Chapter 37 Assessment of Myocardial Viability with Thallium-201 and Technetium-Based Agents
INTRODUCTION (See Chapter 36)
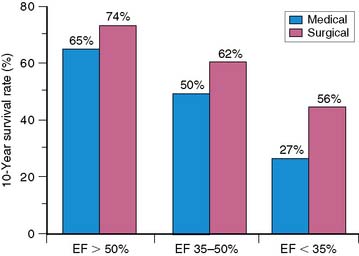
In the current era of revascularization surgery and interventional cardiology, the assessment of myocardial viability has become an integral component of the diagnostic evaluation of patients with coronary artery disease (CAD) and depressed left ventricular (LV) function. It is now well established that LV dysfunction is not always an irreversible process related to previous infarction, as once was widely believed. Regional and global ventricular function may improve substantially and even normalize after reperfusion therapy for acute myocardial infarction1–5 and after myocardial revascularization procedures in patients with chronic CAD.6–9 This potentially reversible form of LV dysfunction is known as myocardial hibernation, indicating a condition in which myocardial contractility has been reduced in the setting of a sustained reduction in myocardial blood supply.9–12 The conceptual framework of hibernation has been challenged in recent years, and an alternative concept has been proposed of persistent LV dysfunction caused by repeated episodes of myocardial ischemia leading to repetitive stunning.13,14 This latter mechanism for reversible LV dysfunction in chronic CAD has gathered support from excellent experimental models of repetitive ischemia in the setting of a chronic coronary artery stenosis.15–18 Independent of the mechanism, which is difficult to determine clinically in individual patients, the important clinical issue is that viable but dysfunctional myocardium in patients with chronic CAD will improve in function only if identified and revascularized. Imaging to evaluate the presence and extent of viable but dysfunctional myocardium has become an important component of the clinical assessment of patients with CAD and impaired LV function,19,20 particularly among patients who are possible candidates for myocardial revascularization. These procedures are often accompanied by high operative morbidity and mortality rates in this subset of patients. On the other hand, the large subgroup of patients with moderate to severe LV dysfunction is at considerable risk for death during the course of medical therapy, and these patients potentially have the most to gain from successful revascularization in terms of survival (Fig. 37-1).21
Although the percentage of patients showing an important reversal of LV dysfunction after revascularization varies among reported series (probably as a result of patient selection factors and revascularization techniques), it is not inconsequential. It is estimated that 25% to 40% of patients with chronic CAD and LV dysfunction have the potential for significant improvement in LV function after revascularization (Fig. 37-2).6–8,22,23 These findings have several implications. First, given the important relationship between LV function and survival (see Fig. 37-1), the improvement in LV function after revascularization may translate into an improvement in survival. Although definitive data tying improved function to improved survival are lacking, many recent retrospective studies have begun to establish this point, as discussed later. Second, the decision to proceed with revascularization in patients with moderate to severe LV dysfunction is often difficult. These patients undergo coronary artery bypass surgery or percutaneous coronary intervention with considerable risk of procedure-related morbidity and mortality. The perioperative mortality rate associated with surgical revascularization of patients with severe LV dysfunction is as high as 10%. Hence, accurate methods to detect viable myocardium distal to a coronary stenosis, with the potential for reversal of LV dysfunction, are essential to select prospective patients in whom these risks are justified.
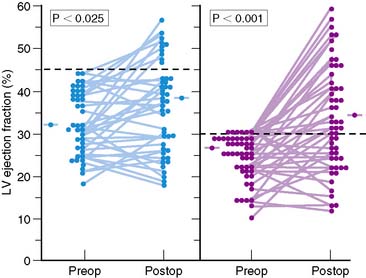
Figure 37-2 Left ventricular (LV) ejection fraction at rest shown by radionuclide ventriculography before (Preop) and after (Postop) coronary artery bypass surgery in patients with preoperative LV dysfunction in two surgical series.8,20 Although surgery resulted in only a small increase in mean ejection fraction, substantial increases were observed in a substantial subset of patients, with normalization of ejection fraction occurring in many patients.
(From Elefteriades JA, Tolis G Jr, Levi E, et al: Coronary artery bypass grafting in severe left ventricular dysfunction: Excellent survival with improved ejection fraction and functional state, J Am Coll Cardiol 22:1411–1417, 1993; and Bonow RO, Dilsizian V: Thallium-201 for assessment of myocardial viability, Semin Nucl Med 21:230–241, 1991.)
Several clinically reliable physiologic markers can be used to assess myocardial viability. Indexes of regional coronary blood flow, regional wall motion, and regional systolic wall thickening are accurate markers of viability if they are normal or near normal. However, these indexes have major limitations in identifying viable myocardium when they are reduced or absent. By definition, regional perfusion and systolic function (regional wall motion and wall thickening) are severely reduced or absent in patients with hibernating myocardium,7,9–11,24 despite maintenance of tissue viability. Other patients have preserved blood flow at rest but recurrent ischemic episodes during stress that lead to persistent contractile dysfunction from repetitive stunning.1,25–29 In these latter patients, indexes of wall motion and wall thickening are also imprecise markers of viability. More recently, cardiac magnetic resonance imaging (MRI) has become a valuable tool for the assessment of myocardial viability. In addition to functional information, areas of hyperenhancement on delayed imaging after gadolinium injection correlate well with areas of scarring.30–32
THALLIUM-201 IMAGING TO ASSESS MYOCARDIAL VIABILITY
Cellular viability requires intact sarcolemmal function to maintain electrochemical gradients across the cell membrane and preserved metabolic activity to generate high-energy phosphates. These processes require adequate myocardial blood flow to deliver substrates and washout metabolites. The retention of 201Tl is an active process that is a function of cell viability and cell membrane activity as well as blood flow. Therefore, in theory, 201Tl should be taken up and retained by viable myocardium regardless of whether systolic function is preserved. Thus, regional thallium activity, even in asynergic regions, should be an accurate marker of viability and should predict improvement of regional contraction after revascularization.19,27,33–37
Stress-Redistribution Imaging
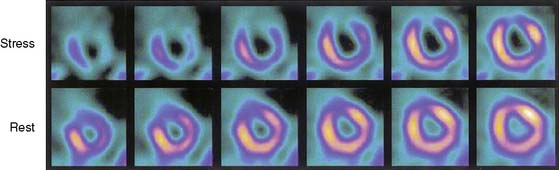
Several recent studies of patients with chronic CAD showed that blood flow under basal conditions may be normal or near normal in myocardial regions, despite severe segmental dysfunction, and that this regional dysfunction improves after myocardial revascularization.1,25,26,28,29 In these patients with perfusion-contraction mismatch, the segmental dysfunction is believed to arise from repeated episodes of myocardial ischemia that lead to repetitive stunning, rather than true myocardial hibernation, which, by definition, requires reduced blood flow under basal conditions. Although the relative prevalence of stunning versus hibernation as a causative mechanism for reversible contractile dysfunction in patients with chronic CAD is uncertain, it appears that both processes occur, but most patients have a form of repetitive stunning. In these patients, the finding of reversible ischemia may be the key to determining the viability of dysfunctional segments, which is readily accomplished with stress thallium imaging. Thus, thallium redistribution in an asynergic region with a defect shown on stress imaging predicts improvement of regional contraction after revascularization (Fig. 37-3).37,38 However, many regions of severely ischemic or hibernating myocardium appear to have irreversible thallium defects on standard exercise-redistribution imaging; hence, the negative predictive value of an irreversible 201Tl defect is relatively poor. Up to 50% of regions with “irreversible” thallium defects improve in function after revascularization.38–43 Thus, standard stress-redistribution thallium imaging has an excellent positive predictive value, but a suboptimal negative predictive value. It is generally accepted that stress 4-hour redistribution imaging does not provide satisfactory precision in differentiating between LV dysfunction arising from infarcted versus hibernating myocardium. This technique often underestimates the presence of viable myocardium and hence the potential for recovery after revascularization.
This concept is supported by studies directly comparing the results of PET imaging and exercise-redistribution thallium scintigraphy. In four studies, between 38% and 47% of apparently irreversible thallium defects (that would have been identified as scar by stress-redistribution thallium imaging) were identified as viable on the basis of regional uptake of fluorine-18 (18F)-fluorodeoxyglucose (FDG).44–47 Although these data suggest that metabolic imaging with PET is superior to thallium imaging in the detection of viable myocardium, two limitations of these studies must be emphasized. First, the severity of the reduction in thallium activity within the irreversible thallium defects was not assessed. The importance of quantitative analysis of regional thallium activity is discussed later. Second, the previous comparative studies of thallium scintigraphy and PET all used postexercise thallium imaging followed by a single redistribution study 3 to 4 hours later. As noted earlier, the limitations of 4-hour redistribution imaging are well established.
Modifications in imaging protocols with 201Tl considerably enhance the ability of 201Tl imaging to detect viable myocardium.12,20,35 These include late redistribution imaging and 201Tl reinjection techniques.
Late Thallium-Redistribution Imaging
In many patients, late imaging at 24 to 72 hours elicits 201Tl redistribution in many defects that appear to be irreversible at 3 to 4 hours. This late 201Tl redistribution is evidence of viable myocardium.39,40,48,49 As many as 54% of defects that appear irreversible at 3 to 4 hours show reversibility at 24 hours, although this number is as low as 22% in some studies. 201Tl redistribution is a continual process,50 and a truly irreversible defect shown on early redistribution images will not reverse later. However, in many viable regions, defect reversal at 3 to 4 hours may be minimal and poorly detected. Hence, the defect may not reverse appreciably on qualitative interpretation, and the increase in relative tracer activity also may not exceed the reproducibility limit on quantitative analysis. In these regions, late redistribution imaging may confirm that defect reversibility has occurred.
Several studies show that late imaging at 8 to 72 hours shows substantial thallium redistribution in many defects that appear to be irreversible at 3 to 4 hours,39,40,48,49 and that this late thallium redistribution is consistent with viable myocardium.40,49 Kiat et al.40 reported in 21 patients undergoing myocardial revascularization procedures that 61% of apparently irreversible defects seen at 4 hours reversed at the time of late (18- to 72-hour) redistribution imaging. The revascularization results in this latter study, as assessed by postintervention repeat thallium imaging, provided additional important insights. Of all regions that appeared irreversible at 4 hours, 72% showed improvement after revascularization, confirming earlier studies that found that standard 3- to 4-hour thallium redistribution imaging overestimates the prevalence and severity of irreversible myocardial damage. However, when these 4-hour irreversible defects were further analyzed with the results of late imaging, 95% of regions with late redistribution improved after revascularization, compared with only 37% of regions that remained irreversible on late imaging.40 These findings indicate that myocardial segments that show late thallium redistribution represent viable myocardium and that late imaging may considerably improve the identification of viable myocardium in thallium defects that appear irreversible at 3 to 4 hours. The implications of late thallium redistribution for myocardial viability are similar to those of 3- to 4-hour redistribution. Many persistent defects seen at 3 to 4 hours show late redistribution. The positive predictive value of late redistribution is excellent, but the negative predictive value remains poor. The positive predictive value of late redistribution is more than 90%40 in predicting improvement after revascularization; thus, defect reversibility with late imaging is an excellent marker of viable myocardium. The negative predictive value of an irreversible defect at 24 hours appears to be only marginally better than that of an irreversible defect at 3 to 4 hours. For example, 37% of irreversible defects seen at 24 hours improve after revascularization,40 and 39% of irreversible defects seen at 24 hours show improvement by quantitative analysis when 201Tl is reinjected at rest.51 Moreover, nearly 50% of these defects are metabolically active on PET imaging.52 This finding suggests that some ischemic regions may never redistribute, even on late imaging, no matter how long the redistribution period, unless serum thallium levels are augmented.
Thallium-Reinjection Techniques
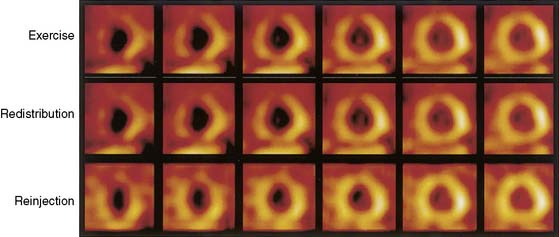
The reinjection of thallium at rest immediately after the standard 4-hour redistribution image may overcome several of these limitations and may be used to assess myocardial viability in apparently irreversible thallium defects on standard early or late-redistribution images (Fig. 37-4).53–55 Up to 49% of apparently irreversible defects on 3- to 4-hour redistribution images53 and 39% of these defects on 24-hour redistribution images51,56,57 show improved or normal uptake after thallium reinjection.53 Additional redistribution imaging obtained hours after the reinjected thallium dose appears to provide no information beyond that obtained by imaging immediately after reinjection.58 Fewer than 5% of myocardial regions with persistent defects on 3- to 4-hour redistribution plus reinjection images show evidence of late redistribution. However, late redistribution imaging is important when reinjection is performed at 4 hours without an intervening redistribution study. A simple exercise-reinjection protocol without redistribution imaging may miss unique and important viability information provided by redistribution. Hence, if reinjection protocols are used, it is essential to perform routine 3- to 4-hour redistribution imaging before the reinjection or to perform late-redistribution imaging in patients with persistent defects on stress-reinjection images.59
The observation that thallium uptake after reinjection represents viable myocardium is substantiated in three subgroups of patients. First, in nine studies43,53,60–66 reporting on 295 patients with LV dysfunction who were evaluated again 3 to 6 months after revascularization, improved wall motion occurred in 69% of segments identified as viable by thallium reinjection before revascularization. Such improvement occurred in only 11% of segments considered nonviable by redistribution or reinjection images (Fig. 37-5). Second, in comparative studies with thallium reinjection and PET imaging with FDG, most myocardial segments that were identified as viable by reinjection had metabolic evidence of myocardial viability.67–69 The concordance between data on thallium reinjection and FDG uptake was excellent, with 51% of regions with severe irreversible thallium defects on 4-hour redistribution studies identified as viable by both thallium reinjection and PET.67 Third, in patients who underwent gated MRI to assess regional systolic function, excellent correlation was observed between regional thallium activity and FDG activity in myocardial regions with severely reduced or absent wall thickening.68
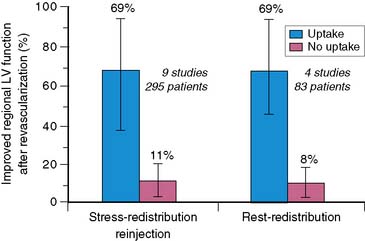
Figure 37-5 Likelihood of improved regional left ventricular (LV) function after revascularization based on thallium-201 single-photon emission tomography imaging. Data are summarized from nine studies done with stress-redistribution-reinjection imaging43,53,60–66 and four studies done with rest-redistribution imaging.71,73–75 The range of values reported in the individual studies is shown by the horizontal bars connected by vertical lines. The blue bars represent the positive predictive value, and the red bars represent the inverse of the negative predictive value.
These PET data are in keeping with previous studies indicating that up to 50% of regions with apparently irreversible thallium defects have metabolic activity by PET and thus evidence of viability.44–47 These data also indicate that thallium reinjection is a convenient, clinically accurate, and relatively inexpensive method with which to identify viable myocardium in patients with chronic CAD and LV dysfunction. Thus, thallium reinjection at rest after 3- to 4-hour redistribution imaging provides most of the clinically relevant information on myocardial viability in regions with apparently irreversible thallium defects. Thallium reinjection may be used instead of 24-hour imaging in most patients with a persistent thallium defect on conventional redistribution images.
Rest-Redistribution Thallium Imaging
The finding of exercise-induced ischemia in a patient with LV dysfunction has important prognostic implications that usually identify the patient as a candidate for revascularization therapy. Thus, exercise-redistribution-reinjection thallium protocols are attractive because they provide important information about both jeopardized myocardium and viable myocardium. However, in many patients, the sole clinical issue is the viability of one or more regions of dysfunctional LV myocardium, not whether there is also inducible ischemia. In others, the coronary anatomy is known to be at high risk, and stress testing is contraindicated, although the determination of viability may establish the potential benefit of revascularization. In these patients, rest-redistribution thallium imaging is a practical approach that can yield accurate viability data. It is essential to obtain both initial images (indicating regional perfusion) and subsequent redistribution images. Although early thallium studies yielded mixed results on the predictive accuracy of rest-redistribution imaging,33,36 subsequent studies show that a quantitative analysis of regional thallium activity in rest-redistribution studies predicts recovery of regional LV function with accuracy that is virtually identical to that achieved with thallium exercise-redistribution-reinjection imaging (see Fig. 37-5).56,70–75 These results are also comparable to those with PET imaging with FDG. PET achieves a higher positive predictive value, and thallium single-photon emission tomography (SPECT) imaging achieves a higher negative predictive value.
The available data comparing rest-redistribution imaging and stress-redistribution-reinjection imaging indicate comparable positive and negative predictive values for recovery of regional function after revascularization. Both techniques have high sensitivity, yielding a negative predictive value of approximately 90% (see Fig. 37-5). However, specificity is much lower, yielding a positive predictive value of less than 70%. A limitation of this analysis is that thallium data are considered binomially, rather than as a continuum.
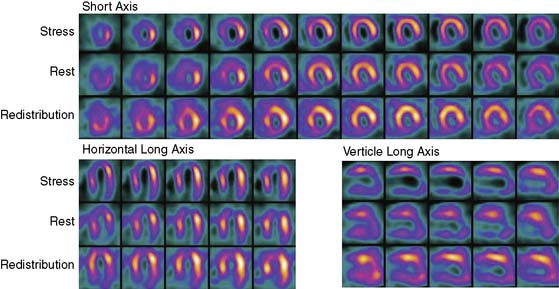
In laboratories using dual-isotope imaging for stress/rest imaging, with a technetium-99m (99mTc) tracer for stress imaging and 201Tl for rest imaging, the rest-redistribution thallium technique for viability assessment can be easily incorporated into the evaluation of patients with LV dysfunction, as discussed subsequently in this chapter. This can be done by performing rest-redistribution thallium imaging before the 99mTc stress test (Fig. 37-6). Alternatively, if irreversible defects are detected after routine stress/rest imaging, late imaging of thallium redistribution at a time when the technetium has decayed provides additional information regarding viability of myocardium with reduced flow under resting conditions.
Quantitative Analysis of Thallium Data
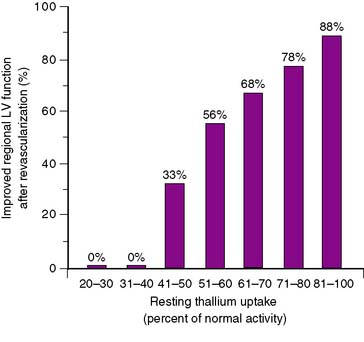
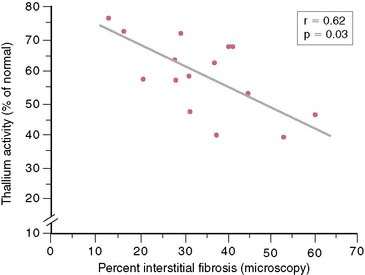
In 12 of the 13 SPECT studies summarized in Figure 37-5, regional thallium activity was analyzed quantitatively rather than with a visual scoring system. It is unclear whether a subjective interpretation of images can replicate these results. However, in most cases, the criteria for viability in these quantitative studies were based on a threshold level of thallium activity such that a thallium level greater than 50% or 60% of the activity in normal myocardial segments was considered viable. This black-and-white approach to the thallium data, classifying myocardial areas simply as viable or nonviable, achieves reasonable results. However, it does not take advantage of one of the greatest strengths of perfusion imaging: the ability to view regional tracer activity as a continuum rather than a simple binary function. Several studies show the nearly linear relationship (Fig. 37-7) between regional thallium activity and the likelihood of recovery of regional function after revascularization.73–75 This continuous relationship between thallium activity and myocardial viability is confirmed by histologic studies of myocardial biopsy specimens obtained at surgery76 that show a significant inverse relationship between thallium uptake and the degree of myocardial interstitial fibrosis (Fig. 37-8). For example, all myocardial segments with thallium activity greater than 50% of normal were considered viable in the study of Perrone-Filardi et al.73 However, 56% of segments with thallium activity of 50% to 60% improved after revascularization, whereas 88% of segments with thallium activity greater than 80% showed functional improvement after revascularization (see Fig. 37-7). This important factor may explain the wide range of positive predictive values of thallium imaging reported in the individual studies summarized in Figure 37-5; there may have been considerable differences in relative thallium activity in regions considered viable in these studies. A corollary to this argument is that relative regional thallium activity provides a high degree of certainty at either end of the thallium-activity spectrum (see Fig. 37-7), but important uncertainty exists for intermediate thallium levels. Recent data suggest that late redistribution imaging (using a rest-redistribution protocol) may provide greater confidence about the potential for recovery of function in a myocardial region with intermediate thallium activity at 3 to 4 hours; 21% of such regions show increased relative thallium activity at 24 hours.74
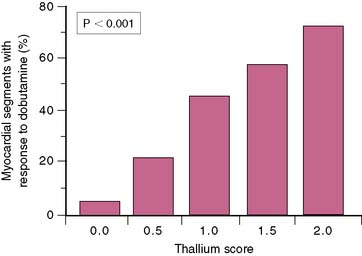
Regional thallium activity also predicts the response of dysfunctional myocardium to dobutamine stimulation. Dobutamine echocardiography to assess inotropic reserve in viable myocardium shows considerable promise in assessing myocardial viability, in keeping with the presence of residual inotropic reserve in stunned or hibernating myocardium that may be elicited through catecholamine stimulation.60,71,73,74,77–84 Data suggest that thallium imaging is more sensitive but less specific in identifying which myocardial regions and which patients will show improved function after revascularization.20 Relative regional thallium activity can be used to determine the likelihood that an asynergic myocardial segment will show contractile reserve with dobutamine (Fig. 37-9).74,85
Several studies show another advantage of quantitative analysis. The level of regional thallium activity on delayed redistribution images appears to be a more important determinant of functional recovery after revascularization and a stronger determinant of dobutamine responsiveness than is the change in regional activity between the initial resting images and the redistribution study.56,74 Thus, the severity of a thallium defect on redistribution images is more predictive of functional outcome than is reversibility of the defect.
Finally, the likelihood of a meaningful increase in LV ejection fraction after revascularization, rather than merely an improvement in regional ventricular function, is dependent on the mass of myocardium with potentially reversible asynergy. In the study of Ragosta and colleagues,56 revascularization of seven or more viable but dysfunctional myocardial segments was required for a postoperative increase in LV ejection fraction. This observation is consistent with previous data derived with preoperative PET imaging with FDG.86,87 Thus, the extent of viable myocardium in a patient with LV dysfunction can be used to predict the magnitude of recovery in global LV function after revascularization.
TECHNETIUM-99m PERFUSION IMAGING TO ASSESS VIABILITY
99mTc sestamibi, like 201Tl, requires intact sarcolemmal and mitochondrial processes for retention. This agent is an excellent marker of cellular viability (see Chapters 2 and 3).88–90 In both experimental and clinical settings in which 99mTc sestamibi delivery is adequate to dysfunctional myocardium, such as after reperfusion of previously ischemic or damaged myocardium, the uptake and retention of 99mTc sestamibi tracks with markers of myocardial viability rather than with pure markers of perfusion.89,91 However, 99mTc sestamibi does not redistribute as rapidly or as completely as 201Tl after its initial uptake, either during exercise or at rest. Thus, compared with 201Tl, 99mTc sestamibi appears to have inherent weaknesses for viability assessment when blood flow is severely impaired and tracer delivery is reduced.92 This observation was supported by initial studies comparing rest/exercise 99mTc sestamibi imaging with exercise-redistribution-reinjection 201Tl imaging that reported that Tc-99m sestamibi underestimates viable myocardium in patients with chronic CAD and LV dysfunction.81,93–95
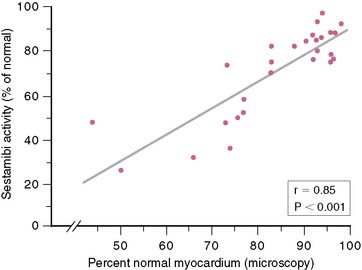
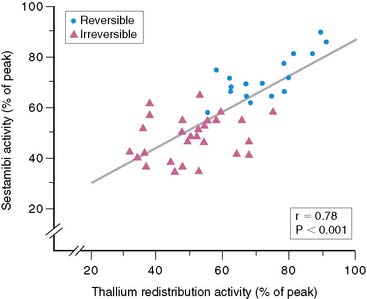
However, many subsequent studies provided consistent, convincing evidence of the potential use of 99mTc sestamibi for viability assessment in patients with LV dysfunction. First, 99mTc sestamibi is well established as an excellent perfusion tracer for detecting inducible myocardial ischemia, determining whether LV function is normal, and assessing the viability of ischemic myocardium. As with 201Tl imaging, myocardial regions with systolic dysfunction and reversible (ischemic) defects on 99mTc sestamibi imaging have a very high likelihood of recovery of function after revascularization. Moreover, many studies show that a quantitative analysis of 99mTc sestamibi activity in regions with perfusion defects at rest provides important insights into the potential for improved function with revascularization.75,96–100 Udelson et al.75 showed a high concordance (87%) between quantitative resting 201Tl and 99mTc sestamibi activity in viable segments of myocardium in patients with regional LV dysfunction. Of greater importance, however, was the finding that 99mTc sestamibi activity at 60% of peak activity had high positive and negative predictive values for the recovery of ventricular function after coronary revascularization (80% and 96%, respectively). These values were similar to those obtained in the same study with 201Tl imaging.75 As with 201Tl imaging, there is a continuous inverse relationship between regional 99mTc sestamibi activity and the extent of interstitial fibrosis measured in myocardial biopsy specimens (Fig. 37-10).98,99 This observation indicates a continuous relationship, similar to that seen with 201Tl imaging, between regional 99mTc sestamibi activity and the likelihood of improvement in regional function after revascularization (Fig. 37-11).75 Regional 99mTc sestamibi activity at rest correlates more strongly with 201Tl redistribution activity than with initial uptake of 201Tl, when resting 99mTc sestamibi imaging is compared directly with rest-redistribution 201Tl imaging in the same patients.75,101,102
Another approach with which to optimize viability assessment with 99mTc sestamibi is nitroglycerin administration (Fig. 37-12). Several studies show that nitrates improve the detection of viable myocardium by reducing the size and intensity of the perfusion defect in most viable regions.100,103–107 Sciagra and colleagues107 showed that a significant decrease in global defect score after nitroglycerin infusion was noted only in patients who had functional recovery after revascularization. There was a strong correlation between changes in ejection fraction after revascularization and change in defect size after nitroglycerin administration. The mechanism of this phenomenon is unclear, but it may be related to improvement in blood flow and contractility, reducing the partial volume effect of thin, poorly contracting myocardium.108,109
Adding ECG gating and dobutamine allows simultaneous assessment of myocardial cellular integrity with perfusion imaging and contractile reserve to potentially reduce the need for echocardiographic assessment.110,111 Leoncini et al.,112 using nitrate rest and dobutamine SPECT perfusion stress imaging, found that the sensitivity of 99mTc sestamibi for assessing viability, as measured by improved wall motion after revascularization, was 85%, with a specificity of 55%. The use of contractile reserve data alone reduced sensitivity to 64%, but specificity rose to 85%. This study showed that in the subgroup with hypokinetic segments, the sensitivity of the contractile reserve assessment alone compared with perfusion was not affected as much as in the dyskinetic segments, but specificity remained unchanged. Consequently, dobutamine-gated SPECT enhanced the reliability of nitrate-enhanced 99mTc sestamibi in hypokinetic segments, whereas perfusion quantification remained superior in the akinetic segments.
Other Technetium-99m-Based Tracers
Studies with 99mTc tetrofosmin to assess myocardial viability are somewhat limited. Both agents require active processes for mitochondrial uptake; therefore, 99mTc tetrofosmin should perform in a similar manner to 99mTc sestamibi. Despite some of the limitations of 99mTc tetrofosmin as a perfusion tracer,113 it can be used in a similar manner to 99mTc sestamibi114–117 to show viability. A study by He and coworkers118 showed that Tc-99m tetrofosmin imaging after nitroglycerin administration correlated well with the results of PET imaging with FDG. Similarly, Giorgetti et al. demonstrated that perfusion defects seen when tetrofosmin was administered during an infusion of nitroglycerin correlated well with the areas of hyperenhancement (infarction) seen on contrast-enhanced MRI.119 Apparently 99mTc tetrofosmin can be used similarly to 99mTc sestamibi for the assessment of myocardial viability.
99mTc NOET is a neutral lipophilic imaging agent that appears to undergo redistribution similar to that of 201Tl.120 In a small clinical study, 99mTc NOET showed similar sensitivity and specificity to 201Tl for the detection of CAD.121 However, animal studies show conflicting data on the potential utility of 99mTc NOET for assessing viability.122,123 Although isolated heart data suggest that 99mTc NOET retention may be affected by myocardial viability,122 a study using a canine model of reperfused myocardial infarction found that 99mTc NOET uptake reflected reperfusion myocardial blood flow rather than viability.123 Larger clinical trials are necessary before conclusions can be drawn.
DUAL-ISOTOPE IMAGING
Dual-isotope imaging with separate acquisition of 201Tl images at rest and 99mTc sestamibi or tetrofosmin for stress imaging is used in many laboratories for the routine evaluation of CAD.124,125 Viability assessments with these protocols use resting thallium images. Viability determination can be performed in two ways. The choice of protocol usually depends on the pretest likelihood of the need for such a determination. When myocardial viability is a concern before the stress test because of known LV dysfunction, the test can be performed so that thallium rest-redistribution imaging is performed before the stress test (see Fig. 37-6). However, more often, the viability of one or more myocardial regions becomes an issue after the stress test is performed and severe, fixed defects are seen. In this case, 24-hour thallium redistribution imaging can be performed after the stress test, because enough of the technetium has decayed or cleared from the myocardium so that it does not contaminate the thallium images (crosstalk). Some laboratories prefer to give an additional dose of thallium before the 24-hour redistribution images, but simply increasing the acquisition time (25 to 40 seconds per stop in our laboratory) increases the counts and improves image quality. Although comparative data126 or data on predictive value127 are limited, results should be similar to those obtained with standard thallium rest-redistribution (4-hour or 24-hour) imaging.
CLINICAL IMPLICATIONS
Viability Assessment and Patient Outcome
The recovery of LV function after revascularization in patients with evidence of myocardial viability also appears to indicate an improved prognosis. PET imaging shows that myocardial revascularization in patients with FDG–blood flow mismatch significantly improves survival, compared with patients who have medical therapy. In two separate studies128,129 involving a total of 87 patients with LV dysfunction (mean ejection fraction, 31%) the 1-year mortality rate in patients treated medically was 33% in one study and 41% in the other. In contrast, the mortality rate was reduced to 4% in the first study and 12% in the second in patients treated with coronary artery bypass surgery or percutaneous coronary intervention. However, these studies have limitations that should be addressed. They are retrospective, nonrandomized studies with relatively small numbers of patients. The factors used to select some patients for revascularization and others for medical therapy are unspecified, and it is unclear if other predictors of outcome, such as severity of angina or inducible myocardial ischemia, were used to guide the selection for revascularization. However, the overall concordance of the results supports the concept that patients with LV dysfunction and evidence of myocardial viability represent a high-risk group with a high cardiac event rate, and that this poor prognosis may be reduced considerably by revascularization of the viable but underperfused myocardium.
Conversely, thallium imaging may identify patients with LV dysfunction who should not undergo myocardial revascularization. Patients with impaired LV function who have no evidence of residual myocardial viability in dysfunctional regions, or who have only a small number of viable regions, appear to have significantly greater short-term and long-term postoperative mortality risk than patients who undergo revascularization with evidence of extensive viable myocardium in the dysfunctional segments.130,131 These data show that assessment of myocardial viability with 201Tl imaging may provide critically important data for risk stratification and revascularization decision making in patients with CAD and LV dysfunction.
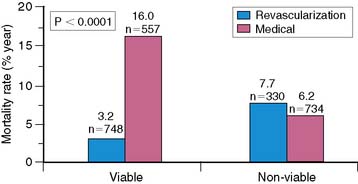
The clinical utility of viability determination was shown in a meta-analysis by Allman and colleagues.132 The authors analyzed 24 studies that used thallium imaging, PET imaging, or dobutamine echocardiography to determine myocardial viability in patients with chronic CAD and LV dysfunction. They showed a strong association between viability as determined by these tests and survival after revascularization (Fig. 37-13). In patients with viable myocardium, the annual mortality rate after revascularization was 3.2% compared with 16.0% per year for patients treated medically. However, there was no significant difference in mortality rate between patients undergoing revascularization and those treated medically who did not have viable myocardium (7.7% versus 6.2%, respectively). When the type of treatment was compared, revascularized patients had greater survival rates if they had viable myocardium (annual mortality rate, 3.2% versus 7.7%, respectively), whereas medically treated patients had worse outcomes if they had viable myocardium (16.0% versus 6.2%, respectively). Although the study has several limitations, it does indicate that using these techniques to determine myocardial viability may help to identify patients who will benefit from revascularization.
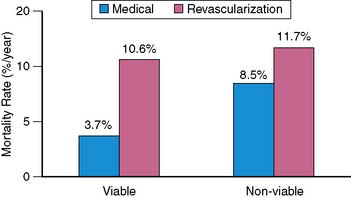
A recent review by Camici et al.133 addressed the phenomena of stunning and hibernation and the assessment of myocardial viability. By pooling data from 11 nonrandomized studies published between 1998 and 2006 that examined long-term survival of patients with LV dysfunction, the authors examined the outcome of four patient groups based on the type of therapy (medical or revascularization) and by the presence or absence of myocardial viability by various techniques (Fig. 37-14). Similar to the results from the meta-analysis of Allman et al., the analysis of Camici et al. demonstrated a survival benefit in patients with viable myocardium who underwent revascularization (weighted average annual mortality 3.71% [95% CI 2.31–5.12] in the viable group versus 10.64% [8.17–13.12] in the nonviable group). However, in those patients without evidence of viable myocardium, there was no significant difference between the treatment groups (weighted average annual mortality 11.69% [8.87–14.51] for medical therapy versus 8.45% [5.80–11.10] for revascularization). In this review of the literature, there was no significant difference in survival between patients with or without evidence of viable myocardium who were treated medically.
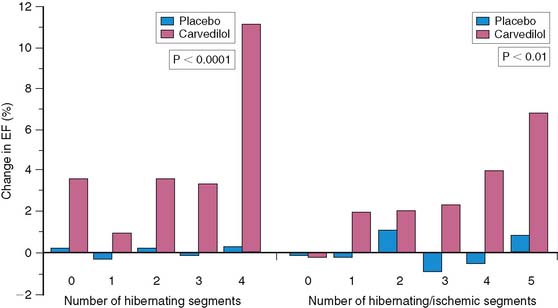
The results from the CHRISTMAS (Carvedilol Hibernating Reversible Ischemia Trial: Marker of Success) study showed the ability of SPECT myocardial perfusion imaging to predict improvement in ejection fraction in patients with ischemic LV dysfunction treated with carvedilol.134 Patients received 99mTc sestamibi after the administration of sublingual nitroglycerin spray. SPECT imaging was performed at rest. On a separate day, stress imaging was performed, and the myocardial regions were assessed for areas of ischemia and hibernating myocardium (dysfunctional but with preserved tracer uptake). The patients were randomized to receive carvedilol or placebo for 6 months. Overall, the mean improvement in ejection fraction was 2.8% in the carvedilol group (compared with −0.4% in the placebo group), and the degree of improvement in ejection fraction was related to the amount of myocardium that was hibernating, ischemic, or both (Fig. 37-15).
Whether a clinically relevant change in ventricular performance occurs after myocardial revascularization, and whether this change translates into improved lifestyle and prognosis, depends on a number of factors, many of which are poorly defined. The amount of dysfunctional but viable myocardium is certainly one such factor, but at the current time, the identification of viable myocardium is not in and of itself an indication for revascularization. Large randomized, controlled clinical trials are needed to address the role of revascularization and viability assessment in the management of patients with ischemic cardiomyopathy. The STICH trial (Surgical Treatment for Ischemic Heart Failure) is a National Heart, Lung and Blood Institute–sponsored study to examine such questions.135 Results regarding viability determination are anticipated to be available in 2010. Based on the currently available data, assessing for viability/ischemia is listed as an appropriate indication (with a score of 8.5) in the American College of Cardiology SPECT Appropriateness Criteria.136 In addition, the assessment of viability for consideration of revascularization in patients with CAD and LV systolic dysfunction who do not have angina is a class I indication for the use of radionuclide imaging with SPECT or PET.137
1. Bolli R. Myocardial “stunning” in man. Circulation. 1992;86:1671-1691.
2. Braunwald E., Kloner R.A. The stunned myocardium: Prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146-1149.
3. Sheehan F.H., Doerr R., Schmidt W.G., et al. Early recovery of left ventricular function after thrombolytic therapy for acute myocardial infarction: An important determinant of survival. J Am Coll Cardiol. 1988;12:289-300.
4. Stack R.S., Phillips H.R.3rd, Grierson D.S., et al. Functional improvement of jeopardized myocardium following intracoronary streptokinase infusion in acute myocardial infarction. J Clin Invest. 1983;72:84-95.
5. Topol E.J., Weiss J.L., Brinker J.A., et al. Regional wall motion improvement after coronary thrombolysis with recombinant tissue plasminogen activator: Importance of coronary angioplasty. J Am Coll Cardiol. 1985;6:426-433.
6. Bonow R.O. The hibernating myocardium: Implications for management of congestive heart failure. Am J Cardiol. 1995;75:17A-25A.
7. Dilsizian V., Bonow R.O., Cannon R.O.3rd, et al. The effect of coronary artery bypass grafting on left ventricular systolic function at rest: Evidence for preoperative subclinical myocardial ischemia. Am J Cardiol. 1988;61:1248-1254.
8. Elefteriades J.A., Tolis G.Jr, Levi E., et al. Coronary artery bypass grafting in severe left ventricular dysfunction: Excellent survival with improved ejection fraction and functional state. J Am Coll Cardiol. 1993;22:1411-1417.
9. Ross J.Jr. Myocardial perfusion-contraction matching: Implications for coronary artery disease and hibernation. Circulation. 1991;83:1076-1083.
10. Braunwald E., Rutherford J.D. Reversible ischemic left ventricular dysfunction: Evidence for “hibernating” myocardium. J Am Coll Cardiol. 1986;8:1467-1470.
11. Rahimtoola S.H. The hibernating myocardium. Am Heart J. 1989;117:211-213.
12. Dilsizian V., Bonow R.O. Current diagnostic techniques of assessing myocardial viability in hibernating and stunned myocardium. Circulation. 1993;87:1-20.
13. Camici P.G., Wijns W., Borgers M., et al. Pathophysiology of chronic reversible left ventricular dysfunction due to coronary artery disease (hibernating myocardium). Circulation. 1997;96:3205-3214.
14. Wijns W., Vatner S.F., Camici P.G. Mechanisms of disease: Hibernating myocardium. N Engl J Med. 1998;339:173-181.
15. Fallovollita J.A., Malm B.J., Canty J.M.Jr. Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ Res. 2003;92:48-55.
16. Thijssen V.L.J.L., Borgers M.PhD, Lenders M.H., et al. Temporal and spatial variations in structural protein expression during the progression from stunned to hibernating myocardium. Circulation. 2004;110:3313-3321.
17. Canty J.M.Jr, Suzuki G., Banas M.D., Verheyen F., Borgers M., Fallavollita J.A. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. 2004;94:1142-1149.
18. Iver V.S., Canty J.M.Jr. Regional desensitization of β-adrenergic receptor signaling in swine with chronic hibernating myocardium. Circ Res. 2005;97:789-795.
19. Beller G.A. Comparison of thallium-201 scintigraphy and low-dose dobutamine echocardiography for the noninvasive assessment of myocardial viability. Circulation. 1996;94:2681-2684.
20. Bonow R.O. Identification of viable myocardium. Circulation. 1996;94:2674-2680.
21. Muhlbaier L.H., Pryor D.B., Rankin J.S., et al. Observational comparison of event-free survival with medical and surgical therapy in patients with coronary artery disease. 20 years of follow-up. Circulation. 1992;86(Suppl 5):II-198–II-204.
22. Brundage B.H., Massie B.M., Botvinick E.H. Improved regional ventricular function after successful surgical revascularization. J Am Coll Cardiol. 1984;3:902-908.
23. Rozanski A., Berman D., Gray R., et al. Preoperative prediction of reversible myocardial asynergy by postexercise radionuclide ventriculography. N Engl J Med. 1982;307:212-213.
24. Rahimtoola S.H. A perspective on the three large multicenter randomized clinical trials of coronary bypass surgery for chronic stable angina. Circulation. 1985;72(6 Pt 2):V123-V135.
25. Buxton D.B. Dysfunction in collateral-dependent myocardium: Hibernation or repetitive stunning? Circulation. 1993;87:1756-1758.
26. Gerber B.L., Vanoverschelde J.L., Bol A., et al. Myocardial blood flow, glucose uptake, and recruitment of inotropic reserve in chronic left ventricular ischemic dysfunction: Implications for the pathophysiology of chronic myocardial hibernation. Circulation. 1996;94:651-659.
27. Hendel R.C., Chaudhry F.A., Bonow R.O. Myocardial viability. Curr Probl Cardiol. 1996;21:145-224.
28. Sawada S., Elsner G., Segar D.S., et al. Evaluation of patterns of perfusion and metabolism in dobutamine-responsive myocardium. J Am Coll Cardiol. 1997;29:55-61.
29. Vanoverschelde J.L., Wijns W., Depre C., et al. Mechanisms of chronic regional postischemic dysfunction in humans: New insights from the study of noninfarcted collateral-dependent myocardium. Circulation. 1993;87:1513-1523.
30. Kim R.J., Wu E., Rafael A., et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453.
31. Bucciarelli-Ducci C., Wu E., Lee D.C., Holly T.A., Klocke F.J., Bonow R.O. Contrast-enhanced cardiac magnetic resonance in the evaluation of myocardial infarction and myocardial viability in patients with ischemic heart disease. Curr Probl Cardiol. 2006;31:125-168.
32. Wu E., Ortiz J., Tejedor P., et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730-736.
33. Berger B.C., Watson D.D., Burwell L.R., et al. Redistribution of thallium at rest in patients with stable and unstable angina and the effect of coronary artery bypass surgery. Circulation. 1979;60:1114-1125.
34. Bonow R.O., Dilsizian V. Thallium-201 for assessing myocardial viability. Semin Nucl Med. 1991;21:230-241.
35. Hendel R.C. Single-photon perfusion imaging for the assessment of myocardial viability. J Nucl Med. 1994;35(Suppl):23S-31S.
36. Iskandrian A.S., Hakki A.H., Kane S.A., et al. Rest and redistribution thallium-201 myocardial scintigraphy to predict improvement in left ventricular function after coronary artery bypass grafting. Am J Cardiol. 1983;51:1312-1316.
37. Rozanski A., Berman D.S., Gray R., et al. Use of thallium-201 redistribution scintigraphy in the preoperative differentiation of reversible and nonreversible myocardial asynergy. Circulation. 1981;64:936-944.
38. Gibson R.S., Watson D.D., Taylor G.J., et al. Prospective assessment of regional myocardial perfusion before and after coronary revascularization surgery by quantitative thallium-201 scintigraphy. J Am Coll Cardiol. 1983;1:804-815.
39. Gutman J., Berman D.S., Freeman M., et al. Time to completed redistribution of thallium-201 in exercise myocardial scintigraphy: Relationship to the degree of coronary artery stenosis. Am Heart J. 1983;106:989-995.
40. Kiat H., Berman D.S., Maddahi J., et al. Late reversibility of tomographic myocardial thallium-201 defects: An accurate marker of myocardial viability. J Am Coll Cardiol. 1988;12:1456-1463.
41. Liu P., Kiess M.C., Okada R.D., et al. The persistent defect on exercise thallium imaging and its fate after myocardial revascularization: Does it represent scar or ischemia? Am Heart J. 1985;110:996-1001.
42. Manyari D.E., Knudtson M., Kloiber R., et al. Sequential thallium-201 myocardial perfusion studies after successful percutaneous transluminal coronary artery angioplasty: Delayed resolution of exercise induced scintigraphic abnormalities. Circulation. 1988;77:86-95.
43. Ohtani H., Tamaki N., Yonekura Y., et al. Value of thallium-201 reinjection after delayed SPECT imaging for predicting reversible ischemia after coronary artery bypass grafting. Am J Cardiol. 1990;66:394-399.
44. Brunken R., Schwaiger M., Grover-McKay M., et al. Positron emission tomography detects tissue metabolic activity in myocardial segments with persistent thallium perfusion defects. J Am Coll Cardiol. 1987;10:557-567.
45. Brunken R.C., Kottou S., Nienaber C.A., et al. PET detection of viable tissue in myocardial segments with persistent defects at Tl-201 SPECT. Radiology. 1989;65:65-73.
46. Tamaki N., Yonekura Y., Yamashita K., et al. Relation of left ventricular perfusion and wall motion with metabolic activity in persistent defects on thallium-201 tomography in healed myocardial infarction. Am J Cardiol. 1988;62:202-208.
47. Tamaki N., Yonekura Y., Yamashita K., et al. SPECT thallium-201 tomography and positron tomography using N-13 ammonia and F-18 fluorodeoxyglucose in coronary artery disease. Am J Cardiac Imaging. 1989;3:3-9.
48. Cloninger K.G., DePuey E.G., Garcia E.V., et al. Incomplete redistribution in delayed thallium-201 single photon emission computed tomographic (SPECT) images: An overestimation of myocardial scarring. J Am Coll Cardiol. 1988;12:955-963.
49. Yang L.D., Berman D.S., Kiat H., et al. The frequency of late reversibility in SPECT thallium-201 stress-redistribution studies. J Am Coll Cardiol. 1990;15:334-340.
50. Watson D.D. Methods for detection of myocardial viability and ischemia. In: Zaret B.L., Beller G.A., editors. Nuclear cardiology. St. Louis: CV Mosby; 1992:65-76.
51. Kayden D.S., Sigal S., Soufer R., et al. Thallium-201 for assessment of myocardial viability: Quantitative comparison of 24-hour redistribution imaging with imaging after reinjection at rest. J Am Coll Cardiol. 1991;18:1480-1486.
52. Brunken R.C., Mody F.V., Hawkins R.A., et al. Positron emission tomography detects metabolic activity in myocardium with persistent 24-hour single photon emission computed tomography 201Tl defects. Circulation. 1992;86:1357-1369.
53. Dilsizian V., Rocco T.P., Freedman N.M., et al. Enhanced detection of ischemic but viable myocardium by the reinjection of thallium after stress-redistribution imaging. N Engl J Med. 1990;323:141-146.
54. Rocco T.P., Dilsizian V., McKusick K.A., et al. Comparison of thallium redistribution with rest “reinjection” imaging for detection of viable myocardium. Am J Cardiol. 1990;66:158-163.
55. Tamaki N., Ohtani H., Yonekura Y., et al. Significance of fill-in after thallium-201 reinjection following delayed imaging: Comparison with regional wall motion and angiographic findings. J Nucl Med. 1990;31:1617-1623.
56. Ragosta M., Beller G.A., Watson D.D., et al. Quantitative planar rest-redistribution 201Tl imaging in detection of myocardial viability and prediction of improvement in left ventricular function after coronary bypass surgery in patients with severely depressed left ventricular function. Circulation. 1993;87:1630-1641.
57. Mori T., Minamiji K., Kurogane H., et al. Rest-injected thallium-201 imaging for assessing viability of severe asynergic regions. J Nucl Med. 1991;32:1718-1724.
58. Dilsizian V., Smeltzer W.R., Freedman N.M., et al. Thallium reinjection after stress-redistribution imaging: Does 24-hour delayed imaging following reinjection enhance detection of viable myocardium? Circulation. 1991;83:1247-1255.
59. Dilsizian V., Bonow R.O. Differential uptake and apparent thallium-201 “washout” after thallium reinjection: Options regarding early redistribution imaging before reinjection or late redistribution imaging after reinjection. Circulation. 1992;85:1032-1038.
60. Arnese M., Cornel J.H., Salustri A., et al. Prediction of improvement of regional left ventricular function after surgical revascularization: A comparison of low-dose dobutamine echocardiography with 201Tl single-photon emission computed tomography. Circulation. 1995;91:2748-2752.
61. Bartenstein P. Tl-201 reinjection and improvement of left ventricular function following revascularization (abstract). J Nucl Med. 1993;34:45P.
62. Bax J.J., Cornel J.H., Visser F.C., et al. Prediction of recovery of regional ventricular dysfunction following revascularization: Comparison of fluorine-18 fluorodeoxyglucose/thallium-201 SPECT, thallium-201 stress-reinjection SPECT and dobutamine echocardiography. J Am Coll Cardiol. 1996;28:558-564.
63. Haque T., Furukawa T., Takahashi M., et al. Identification of hibernating myocardium by dobutamine stress echocardiography: Comparison with thallium-201 reinjection imaging. Am Heart J. 1995;130(3 Pt 1):553-563.
64. Nienaber C.A., de la Roche J., Camarius H., et al. Impact of 201thallium reinjection imaging to identify myocardial viability after vasodilation-redistribution SPECT (abstract). J Am Coll Cardiol. 1993;21:283A.
65. Skopicki H.A., Weissman N.J., Rose G.A., et al. Thallium imaging, dobutamine echocardiography, and positron emission tomography for the assessment of myocardial viability (abstract). J Am Coll Cardiol. 1996;27:162A.
66. Vanoverschelde J.L.J., D’Hondt A.M., Marwick T., et al. Head-to- head comparison of exercise-redistribution-reinjection thallium single-photon emission computed tomography and low dose dobutamine echocardiography for prediction of reversibility of chronic left ventricular ischemic dysfunction. J Am Coll Cardiol. 1996;28:432-442.
67. Bonow R.O., Dilsizian V., Cuocolo A., et al. Identification of viable myocardium in patients with chronic coronary artery disease and left ventricular dysfunction: Comparison of thallium scintigraphy with reinjection and PET imaging with 18F-fluorodeoxyglucose. Circulation. 1991;83:26-37.
68. Perrone-Filardi P., Bacharach S.L., Dilsizian V., et al. Regional left ventricular wall thickening: Relation to regional uptake of 18fluorodeoxyglucose and 201Tl in patients with chronic coronary artery disease and left ventricular dysfunction. Circulation. 1992;86:1125-1137.
69. Tamaki N., Ohtani H., Yamashita K., et al. Metabolic activity in the areas of new fill-in after thallium-201 reinjection: Comparison with positron emission tomography using fluorine-18-deoxyglucose. J Nucl Med. 1991;32:673-678.
70. Bax J.J., Cornel J.H., Visser F.C., et al. Comparison of fluorine-18-FDG with rest-redistribution thallium-201 SPECT to delineate viable myocardium and predict functional recovery after revascularization. J Nucl Med. 1998;39:1481-1486.
71. Charney R., Schwinger M.E., Chun J., et al. Dobutamine echocardiography and resting-redistribution thallium-201 scintigraphy predicts recovery of hibernating myocardium after coronary revascularization. Am Heart J. 1994;128:864-869.
72. Dilsizian V., Perrone-Filardi P., Arrighi J.A., et al. Concordance and discordance between stress-redistribution-reinjection and rest-redistribution thallium imaging for assessing viable myocardium: Comparison with metabolic activity by positron emission tomography. Circulation. 1993;88:941-952.
73. Perrone-Filardi P., Pace L., Prastaro M., et al. Dobutamine echocardiography predicts improvement of hypoperfused dysfunctional myocardium after revascularization in patients with coronary artery disease. Circulation. 1995;91:2556-2565.
74. Perrone-Filardi P., Pace L., Prastaro M., et al. Assessment of myocardial viability in patients with chronic coronary artery disease: Rest-4 hour-24-hour 201thallium tomography versus dobutamine echocardiography. Circulation. 1996;94:2712-2719.
75. Udelson J.E., Coleman P.S., Metherall J., et al. Predicting recovery of severe regional ventricular dysfunction: Comparison of resting scintigraphy with 201Tl and 99Tc-sestamibi. Circulation. 1994;89:2552-2561.
76. Zimmermann R., Mall G., Rauch B., et al. Residual 201Tl activity in irreversible defects as a marker of myocardial viability: Clinicopathological study. Circulation. 1995;91:1016-1021.
77. Afridi I., Kleiman N.S., Raizner A.E., et al. Dobutamine echocardiography in myocardial hibernation: Optimal dose and accuracy in predicting recovery of ventricular function after coronary revascularization. Circulation. 1995;91:663-670.
78. Barilla F., Gheorghiade M., Alam M., et al. Low-dose dobutamine in patients with acute myocardial infarction identifies viable but not contractile myocardium and predicts the magnitude of improvement in wall motion abnormalities in response to coronary revascularization. Am Heart J. 1991;122:1522-1531.
79. Cigarroa C.G., deFilippi C.R., Brickner M.E., et al. Dobutamine stress echocardiography identifies hibernating myocardium and predicts recovery of left ventricular function after coronary revascularization. Circulation. 1993;88:430-436.
80. La Canna G., Alfieri O., Giubbini R., et al. Echocardiography during infusion of dobutamine for identification of reversible dysfunction in patients with chronic coronary artery disease. J Am Coll Cardiol. 1994;23:617-626.
81. Marzullo P., Parodi O., Reisenhofer B., et al. Value of rest thallium-201/technetium-99m sestamibi scans and dobutamine echocardiography for detecting myocardial viability. Am J Cardiol. 1993;71:166-172.
82. Pierard L.A., De Landsheere C.M., Berthe C., et al. Identification of viable myocardium by echocardiography during dobutamine infusion in patients with myocardial infarction after thrombolytic therapy: Comparison with positron emission tomography. J Am Coll Cardiol. 1990;15:1021-1031.
83. Smart S.C., Sawada S., Ryan T., et al. Low-dose dobutamine echocardiography detects reversible dysfunction after thrombolytic therapy of acute myocardial infarction. Circulation. 1993;88:405-415.
84. Smart S.C. The clinical utility of echocardiography in the assessment of myocardial viability. J Nucl Med. 1994;35(Suppl 4):49S-58S.
85. Panza J.A., Dilsizian V., Laurienzo J.M., et al. Relation between thallium uptake and contractile response to dobutamine: Implications regarding myocardial viability in patients with chronic coronary artery disease and left ventricular dysfunction. Circulation. 1995;91:990-998.
86. Nienaber C.A., Brunken R.C., Sherman C.T., et al. Metabolic and functional recovery of ischemic human myocardium after coronary angioplasty. J Am Coll Cardiol. 1991;18:966-978.
87. Tillisch J., Brunken R., Marshall R., et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884-888.
88. Freeman I., Grunwald A.M., Hoory S., et al. Effect of coronary occlusion and myocardial viability on myocardial activity of technetium-99m-sestamibi. J Nucl Med. 1991;32:292-298.
89. Sinusas A.J., Watson D.D., Cannon J.M., et al. Effect of ischemia and postischemic dysfunction on myocardial uptake of technetium-99m-labeled methoxyisobutyl isonitrile and thallium-201. J Am Coll Cardiol. 1989;14:1785-1793.
90. Beanlands R.S.B., Dawood F., Wen W.H., et al. Are the kinetics of technetium-99m methoxyisobutyl isonitrile affected by cell metabolism and viability? Circulation. 1990;82:1802-1814.
91. Christian T.F., Behrenbeck T., Pellikka P.A., et al. Mismatch of left ventricular function and infarct size demonstrated by Tc-99m isonitrile imaging after reperfusion therapy for acute myocardial infarction: Identification of myocardial stunning and hyperkinesia. J Am Coll Cardiol. 1990;16:1632-1638.
92. Bonow R.O., Dilsizian V. Thallium-201 and technetium-99m-sestamibi for assessing viable myocardium. J Nucl Med. 1992;33:815-818.
93. Cuocolo A., Pace L., Ricciardelli B., et al. Identification of viable myocardium in patients with chronic coronary artery disease: Comparison of thallium-201 scintigraphy with reinjection and technetium-99m-methoxyisobutyl isonitrile. J Nucl Med. 1992;33:505-511.
94. Marzullo P., Sambuceti G., Parodi O. The role of sestamibi scintigraphy in the radioisotopic assessment of myocardial viability. J Nucl Med. 1992;33:1925-1930.
95. Sawada S.G., Allman K.C., Muzik O., et al. Positron emission tomography detects evidence of viability in rest technetium-99m sestamibi defects. J Am Coll Cardiol. 1994;23:92-98.
96. Maes A.F., Borgers M., Flameng W., et al. Assessment of myocardial viability in chronic coronary artery disease using technetium-99m sestamibi SPECT: Correlation with histologic and positron emission tomographic studies and functional follow-up. J Am Coll Cardiol. 1997;29:62-68.
97. Dilsizian V., Arrighi J.A., Diodati J.G., et al. Myocardial viability in patients with chronic ischemic left ventricular dysfunction: Comparison of 99mTc-sestamibi, thallium reinjection, and [18F]fluorodeoxyglucose. Circulation. 1994;89:578-587.
98. Medrano R., Lowry R.W., Young J.B., et al. Assessment of myocardial viability with 99mTc sestamibi in patients undergoing cardiac transplantation: A scintigraphic/pathological study. Circulation. 1996;94:1010-1017.
99. Dakik H.A., Howell J.F., Lawria G.M., et al. Assessment of myocardial viability with 99mTc-sestamibi tomography before coronary bypass graft surgery: Correlation with histopathology and postoperative improvement in cardiac function. Circulation. 1997;96:2892-2898.
100. Schneider C.A., Voth E., Gawlich S., et al. Significance of rest technetium-99m sestamibi imaging for the prediction of improvement of left ventricular dysfunction after Q wave myocardial infarction: Importance of infarct location adjusted thresholds. J Am Coll Cardiol. 1998;32:648-654.
101. Kauffman G.J., Boyne T.S., Watson D.D., et al. Comparison of rest thallium-201 imaging and rest technetium-99m sestamibi for assessment of myocardial viability in patients with coronary artery disease and severe left ventricular dysfunction. J Am Coll Cardiol. 1996;27:1592-1597.
102. Sansoy V., Glover D.K., Watson D.D., et al. Comparison of thallium-201 resting redistribution with technetium-99m-sestamibi uptake and functional response to dobutamine for assessment of myocardial viability. Circulation. 1995;92:994-1004.
103. Bisi G., Sciagra R., Santoro G.M., et al. Rest technetium-99m sestamibi in combination with short-term administration of nitrates: Feasibility and reliability for prediction of postrevascularization outcome of asynergic territories. J Am Coll Cardiol. 1994;24:1282-1289.
104. Galli M., Marcassa C., Imperato A., et al. Effects of nitroglycerin by technetium-99m sestamibi tomoscintigraphy on resting global myocardial hypoperfusion in stable patients with healed myocardial infarction. Am J Cardiol. 1994;74:843-848.
105. Maurea S., Cuocolo A., Soricelli A., et al. Enhanced detection of viable myocardium by technetium-99m-MIBI imaging after nitrate administration in chronic coronary artery disease. J Nucl Med. 1995;36:1945-1952.
106. Bisi G., Sciagra R., Santoro G.M., et al. Technetium-99m-sestamibi imaging with nitrate infusion to detect hibernating myocardium and predict postrevascularization recovery. J Nucl Med. 1995;36:1994-2000.
107. Sciagra R., Bisi G., Santoro G.M., et al. Comparison of baseline-nitrate technetium-99m sestamibi with rest-redistribution thallium-201 tomography in detecting viable hibernating myocardium and predicting post-revascularization recovery. J Am Coll Cardiol. 1997;30:384-391.
108. Eisner R.L., Schmarkey L.S., Martin S.E., et al. Defects on SPECT “perfusion” images can occur due to abnormal segmental contraction. J Nucl Med. 1994;35:638-643.
109. Sinusas A.J., Shi Q., Vitols P.J., et al. Impact of regional ventricular function, geometry, and dobutamine stress on quantitative 99mTc-sestamibi defect size. Circulation. 1993;88:2224-2234.
110. Amanullah A.M., Chaudhry F.A., Heo J., et al. Comparison of dobutamine echocardiography, dobutamine sestamibi, and rest-redistribution thallium-201 single-photon emission computed tomography for determining contractile reserve and myocardial ischemia in ischemic cardiomyopathy. Am J Cardiol. 1999;84:626-631.
111. Leoncini M., Sciagra R., Bellandi F., et al. Low-dose dobutamine nitrate-enhanced technetium 99m sestamibi gated SPECT versus low-dose dobutamine echocardiography for detecting reversible dysfunction in ischemic cardiomyopathy. J Nucl Cardiol. 2002;9:402-406.
112. Leoncini M., Marcucci G., Sciagra R., et al. Prediction of functional recovery in patients with chronic coronary artery disease and left ventricular dysfunction combining the evaluation of myocardial perfusion and of contractile reserve using nitrate-enhanced technetium-99m sestamibi gated single-photon emission computed tomography and dobutamine stress. Am J Cardiol. 2001;87:1346-1350.
113. Soman P., Taillefer R., DePuey E.G., et al. Enhanced detection of reversible perfusion defects by Tc-99m sestamibi compared to Tc-99m tetrofosmin during vasodilator stress SPECT imaging in mild-to-moderate coronary artery disease. J Am Coll Cardiol. 2001;37:458-462.
114. Gunning M.G., Kaprielian R.R., Pepper J., et al. The histology of viable and hibernating myocardium in relation to imaging characteristics. J Am Coll Cardiol. 2002;39:428-435.
115. Acampa W., Cuocolo A., Petretta M., et al. Tetrofosmin imaging in the detection of myocardial viability in patients with previous myocardial infarction: Comparison with sestamibi and Tl-201 scintigraphy. J Nucl Cardiol. 2002;9:33-40.
116. Gunning M.G., Anagnostopoulos C., Knight C.J., et al. Comparison of 201Tl, 99mTc-tetrofosmin, and dobutamine magnetic resonance imaging for identifying hibernating myocardium. Circulation. 1998;98:1869-1874.
117. Yoshinaga K., Morita K., Yamada S., et al. Low-dose dobutamine electrocardiograph-gated myocardial SPECT for identifying viable myocardium: Comparison with dobutamine stress echocardiography and PET. J Nucl Med. 2001;42:838-844.
118. He W., Acampa W., Mainolfi C., et al. Tc-99m tetrofosmin tomography after nitrate administration in patients with ischemic left ventricular dysfunction: Relation to metabolic imaging by PET. J Nucl Cardiol. 2003;10:599-606.
119. Giorgetti A., Pingitore A., Favilli B., et al. Baseline/postnitrate tetrofosmin SPECT for myocardial viability assessment in patients with postischemic severe left ventricular dysfunction: New evidence from MRI. J Nucl Med. 2005;46:1285-1293.
120. Ghezzi C., Fagret D., Arvieux C.C., et al. Myocardial kinetics of TcN-NOET: A neutral lipophilic complex tracer of regional myocardial blood flow. J Nucl Med. 1995;36:1069-1077.
121. Fagret D., Marie P.Y., Brunotte F., et al. Myocardial perfusion imaging with technetium-99m-Tc NOET: Comparison with thallium-201 and coronary angiography. J Nucl Med. 1995;36:936-943.
122. Holly T.A., Leppo J.A., Gilmore M.P., et al. The effect of ischemic injury on the cardiac transport of Tc-99m N-NOET in the isolated rabbit heart. J Nucl Cardiol. 1999;6:633-640.
123. Vanzetto G., Glover D.K., Ruiz M., et al. 99mTc-N-NOET myocardial uptake reflects myocardial blood flow and not viability in dogs with reperfused acute myocardial infarction. Circulation. 2000;101:2424-2430.
124. Berman D.S., Kiat H., Friedman J.D., et al. Separate acquisition rest thallium-201/stress technetium-99m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography: A clinical validation study. J Am Coll Cardiol. 1993;22:1455-1464.
125. Heo J., Wolmer I., Kegel J., et al. Sequential dual-isotope SPECT imaging with thallium-201 and technetium-99m-sestamibi. J Nucl Med. 1994;35:549-553.
126. Matsuno K., Kuwabara Y., Watanabe S., et al. Detection of myocardial viability using rest-redistribution thallium-201 imaging in a stress 99Tcm-tetrofosmin/rest thallium-201 dual-isotope protocol. Nucl Med Commun. 2001;22:165-173.
127. Fukuzawa S., Inagaki M., Morooka S., et al. Evaluation of myocardial viability using sequential dual-isotope single photon emission tomography imaging with rest TI-201/stress Tc-99m tetrofosmin in the prediction of wall motion recovery after revascularization. Jpn Circ J. 1997;61:481-487.
128. Di Carli M.F., Davidson M., Little R., et al. Value of metabolic imaging with positron emission tomography for evaluating prognosis in patients with coronary artery disease and left ventricular dysfunction. Am J Cardiol. 1994;73:527-533.
129. Eitzman D., al-Aouar Z., Kanter H.L., et al. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. J Am Coll Cardiol. 1992;20:559-565.
130. Pagley P.R., Beller G.A., Watson D.D., et al. Improved outcome after coronary artery bypass surgery in patients with ischemic cardiomyopathy and residual myocardial viability. Circulation. 1997;96:793-800.
131. Gimelli A., L’Abbate A., Glauber M., Ripoli A., Giorgetti A., Marzullo P. Cardiac imaging improves risk stratification in high-risk patients undergoing surgical revascularization. J Cardiovasc Med. 2006;7:51-56.
132. Allman K.C., Shaw L.J., Hachamovitch R., et al. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: A meta-analysis. J Am Coll Cardiol. 2002;39:1151-1158.
133. Camici, Prasad S.J., Rimoldi O.E. Stunning, hibernation, and the assessment of myocardial viability. Circulation. 2008;117:103-114.
134. Cleland J.G., Pennell D.J., Ray S.G., et al. Carvedilol hibernating reversible ischaemia trial: Marker of success investigators. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): Randomised controlled trial. Lancet. 2003;362:14-21.
135. Velazquez E.J., Lee K.L., O’Connor C.M., et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. J Thorac Cardiovasc Surg. 2007;134:1540-1547.
136. Brindis R.G., Douglas P.S., Hendel R.C., et al. ACCF/ASNC Appropriateness criteria for single-photon computed emission tomography myocardial perfusion imaging (SPECT MPI). J Am Coll Cardiol. 2005;46:1587-1605.
137. Klocke F.J., Baird M.G., Lorell B.H., et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol. 2003;42:1318-1333.