1 Assessment of Cardiac Risk and the Cardiology Consultation
In the early 1980s, coronary artery bypass graft surgery (CABG) was characterized by operative mortality rates in the range of 1% to 2%. Over the ensuing years, however, urgent and emergent operations and “redo” procedures became common, and greater morbidity and mortality rates were observed. Percutaneous coronary interventions (PCIs) absorbed low-risk patients from the surgery pool, with the net result being that the operative mortality rate increased to the range of 5% to 6%. The trend toward PCI has continued, with recent trials demonstrating the safety of stenting even left main coronary artery disease (CAD).1 This demographic shift has led hospital administrators to ask for justification of the observed increase in CABG mortality. This often has prompted a time-consuming and expensive chart review to identify the differences in the patient populations that led to the greater morbidity. Even with this information, it was difficult to objectively determine the impact of these new and compelling factors on mortality. The impetus for the development of a risk-adjusted outcome assessment/appropriate risk adjustment scoring system was the need to compare adult cardiac surgery results in different institutions and to benchmark the observed complication rates.2 With the passage of healthcare reform, there is increased interest in publicly reporting perioperative outcomes, which requires optimal risk adjustment.
The first risk-scoring scheme for cardiac surgery was introduced by Paiement et al3 at the Montreal Heart Institute in 1983. Since then, multiple preoperative cardiac surgery risk indices have been developed. The patient characteristics that affected the probability of specific adverse outcomes were identified and weighed, and the resultant risk indices have been used to adjust for case-mix differences among surgeons and centers where performance profiles have been compiled. In addition to comparisons among centers, the preoperative cardiac risk indices have been used to counsel patients and their families in resource planning, in high-risk group identification for special care or research, to determine cost-effectiveness, to determine effectiveness of intervention, to improve provider practice, and to assess costs related to severity of disease.4,5
 Sources of Perioperative Myocardial Injury in Cardiac Surgery
Sources of Perioperative Myocardial Injury in Cardiac Surgery
Myocardial injury, manifested as transient cardiac contractile dysfunction (“stunning”) or acute myocardial infarction (AMI), or both, is the most frequent complication after cardiac surgery and is the single-most important cause of hospital complications and death. Furthermore, patients who have a perioperative myocardial infarction (MI) have poor long-term prognosis; only 51% of such patients remain free from adverse cardiac events after 2 years, compared with 96% of patients without MI.6
Myocardial necrosis is the result of progressive pathologic ischemic changes that start to occur in the myocardium within minutes after the interruption of its blood flow, as seen in cardiac surgery (Box 1-1). The duration of the interruption of blood flow, either partial or complete, determines the extent of myocardial necrosis. This is consistent with the finding that both the duration of the period of aortic cross-clamping (AXC) and the duration of cardiopulmonary bypass (CPB) consistently have been shown to be the main determinants of postoperative outcomes in virtually all studies. This was further supported in a study with an average follow-up of 10 years after complex cardiac surgery in which Khuri7 observed a direct relation between the lowest mean myocardial pH recorded both during and after the period of AXC and long-term patient survival. Patients who experienced acidosis (pH < 6.5) had decreased survival compared with those who did not. Because myocardial acidosis reflects both myocardial ischemia and poor myocardial protection during CPB, this study demonstrated the relation of the adequacy of intraoperative myocardial protection to long-term outcome (see Chapters 3, 6, 18, and 28).
Reperfusion of an Ischemic Myocardium
Surgical interventions requiring interruption of blood flow to the heart must, out of necessity, be followed by restoration of perfusion. Numerous experimental studies have provided compelling evidence that reperfusion, although essential for tissue or organ survival, or both, is not without risk because of the extension of cell damage as a result of reperfusion itself. Myocardial ischemia of limited duration (< 20 minutes), followed by reperfusion, are accompanied by functional recovery without evidence of structural injury or biochemical evidence of tissue injury.8,9
Paradoxically, reperfusion of cardiac tissue, which has been subjected to an extended period of ischemia, results in a phenomenon known as myocardial reperfusion injury.10–12 Thus, a paradox exists in that tissue viability can be maintained only if reperfusion is instituted within a reasonable time period, but only at the risk for extending the injury beyond that caused by the ischemic insult itself. This is supported by the observation that ventricular fibrillation was prominent when the regionally ischemic canine heart was subjected to reperfusion.13 Jennings et al14 reported adverse structural and electrophysiologic changes associated with reperfusion of the ischemic canine heart, and Hearse15 introduced the concept of an oxygen paradox in noting cardiac muscle enzyme release and alterations in ultrastructure when isolated hearts were reoxygenated after a period of hypoxic perfusion.
Myocardial reperfusion injury is defined as the death of myocytes, alive at the time of reperfusion, as a direct result of one or more events initiated by reperfusion. Myocardial cell damage results from the restoration of blood flow to the previously ischemic heart, thereby extending the region of irreversible injury beyond that caused by the ischemic insult alone. The cellular damage that results from reperfusion can be reversible or irreversible, depending on the length of the ischemic insult. If reperfusion is initiated within 20 minutes after the onset of ischemia, the resulting myocardial injury is reversible and is characterized functionally by depressed myocardial contractility, which eventually recovers completely. Myocardial tissue necrosis is not detectable in the previously ischemic region, although functional impairment of contractility may persist for a variable period, a phenomenon known as myocardial stunning. Initiating reperfusion after a duration of ischemia of longer than 20 minutes, however, results in irreversible myocardial injury or cellular necrosis. The extent of tissue necrosis that develops during reperfusion is directly related to the duration of the ischemic event. Tissue necrosis originates in the subendocardial regions of the ischemic myocardium and extends to the subepicardial regions of the area at risk, often referred to as the wavefront phenomenon. The cell death that occurs during reperfusion can be characterized microscopically by explosive swelling, which includes disruption of the tissue lattice, contraction bands, mitochondrial swelling, and calcium phosphate deposits within mitochondria.13
The magnitude of reperfusion injury is directly related to the magnitude of the ischemic injury that precedes it. In its most severe form, it manifests in a “no-reflow” phenomenon. In cardiac surgery, prevention of myocardial injury after the release of the AXC, including the prevention of no reflow, is directly dependent on the adequacy of myocardial protection during the period of aortic clamping. The combination of ischemic and reperfusion injury is probably the most frequent and serious type of injury that leads to poor outcomes in cardiac surgery today (see Chapters 2, 3, 6, 12 to 14, 18, and 28).
Basic science investigations (in mouse, human, and porcine hearts) have implicated acidosis as a primary trigger of apoptosis. Acidosis, reoxygenation, and reperfusion, but not hypoxia (or ischemia) alone, are strong stimuli for programmed cell death, as well as the demonstration that cardiac apoptosis can lead to heart failure.16,17 This suggests that apoptotic changes might be triggered in the course of a cardiac operation, thus effecting an injurious cascade of adverse clinical events that manifest late in the postoperative course.
Adverse Systemic Effects of Cardiopulmonary Bypass
In addition to the effects of disruption and restoration of myocardial blood flow, cardiac morbidity may result from many of the components used to perform cardiovascular operations, which lead to systemic insults that result from CPB circuit-induced contact activation. Inflammation in cardiac surgical patients is produced by complex humoral and cellular interactions, including activation, generation, or expression of thrombin, complement, cytokines, neutrophils, adhesion molecules, mast cells, and multiple inflammatory mediators.18 Because of the redundancy of the inflammatory cascades, profound amplification occurs to produce multiorgan system dysfunction that can manifest as coagulopathy, respiratory failure, myocardial dysfunction, renal insufficiency, and neurocognitive defects. Coagulation and inflammation also are linked closely through networks of both humoral and cellular components, including proteases of the clotting and fibrinolytic cascades, as well as tissue factor. Vascular endothelial cells mediate inflammation and the cross-talk between coagulation and inflammation. Surgery alone activates specific hemostatic responses, activation of immune mechanisms, and inflammatory responses mediated by the release of various cytokines and chemokines (see Chapters 8 and 28 to 31). This complex inflammatory reaction can lead to death from nonischemic causes and suggests that preoperative risk factors may not predict morbidity. The ability to risk-adjust populations is critical to study interventions that may influence these responses to CPB.
 Assessment of Perioperative Myocardial Injury in Cardiac Surgery
Assessment of Perioperative Myocardial Injury in Cardiac Surgery
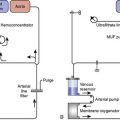
Unfortunately, the current clinical armamentarium is devoid of a means by which perioperative cardiac injury can be reliably monitored in real time, leading to the use of indicators of AMI after the event occurs. Generally, there is a lack of consensus regarding how to measure myocardial injury in cardiac surgery because of the continuum of cardiac injury. Electrocardiographic (ECG) changes, biomarker elevations, and measures of cardiac function have all been used, but all assessment modalities are affected by the direct myocardial trauma of surgery. The American College of Cardiology/European Society of Cardiology (ACC/ESC) published a definition of AMI in 2000, which includes a characteristic rise and fall in blood concentrations of cardiac troponins or creatine kinase (CK)-MB, or both, in the context of a coronary intervention, whereas other modalities are less sensitive and specific (Figure 1-1).19 Subsequently, the Joint ESC/ACCF/American Heart Association/World Heart Federation Task Force’s Universal Definition of Myocardial Infarction published a new “Universal Definition of Myocardial Infarction” in 2007.20 Any of the following criteria meet the diagnosis for MI: Detection of rise/fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit (URL), together with evidence of myocardial ischemia with at least one of the following: symptoms of ischemia, ECG changes indicative of new ischemia (new ST-T changes or new left bundle branch block), development of pathologic Q waves in the ECG, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality (RWMA).
Figure 1-1 Timing of release of various biomarkers after acute ischemic myocardial infarction.
(From Apple FS, Gibler WB: National Academy of Clinical Biochemistry Standards of Laboratory Practice: Recommendations for the use of cardiac markers in coronary artery disease. Clin Chem 45:1104, 1999.)
Traditionally, AMI was determined electrocardiographically (see Chapters 15 and 18). Biochemical measures have not been widely accepted because exact thresholds for myocardial injury have not been clearly defined. Cardiac biomarkers are increased after surgery and can be used for postoperative risk stratification, in addition to being used to diagnose acute morbidity (Box 1-2).
Assessment of Cardiac Function
RWMAs follow the onset of ischemia in 10 to 15 seconds. Echocardiography can, therefore, be a sensitive and rapid monitor for cardiac ischemia/injury.21 If the RWMA is irreversible, this indicates irreversible myocardial necrosis (see Chapters 11 through 14). The importance of TEE assessment of cardiac function is further enhanced by its value as a predictor of long-term survival.22 In patients undergoing CABG, a postoperative decrease in left ventricular ejection fraction (LVEF) compared with preoperative baseline predicts decreased long-term survival.23
The use of TEE is complicated because myocardial stunning (postischemic transient ventricular dysfunction) is a common cause of new postoperative RWMAs, which are transient. However, the appearance of a new ventricular RWMA in the postoperative period, whether caused by irreversible AMI or by reversible myocardial stunning, is an indication of some form of inadequate myocardial protection during the intraoperative period and, therefore, of interest for the assessment of new interventions. Echocardiographic and Doppler systems also have the limitation of being sensitive to alterations in loading conditions, similar to the need for inotropic support and CO determinations.24 The interpretation of TEE images is also operator dependent.25 In addition, there are nonischemic causes of RWMAs, such as conduction abnormalities, ventricular pacing, and myocarditis, which confound the use of this outcome measure for the assessment of ischemic morbidity.
Electrocardiography Monitoring
The presence of new persistent Q waves of at least 0.03-second duration, broadening of preexisting Q waves, or new QS deflections on the postoperative ECG have been considered evidence of perioperative AMI.26 However, new Q waves also may be caused by unmasking of an old MI and therefore not indicative of a new AMI. Crescenzi et al27 demonstrated that the association of a new Q wave and high levels of biomarkers was strongly associated with postoperative cardiac events, whereas the isolated appearance of a new Q wave had no impact on the postoperative cardiac outcome. In addition, new Q waves may actually disappear over time.28 Signs of non–Q-wave MI, such as ST-T wave changes, are even less reliable signs of AMI after cardiac surgery in the absence of biochemical evidence. ST-segment changes are even less specific for perioperative MI because they can be caused by changes in body position, hypothermia, transient conduction abnormalities, and electrolyte imbalances (see Chapter 15).
Serum Biochemical Markers to Detect Myocardial Injury
Serum biomarkers have become the primary means of assessing the presence and extent of AMI after cardiac surgery. Serum biomarkers that are indicative of myocardial damage include the following (with post-insult peak time given in parentheses): myoglobin (4 hours), total CK (16 hours), CK-MB isoenzyme (24 hours), troponins I and T (24 hours), and lactate dehydrogenase (LDH) (76 hours). The CK-MB isoenzyme has been used most widely, but studies have suggested that troponin I is the most sensitive and specific in depicting myocardial ischemia and infarction.29–34
With respect to CK-MB, the definition of an optimal cutoff has been defined best by the correlation of multiples of the upper limit of normal (ULN) for the laboratory and medium- and long-term outcomes. For example, Klatte et al35 reported on the implications of CK-MB in 2918 high-risk CABG patients enrolled in a clinical trial of an anti-ischemic agent. The unadjusted 6-month mortality rates were 3.4%, 5.8%, 7.8%, and 20.2% for patients with a postoperative peak CK-MB ratio (peak CK-MB value/ULN for laboratory test) of less than 5, ≥5 to <10, ≥10 to < 20, and ≥20 ULN, respectively.35 The relation remained statistically significant after adjustment for ejection fraction (EF), congestive heart failure (CHF), cerebrovascular disease, peripheral vascular disease, cardiac arrhythmias, and the method of cardioplegia delivery. In the Arterial Revascularization Therapies Study (ARTS), 496 patients with multivessel CAD undergoing CABG were evaluated by CK-MB testing and followed after surgery at 30 days and 1 year.36 Patients with increased cardiac enzyme levels after CABG were at increased risk for both death and repeat AMI within the first 30 days. CK-MB increase also was independently related to late adverse outcome.
Studies suggest that postcardiac surgery monitoring of troponins can be used to assess myocardial injury and risk stratification. Increased cardiac-specific troponin I or T in patients after CABG has been associated with a cardiac cause of death and with major postoperative complications within 2 years after CABG.37,38 The ACC/ESC definition includes biomarkers but does not include specific criteria for diagnosing post-CABG AMI using cardiac biomarkers.19
There are a few new biomarkers of perioperative cardiac injury or ischemia under development. Brain natriuretic peptide (BNP) could be detected in the early stages of ischemia and decreases shortly after ischemic insult, allowing better detection of reinjury.39 BNP concentrations after CABG in the patients who had cardiac events within 2 years were significantly greater than those in the patients free of cardiac events.40 Soluble CD40 ligand (sCD40L) is another early biomarker of myocardial ischemia,41 and CPB causes an increase in the concentration of plasma sCD40L. A corresponding decrease in platelet CD40L suggests that this prothrombotic and proinflammatory protein was derived primarily from platelets and may contribute to the thrombotic and inflammatory complications associated with CPB.42 Future research will be required to determine how these biomarkers will be used to assess outcome after cardiac surgery.
Variability in Diagnosis of Perioperative Myocardial Infarction
The variability in diagnosing perioperative AMI has been studied by Jain and colleagues,43 who evaluated data from 566 patients at 20 clinical sites, collected as part of a clinical trial. The occurrence of AMI by Q-wave, CK-MB, or autopsy criteria was determined. Of the 25% of patients who met the Q-wave, CK-MB, or autopsy criteria for AMI, 19% had increased CK-MB concentrations, as well as ECG changes. Q-wave and CK-MB or autopsy criteria for AMI were met by 4% of patients. Multicenter data collection showed a substantial variation in the incidence of AMI and an overall incidence rate of up to 25%. The definition of perioperative AMI was highly variable depending on the definitions used.
 Cardiac Risk Assessment and Cardiac Risk Stratification Models
Cardiac Risk Assessment and Cardiac Risk Stratification Models
In defining important risk factors and developing risk indices, each of the studies has used different primary outcomes. Postoperative mortality remains the most definitive outcome that is reflective of patient injury in the perioperative period. It is important to note that death can be cardiac and noncardiac, and if cardiac, may be ischemic or nonischemic in origin. Postoperative mortality rate is reported as either in-hospital or 30-day rate. The latter represents a more standardized definition, although more difficult to capture because of the cost-cutting push to discharge patients early after surgery. The value of developing risk-adjusted postoperative mortality models is the assessment of the comparative efficacy of various techniques in preventing myocardial damage, but it does not provide information that is useful in preventing the injury in real time.44 The postoperative mortality rate also has been used as a comparative measure of quality of cardiac surgical care.45,46
Postoperative morbidity includes AMI and reversible events such as CHF and need for inotropic support. The problems of using AMI as an outcome of interest were described earlier. Because resource utilization has become such an important financial consideration for hospitals, length of intensive care unit (ICU) stay increasingly has been used in the development of risk indices (see Chapter 33).
Predictors of Postoperative Morbidity and Mortality
Clinical and angiographic predictors of operative mortality were initially defined from the Coronary Artery Surgery Study (CASS).47,48 A total of 6630 patients underwent isolated CABG between 1975 and 1978. Women had a significantly greater mortality rate than men; mortality increased with advancing age in men, but this was not a significant factor in women. Increasing severity of angina, manifestations of heart failure, and number and extent of coronary artery stenoses all correlated with greater mortality, whereas EF was not a predictor. Urgency of surgery was a strong predictor of outcome, with those patients requiring emergency surgery in the presence of a 90% left main coronary artery stenosis sustaining a 40% mortality rate.
A risk-scoring scheme for cardiac surgery (CABG and valve) was introduced by Paiement et al3 at the Montreal Heart Institute in 1983. Eight risk factors were identified: (1) poor left ventricular (LV) function, (2) CHF, (3) unstable angina or recent (within 6 weeks) MI, (4) age greater than 65 years, (5) severe obesity (body mass index > 30 kg/m2), (6) reoperation, (7) emergency surgery, and (8) other significant or uncontrolled systemic disturbances. Three classifications were identified: patients with none of these factors (normal), those presenting with one risk factor (increased risk), and those with more than one factor (high risk). In a study of 500 consecutive cardiac surgical patients, it was found that operative mortality increased with increasing risk (confirming their scoring system).
One of the most commonly used scoring systems for CABG was developed by Parsonnet and colleagues (Table 1-1).49 Fourteen risk factors were identified for in-hospital or 30-day mortality after univariate regression analysis of 3500 consecutive operations. An additive model was constructed and prospectively evaluated in 1332 cardiac procedures. Five categories of risk were identified with increasing mortality rates, complication rates, and length of stay at the Newark Beth Israel Medical Center. The Parsonnet Index frequently is used as a benchmark for comparison among institutions. However, the Parsonnet model was created earlier than the other models and may not be representative of the current practice of CABG. During the period after publication of the Parsonnet model, numerous technical advances now in routine use have diminished CABG mortality rates.
| Risk Factor | Assigned Weight |
|---|---|
| Female sex | 1 |
| Morbid obesity (≥ 1.5 × ideal weight) | 3 |
| Diabetes (unspecified type) | 3 |
| Hypertension (systolic BP > 140 mm Hg) | 3 |
| Ejection fraction (%): | |
| Good > 50) | 0 |
| Fair (30–49) | 2 |
| Poor (< 30) | 4 |
| Age (yr): | |
| 70–74 | 7 |
| 75–79 | 12 |
| ≥ 80 | 20 |
| Reoperation | |
| First | 5 |
| Second | 10 |
| Preoperative IABP | 2 |
| Left ventricular aneurysm | 5 |
| Emergency surgery after PTCA or catheterization complications | 10 |
| Dialysis dependency (PD or Hemo) | 10 |
| Catastrophic states (e.g., acute structural defect, cardiogenic shock, acute renal failure)* | 10–50† |
| Other rare circumstances (e.g., paraplegia, pacemaker dependency, congenital HD in adult, severe asthma)* | 2–10† |
| Valve surgery | |
| Mitral | 5 |
| PA pressure ≥ 60 mm Hg | 8 |
| Aortic | 5 |
| Pressure gradient > 120 mm Hg | 7 |
| CABG at the time of valve surgery | 2 |
BP, blood pressure; CABG, coronary artery bypass graft; HD, heart disease; Hemo, hemodialysis; IABP, intra-aortic balloon pump; PA, pulmonary artery; PD, peritoneal dialysis; PTCA, percutaneous transluminal coronary angioplasty.
* On the actual worksheet, these risk factors require justification.
† Values were predictive of increased risk for operative mortality in univariate analysis.
From Parsonnet V, Dean D, Bernstein A: A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation 79:I3, 1989, by permission.
Bernstein and Parsonnet50 simplified the risk-adjusted scoring system in 2000 to provide a handy tool in preoperative discussions with patients and their families, and for preoperative risk stratification calculation. The authors developed a logistic regression model in which 47 potential risk factors were considered, and a method requiring only simple addition and graphic interpretation was designed for relatively easily approximating the estimated risk. The final estimates provided by the simplified model correlated well with the observed mortality (Figure 1-2).
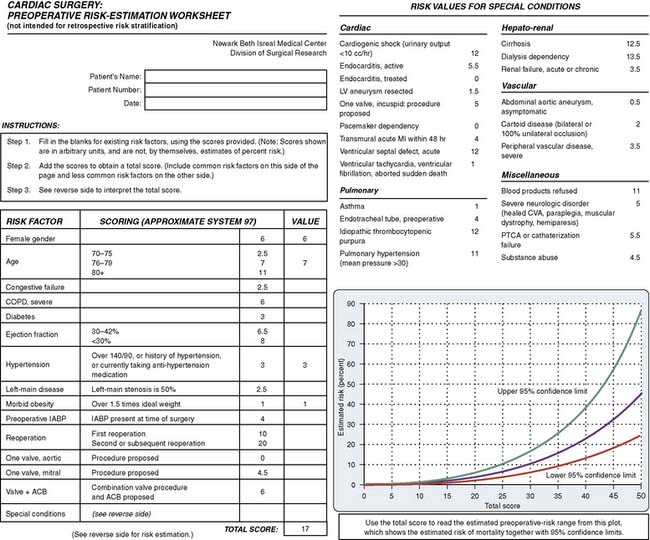
Figure 1-2 Preoperative Risk-Estimation Worksheet.
(From Bernstein AD, Parsonnet V: Bedside estimation of risk as an aid for decision-making in cardiac surgery. Ann Thorac Surg 69:823, 2000, by permission from the Society of Thoracic Surgeons.)
O’Connor et al51 used data collected from 3055 patients undergoing isolated CABG at five clinical centers between 1987 and 1989 to develop a multivariate numerical score. A regression model was developed in a training set and subsequently validated in a test set. Independent predictors of in-hospital mortality included patient age, body surface area, comorbidity score, prior CABG, EF, LV end-diastolic pressure, and priority of surgery. The validated multivariate prediction rule was robust in predicting the in-hospital mortality for an individual patient, and the authors proposed that it could be used to contrast observed and expected mortality rates for an institution or a particular clinician.
Higgins et al52 developed a Clinical Severity Score for CABG at The Cleveland Clinic. A multivariate logistic regression model to predict perioperative risk was developed in 5051 patients undergoing CABG between 1986 and 1988, and subsequently validated in a cohort of 4069 patients. Independent predictors of in-hospital and 30-day mortality were emergency procedure, preoperative serum creatinine level of greater than 168 μmol/L, severe LV dysfunction, preoperative hematocrit of less than 34%, increasing age, chronic pulmonary disease, prior vascular surgery, reoperation, and mitral valve insufficiency. Predictors of morbidity (AMI and use of the intra-aortic balloon pump [IABP], mechanical ventilation for ≥3 days, neurologic deficit, oliguric or anuric renal failure, or serious infection) included diabetes mellitus, body weight of 65 kg or less, aortic stenosis, and cerebrovascular disease. Each independent predictor was assigned a weight or score, with increasing mortality and morbidity associated with an increasing total score.
The New York State model of Hannan et al53 collected data over the years of 1989 through 1992 with 57,187 patients in a study with 14 variables. It was validated in 30 institutions. The mortality definition was “in-hospital.” The crude mortality rate was 3.1%; the receiver operating characteristic (ROC) curve was 0.7, with the Hosmer-Lemeshow (H-L) statistic less than 0.005. Observed mortality was 3.7%, and the expected mortality rate was 2.8%. They included only isolated CABG operations.
The Society of Thoracic Surgeons (STS) national database represents the most robust source of data for calculating risk-adjusted scoring systems. Established in 1989, the database has grown to include 892 participating hospitals in 2008. This provider-supported database allows participants to benchmark their risk-adjusted results against regional and national standards. This National Adult Cardiac Surgery Database (STS NCD) has become one of the largest in the world. New patient data are brought into the STS database on an annual and now semiannual basis. These new data have been analyzed, modeled, and tested using a variety of statistical algorithms. Since 1990, when more complete data collection was achieved, risk stratification models were developed for both CABG and valve replacement surgery. Models developed in 1995 and 1996 were shown to have good predictive value (Table 1-2; Figure 1-3).54,55 In 1999, the STS analyzed the database for valve replacement with and without CABG to determine trends in risk stratification. Between 1986 and 1995, 86,580 patients were analyzed. The model evaluated the influence of 51 preoperative variables on operative mortality by univariate and multivariate analyses for the overall population and for each subset. After the significant risk factors were determined by univariate analysis, a standard logistic regression analysis was performed using the training-set population to develop a formal model. The test-set population then was used to determine the validity of the model. The preoperative risk factors associated with greatest operative mortality rates were salvage status, renal failure (dialysis dependent and nondialysis dependent), emergent status, multiple reoperations, and New York Heart Association class IV. The multivariate logistic regression analysis identified 30 independent preoperative risk factors among the 6 valvular models, isolated or in combination with CABG. The addition of CABG increased the mortality rate significantly for all age groups and for all subset models.56
| Variable | Odds Ratio |
|---|---|
| Age (in 10-year increments) | 1.640 |
| Female sex | 1.157 |
| Non-white | 1.249 |
| Ejection fraction | 0.988 |
| Diabetes | 1.188 |
| Renal failure | 1.533 |
| Serum creatinine (if renal failure is present) | 1.080 |
| Dialysis dependence (if renal failure is present) | 1.381 |
| Pulmonary hypertension | 1.185 |
| Cerebrovascular accident timing | 1.198 |
| Chronic obstructive pulmonary disease | 1.296 |
| Peripheral vascular disease | 1.487 |
| Cerebrovascular disease | 1.244 |
| Acute evolving, extending myocardial infarction | 1.282 |
| Myocardial infarction timing | 1.117 |
| Cardiogenic shock | 2.211 |
| Use of diuretics | 1.122 |
| Hemodynamic instability | 1.747 |
| Triple-vessel disease | 1.155 |
| Left main disease > 50% | 1.119 |
| Preoperative intra-aortic balloon pump | 1.480 |
| Status | |
| Urgent or emergent | 1.189 |
| Emergent salvage | 3.654 |
| First reoperation | 2.738 |
| Multiple reoperations | 4.282 |
| Arrhythmias | 1.099 |
| Body surface area | 0.488 |
| Obesity | 1.242 |
| New York Heart Association Class IV | 1.098 |
| Use of steroids | 1.214 |
| Congestive heart failure | 1.191 |
| Percutaneous transluminal coronary angioplasty within 6 hours of surgery | 1.332 |
| Angiographic accident with hemodynamic instability | 1.203 |
| Use of digitalis | 1.168 |
| Use of intravenous nitrates | 1.088 |
From Shroyer AL, Plomondon ME, Grover FL, et al: The 1996 coronary artery bypass risk model: The Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg 67:1205, 1999, by permission of Society of Thoracic Surgeons.
There are currently three general STS risk models: CABG, valve (aortic or mitral), and valve plus CABG. These apply to seven specific, precisely defined procedures: the CABG model refers to an isolated CABG; the valve model includes isolated aortic or mitral valve replacement and mitral valve repair; and the valve and CABG model includes aortic valve replacement and CABG, mitral valve replacement and CABG, and mitral valve repair and CABG. Besides operative mortality, these models were developed for eight additional end points: reoperation, permanent stroke, renal failure, deep sternal wound infection, prolonged (> 24 hours) ventilation, major morbidity, and operative death, and finally short (< 6 days) and long (> 14 days) postoperative length of stay.57–59 These models are updated periodically, every few years, and calibrated annually to provide an immediate and accurate tool for regional and national benchmarking, and have been proposed for public reporting. The calibration of the risk factors is based on the observed/expected (O/E) ratio, and calibration factors are updated quarterly. The expected mortality (E) is calibrated to obtain the national E/O ratio.
Tu et al60 collected data from 13,098 patients undergoing cardiac surgery between 1991 and 1993 at all nine adult cardiac surgery institutions in Ontario, Canada. Six variables (age, sex, LV function, type of surgery, urgency of surgery, and repeat operation) predicted in-hospital mortality, ICU stay, and postoperative stay in days after cardiac surgery. Subsequently, the Working Group Panel on the Collaborative CABG Database Project categorized 44 clinical variables into 7 core, 13 level 1, and 24 level 2 variables, to reflect their relative importance in determining short-term mortality after CABG. Using data from 5517 patients undergoing isolated CABG at 9 institutions in Ontario in 1993, a series of models were developed. The incorporation of additional variables beyond the original six added little to the prediction of in-hospital mortality.
Spivack et al61 collected data during 1991 and 1992 and included 513 patients with 15 variables, validated only in their institution. They used only an isolated CABG population, and the outcomes measured were mortality and morbidity. The morbidity definition was ventilator time and ICU days. Both prolonged mechanical ventilation and death were rare events (8.3% and 2.0%, respectively). The combination of reduced LVEF and the presence of selected preexisting comorbid conditions (clinical CHF, angina, current smoking, diabetes) served as modest risk factors for prolonged mechanical ventilation; their absence strongly predicted an uncomplicated postoperative respiratory course.
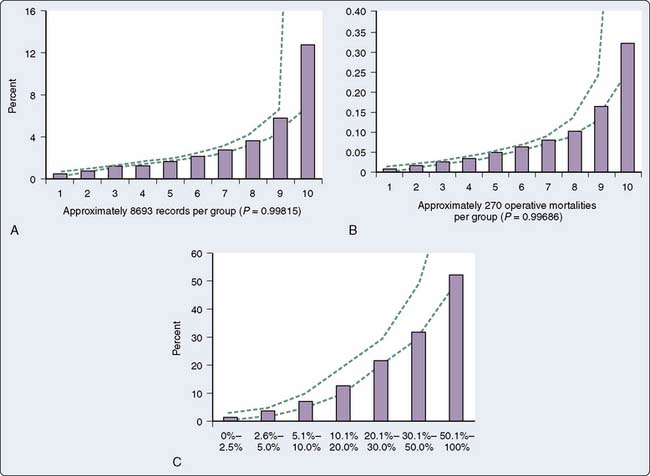
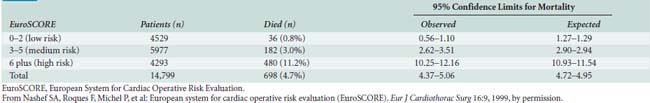
The European System for Cardiac Operative Risk Evaluation (EuroSCORE) for cardiac operative risk evaluation was constructed from an analysis of 19,030 patients undergoing a diverse group of cardiac surgical procedures from 128 centers across Europe (Tables 1-3 and 1-4).62,63 The following risk factors were associated with increased mortality: age, female sex, serum creatinine, extracardiac arteriopathy, chronic airway disease, severe neurologic dysfunction, previous cardiac surgery, recent MI, LVEF, chronic CHF, pulmonary hypertension, active endocarditis, unstable angina, procedure urgency, critical preoperative condition, ventricular septal rupture, noncoronary surgery, and thoracic aortic surgery.
TABLE 1-3 Risk Factors, Definitions, and Weights (Score)
| Risk Factors | Definition | Score |
|---|---|---|
| Patient-Related Factors | ||
| Age | Per 5 years or part thereof over 60 years | 1 |
| Sex | Female | 1 |
| Chronic pulmonary disease | Long-term use of bronchodilators or steroids for lung disease | 1 |
| Extracardiac arteriopathy | Any one or more of the following: claudication, carotid occlusion or > 50% stenosis, previous or planned intervention on the abdominal aorta, limb arteries, or carotids | 2 |
| Neurologic dysfunction | Disease severely affecting ambulation or day-to-day functioning | 2 |
| Previous cardiac surgery | Requiring opening of the pericardium | 3 |
| Serum creatinine | > 200 μmol/L before surgery | 2 |
| Active endocarditis | Patient still under antibiotic treatment for endocarditis at the time of surgery | 3 |
| Critical preoperative state | Any one or more of the following: ventricular tachycardia or fibrillation or aborted sudden death, preoperative cardiac massage, preoperative ventilation before arrival in the anesthetic room, preoperative inotropic support, intra-aortic balloon counterpulsation or preoperative acute renal failure (anuria or oliguria < 10 mL/hr) | 3 |
| Cardiac-Related Factors | ||
| Unstable angina | Rest angina requiring IV nitrates until arrival in the anesthetic room | 2 |
| Left ventricular dysfunction | Moderate or LVEF 30–50% | 1 |
| Poor or LVEF > 30% | 3 | |
| Recent myocardial infarct (< 90 days) | 2 | |
| Pulmonary hypertension | Systolic pulmonary artery pressure > 60 mm Hg | 2 |
| Surgery-Related Factors | ||
| Emergency | Carried out on referral before the beginning of the next working day | 2 |
| Other than isolated CABG | Major cardiac procedure other than or in addition to CABG | 2 |
| Surgery on thoracic aorta | For disorder of ascending aorta, arch or descending aorta | 3 |
| Postinfarct septal rupture | 4 | |
CABG, coronary artery bypass graft surgery; LVEF, left ventricular ejection fraction.
From Nashef SA, Roques F, Michel P, et al: European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 16:9, 1999.
During the 2000s, this additive EuroSCORE has been used widely and validated across different centers in Europe and across the world, making it a primary tool for risk stratification in cardiac surgery.64–75 Although its accuracy has been well established for CABG and isolated valve procedures, its predictive ability in combined CABG and valve procedures has been less well studied. Karthik et al66 showed that, in patients undergoing combined procedures, the additive EuroSCORE significantly underpredicted the risk compared with the observed mortality. In this subset, they determined that the logistic EuroSCORE is a better and more accurate method of risk assessment.
Dupuis et al76 attempted to simplify the approach to risk of cardiac surgical procedures in a manner similar to the original American Society of Anesthesiologists (ASA) physical status classification. They developed a score that uses a simple continuous categorization, using five classes plus an emergency status (Table 1-5). The Cardiac Anesthesia Risk Evaluation (CARE) score model collected data from 1996 to 1999 and included 3548 patients to predict both in-hospital mortality and a diverse group of major morbidities. It combined clinical judgment and the recognition of three risk factors previously identified by multifactorial risk indices: comorbid conditions categorized as controlled or uncontrolled, the surgical complexity, and the urgency of the procedure. The CARE score demonstrated similar or superior predictive characteristics compared with the more complex indices.
TABLE 1-5 Cardiac Anesthesia Risk Evaluation Score
| 1 = Patient with stable cardiac disease and no other medical problem. A noncomplex surgery is undertaken. |
| 2 = Patient with stable cardiac disease and one or more controlled medical problems.* A noncomplex surgery is undertaken. |
| 3 = Patient with any uncontrolled medical problem† or patient in whom a complex surgery is undertaken.‡ |
| 4 = Patient with any uncontrolled medical problem and in whom a complex surgery is undertaken. |
| 5 = Patient with chronic or advanced cardiac disease for whom cardiac surgery is undertaken as a last hope to save or improve life. |
| E = Emergency: surgery as soon as diagnosis is made and operating room is available. |
* Examples: controlled hypertension, diabetes mellitus, peripheral vascular disease, chronic obstructive pulmonary disease, controlled systemic diseases, others as judged by clinicians.
† Examples: unstable angina treated with intravenous heparin or nitroglycerin, preoperative intra-aortic balloon pump, heart failure with pulmonary or peripheral edema, uncontrolled hypertension, renal insufficiency (creatinine level > 140 μmol/L, debilitating systemic diseases, others as judged by clinicians).
‡ Examples: reoperation, combined valve and coronary artery surgery, multiple valve surgery, left ventricular aneurysmectomy, repair of ventricular septal defect after myocardial infarction, coronary artery bypass of diffuse or heavily calcified vessels, others as judged by clinicians.
From Dupuis JY, Wang F, Nathan H, et al: The cardiac anesthesia risk evaluation score: A clinically useful predictor of mortality and morbidity after cardiac surgery. Anesthesiology 94:194, 2001, by permission.
Nowicki et al77 used data on 8943 cardiac valve surgery patients aged 30 years and older from eight northern New England medical centers from 1991 through 2001 to develop a model to predict in-hospital mortality. In the multivariate analysis, 11 variables in the aortic model (older age, lower body surface area, prior cardiac operation, increased creatinine, prior stroke, NYHA class IV, CHF, atrial fibrillation, acuity, year of surgery, and concomitant CABG) and 10 variables in the mitral model (female sex, older age, diabetes, CAD, prior cerebrovascular accident, increased creatinine, NYHA class IV, CHF, acuity, and valve replacement) remained independent predictors of the outcome. They developed a look-up table for mortality rate based on a simple scoring system.
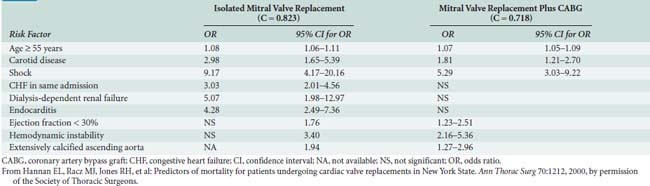
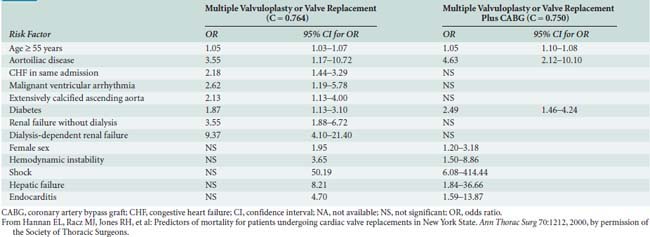
Hannan and colleagues78 also evaluated predictors of mortality after valve surgery but used data from 14,190 patients from New York State. A total of 18 independent risk factors were identified in the 6 models of differing combinations of valve and CABG. Shock and dialysis-dependent renal failure were among the most significant risk factors in all models. The risk factors and odds ratios are shown in Tables 1-6, 1-7, and 1-8. They also studied which risk factors are associated with early readmission (within 30 days) after CABG. Of 16,325 total patients, 2111 (12.9%) were readmitted within 30 days for reasons related to CABG. Eleven risk factors were found to be independently associated with greater readmission rates: older age, female sex, African American race, greater body surface area, previous AMI within 1 week, and six comorbidities. After controlling for these preoperative patient-level risk factors, two provider characteristics (annual surgeon CABG volume < 100 and hospital risk-adjusted mortality rate in the highest decile) and two postoperative factors (discharge to nursing home or rehabilitation/acute care facility and length of stay during index CABG admission of ≥5 days) also were related to greater readmission rates. The development of several excellent risk models for cardiac valve surgery provides a powerful new tool to improve patient care, select procedures, counsel patients, and compare outcomes (see Chapter 19).79
TABLE 1-6 Significant Independent Risk Factors for In-Hospital Mortality for Isolated Aortic Valve Replacement and for Aortic Valvuloplasty or Valve Replacement Plus Coronary Artery Bypass Graft Surgery
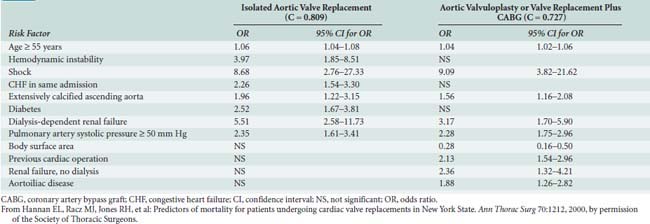
Consistency Among Risk Indices
Many different variables have been found to be associated with the increased risk during cardiac surgery, but only a few variables consistently have been found to be major risk factors across multiple and very diverse study settings. Age, female sex, LV function, body habitus, reoperation, type of surgery, and urgency of surgery were some variables consistently present in most of the models (Box 1-3).
Although a variety of investigators have found different comorbid diseases to be significant risk factors, no diseases have been shown to be consistent risk factors, with the possible exception of renal dysfunction and diabetes. These two comorbidities have been shown to be important risk factors in a majority of the studies (Box 1-4).
Applicability of Risk Indices to a Given Population
One critical factor in the choice of model to use for a given practice is to understand the clinical goals used in the original development process. In addition, despite extensive research and widespread use of risk models in cardiac surgery, there are methodologic problems. The extent of the details in the reports varies greatly. Different conclusions can be reached depending on the risk model used. Processes critical to the development of risk models are shown in Figure 1-4.
 Specific Risk Conditions
Specific Risk Conditions
Renal Dysfunction
Renal dysfunction has been shown to be an important risk factor for surgical mortality in patients undergoing cardiac surgery.80–82 However, the spectrum of what constitutes renal dysfunction is broad, with some models defining it as increased creatinine levels and others defining it as dialysis dependency.
The Northern New England Cardiovascular Study Group reported a 12.2% in-hospital mortality rate after CABG in patients on chronic dialysis versus a 3.0% mortality rate in patients not on dialysis.83 However, the incidence of dialysis dependency in the cardiac surgical population is sufficiently low (e.g., 0.5% in New York State) so that it may not enter into many of the models developed.
Acute kidney injury (AKI) after cardiac surgery carries significant morbidity and mortality. Patients who experienced development of severe renal dysfunction (defined as glomerular filtration rate [GFR] < 30 mL/min) after CABG had an almost 10% mortality rate compared with 1% mortality in those with normal renal function.84 Poor outcome associated with perioperative AKI has led to development of predictive models of AKI to identify patients at risk. One of the recent models predicts need for renal replacement therapy (RRT) after cardiac surgery. Wijeysundera et al85 retrospectively studied a cohort of 20,131 cardiac surgery patients at 2 hospitals in Ontario, Canada. Multivariate predictors of RRT were preoperative estimated GFR, diabetes mellitus requiring medication, LVEF, previous cardiac surgery, procedure, urgency of surgery, and preoperative IABP. An estimated GFR less than or equal to 30 mL/min was assigned 2 points; other components were assigned 1 point each: estimated GFR of 31 to 60 mL/min, diabetes mellitus, EF less than or equal to 40%, previous cardiac surgery, procedure other than CABG, IABP, and nonelective case. Among the 53% of patients with low risk scores (≤1), the risk for RRT was 0.4%; by comparison, this risk was 10% among the 6% of patients with high-risk scores (≥4). Another group developed a robust prediction rule to assist clinicians in identifying patients with normal, or near-normal, preoperative renal function who are at high risk for development of severe renal insufficiency.86 In a multivariate model, the preoperative patient characteristics most strongly associated with postoperative severe renal insufficiency included age, sex, white blood cell count > 12,000, prior CABG, CHF, peripheral vascular disease, diabetes, hypertension, and preoperative IABP.
A major issue with respect to the development of indices to predict perioperative renal failure is that the pathophysiology of perioperative AKI includes inflammatory, nephrotoxic, and hemodynamic insults. This multifactorial nature of AKI might be one of the reasons that a limited single-strategy approach has not been successful.87 Contrast agents used for angiography before cardiac surgery represent one of the modifiable nephrotoxic factors perioperatively. Delaying cardiac surgery beyond 24 hours after the exposure and minimizing the contrast agent load can decrease the incidence of AKI in elective cardiac surgery cases.88
Uniformity of AKI definition (Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease; RIFLE) improved risk stratification models and utilization of early biomarkers of AKI hopefully will provide tools to design clinical trials addressing this important issue.89,90
Diabetes
The association between diabetes and mortality with cardiac surgery has been inconsistent, with some studies supporting the association, whereas other studies do not.91–98 Several recent trials have evaluated outcome between CABG and PCI in patients with diabetes. In the CARDia (Coronary Artery Revascularization in Diabetes) trial,99 a total of 510 patients with diabetes with multivessel or complex single-vessel CAD from 24 centers were randomized to PCI plus stenting (and routine abciximab) or CABG. At 1 year of follow-up, the composite rate of death, MI, and stroke was 10.5% in the CABG group and 13.0% in the PCI group (hazard ratio [HR]: 1.25; 95% CI: 0.75 to 2.09; P = 0.39), all-cause mortality rates were 3.2% and 3.2%, and the rates of death, MI, stroke, or repeat revascularization were 11.3% and 19.3% (HR: 1.77; 95% CI: 1.11 to 2.82; P = 0.02), respectively. The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial randomized 2368 patients with both type 2 diabetes and heart disease to undergo either prompt revascularization with intensive medical therapy or intensive medical therapy alone, and to undergo either insulin-sensitization or insulin-provision therapy.100 In patients with more extensive CAD, similar to those enrolled in the CABG stratum, prompt CABG, in the absence of contraindications, intensive medical therapy, and an insulin sensitization strategy appears to be a preferred therapeutic strategy to reduce the incidence of MI.101
Acute Coronary Syndrome
Patients with a recent episode of non–ST-segment elevation acute coronary syndrome before CABG have greater rates of operative morbidity and mortality than do patients with stable coronary syndromes.102 However, a recent report of the American College of Cardiology Foundation, in collaboration with numerous other societies, has published appropriateness for coronary revascularization.103 There are numerous Class A recommendations for revascularization and, therefore, many patients may come to the operating room directly after coronary angiography and potentially after attempted stent placement with antiplatelet agents. There is evidence to suggest that delaying CABG for 3 to 7 days in patients after ST-elevation myocardial infarction (STEMI) or non–ST-elevation myocardial infarction (NSTEMI) is beneficial in selected stable patients with contraindications to PCI. In addition, patients with a hemodynamically significant right ventricular MI should be allowed to recover the injured ventricle.104
 Cardiovascular Testing
Cardiovascular Testing
Patients who present for cardiac surgery have extensive cardiovascular imaging before surgery to guide the procedure. Coronary angiography provides a static view of the coronary circulation, whereas exercise and pharmacologic testing provide a more dynamic view. Because both tests may be available, it is useful to review some basics of cardiovascular imaging (Box 1-5) (see Chapters 2, 3, 6, 11 to 14, and 18).
In patients with a normal baseline ECG without a prior history of CAD, the exercise ECG response is abnormal in up to 25% and increases up to 50% in those with a prior history of MI or an abnormal resting ECG. In the general population, the usefulness of an exercise ECG test is somewhat limited. The mean sensitivity and specificity are 68% and 77%, respectively, for detection of single-vessel disease, 81% and 66% for detection of multivessel disease, and 86% and 53% for detection of three-vessel or left main CAD.105–108
The level at which ischemia is evident on exercise ECG can be used to estimate an “ischemic threshold” for a patient to guide perioperative medical management, particularly in the prebypass period.109,110 This may support further intensification of perioperative medical therapy in high-risk patients, which may have an impact on perioperative cardiovascular events (see Chapters 2, 3, 6, 10, 12 to 15, and 18).
Nonexercise (Pharmacologic) Stress Testing
Pharmacologic stress testing has been advocated for patients in whom exercise tolerance is limited, both by comorbid diseases and by symptomatic peripheral vascular disease. Often, these patients may not stress themselves sufficiently during daily life to provoke symptoms of myocardial ischemia or CHF. Pharmacologic stress testing techniques either increase myocardial oxygen demand (dobutamine)111 or produce coronary vasodilatation leading to coronary flow redistribution (dipyridamole/adenosine).112 Echocardiographic or nuclear scintigraphic imaging (SPECT) are used in conjunction with the pharmacologic therapy to perform myocardial perfusion imaging for risk stratification and myocardial viability assessment (Box 1-6) (see Chapters 2, 3, 6, 11 to 15, and 18).
Dipyridamole-Thallium Scintigraphy
Dipyridamole works by blocking adenosine reuptake and increasing adenosine concentration in the coronary vessels. Adenosine is a direct coronary vasodilator. After infusion of the vasodilator, flow is preferentially distributed to areas distal to normal coronary arteries, with minimal flow to areas distal to a coronary stenosis.113,114 A radioisotope, such as thallium or 99-technetium sestamibi, then is injected. Normal myocardium will show up on initial imaging, whereas areas of either myocardial necrosis or ischemia distal to a significant coronary stenosis will demonstrate a defect. After a delay of several hours, or after infusion of a second dose of 99-technetium sestamibi, the myocardium is again imaged. Those initial defects that remain as defects are consistent with old scar, whereas those defects that demonstrate normal activity on subsequent imaging are consistent with areas at risk for myocardial ischemia. Several strategies have been suggested to increase the predictive value of the test. The redistribution defect can be quantitated, with larger areas of defect being associated with increased risk.114 In addition, both increased lung uptake and LV cavity dilation have been shown to be markers of ventricular dysfunction with ischemia (Box 1-7).
Dobutamine Stress Echocardiography
Dobutamine stress echocardiography (DSE) involves the identification of new or worsening RWMAs using two-dimensional echocardiography during infusion of intravenous dobutamine. It has been shown to have the same accuracy as dipyridamole thallium scintigraphy for the detection of CAD.115,116 There are several advantages to DSE compared with dipyridamole thallium scintigraphy: the DSE study also can assess LV function and valvular abnormalities, the cost of the procedure is significantly lower, there is no radiation exposure, the duration of the study is significantly shorter, and results are immediately available.
 Conclusions
Conclusions
Clinicians are unable to reliably monitor cardiac injury intraoperatively or in real time. There is also a lack of consensus regarding the definition and quantification of AMI in the perioperative and early postoperative periods. In contrast, postoperative mortality is easy to define. Therefore, deviation of expected mortality from observed mortality has been used as a “gold standard.” However, it is important to recognize that late outcome and survival may also be reflective of intraoperative events. Preoperative cardiac risk assessment of patients undergoing cardiac surgery would ideally lead to identification of a group of patients at risk for increased morbidity and mortality because of perioperative myocardial injury. Based on individual risk factors, perioperative care would then be modified to improve the patient’s outcome. To achieve this goal, a clear definition and quantification of myocardial injury in cardiac surgery patients are required. Clinicians need to be able to monitor intraoperative ischemia and intervene to prevent loss of myocardium. Anesthesiologists also need to follow both short- and long-term outcomes of cardiac surgical patients, as well as the impact of different preoperative and intraoperative strategies, on short- and long-term outcomes. Evidence-based medicine has led to an unprecedented growth in the scientific approach to decision making in the belief that it will translate into benefits for patients to decrease their risk and improve outcomes.117
1 Kang S.H., Park K.H., Choi D.J., et al. Coronary artery bypass grafting versus drug-eluting stent implantation for left main coronary artery disease (from a two-center registry). Am J Cardiol. 2010;105:343.
2 Kouchoukos N.T., Ebert P.A., Grover F.L., et al. Report of the Ad Hoc Committee on Risk Factors for Coronary Artery Bypass Surgery. Ann Thorac Surg. 1988;45:348.
3 Paiement B., Pelletier C., Dyrda I., et al. A simple classification of the risk in cardiac surgery. Can Anaesth Soc J. 1983;30:61.
4 Pinna-Pintor P., Bobbio M., Sandrelli L., et al. Risk stratification for open heart operations: Comparison of centers regardless of the influence of the surgical team. Ann Thorac Surg. 1997;64:410.
5 Smith P.K., Smith L.R., Muhlbaier L.H. Risk stratification for adverse economic outcomes in cardiac surgery. Ann Thorac Surg. 1997;64:S61. discussion S80
6 Guiteras Val P., Pelletier L.C., Hernandez M.G., et al. Diagnostic criteria and prognosis of perioperative myocardial infarction following coronary bypass. J Thorac Cardiovasc Surg. 1983;86:878.
7 Khuri S.F. Evidence, sources, and assessment of injury during and following cardiac surgery. Ann Thorac Surg. 2001;72:S2205.
8 Heyndrickx G.R., Millard R.W., McRitchie R.J., et al. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975;56:978.
9 Bolli R. Mechanism of myocardial “stunning,”. Circulation. 1990;82:723.
10 Hearse D.J., Bolli R. Reperfusion-induced injury: Manifestations, mechanisms, and clinical relevance. Cardiovasc Res. 1992;26:101.
11 Opie L.H. Reperfusion injury and its pharmacologic modification. Circulation. 1989;80:1049.
12 Braunwald E., Kloner R.A. Myocardial reperfusion: A double-edged sword? J Clin Invest. 1985;76:1713.
13 Park J.L., Lucchesi B.R. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1999;68:1905.
14 Jennings R.B., Sommers H.M., Smyth G.A., et al. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68.
15 Hearse D.J. Ischemia, reperfusion, and the determinants of tissue injury. Cardiovasc Drugs Ther. 1990;4(Suppl 4):767.
16 Webster K.A., Discher D.J., Kaiser S., et al. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Invest. 1999;104:239.
17 Thatte H.S., Rhee J.H., Zagarins S.E., et al. Acidosis-induced apoptosis in human and porcine heart. Ann Thorac Surg. 2004;77:1376.
18 Levy J.H., Tanaka K.A. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715.
19 Alpert J.S., Thygesen K., Antman E., et al. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959.
20 Thygesen K., Alpert J.S., White H.D., et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634.
21 Comunale M.E., Body S.C., Ley C., et al. The concordance of intraoperative left ventricular wall-motion abnormalities and electrocardiographic S-T segment changes: Association with outcome after coronary revascularization. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology. 1998;88:945.
22 Royster R.L., Butterworth J.F., Prough D.S., et al. Preoperative and intraoperative predictors of inotropic support and long-term outcome in patients having coronary artery bypass grafting. Anesth Analg. 1991;72:729.
23 Jacobson A., Lapsley D., Tow D.E., et al. Prognostic significance of change in resting left ventricular ejection fraction early after successful coronary artery bypass surgery: A long-term follow-up study. J Am Coll Cardiol. 1995:184A.
24 Fleisher L.A., Tuman K.J. What can we learn from provoking ischemia? Anesth Analg. 1997;84:1177.
25 Griffin M., Edwards B., Judd J., et al. Field-by-field evaluation of intraoperative transoesophageal echocardiography interpretative skills. Physiol Meas. 2000;21:165.
26 Brewer D.L., Bilbro R.H., Bartel A.G. Myocardial infarction as a complication of coronary bypass surgery. Circulation. 1973;47:58.
27 Crescenzi G., Bove T., Pappalardo F., et al. Clinical significance of a new Q wave after cardiac surgery. Eur J Cardiothorac Surg. 2004;25:1001.
28 Sztajzel J., Urban P. Early and late Q wave regression in the setting of acute myocardial infarction. Heart. 2000;83:708.
29 Alyanakian M.A., Dehoux M., Chatel D., et al. Cardiac troponin I in diagnosis of perioperative myocardial infarction after cardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:288.
30 Carrier M., Pellerin M., Perrault L.P., et al. Troponin levels in patients with myocardial infarction after coronary artery bypass grafting. Ann Thorac Surg. 2000;69:435.
31 Etievent J.P., Chocron S., Toubin G., et al. Use of cardiac troponin I as a marker of perioperative myocardial ischemia. Ann Thorac Surg. 1995;59:1192.
32 Greenson N., Macoviak J., Krishnaswamy P., et al. Usefulness of cardiac troponin I in patients undergoing open heart surgery. Am Heart J. 2001;141:447.
33 Mair J., Larue C., Mair P., et al. Use of cardiac troponin I to diagnose perioperative myocardial infarction in coronary artery bypass grafting. Clin Chem. 1994;40:2066.
34 Vermes E., Mesguich M., Houel R., et al. Cardiac troponin I release after open heart surgery: A marker of myocardial protection? Ann Thorac Surg. 2000;70:2087.
35 Klatte K., Chaitman B.R., Theroux P., et al. Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: Results from the GUARDIAN trial. J Am Coll Cardiol. 2001;38:1070.
36 Costa M.A., Carere R.G., Lichtenstein S.V., et al. Incidence, predictors, and significance of abnormal cardiac enzyme rise in patients treated with bypass surgery in the Arterial Revascularization Therapies Study (ARTS). Circulation. 2001;104:2689.
37 Fellahi J.L., Gue X., Richomme X., et al. Short- and long-term prognostic value of postoperative cardiac troponin I concentration in patients undergoing coronary artery bypass grafting. Anesthesiology. 2003;99:270.
38 Lehrke S., Steen H., Sievers H.H., et al. Cardiac troponin T for prediction of short- and long-term morbidity and mortality after elective open heart surgery. Clin Chem. 2004;50:1560.
39 Baxter G.F. Natriuretic peptides and myocardial ischaemia. Basic Res Cardiol. 2004;99:90.
40 Watanabe M., Egi K., Hasegawa S., et al. Significance of serum atrial and brain natriuretic peptide release after coronary artery bypass grafting. Surg Today. 2003;33:671.
41 Vishnevetsky D., Kiyanista V.A., Gandhi P.J. CD40 ligand: A novel target in the fight against cardiovascular disease. Ann Pharmacother. 2004;38:1500.
42 Nannizzi-Alaimo L., Rubenstein M.H., Alves V.L., et al. Cardiopulmonary bypass induces release of soluble CD40 ligand. Circulation. 2002;105:2849.
43 Jain U., Laflamme C.J., Aggarwal A., et al. Electrocardiographic and hemodynamic changes and their association with myocardial infarction during coronary artery bypass surgery. A multicenter study. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology. 1997;86:576.
44 Fleisher L.A. Risk indices: What is their value to the clinician and patient? Anesthesiology. 2001;94:191.
45 Hannan E.L., Kilburn H.Jr, Racz M., et al. Improving the outcomes of coronary artery bypass surgery in New York State. JAMA. 1994;271:761.
46 Mukamel D.B., Mushlin A.I. Quality of care information makes a difference: An analysis of market share and price changes after publication of the New York State Cardiac Surgery Mortality Reports. Med Care. 1998;36:945.
47 Coronary Artery Surgery Study (CASS). A randomized trial of coronary artery bypass surgery. Survival data. Circulation. 1983;68:939.
48 Alderman E.L., Fisher L.D., Litwin P., et al. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation. 1983;68:785.
49 Parsonnet V., Dean D., Bernstein A. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation. 1989;79:I-13.
50 Bernstein A.D., Parsonnet V. Bedside estimation of risk as an aid for decision-making in cardiac surgery. Ann Thorac Surg. 2000;69:823.
51 O’Connor G., Plume S., Olmstead E., et al. Multivariate prediction of in-hospital mortality associated with coronary artery by-pass graft surgery. Circulation. 1992;85:2110.
52 Higgins T., Estafanous F., Loop F., et al. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. JAMA. 1992;267:2344.
53 Hannan E.L., Kilburn H.Jr, O’Donnell J.F., et al. Adult open heart surgery in New York State. An analysis of risk factors and hospital mortality rates. JAMA. 1990;264:2768.
54 Shroyer A.L., Grover F.L., Edwards F.H. 1995 Coronary artery bypass risk model: The Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg. 1998;65:879.
55 Shroyer A.L., Plomondon M.E., Grover F.L., et al. The 1996 coronary artery bypass risk model: The Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg. 1999;67:1205.
56 Jamieson W.R., Edwards F.H., Schwartz M., et al. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg. 1999;67:943.
57 Shahian D.M., O’Brien S.M., Filardo G., et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 1—coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2.
58 O’Brien S.M., Shahian D.M., Filardo G., et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 2—isolated valve surgery. Ann Thorac Surg. 2009;88:S23.
59 Shahian D.M., O’Brien S.M., Filardo G., et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 3—valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S43.
60 Tu J.V., Jaglal S.B., Naylor C.D. Multicenter validation of a risk index for mortality, intensive care unit stay, and overall hospital length of stay after cardiac surgery. Steering Committee of the Provincial Adult Cardiac Care Network of Ontario. Circulation. 1995;91:677.
61 Spivack S.D., Shinozaki T., Albertini J.J., et al. Preoperative prediction of postoperative respiratory outcome. Coronary artery bypass grafting. Chest. 1996;109:1222.
62 Nashef S.A., Roques F., Michel P., et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9.
63 Roques F., Nashef S.A., Michel P., et al. Risk factors and outcome in European cardiac surgery: Analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816. discussion 822
64 Al-Ruzzeh S., Nakamura K., Athanasiou T., et al. Does off-pump coronary artery bypass (OPCAB) surgery improve the outcome in high-risk patients? A comparative study of 1398 high-risk patients. Eur J Cardiothorac Surg. 2003;23:50.
65 Ghosh P., Djordjevic M., Schistek R., et al. Does gender affect outcome of cardiac surgery in octogenarians? Asian Cardiovasc Thorac Ann. 2003;11:28.
66 Karthik S., Srinivasan A.K., Grayson A.D., et al. Limitations of additive EuroSCORE for measuring risk stratified mortality in combined coronary and valve surgery. Eur J Cardiothorac Surg. 2004;26:318.
67 Kasimir M.T., Bialy J., Moidl R., et al. EuroSCORE predicts mid-term outcome after combined valve and coronary bypass surgery. J Heart Valve Dis. 2004;13:439.
68 Kurki T.S., Jarvinen O., Kataja M.J., et al. Performance of three preoperative risk indices CABDEAL, EuroSCORE and Cleveland models in a prospective coronary bypass database. Eur J Cardiothorac Surg. 2002;21:406.
69 Nakamura Y., Nakano K., Nakatani H., et al. Hospital and mid-term outcomes in elderly patients undergoing off-pump coronary artery bypass grafting—comparison with younger patients. Circ J. 2004;68:1184.
70 Nilsson J., Algotsson L., Hoglund P., et al. Early mortality in coronary bypass surgery: The EuroSCORE versus The Society of Thoracic Surgeons risk algorithm. Ann Thorac Surg. 2004;77:1235. discussion 1239
71 Riha M., Danzmayr M., Nagele G., et al. Off pump coronary artery bypass grafting in EuroSCORE high and low risk patients. Eur J Cardiothorac Surg. 2002;21:193.
72 Swart M.J., Joubert G. The EuroSCORE does well for a single surgeon outside Europe. Eur J Cardiothorac Surg. 2004;25:145. author reply 146
73 Toumpoulis I.K., Anagnostopoulos C.E., DeRose J.J., et al. European system for cardiac operative risk evaluation predicts long-term survival in patients with coronary artery bypass grafting. Eur J Cardiothorac Surg. 2004;25:51.
74 Toumpoulis I.K., Anagnostopoulos C.E., Swistel D.G., et al. Does EuroSCORE predict length of stay and specific postoperative complications after cardiac surgery? Eur J Cardiothorac Surg. 2005;27:128.
75 Ugolini C., Nobilio L. Risk adjustment for coronary artery bypass graft surgery: An administrative approach versus EuroSCORE. Int J Qual Health Care. 2004;16:157.
76 Dupuis J.Y., Wang F., Nathan H., et al. The cardiac anesthesia risk evaluation score: A clinically useful predictor of mortality and morbidity after cardiac surgery. Anesthesiology. 2001;94:194.
77 Nowicki E.R., Birkmeyer N.J., Weintraub R.W., et al. Multivariable prediction of in-hospital mortality associated with aortic and mitral valve surgery in Northern New England. Ann Thorac Surg. 2004;77:1966.
78 Hannan E.L., Racz M.J., Jones R.H., et al. Predictors of mortality for patients undergoing cardiac valve replacements in New York State. Ann Thorac Surg. 2000;70:1212.
79 Gardner S., Grunwald G., Rumsfeld J., et al. Comparison of short-term mortality risk factors for valve replacement vs. coronary artery bypass graft surgery. Ann Thorac Surg. 2004;77:549.
80 Brandrup-Wognsen G., Haglid M., Karlsson T., et al. Preoperative risk indicators of death at an early and late stage after coronary artery bypass grafting. Thorac Cardiovasc Surg. 1995;43:77.
81 Conlon P.J., Little M.A., Pieper K., et al. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001;60:1490.
82 Hayashida N., Chihara S., Tayama E., et al. Coronary artery bypass grafting in patients with mild renal insufficiency. Jpn Circ J. 2001;65:28.
83 Liu J.Y., Birkmeyer N.J., Sanders J.H., et al. Risks of morbidity and mortality in dialysis patients undergoing coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 2000;102:2973.
84 Cooper W.A., O’Brien S.M., Thourani V.H., et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006;113:1063.
85 Wijeysundera D.N., Karkouti K., Dupuis J.Y., et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801.
86 Brown J.R., Cochran R.P., Leavitt B.J., et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116:I139.
87 Rosner M.H., Portilla D., Okusa M.D. Cardiac surgery as a cause of acute kidney injury: Pathogenesis and potential therapies. J Intensive Care Med. 2008;23:3.
88 Ranucci M., Ballotta A., Kunkl A., et al. Influence of the timing of cardiac catheterization and the amount of contrast media on acute renal failure after cardiac surgery. Am J Cardiol. 2008;101:1112.
89 Bellomo R., Ronco C., Kellum J.A., et al. Acute renal failure: Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204.
90 Bennett M., Dent C.L., Ma Q., et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin J Am Soc Nephrol. 2008;3:665.
91 Yamamoto T., Hosoda Y., Takazawa K., et al. Is diabetes mellitus a major risk factor in coronary artery bypass grafting? The influence of internal thoracic artery grafting on late survival in diabetic patients. Jpn J Thorac Cardiovasc Surg. 2000;48:344.
92 Clement R., Rousou J.A., Engelman R.M., et al. Perioperative morbidity in diabetics requiring coronary artery bypass surgery. Ann Thorac Surg. 1988;46:321.
93 Devineni R., McKenzie F.N. Surgery for coronary artery disease in patients with diabetes mellitus. Can J Surg. 1985;28:367.
94 Engelman R.M., Bhat J.G., Glassman E., et al. The influence of diabetes and hypertension on the results of coronary revascularization. Am J Med Sci. 1976;271:4.
95 Herlitz J., Wognsen G.B., Emanuelsson H., et al. Mortality and morbidity in diabetic and nondiabetic patients during a 2-year period after coronary artery bypass grafting. Diabetes Care. 1996;19:698.
96 Magee M.J., Dewey T.M., Acuff T., et al. Influence of diabetes on mortality and morbidity: Off-pump coronary artery bypass grafting versus coronary artery bypass grafting with cardiopulmonary bypass. Ann Thorac Surg. 2001;72:776. discussion 780
97 Salomon N.W., Page U.S., Okies J.E., et al. Diabetes mellitus and coronary artery bypass. Short-term risk and long-term prognosis. J Thorac Cardiovasc Surg. 1983;85:264.
98 Thourani V.H., Weintraub W.S., Stein B., et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045.
99 Kapur A., Hall R.J., Malik I.S., et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients: 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol. 2010;55:432.
100 Frye R.L., August P., Brooks M.M., et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503.
101 Chaitman B.R., Hardison R.M., Adler D., et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: Impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529.
102 Marso S.P., Bhatt D.L., Roe M.T., et al. Enhanced efficacy of eptifibatide administration in patients with acute coronary syndrome requiring in-hospital coronary artery bypass grafting. PURSUIT Investigators. Circulation. 2000;102:295.
103 Patel M.R., Dehmer G.J., Hirshfeld J.W., et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: A Report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology: Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. Circulation. 2009;119:1330.
104 Eagle K.A., Guyton R.A., Davidoff R., et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:e340.
105 Connolly H.M., Oh J.K., Schaff H.V., et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: Result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940.
106 Horacek B.M., Wagner G.S. Electrocardiographic ST-segment changes during acute myocardial ischemia. Cardiol Electrophysiol Rev. 2002;6:196.
107 Schneider R.M., Seaworth J.F., Dohrmann M.L., et al. Anatomic and prognostic implications of an early treadmill exercise test. Am J Cardiol. 1982;50:682.
108 Weiner D.A., McCabe C.H., Ryan T.J. Prognostic assessment of patients with coronary artery disease by exercise testing. Am Heart J. 1983;105:749.
109 Myers J., Prakash M., Froelicher V., et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793.
110 Balady G.J. Survival of the fittest: More evidence. N Engl J Med. 2002;346:852.
111 Carstensen S. Dobutamine-atropine stress echocardiography. Heart Drug. 2005;5:101.
112 Grossman G.B., Alazraki N. Myocardial perfusion imaging in coronary artery disease. Cardiology. 2004;10:1.
113 Klocke F.J., Baird M.G., Bateman T.M., et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionucleotide imaging: Executive summary. Circulation. 2003;108:1404.
114 Beller G.A. Clinical value of myocardial perfusion imaging in coronary artery disease. J Nucl Cardiol. 2003;10:529.
115 Armstrong W.F., Pellikka P.A., Ryan T., et al. Stress echocardiography: Recommendations for performance and interpretation. J Am Soc Echocardiogr. 1998;11:97.
116 Marwick T.H. Quantitative techniques for stress echocardiography. Eur J Echocardiogr. 2002;3:171.
117 Cheng D.C., Martin J.E. Raising the bar: A primer on evidence-based decision-making. Semin Cardiothoracic Vasc Anesth. 2005;9:1.