic subluxation of the cervical spine during childhood. Orthop Clin North Am. 1978;9:945-954.
230 Cramer F, McGowan F. The role of the nucleus pulposus in the pathogenesis of so called “recoil” injuries of the spinal cord. Surg Gynecol Obstet. 1944;79:516-521.
231 Crooks F, Birkett A. Fractures and dislocations of the cervical spine. Br J Surg. 1944;31:252-265.
232 Alexander EJ, Davis CJ, Field C. Hyperextension injuries of the cervical spine. Arch Neurol Psychiatry. 1958;79:146-150.
233 Taylor A. The mechanism of injury to the spinal cord in the neck without injury to the vertebral column. J Bone Joint Surg Br. 1951;33:543-547.
234 Hendy GW, Wolfson AB, Mower WR, et al. Spinal cord injury without radiographic abnormality: results of the National Emergency X-radiography Utilization study in blunt cervical trauma. J Trauma. 2002;53:1-4.
235 Cothren CC, Moore EE, Biffl WL, et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55:811-813.
236 Cothren CC, Moore EE, Ray CEJr, et al. Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury. Surgery. 2007;141:76-82.
237 Weller SJ, Rossitch EJr, Malek AM. Detection of vertebral artery injury after cervical spine trauma using magnetic resonance angiography. J Trauma. 1999;46:660-666.
238 Sack JA, Etame AB, Shah AB, et al. Management and outcomes of patients undergoing surgery for traumatic cervical fracture-subluxation associated with an asymptomatic vertebral artery injury. J Spinal Disord Tech. 2009;22:86-90.
239 Eastman AL, Chason DP, Perez CL, et al. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for prime time? J Trauma. 2006;60:925-929.