Chapter 38 Assessment of Myocardial Viability with Positron Emission Tomography
INTRODUCTION
Positron emission tomography (PET) enables quantification of myocardial perfusion, glucose utilization, fatty acid uptake and oxidation, oxygen consumption, contractile function, and presynaptic and postsynaptic neuronal activity. One of the first clinical applications of PET was the assessment of myocardial viability, and it has been generally regarded as the gold standard technique for this indication. The most common approach with PET is comparing myocardial metabolism and perfusion using FDG and ammonia or other perfusion tracers.1 It has been shown that PET can predict regional and global recovery of wall motion, symptoms, functional outcome, and prognosis after revascularization. Also, other tracers and newer techniques are available, such as gating and hybrid imaging using PET/computed tomography (CT) scanners. However, there are less data about their clinical value in the assessment of viability.
METHODS FOR ASSESSING MYOCARDIAL VIABILITY WITH PET
Myocardial Perfusion Tracers
Estimates of regional perfusion themselves have also been used for predicting functional recovery of dysfunctional myocardium. Several studies have investigated the use of nitrogen-13 (13N)-labeled ammonia2 and rubidium-82 (82Rb) in the assessment of viability.3,4 82Rb is a potassium analog that is similar to thallium-201 (201Tl), and its uptake has been shown to be a function of both blood flow and myocardial cell integrity. Oxygen-15 (15O)-labeled water offers a unique feature, since the fraction of tissue that can exchange water can be measured, and this has been used as a marker of myocardial viability.5,6 These techniques, however, are not widely used clinically.
Fluorine-18-Deoxyglucose (See Chapter 40)
Physiologic Basis
Normal myocardium uses a variety of energy-producing substrates to fulfill its energy requirements.7 In the fasting state, free fatty acids (FFA) are mobilized in relatively large quantities from triglycerides stored in adipose tissue. Thus, the increased availability of FFA in plasma makes them the preferred energy-producing fuel in the myocardium.7 In the fed state, however, the increase in plasma glucose and the subsequent rise in insulin levels significantly reduce FFA release from adipose tissue and, consequently, its availability in plasma. This leads to an increased utilization of exogenous glucose by the myocardium.8
FFA metabolism via β-oxidation in the mitochondria is highly dependent on oxygen availability, and thus it declines sharply during myocardial ischemia.9,10 Under this condition, studies in animal experiments9 and in humans11 have shown that the uptake and subsequent metabolism of glucose by the ischemic myocardium is markedly increased. This shift to preferential glucose uptake may play a critical role in the survival of functionally compromised myocytes (i.e., stunned and hibernating), inasmuch as glycolytically derived high-energy phosphates are thought to be critical for maintaining basic cellular functions. Consequently, noninvasive approaches that can assess the magnitude of exogenous glucose utilization play an important role in the evaluation of tissue viability in patients with myocardial dysfunction due to coronary artery disease (CAD). For these metabolic adaptations to occur, sufficient nutrient perfusion is required to supply energy-rich substrates (e.g., glucose) and oxygen, and for removal of the byproducts of glycolysis (e.g., lactate and hydrogen ion). A prolonged and severe reduction of myocardial blood flow rapidly precipitates depletion of high-energy phosphate, cell membrane disruption, and cellular death. Therefore, assessment of regional blood flow also provides important information regarding the presence of tissue viability within dysfunctional myocardial regions.
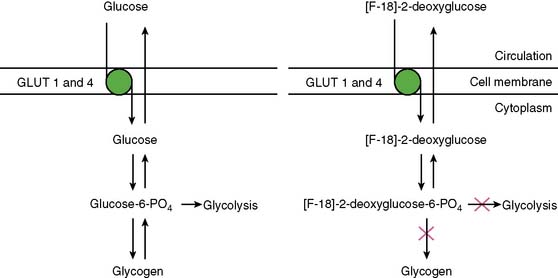
With PET, regional glucose uptake is assessed with fluorine-18 deoxyglucose (FDG), a marker of exogenous glucose uptake that provides an index of myocardial metabolism and thus cell viability. The technique was first described by Tillisch et al.1 After intravenous administration, FDG traces the initial transport of glucose across the myocyte membrane and its subsequent hexokinase-mediated phosphorylation to FDG-6-phosphate (Fig. 38-1).12 Since the latter is a poor substrate for further metabolism and is rather impermeable to the cell membrane, it becomes virtually trapped in the myocardium.
Protocols
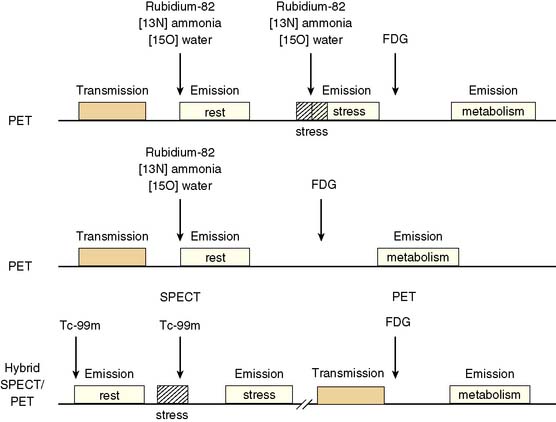
Because dysfunctional myocardium that improves functionally after revascularization must retain sufficient blood flow and metabolic activity to sustain myocyte viability, the combined assessment of regional blood flow and glucose metabolism appears most attractive for delineating myocardial viability. With this approach, regional myocardial perfusion is first evaluated following the administration of 13N-ammonia,82Rb, or 15O-water. Since information regarding the magnitude of both stress-induced ischemia and resting viability is important for management decisions, the ideal approach should include both rest and stress perfusion imaging. However, the selection of the approach (i.e., rest versus stress/rest) should be tailored to the clinical question being addressed in an individual patient (Fig. 38-2). Regional glucose uptake is then assessed with FDG.
Some investigators have also used absolute quantification of glucose uptake.13,14 These studies have documented reasonable results, but absolute quantification of glucose uptake using dynamic imaging and kinetic modeling does not appear to improve diagnostic accuracy, likely because of high variability in glucose utilization rates between individual patients.
Patient Preparation for FDG Imaging
As mentioned, utilization of energy-producing substrates by the heart muscle is largely a function of their concentration in plasma and hormone levels (especially plasma insulin, insulin/glucagon ratio, growth hormone, and catecholamines) and oxygen availability for oxidative metabolism. For a detailed step-by-step description of the available methods for FDG imaging, the reader should review the Guidelines for PET Imaging published by the American Society of Nuclear Cardiology and the Society of Nuclear Medicine15 or European Council of Nuclear Cardiology.16 These approaches for FDG imaging include:
Myocardial Perfusion and Glucose-Loaded FDG Patterns
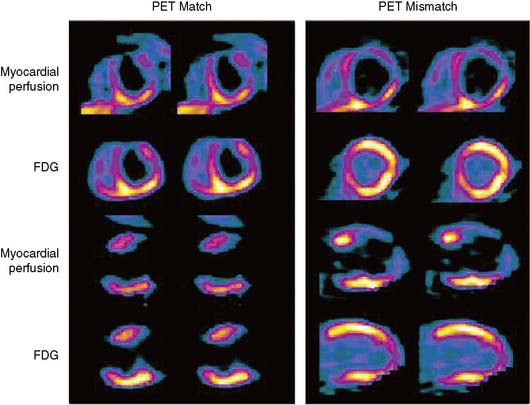
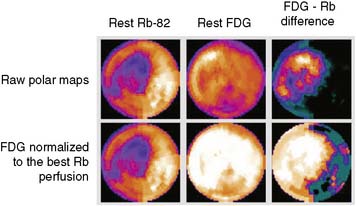
The patterns of normal perfusion and metabolism or of a PET mismatch identify potentially reversible myocardial dysfunction, whereas the PET match pattern identifies irreversible myocardial dysfunction. The reversed perfusion-FDG mismatch has been described in the context of repetitive myocardial stunning22 and in patients with left bundle branch block.23 Images are usually interpreted semiquantitatively, but relative quantitation of regional myocardial perfusion and FDG tracer uptake and their difference can be helpful to objectively assess the magnitude of viability (Fig. 38-6).
Special Considerations for the Hybrid Myocardial Perfusion SPECT and FDG PET Approach
In current clinical practice, FDG PET images are often performed and interpreted in combination with SPECT myocardial perfusion images (see Fig. 38-2). The interpretation of the specific viability patterns shown in Figures 38-3 and 38-4 should be performed carefully, especially when comparing non–attenuation-corrected SPECT myocardial perfusion images with attenuation-corrected FDG PET images. Myocardial regions showing an excessive reduction in tracer concentration due to attenuation artifacts on the perfusion images, such as the inferior wall or the anterior wall in females, may result in falsely positive perfusion-FDG mismatches. Two approaches have proved useful for overcoming this limitation. First, because assessment of viability is relevant only in myocardium with regional contractile dysfunction, gated SPECT or gated PET images offer means for determining whether apparent perfusion defects are associated with abnormal regional wall motion. Second, quantitative analysis of regional myocardial perfusion using polar map displays that are compared to tracer-specific and gender-specific (for SPECT images) databases may be a useful aid to the visual interpretation.
Gated FDG Study
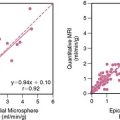
Parameters of global and regional left ventricular (LV) function derived from gated FDG PET images correlate closely with those obtained by MRI.24 As mentioned earlier, gated images are particularly useful when FDG PET patterns are interpreted in relation to non-attenuation-corrected SPECT perfusion images.25 In addition, measures of global LV function and remodeling (i.e., LVEF and volumes) are also useful for predicting improvement in LV function after revascularization.26 Myocardial infarction (MI), especially one that is large and transmural, can produce alterations in both the infarcted and noninfarcted regions that result in changes in LV architecture known as remodeling.27 Increased LV volumes and cavity size are important predictors of poor outcome in patients after infarction28 and may offset the potential benefits from revascularization on ventricular function and survival even if there is evidence of viable (ischemic) myocardium.29–32
Hybrid FDG Positron Emission Tomography and Computed Tomography
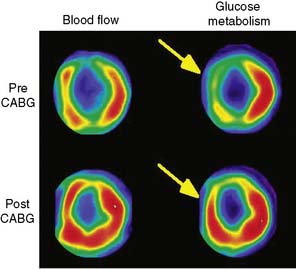
Since modern PET scanners now include a multislice CT scanner, an attractive approach could be performing FDG PET imaging with CT (Fig. 38-7). CT could be used for attenuation correction, calcium score measurement, and visualization of coronary arteries and accompanying stenoses. Recently, multislice CT has been found to have sufficient accuracy to image coronary arteries and assess the coronary stenoses.30 An example of such a viability study using FDG and PET/CT hybrid imaging is displayed in Figure 38-7. The application of contrast-enhanced CT angiography in heart failure patients who need viability investigation may be problematic, owing to advanced coronary atherosclerosis and concomitant problems such as kidney disease. The clinical value of hybrid imaging is at this time still open, and feasibility needs to be explored.
Carbon-11-Acetate
Physiologic Basis
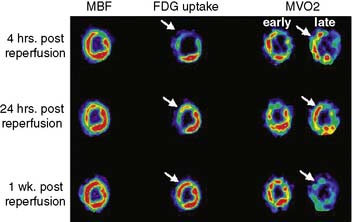
The consumption of oxygen by the myocardium is required for the continued generation of high-energy phosphates to maintain contractile function and other cellular processes. As mentioned, the heart uses a variety of substrates to support overall oxidative metabolism and thus fulfill its energy requirements.7 PET imaging with 11C-acetate offers a direct approach to evaluating myocardial oxygen consumption (MVO2) and oxidative metabolism.31,32 Indeed, the clearance of 11C-acetate from myocardium on PET imaging reflects overall regional myocardial oxidative metabolism. Acetate is a short-chained fatty acid that is readily extracted by myocardial tissue. The extracted acetate is then converted into acetyl-CoA within the mitochondria and undergoes near-complete oxidation by the tricarboxylic acid (TCA) cycle and oxidative phosphorylation.
Quantification of Myocardial Carbon-11-Acetate Kinetics (See Chapter 40)
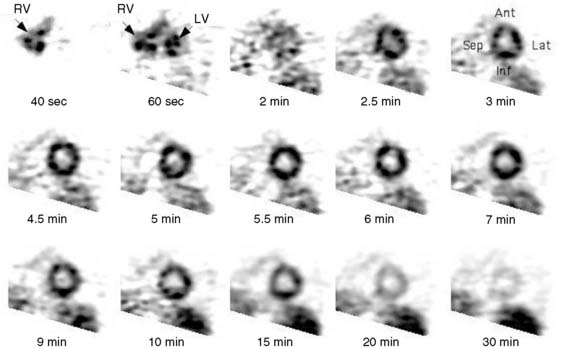
Dynamic imaging following the intravenous administration of [11C]acetate demonstrates the passage of the radioactive bolus through the cardiac chambers, followed by the extraction and accumulation of the radiotracer in the myocardium and its clearance from the blood pool, and finally the clearance of the radiotracer from the myocardial tissue, reflecting the rate of oxidative metabolism (Fig. 38-8). The rapid tissue clearance rate (Kmono) is determined by monoexponential least-squares fitting of the initial portion of the time-activity curve. Kmono values are then compared to reference normal values and used to determine the amount of residual viability within a dysfunctional myocardial segment.33
ACCURACY AND IMPACT OF VIABILITY ASSESSMENT BY PET
The term viable commonly refers to the myocardial region that has compromised function but is alive and has potential to recover function after revascularization. In patients with chronic LV dysfunction, the frequency of segmental recovery of function after revascularization, as well as the proportion of patients showing functional recovery, has been estimated to be 50% to 60%, even in patients with baseline ejection fraction below 40%.34,35 However, the true prevalence of recoverable dysfunction is likely even higher, because the revascularization is seldom complete, and it may take up to a year to recover function.36–38
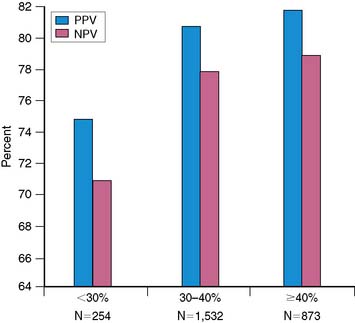
The goal of viability assessment in the setting of severe LV dysfunction after MI is to identify patients in whom revascularization can potentially improve LV function, symptoms, and survival. Then viability assessment would be of critical clinical importance in patients with the highest clinical risk after MI (i.e., LVEF < 30%), those who would derive the highest potential benefit from revascularization. Even in patients being considered for cardiac transplantation, viability assessment can alter the initial choice of treatment in majority of the patients.39 However, the vast majority of the published data documenting the accuracy of noninvasive methods for diagnosing viability and predicting functional recovery have been obtained in patients with normal, mild, or moderate LV dysfunction (i.e., LVEF > 30%).34 This is important because the accuracy of noninvasive methods for predicting functional recovery after revascularization appears to decrease significantly with worsening LV function (Fig. 38-9). Consequently, the direct extrapolation of the excellent results obtained in patients with regional or mild to moderate LV dysfunction to patients with very low EF is problematic, and it often results in suboptimal clinical results.
It is important to also realize that various imaging techniques measure different characteristics of viability, such as nuclear function, metabolic function, contractile function, membrane function, and so forth. It is also important to note that although individual myocytes may only be viable or nonviable, the macroscopic myocardium has a continuum from fully viable, through areas of partial infarction, to areas of scar with no remaining myocytes. It should also be kept in mind that although a large majority of studies have assessed the accuracy of the imaging technique against the recovery of regional function, clinically more important parameters are global LV function, exercise capacity, quality of life, and long-term survival, and these are less often assessed (Table 38-1).40
Table 38-1 End points Used in Various Studies in Assessing the Accuracy of Viability Imaging
CHF, congestive heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction.
The focus on viability information alone seems to ignore the multifactorial influences on improvement in LV function after revascularization.41 From a clinical standpoint, it is likely that relying even on the most accurate of these multiple indexes of tissue viability or its absence in isolation will lead to suboptimal prediction of outcomes.42 It is now evident that multiple other factors—including the presence and magnitude of stress-induced ischemia, the stage of cellular degeneration within viable myocytes, mitral insufficiency, the degree of LV remodeling, the timing and success of revascularization procedures, the adequacy of the target coronary vessels, and the timing of LV functional assessment after revascularization—can affect functional outcome after revascularization. Indeed, recent evidence suggests that integrating many of these factors influencing functional recovery after revascularization in multivariable models results in improved clinical predictions, as compared to approaches that are based only on a single index of tissue viability.43
PREDICTING IMPROVEMENT IN REGIONAL LEFT VENTRICULAR FUNCTION
Myocardial Perfusion
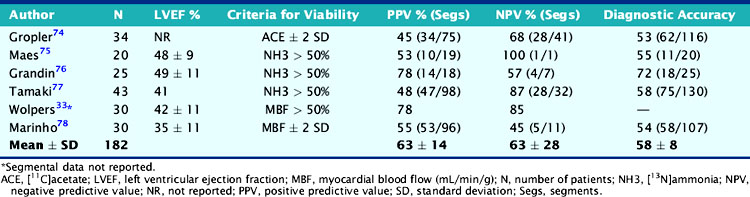
The experience using PET tracers of blood flow, including 15O-water, 11C-acetate, and 13N-ammonia, for predicting recovery of function has been documented in 6 studies including 182 patients with LV dysfunction (Table 38-2).44 Contractile dysfunction was predicted to be reversible after revascularization when regional blood flow was only mild or moderately reduced (>50% of normal) and irreversible when regional blood flow was severely reduced (<50% of normal). Using these criteria, the average positive predictive accuracy of blood flow estimates for predicting functional recovery after revascularization is 63% (range, 45% to 78%), whereas the average negative predictive accuracy is 63% (range, 45% to 100%). While normal or near-normal blood flow in a dysfunctional region served by a stenosed coronary artery indicates tissue viability and that function can be improved with revascularization, a severe blood flow deficit generally (although not always) reflects mostly nonviable myocardium that is unlikely to show improved function with revascularization. However, blood flow deficits of intermediate severity are more difficult to interpret. They may represent the coexistence of extensive subendocardial necrosis with normal myocardium, a condition unlikely to show improved function with revascularization. Alternatively, they may reflect the coexistence of extensive areas of ischemic but viable with normal and/or scar tissue, a condition likely to show improved function with revascularization. Patients with low ejection fraction and relatively normal perfusion at rest should undergo stress imaging to determine whether reversible ischemia (followed by stunning) is the likely cause of LV dysfunction.
FDG With and Without Myocardial Perfusion
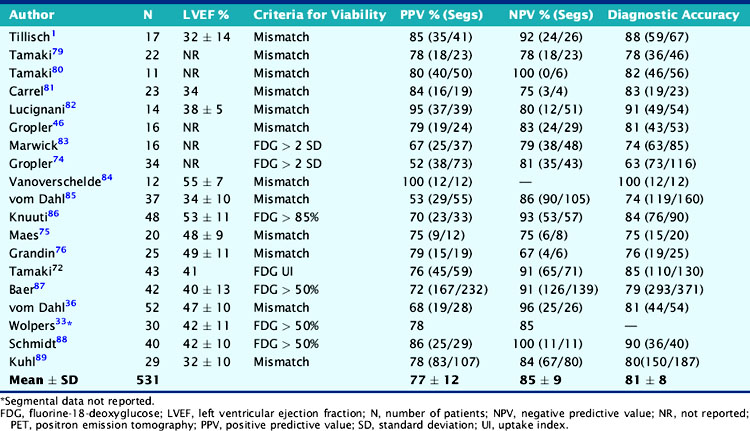
Experience with the combined blood flow–FDG approach using PET or the PET-SPECT hybrid technique (SPECT perfusion with PET FDG imaging) has been extensively documented in 19 studies including 531 patients (Table 38-3). Contractile dysfunction was predicted to be reversible after revascularization in regions with increased FDG uptake or a perfusion-metabolism mismatch, and irreversible in those with reduced FDG uptake or a perfusion-metabolism match pattern. Using these criteria, the average positive predictive accuracy for predicting improved segmental function after revascularization is 77% (range, 52% to 100%), whereas the average negative predictive accuracy is 85% (range, 67% to 100%). In a recent meta-analysis by Schinkel et al.,45 very similar values were reported (weighted positive predictive value was 74% and negative predictive value 87%).
Measures of Myocardial Oxidative Metabolism
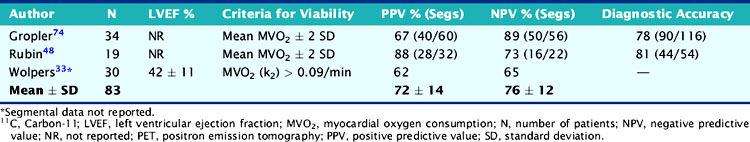
The experience with 11C-acetate and PET for identifying viability and predicting functional recovery after revascularization has been documented in 5 studies, of which only 3 studies including 83 patients provided sufficient data to estimate predictive accuracies for regional functional recovery (Table 38-4). Of note, most of these studies come from the same institution,46,47,48 indicating that assessment of myocardial viability using 11C-acetate has had less widespread validation compared to the FDG PET approach. Relatively preserved quantitative measures of oxidative metabolism in dysfunctional myocardial regions were predictive of improved function after revascularization; severely decreased oxidative metabolism was predictive of irreversible damage. Using these criteria, the average positive predictive accuracy for predicting improved segmental function after revascularization is 72% (range, 62% to 88%), whereas the average negative predictive accuracy is 76% (range, 65% to 89%) (see Table 38-4). Similar to regional perfusion measures, estimates of MVO2 (which are closely coupled with myocardial perfusion) accurately predict functional outcome when they are either normal or severely decreased. In individual patients, intermediate reductions in estimates of MVO2 are more difficult to interpret because they could represent nontransmural scar or hibernating myocardium. To overcome this limitation, it has been suggested that the magnitude of improvement (from baseline) in estimates for MVO2 during low-dose dobutamine stimulation (a measure of myocardial “oxidative reserve”) may be a more accurate predictor of functional recovery than PET measurements of MVO2 performed at rest.49 In 28 patients with chronic MI and mild to moderate LV dysfunction, Hata et al. demonstrated excellent discrimination (with virtually no overlap) between viable and nonviable myocardium using estimates of MVO2 in response to low-dose dobutamine stimulation, assessed by 11C-acetate and PET.49
PREDICTING IMPROVEMENT IN GLOBAL LEFT VENTRICULAR FUNCTION
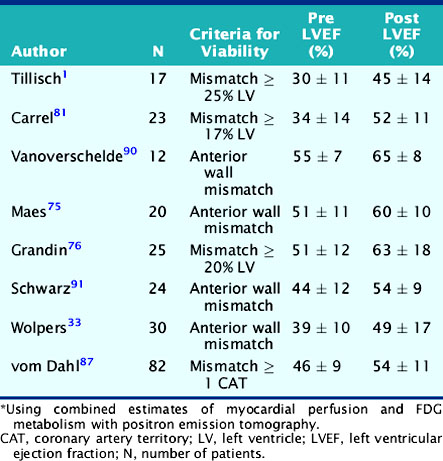
An important question in patients with severely depressed LV function is whether revascularization will provide a clinically meaningful improvement in global cardiac function that can translate into improved exercise capacity, symptoms, and survival. Several studies using different PET approaches have shown that the gain in global LV systolic function after revascularization is related to the magnitude of viable myocardium assessed preoperatively (Table 38-5).44 These data demonstrate that clinically meaningful changes in global LV function can be expected after revascularization only in patients with relatively large areas of hibernating and/or stunned myocardium (≥17% of the LV mass). Similar results have been reported using estimates of myocardial scar with PET.43 These results are in agreement with those obtained with SPECT,50 dobutamine echocardiography,51 and contrast-enhanced MRI.52 In a recent meta-analysis,45 the positive predictive value of FDG PET for improvement of global LV function was 68%, with negative predictive value of 80%.
Predicting Improvement in Symptoms and Exercise Capacity
An important challenge in the management of patients with poor cardiac function under consideration for bypass surgery is to identify those in whom revascularization can provide a significant alleviation of anginal and, especially, heart failure symptoms, which is often their primary functional limitation.53 Several studies have documented the association of viability with improvement of symptoms (NYHA class).54–56 The magnitude of post revascularization improvement in heart failure symptoms correlates with the preoperative extent of viable myocardium.57
A few studies have also documented that improvement in exercise capacity after revascularization is linked with viability in FDG PET study.57,58,59 In one study of 23 patients with LV dysfunction (LVEF: 35% ± 14%) and impaired functional capacity (70% in NYHA classes II-III), investigators have shown that the amount of viable myocardium before revascularization was predictive of a significant improvement in exercise parameters after revascularization.58 In this study, peak rate-pressure product, maximal heart rate, and exercise capacity increased significantly after revascularization only in patients with multiple viable regions on preoperative PET imaging.
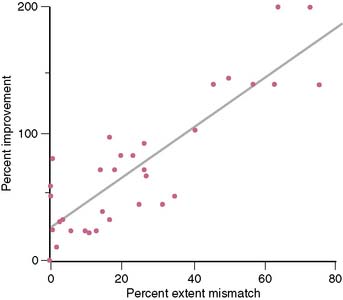
Di Carli et al. demonstrated a significant linear correlation between the global extent of a preoperative perfusion-metabolism PET mismatch and the percent improvement in functional capacity after CABG in 36 patients with ischemic cardiomyopathy (LVEF: 28% ± 6%) (Fig. 38-10).57 In this study, a perfusion-metabolic PET mismatch involving ≥ 18% of the LV on quantitative analysis was associated with a sensitivity of 76% and a specificity of 78% for predicting a significant improvement in heart failure class following bypass surgery. In these patients, exercise capacity improved by 107% after CABG, compared to only 34% improvement in patients without significant viability (<5% of the left ventricle).
Predicting a Reduction in Hospital Readmissions for Congestive Heart Failure
Frequent hospital readmissions for decompensated CHF are a major source of morbidity and increased health care costs in patients with CHF. Thus, the notion that preoperative viability imaging may be able to identify patients with a high likelihood of improvement in LV function and CHF symptoms is of great clinical importance because it would also prevent costly hospitalizations for CHF. A study by Rohatgi et al. examined the interaction between the extent of preoperative viability, the mode of treatment (i.e., medical therapy versus CABG), and the frequency of hospital readmissions during a mean follow-up of 25 months after the PET scan in 99 patients with severe LV dysfunction (LVEF: 22% ± 6%).60 They found that among patients with relatively large areas of viable myocardium preoperatively, high-risk revascularization provided a significant reduction in hospital readmissions for CHF compared to medical therapy alone (3% versus 31%, respectively). In contrast, hospital admissions for CHF were similarly high regardless of the mode of treatment in patients without significant amounts of viability by PET (50% versus 48% for revascularization and medical therapy, respectively).
Predicting Improvement in Survival
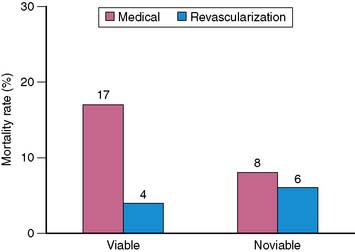
A major goal of viability imaging is to identify patients with low EF who are at the highest clinical risk, in whom revascularization may offer the greatest survival benefit.44 Table 38-6 summarizes the results of five published reports61–65 evaluating the risk of cardiovascular events in patients with viable myocardium treated medically compared to those without viability. In 288 patients with CAD and moderate or severe LV dysfunction, patients with viable myocardium had a consistently higher event rate than those without viability. The odds ratios were consistently greater than 1 in all reports, suggesting an increased risk of a cardiac event for those subjects with viable myocardium treated medically. In a recent meta-analysis,45 annualized mortality rates in patients with viable and nonviable myocardium assessed by PET and FDG of 10 studies and 1046 patients were reported. In patients with viability and who were revascularized, annual mortality rate was 4% but it was 17% in patients who were not revascularized (Fig. 38-11). The corresponding values in patients without viability were 6% and 8%. These data suggest that the presence of ischemic but viable myocardium as assessed by FDG PET is able to identify patients with LV dysfunction who are at high risk for cardiac events when treated with medical therapy alone.
Table 38-6 Risk of Cardiac Events for Patients with Moderate to Severe LV Dysfunction and Hibernating Myocardium*
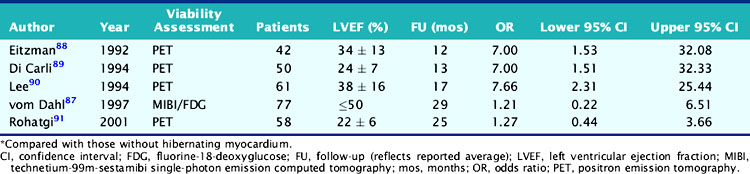
These findings with FDG PET have been confirmed by virtually all subsequent studies using noninvasive imaging with either nuclear testing or echocardiography. Indeed, Allman and colleagues recently reported a meta-analysis of 24 studies that documented long-term patient outcomes after viability imaging by SPECT, PET, or dobutamine echocardiography in 3088 patients (2228 men, 860 women) with a mean ejection fraction of 32% ± 8% and follow-up for 25 ± 10 months.66 The results demonstrated that in patients with evidence of viable myocardium, a strong association was present between revascularization and improved outcomes, particularly in patients with severe LV dysfunction. There was no apparent benefit for revascularization over medical therapy in the absence of demonstrated viability, and there was a trend toward higher death and nonfatal event rates with revascularization, which could reflect the higher procedural risk for patients with severe LV impairment associated with the revascularization itself in the absence of a balancing clinical benefit. Multivariate modeling (meta-regression) of the pooled study data in patients with viable myocardium demonstrated an inverse relationship between ejection fraction and the prognostic benefit associated with revascularization. That is, as the severity of LV dysfunction increased, the potential benefit (reduction in risk of death as well as nonfatal events) associated with revascularization of patients with viable myocardium increased as well. This finding implies that despite an increasing risk of revascularization with worsening LV dysfunction, noninvasive imaging evidence of preserved viability may provide information on clinical benefit to balance against that risk, informing clinical decision making.
In a recent study,67 it was found that the extent of perfusion-metabolism mismatch of greater than 20% was predictive for cardiac death, but when the extent was smaller, no difference was detected in prognosis, indicating that large areas of viable myocardium on PET indicate a high risk for cardiac death. Zhang et al.68 studied the prognostic value of myocardial viability in the LV aneurysms in 70 patients. It was detected that viable aneurysm predicted cardiac events if treated medically, as compared with patients with a viable aneurysm treated surgically.
In a recent randomized trial, the effectiveness of FDG PET–assisted management in patients with severe ventricular dysfunction was studied in 430 patients.69 The study could not demonstrate a significant reduction in cardiac events in patients with LV dysfunction and suspected coronary disease for FDG PET–assisted management versus standard care in a general population. However, in those who adhered to PET recommendations, significant benefits were observed (hazard ratio 0.62; 95% CI 0.42 to 0.93; P = 0.019).
RELATIVE VALUE OF FDG PET VERSUS SPECT IN MANAGEMENT DECISIONS
In a relatively small prospective randomized clinical trial, Siebelink et al. compared management decisions and clinical outcomes based on either PET or SPECT image-guided management in patients with chronic CAD and LV dysfunction.70 Although all patients underwent both PET and SPECT imaging, each patient was randomized to have information from only one of the modalities revealed to the clinical decision-making physicians. Management decisions were made on the basis of the data provided, and the patients were followed for an average of 28 months. The end point in this study was cardiac event-free survival. Events recorded were cardiac death, MI, and unintended revascularization. The results demonstrated no significant differences in the proportions of patients sent to revascularization (PCI or CABG) or medical therapy, or in event-free survival between the groups managed based on results from the two techniques. One of the important limitations of the study by Siebelink and colleagues is that only one-third of the patients had severe LV dysfunction (i.e., LVEF ≤ 30%), which in practice comprises the majority of patients in whom the viability question is of most clinical relevance. Although they reported no difference in outcomes in the subgroup of patients with very low LVEF using the two different imaging modalities, the numbers in each group were too small to provide a definite answer as to relative value of each technique. This is important because FDG imaging appears to improve the detection of viability in patients with very low EF (<25%) when compared to thallium SPECT scintigraphy.71
In a more recent study,72 dual-isotope simultaneous acquisition (DISA) SPECT was compared against PET. PET and DISA SPECT had comparable predictive values for improvement in regional and global LV function and for the prediction of LV reverse remodeling after revascularization.
CONCLUSIONS AND FUTURE DIRECTIONS
Despite the robust and extensive body of evidence supporting the use of viability information for selecting patients for high-risk revascularization, several issues remain unresolved. There appears to be a rather significant reduction in the accuracy of viability testing, including PET, for predicting functional recovery in patients with severely depressed LV function (LVEF < 30%). As discussed, this is likely related to the fact that clinical predictions of functional recovery based on viability information alone are inadequate because they ignore the multifactorial influences affecting changes in LV function after revascularization.41 Clinically important factors such as the degree of LV remodeling and the severity of morphologic alterations within viable but dysfunctional myocytes (and likely others) may influence clinical outcomes, even in the presence of relatively large areas of viable myocardium.26,29,54,73 Future studies should focus on understanding the complex links between viability and ischemia, as well as the molecular changes leading to altered myocyte function, progressive tissue damage, and LV remodeling. This type of information will be of great pathophysiologic interest and will likely have important diagnostic implications.
1. Tillisch J., Brunken R., Marshall R., et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884-888.
2. Kitsiou A.N., Bacharach S.L., Bartlett M.L., et al. [13N]ammonia myocardial blood flow and uptake: relation to functional outcome of asynergic regions after revascularization. J Am Coll Cardiol. 1999;33(3):678-686.
3. Gould K.L. Identifying and measuring severity of coronary artery stenosis. Quantitative coronary arteriography and positron emission tomography. Circulation. 1988;78(2):237-245. 72
4. Grover-McKay M., Ratib O., Schwaiger M., et al. Detection of coronary artery disease with positron emission tomography and rubidium 82. Am Heart J. 1992;123(3):646-652.
5. De Silva R., Yamamoto Y., Rhodes C.G., et al. Preoperative prediction of the outcome of coronary revascularization using positron emission tomography. Circulation. 1992;86(6):1738-1742.
6. Yamamoto Y., De Silva R., Rhodes C.G., et al. A new strategy for the assessment of viable myocardium and regional myocardial blood flow using 15O-water and dynamic positron emission tomography. Circulation. 1992;86(1):167-178.
7. Opie L.H. The heart. Physiology and metabolism, ed 2. New York: Raven Press, 1991.
8. Young L.H., Coven D.L., Russell R.R.3rd. Cellular and molecular regulation of cardiac glucose transport. J Nucl Cardiol. 2000;7:267-276.
9. Opie L.H. Effects of regional ischemia on metabolism of glucose and fatty acids. Circ Res. 1976;38:152-174.
10. Liedtke A.J. Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis. 1981;23:321-336.
11. Camici P., Araujo L.I., Spinks T., et al. Increased uptake of 18F-fluorodeoxyglucose in postischemic myocardium of patients with exercise-induced angina. Circulation. 1986;74:81-88.
12. Phelps M.E., Hoffman E.J., Selin C., et al. Investigation of [18F]2-fluoro-2-deoxyglucose for the measure of myocardial glucose metabolism. J Nucl Med. 1978;19:1311-1319.
13. Knuuti M.J., Nuutila P., Ruotsalainen U., Teräs M., Saraste M., Härkönen R., Ahonen A., Wegelius U., Haapanen A., Bergman J., Haaparanta M., Voipio-Pulkki L.-M. The Value of Quantitative Analysis of Glucose Utilization in Detection of Myocardial Viability by PET. J Nucl Med. 1993;34:2068-2075.
14. Gerber B.L., Ordoubadi F.F., Wijns W., et al. Positron emission tomography using(18)F-fluoro-deoxyglucose and euglycaemic hyperinsulinaemic glucose clamp: optimal criteria for the prediction of recovery of post-ischaemic left ventricular dysfunction. Results from the European Community Concerted Action Multicenter study on use of (18)F-fluoro-deoxyglucose Positron Emission Tomography for the Detection of Myocardial Viability. Eur Heart J. 2001;22(18):1691-1701.
15. Bacharach S.L., Bax J.J., Case J., et al. PET myocardial glucose metabolism and perfusion imaging with 18FDG, 13NH3 and 82Rb. Part 1—Guidelines for data acquisition and patient preparation. J Nucl Cardiol. 2003;10(5):543-556.
16. Hesse B., Tagil K., Cuocolo A., Anagnostopoulos C., Bardies M., Bax J., Bengel F., Busemann Sokole E., Davies G., Dondi M., Edenbrandt L., Franken P., Kjaer A., Knuuti J., Lassmann M., Ljungberg M., Marcassa C., Marie P.Y., McKiddie F., O’Connor M., Prvulovich E., Underwood R., van Eck-Smit B. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging. 2005;32(7):855-897.
17. Schoder H., Campisi R., Ohtake T., et al. Blood flow-metabolism imaging with positron emission tomography in patients with diabetes mellitus for the assessment of reversible left ventricular contractile dysfunction. J Am Coll Cardiol. 1999;33:1328-1337.
18. Knuuti M.J., Nuutila P., Ruotsalainen U., Saraste M., Härkönen R., Ahonen A., Teräs M., Haaparanta M., Wegelius U., Haapanen A., Hartiala J., Voipio-Pulkki L.-M. Euglycemic Hyperinsulinemic Clamp and Oral Glucose Load in Stimulating Myocardial Glucose Utilization During Positron Emission Tomography. J Nucl Med. 1992;33:1255-1256.
19. Bax J.J., Veening M.A., Visser F.C., et al. Optimal metabolic conditions during fluorine-18 fluorodeoxyglucose imaging; a comparative study using different protocols. Eur J Nucl Med. 1997;24:35-41.
20. Knuuti M.J., Yki-Järvinen H., Voipio-Pulkki L.-M., Mäki M.M.D., Ruotsalainen U., Härkönen R., Teräs M., Haaparanta M., Bergman J., Hartiala J., Wegelius U., Nuutila P. Enhancement of Myocardial 18-FDG Uptake by Nicotinic Acid Derivative. J Nucl Med. 1994;35:989-998.
21. Stone C.K., Holden J.E., Stanley W., Perlman S.B. Effect of nicotinic acid on exogenous myocardial glucose utilization. J Nucl Med. 1995;36(6):996-1002.
22. Di Carli M.F., Prcevski P., Singh T.P., et al. Myocardial blood flow, function, and metabolism in repetitive stunning. J Nucl Med. 2000;41:1227-1234.
23. Nowak B., Sinha A.M., Schaefer W.M., et al. Cardiac resynchronization therapy homogenizes myocardial glucose metabolism and perfusion in dilated cardiomyopathy and left bundle branch block. J Am Coll Cardiol. 2003;41:1523-1528.
24. Schaefer W.M., Lipke C.S., Nowak B., et al. Validation of an evaluation routine for left ventricular volumes, ejection fraction and wall motion from gated cardiac FDG PET: a comparison with cardiac magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2003;30:545-553.
25. Slart R.H., Bax J.J., van Veldhuisen D.J., et al. Prediction of functional recovery after revascularization in patients with coronary artery disease and left ventricular dysfunction by gated FDG-PET. J Nucl Cardiol. 2006;13(2):210-219.
26. Yamaguchi A., Ino T., Adachi H., Mizuhara A., Murata S., Kamio H. Left ventricular end-systolic volume index in patients with ischemic cardiomyopathy predicts postoperative ventricular function. Ann Thorac Surg. 1995;60:1059-1062.
27. Pfeffer M.A., Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161-1172.
28. White H.D., Norris R.M., Brown M.A., Brandt P.W., Whitlock R.M., Wild C.J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44-51.
29. Yamaguchi A., Ino T., Adachi H., et al. Left ventricular volume predicts postoperative course in patients with ischemic cardiomyopathy. Ann Thorac Surg. 1998;65:434-438.
30. Schroeder S., Achenbach S., Bengel F., et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: Report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29(4):531-556.
31. Armbrecht J.J., Buxton D.B., Schelbert H.R. Validation of [1–11C]acetate as a tracer for noninvasive assessment of oxidative metabolism with positron emission tomography in normal, ischemic, postischemic, and hyperemic canine myocardium. Circulation. 1990;81:1594-1605.
32. Sun K.T., Chen K., Huang S.C., et al. Compartment model for measuring myocardial oxygen consumption using [1–11C]acetate. J Nucl Med. 1997;38:459-466.
33. Wolpers H.G., Burchert W., van den Hoff J., Weinhardt R., Meyer G.J., Lichtlen P.R. Assessment of myocardial viability by use of 11C-acetate and positron emission tomography. Threshold criteria of reversible dysfunction. Circulation. 1997;95:1417-1424.
34. Bax J.J., Poldermans D., Elhendy A., Boersma E., Rahimtoola S.H. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr Probl Cardiol. 2001;26:141-186.
35. Schinkel A.F., Bax J.J., Sozzi F.B., Boersma E., Valkema R., Elhendy A., Roelandt J.R., Poldermans D. Prevalence of myocardial viability assessed by single photon emission computed tomography in patients with chronic ischaemic left ventricular dysfunction. Heart. 2002;88(2):125-130.
36. vom Dahl J., Altehoefer C., Sheehan F.H., et al. Recovery of regional left ventricular dysfunction after coronary revascularization. Impact of myocardial viability assessed by nuclear imaging and vessel patency at follow-up angiography. J Am Coll Cardiol. 1996;28:948-958.
37. Vanoverschelde J.L., Depre C., Gerber B.L., et al. Time course of functional recovery after coronary artery bypass graft surgery in patients with chronic left ventricular ischemic dysfunction. Am J Cardiol. 2000;85:1432-1439.
38. Auerbach M.A., Sch€oder H., Hoh C., et al. Prevalence of myocardial viability as detected by positron emission tomography in patients with ischemic cardiomyopathy. Circulation. 1999;99:2921-2926.
39. Beanlands R.S., deKemp R.A., Smith S., et al. F-18 fluorodeoxyglucose PET imaging alters clinical decision making in patients with impaired ventricular function. Am J Cardiol. 1997;79:1092-1095.
40. Underwood S.R., Bax J.J., vom Dahl J., et al. Study Group of the European Society of Cardiology. Imaging techniques for the assessment of myocardial hibernation. Report of a Study Group of the European Society of Cardiology. Eur Heart J. 2004;25(10):815-836.
41. Di Carli M.F., Hachamovitch R., Berman D. The Art and Science of Predicting Post-revascularization Improvement in LV Function in Patients With Severely Depressed LV Function (Editorial). J Am Coll Cardiol. 2002;40:1744-1747.
42. Di Carli M.F., Hachamovitch R., Berman D.S. The art and science of predicting postrevascularization improvement in left ventricular (LV) function in patients with severely depressed LV function. J Am Coll Cardiol. 2002;40:1744-1747.
43. Beanlands R.S., Ruddy T.D., deKemp R.A., et al. Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J Am Coll Cardiol. 2002;40:1735-1743.
44. Di Carli M.F. Predicting improved function after myocardial revascularization. Curr Opin Cardiol. 1998;13:415-424.
45. Schinkel A.F., Bax J.J., Poldermans D., Elhendy A., Ferrari R., Rahimtoola S.H. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32(7):375-410.
46. Gropler R.J., Geltman E.M., Sampathkumaran K., et al. Functional recovery after coronary revascularization for chronic coronary artery disease is dependent on maintenance of oxidative metabolism. J Am Coll Cardiol. 1992;20:569-577.
47. Gropler R.J., Bergmann S.R. Flow and metabolic determinants of myocardial viability assessed by positron-emission tomography. Coron Artery Dis. 1993;4:495-504.
48. Rubin P.J., Lee D.S., Davila-Roman V.G., et al. Superiority of C-11 acetate compared with F-18 fluorodeoxyglucose in predicting myocardial functional recovery by positron emission tomography in patients with acute myocardial infarction. Am J Cardiol. 1996;78:1230-1235.
49. Hata T., Nohara R., Fujita M., et al. Noninvasive assessment of myocardial viability by positron emission tomography with 11C acetate in patients with old myocardial infarction. Circulation. 1996;94:1834-1841.
50. Ragosta M., Beller G.A., Watson D.D., Kaul S., Gimple L.W. Quantitative planar rest-redistribution 201Tl imaging in detection of myocardial viability and prediction of improvement in left ventricular function after coronary bypass surgery in patients with severely depressed left ventricular function. Circulation. 1993;87:1630-1641.
51. Perrone-Filardi P., Pace L., Prastaro M., et al. Dobutamine echocardiography predicts improvement of hypoperfused dysfunctional myocardium after revascularization in patients with coronary artery disease. Circulation. 1995;91:2556-2565.
52. Kim R.J., Wu E., Rafael A., et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453.
53. Dreyfus G.D., Duboc D., Blasco A., et al. Myocardial viability assessment in ischemic cardiomyopathy: benefits of coronary revascularization. Ann Thorac Surg. 1994;57:1402-1408.
54. Beanlands R.S., Hendry P.J., Masters R.G., deKemp R.A., Woodend K., Ruddy T.D. Delay in revascularization is associated with increased mortality rate in patients with severe left ventricular dysfunction and viable myocardium on fluorine 18-fluorodeoxyglucose positron emission tomography imaging. Circulation. 1998;98:II51-II56.
55. Haas F., Haehnel C.J., Picker W., et al. Preoperative positron emission tomographic viability assessment and perioperative and postoperative risk in patients with advanced ischemic heart disease. J Am Coll Cardiol. 1997;30:1693-1700.
56. Schwarz E.R., Schoendube F.A., Kostin S., et al. Prolonged myocardial hibernation exacerbates cardiomyocyte degeneration and impairs recovery of function after revascularization. J Am Coll Cardiol. 1998;31:1018-1026.
57. Di Carli M.F., Asgarzadie F., Schelbert H.R., et al. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation. 1995;92:3436-3444.
58. Marwick T.H., Nemec J.J., Lafont A., Salcedo E.E., MacIntyre W.J. Prediction by postexercise fluoro-18 deoxyglucose positron emission tomography of improvement in exercise capacity after revascularization. Am J Cardiol. 1992;69:854-859.
59. Marwick T.H., Zuchowski C., Lauer M.S., et al. Functional status and quality of life in patients with heart failure undergoing coronary bypass surgery after assessment of myocardial viability. J Am Coll Cardiol. 1999;33:750-758.
60. Rohatgi R., Epstein S., Henriquez J., et al. Utility of positron emission tomography in predicting cardiac events and survival in patients with coronary artery disease and severe left ventricular dysfunction. Am J Cardiol. 2001;87:1096-1099. A6
61. vom Dahl J., Altehoefer C., Sheehan F.H., et al. Effect of myocardial viability assessed by technetium-99m-sestamibi SPECT and fluorine-18-FDG PET on clinical outcome in coronary artery disease. J Nucl Med. 1997;38:742-748.
62. Eitzman D., al-Aouar Z., Kanter H.L., et al. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography [commentary]. J Am Coll Cardiol. 1992;20:559-565.
63. Di Carli M.F., Davidson M., Little R., et al. Value of metabolic imaging with positron emission tomography for evaluating prognosis in patients with coronary artery disease and left ventricular dysfunction. Am J Cardiol. 1994;73:527-533.
64. Lee K.S., Marwick T.H., Cook S.A., et al. Prognosis of patients with left ventricular dysfunction, with and without viable myocardium after myocardial infarction. Relative efficacy of medical therapy and revascularization. Circulation. 1994;90:2687-2694.
65. Rohatgi R., Epstein S., Henriquez J., et al. Utility of positron emission tomography in predicting cardiac events and survival in patients with coronary artery disease and severe left ventricular dysfunction. Am J Cardiol. 2001;87:1096-1099. A6
66. Allman K., Shaw L.J., Hachamovitch R., Udelson J.E. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39(7):1151-1158.
67. Desideri A., Cortigiani L., Christen A.I., et al. The extent of perfusion-F18-fluorodeoxyglucose positron emission tomography mismatch determines mortality in medically treated patients with chronic ischemic left ventricular dysfunction. J Am Coll Cardiol. 2005;46(7):1264-1269.
68. Zhang X., Liu X.J., Hu S., et al. Long-term survival of patients with viable and nonviable aneurysms assessed by 99mTc-MIBI SPECT and 18F-FDG PET: a comparative study of medical and surgical treatment. J Nucl Med. 2008;49(8):1288-1298. Epub 2008 Jul 16
69. Beanlands R.S., Nichol G., Huszti E., et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol. 2007;50(20):2002-2012. Epub 2007 Oct 1
70. Siebelink H.M., Blanksma P.K., Crijns H.J., et al. No difference in cardiac event-free survival between positron emission tomography-guided and single-photon emission computed tomography-guided patient management: a prospective, randomized comparison of patients with suspicion of jeopardized myocardium. J Am Coll Cardiol. 2001;37:81-88.
71. Srinivasan G., Kitsiou A.N., Bacharach S.L., Bartlett M.L., Miller-Davis C., Dilsizian V. 18F-Deoxyglucose SPECT: can it replace PET and thallium SPECT for the assessment of myocardial viability? Circulation. 1998;97:843-850.
72. Slart R.H., Bax J.J., van Veldhuisen D.J., et al. Prediction of functional recovery after revascularization in patients with chronic ischaemic left ventricular dysfunction: head-to-head comparison between 99mTc-sestamibi/18F-FDG DISA SPECT and 13N-ammonia/ 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2006;33(6):716-723. Epub 2006 Mar 8
73. Elsasser A., Schlepper M., Klovekorn W.P., et al. Hibernating myocardium: an incomplete adaptation to ischemia. Circulation. 1997;96:2920-2931.
74. Gropler R.J., Geltman E.M., Sampathkumaran K., et al. Comparison of carbon-11-acetate with fluorine-18-fluorodeoxyglucose for delineating viable myocardium by positron emission tomography. J Am Coll Cardiol. 1993;22:1587-1597.
75. Maes A., Borgers M., Flameng W., Nuyts J.L., van de Werf F., Ausma J.J., Sergeant P., Mortelmans L.A. Assessment of myocardial viability in chronic coronary artery disease using technetium-99m sestamibi SPECT. J Am Coll Cardiol. 1997;29:62-68.
76. Grandin C., Wijns W., Melin J.A., et al. Delineation of myocardial viability with PET. J Nucl Med. 1995;36:1543-1552.
77. Tamaki N., Kawamoto M., Tadamura E., et al. Prediction of reversible ischemia after revascularization. Perfusion and metabolic studies with positron emission tomography. Circulation. 1995;91:1697-1705.
78. Marinho N.V., Keogh B.E., Costa D.C., Lammerstma A.A., Ell P.J., Camici P.G. Pathophysiology of chronic left ventricular dysfunction. New insights from the measurement of absolute myocardial blood flow and glucose utilization. Circulation. 1996;93:737-744.
79. Tamaki N., Yonekura Y., Yamashita K., et al. Positron emission tomography using fluorine-18 deoxyglucose in evaluation of coronary artery bypass grafting. Am J Cardiol. 1989;64:860-865.
80. Tamaki N., Ohtani H., Yamashita K., et al. Metabolic activity in the areas of new fill-in after thallium-201 reinjection: comparison with positron emission tomography using fluorine-18-deoxyglucose. J Nucl Med. 1991;32:673-678.
81. Carrel T., Jenni R., Haubold-Reuter S., Von Schulthess G., Pasic M., Turina M. Improvement in severely reduced left ventricular function after surgical revascularization in patients with preoperative myocardial infarction. Eur J Cardiothorac Surg. 1992;6:479-484.
82. Lucignani G., Schwaiger M., Melin J., Fazio F. Assessing hibernating myocardium: an emerging cost-effectiveness issue [editorial]. Eur J Nucl Med. 1997;24:1337-1341.
83. Marwick T.H., MacIntyre W.J., Lafont A., Nemec J.J., Salcedo E.E. Metabolic responses of hibernating and infarcted myocardium to revascularization. A follow-up study of regional perfusion, function, and metabolism. Circulation. 1992;85:1347-1353.
84. Vanoverschelde J.L., Wijns W., Depre C., et al. Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium [commentary]. Circulation. 1993;87:1513-1523.
85. vom Dahl J., Eitzman D.T., al-Aouar Z.R., et al. Relation of regional function, perfusion, and metabolism in patients with advanced coronary artery disease undergoing surgical revascularization. Circulation. 1994;90:2356-2366.
86. Knuuti M.J., Saraste M., Nuutila P., et al. Myocardial viability: fluorine-18-deoxyglucose positron emission tomography in prediction of wall motion recovery after revascularization. Am Heart J. 1994;127:785-796.
87. Baer F.M., Voth E., Deutsch H.J., et al. Predictive value of low dose dobutamine transesophageal echocardiography and fluorine-18 fluorodeoxyglucose positron emission tomography for recovery of regional left ventricular function after successful revascularization. J Am Coll Cardiol. 1996;28:60-69.
88. Schmidt M., Voth E., Schneider C.A., et al. F-18-FDG uptake is a reliable predictor of functional recovery of akinetic but viable infarct regions as defined by magnetic resonance imaging before and after revascularization. Magn Reson Imaging. 2004;22:229-236.
89. Kuhl H.P., Lipke C.S., Krombach G.A., et al. Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography single photon emission computed tomography imaging protocol. Eur Heart J. 2006;27:846-853.
90. Vanoverschelde J.L., Wijns W., Depre C., et al. Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation. 1993;87:1513-1523.
91. Schwarz E.R., Schaper J., vom Dahl J., et al. Myocyte degeneration and cell death in hibernating human myocardium. J Am Coll Cardiol. 1996;27:1577-1585.