CHAPTER 91 Ascites and Spontaneous Bacterial Peritonitis
PATHOGENESIS OF ASCITES
CIRRHOTIC ASCITES
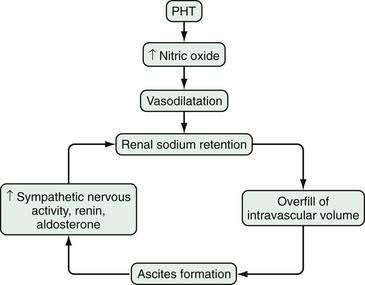
Ascites occurs in the setting of cirrhosis as a result of the sequence of events detailed in Figure 91-1. The most recent theory of ascitic fluid formation, the “peripheral arterial vasodilation hypothesis,” proposes that both older hypotheses, the underfill and overflow theories, are correct, but that each is operative at a different stage.1 The first abnormality that develops appears to be portal hypertension. Portal pressure increases above a critical threshold, and circulating nitric oxide levels increase. Nitric oxide leads to vasodilatation. As the state of vasodilatation worsens, plasma levels of vasoconstrictor, sodium-retentive hormones increase, renal function deteriorates, and ascitic fluid forms—that is, decompensation occurs.
NONCIRRHOTIC ASCITES
The mechanism of fluid retention in patients with malignancy-related ascites depends on the location of the tumor. Peritoneal carcinomatosis appears to cause ascites through the production of proteinaceous fluid by tumor cells lining the peritoneum. Extracellular fluid enters the peritoneal cavity to reestablish oncotic balance. Fluid accumulates in patients with massive liver metastases because of portal hypertension caused by stenosis or occlusion of portal veins by tumor nodules or tumor emboli.2 In patients with hepatocellular carcinoma, ascites arises because of the underlying cirrhosis-related portal hypertension, tumor-induced portal vein thrombosis, or both. Chylous ascites in patients with malignant lymphoma appears to be caused by lymph node obstruction by tumor and rupture of chyle-containing lymphatics.
Ascites can complicate high-output or low-output heart failure or nephrotic syndrome. As in cirrhosis, effective arterial blood volume appears to be decreased, and the vasopressin, renin-aldosterone, and sympathetic nervous systems are activated.3 These changes lead to renal vasoconstriction and sodium and water retention. Fluid then “weeps” from the congested hepatic sinusoids as lymph, as in cirrhotic ascites. Tuberculosis, Chlamydia infection, and coccidioidomycosis probably cause ascites through the production of proteinaceous fluid, as in peritoneal carcinomatosis. Spontaneous bacterial peritonitis does not appear to cause fluid to accumulate; infection develops only in preexisting ascites.
CLINICAL FEATURES
HISTORY
Most patients (approximately 85%) with ascites in the United States have cirrhosis. The three most common causes of cirrhosis are excess alcohol use, chronic hepatitis C, and nonalcoholic steatohepatitis (NASH) related in many cases to obesity. As the obesity epidemic evolves, NASH could become the most common cause of cirrhosis. Many patients have two of these conditions, and some have all three.4 In approximately 15% of patients with ascites, a nonhepatic cause of fluid retention is identified (Table 91-1).
| CAUSE | % |
|---|---|
| Cirrhosis (with or without infection) | 85 |
| Miscellaneous portal hypertension-related disorder (including 5% with two causes) | 8 |
| Cardiac disease | 3 |
| Peritoneal carcinomatosis | 2 |
| Miscellaneous nonportal hypertension-related disorders | 2 |
Data from Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117:215-20.
Evidence is accumulating that cirrhosis unrelated to alcohol use can also be reversible with effective therapy.5 Whether a decompensated cirrhotic liver can revert to a normal liver, however, remains to be seen. Many patients with cirrhosis and ascites will ultimately require liver transplantation.
Patients with ascites should be questioned about risk factors for liver disease other than alcohol, such as injection drug use, blood transfusions, sex with a same-gender partner, acupuncture, tattoos, ear piercing, and country of origin. Commonly, the cause of ascites in a middle-aged or elderly woman is viral hepatitis–induced cirrhosis resulting from a remote, often forgotten blood transfusion. Another cause of “cryptogenic” cirrhosis and ascites is NASH from long-standing obesity.6 Many patients who have been obese will spontaneously lose 50 or even 100 pounds after their liver disease decompensates. Unless the patient is questioned about lifetime maximum body weight and usual adult body weight, the possibility of NASH-related cirrhosis may not be considered. With careful history-taking and appropriate laboratory testing, the percentage of patients with cirrhosis who are now labeled cryptogenic is approaching zero.6
Patients with a long history of stable cirrhosis and the sudden development of ascites should be suspected of harboring a hepatocellular carcinoma that has precipitated the decompensation. Patients with ascites who have a history of cancer should be suspected of having malignancy-related ascites. Cancer in the past, however, does not guarantee a malignant cause of ascites. For example, patients with tobacco-related lung cancer and a history of alcohol abuse may have ascites due to cirrhosis. Breast, lung, colon, and pancreatic cancers are regularly complicated by ascites.2 Abdominal pain is a helpful distinguishing feature. Malignancy-related ascites frequently is painful, whereas cirrhotic ascites usually is not, unless bacterial peritonitis or alcoholic hepatitis is superimposed.
Ascites may occur in patients with acute pancreatitis with necrosis or a ruptured pancreatic duct from chronic pancreatitis or trauma. Often troublesome ascites also may develop in a small percentage of patients undergoing hemodialysis. Fitz-Hugh–Curtis syndrome caused by Chlamydia or gonorrhea may cause inflammatory ascites in a sexually active woman. Patients in whom ascites and anasarca develop in the setting of diabetes mellitus should be suspected of having nephrotic ascites. Ascites in a patient with symptoms and signs of myxedema should prompt assessment of thyroid function. Serositis in a patient with a connective tissue disease may be complicated by ascites.7
PHYSICAL EXAMINATION
On the basis of the history and the appearance of the abdomen, the diagnosis of ascites is readily suspected and usually confirmed easily on physical examination. The presence of a full, bulging abdomen should lead to percussion of the flanks. If the degree of flank dullness is greater than usual (i.e., if the percussed air-fluid level is higher than that normally found on the lateral aspect of the abdomen with the patient supine), the examiner should check for “shifting.” If flank dullness is absent, checking for shifting is unnecessary. Approximately 1500 mL of fluid must be present before dullness is detected.8 If flank dullness is not present, the chance that the patient has ascites is less than 10%.8 A fluid wave is not worth testing for.8
Gaseous distention of the bowel, a thick panniculus, and an ovarian mass can mimic ascites. Gaseous distention should be readily apparent on percussion. Ovarian masses usually cause tympanitic flanks with central dullness. Also, the speed of increase in abdominal girth can be helpful; ascites develops in days to weeks, whereas thickening of omentum and panniculus takes months to years. An obese abdomen may be diffusely dull to percussion, and abdominal ultrasonography may be required to determine if fluid is present. Ultrasonography can detect as little as 100 mL of fluid in the abdomen.9
DIAGNOSIS
ABDOMINAL PARACENTESIS
Indications
Abdominal paracentesis with appropriate ascitic fluid analysis is probably the most rapid and cost-effective method of diagnosing the cause of ascites. Also, because of the possibility of ascitic fluid infection in a cirrhotic patient admitted to the hospital, a surveillance paracentesis performed on admission may detect unexpected infection.9 Not all patients with ascitic fluid infection are symptomatic; many have subtle symptoms, such as mild confusion noticed only by the family. Detection of infection at an early asymptomatic stage may reduce mortality. Therefore, ascitic fluid should be sampled in all inpatients and outpatients with new-onset ascites and in all patients with ascites who are admitted to the hospital. Paracentesis should be repeated in patients (whether hospitalized or not) in whom symptoms, signs, or laboratory abnormalities suggestive of infection develop (e.g., abdominal pain or tenderness, fever, encephalopathy, hypotension, renal failure, acidosis, peripheral leukocytosis).
Contraindications
Few contraindications to paracentesis have been recognized. Coagulopathy is a potential contraindication; however, most patients with cirrhotic ascites have coagulopathy, and if mild to moderate coagulopathy were viewed as a contraindication to paracentesis, few patients with cirrhosis would undergo this procedure.10 Coagulopathy should preclude paracentesis only when clinically evident fibrinolysis or disseminated intravascular coagulation is present.10 These conditions occur in fewer than 1 per 1000 paracenteses. No data are available to support cutoff values for coagulation parameters beyond which paracentesis should be avoided. Global coagulation is usually normal in the setting of cirrhosis despite abnormal tests of coagulation because there is a balanced deficiency of procoagulants and anticoagulants.11 Even after multiple paracenteses, bloody ascites usually does not develop in patients with severe prolongation of the prothrombin time. Patients with cirrhosis and without clinically obvious coagulopathy simply do not bleed excessively from needlesticks unless a blood vessel is entered.10
Studies regarding complications of paracentesis in patients with ascites have documented no deaths or infections caused by paracentesis.9,10 No episodes of hemoperitoneum or entry of the paracentesis needle into the bowel have been reported in these studies. Complications have included only abdominal wall hematomas in approximately 2% of paracenteses, even though 71% of the patients had an abnormal prothrombin time and 21% had a prothrombin time prolonged by more than five seconds.10 Complication rates may be higher when paracentesis is performed by an inexperienced operator.
Transfusion of blood products (fresh frozen plasma or platelets) routinely before paracentesis in cirrhotic patients with coagulopathy, presumably to prevent hemorrhagic complications, is not supported by data. Because a hematoma that necessitates blood transfusion develops in only approximately 1% of patients who undergo paracentesis without prophylactic transfusion of plasma or platelets, approximately 100 to 200 units of fresh frozen plasma or platelets would have to be given to prevent the transfusion of approximately 2 units of red blood cells. In a prospective study of 1100 therapeutic paracenteses, no blood products were given prior to the procedure nor were they needed after the procedure despite a platelet count as low as 19,000 cells/mm3 [0.25 × 109/L]) and international normalized ratio (INR) as high as 8.7.12
Patient Position and Choice of Needle and Entry Site
The volume of fluid in the abdomen and the thickness of the abdominal wall determine, in part, how the patient should be positioned in preparation for paracentesis. Patients with a large volume of ascites and thin abdominal wall can be “tapped” successfully in the supine position, with the head of the bed or examining table elevated slightly. Patients with less fluid can be placed in the lateral decubitus position and tapped in the midline or in the right or left lower quadrant while supine (see later). Patients with small amounts of fluid may be tapped successfully only in the face-down position or with ultrasound guidance.13
The choice of the site for inserting the needle has changed over the years because of the increasing prevalence of obesity and frequency of therapeutic paracentesis. Paracentesis in obese patients poses special challenges. In obese patients, the abdominal wall usually is substantially thicker in the midline than in the lower quadrants on ultrasound examination.13 The abdominal wall may be even thicker than the length of a 3.5-inch paracentesis needle. Also, on physical examination, determining whether ascites is present or absent in the obese patient is frequently difficult. Ultrasound examination is helpful in confirming the presence of fluid and in guiding the paracentesis needle. Preferably, the needle is inserted into the left lower quadrant, rather than the right lower quadrant because the cecum may be distended with gas from lactulose therapy. Also, the right lower quadrant is more likely than the left to have a surgical scar (e.g., from an appendectomy). When therapeutic paracentesis is performed, more fluid can be obtained using a lower quadrant needle insertion site than a midline site.
The needle must be placed several centimeters from a surgical scar. The bowel may be adherent to the peritoneal surface of the abdomen near a scar, and a needle inserted there may enter the bowel.9 A long midline scar precludes midline paracentesis. An appendectomy scar precludes a right lower quadrant site, in general.
I usually choose a site in the left lower quadrant two fingerbreadths (3 cm) cephalad and two fingerbreadths medial to the anterior superior iliac spine.13 In a patient with multiple abdominal scars, ultrasound guidance may be required.
Technique
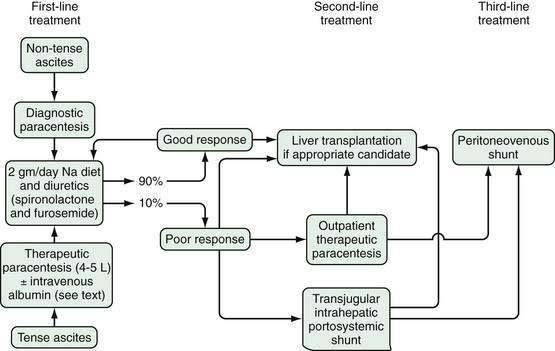
Therapeutic Paracentesis
After samples of fluid are obtained for testing, 2 to 4 L of fluid is removed to relieve the pressure of tense ascites in patients with new or diuretic-sensitive ascites. A sodium-restricted diet and diuretics are prescribed to reduce the fluid further (see later). If a patient is known to be diuretic-resistant, a “total tap” is performed—that is, all of the fluid that is accessible is removed. If less is removed, the tap will need to be repeated soon (see later—“Refractory Ascites”).
ASCITIC FLUID ANALYSIS
Gross Appearance
Non-neutrocytic (i.e., ascitic fluid polymorphonuclear neutrophil [PMN] count less than 250/mm3 [0.25 × 109/L]) ascitic fluid is transparent and usually slightly yellow (Fig. 91-2). Ascitic fluid with a very low protein concentration may have no pigment and look like water. The opacity of many cloudy ascitic fluid specimens is caused by neutrophils. The presence of neutrophils leads to a shimmering effect when a glass tube containing the fluid is rocked back and forth in front of a light. Fluid with an absolute neutrophil count less than 1000/mm3 (1.0 × 109/L) may be nearly clear. Fluid with a count greater than 5000/mm3 (5.0 × 109/L) is quite cloudy, and fluid with a count greater than 50,000/mm3 (50.0 × 109/L) resembles mayonnaise.
Ascitic fluid specimens frequently are blood-tinged or frankly bloody. A red blood cell count of 10,000/mm3 (10.0 × 109/L) is the threshold for a pink appearance; lower concentrations result in clear or turbid fluid. Ascitic fluid with a red blood cell count greater than 20,000/mm3 (20.0 × 109/L) is distinctly red. Many ascitic fluid specimens are bloody because of a traumatic tap; these specimens are blood-streaked and frequently clot unless the fluid is transferred immediately to the anticoagulant-containing tube for the cell count. By contrast, nontraumatic or remotely traumatic blood-tinged ascitic fluid is homogeneous and does not clot because it has already clotted and the clot has lysed. Some patients with portal hypertension have bloody hepatic lymph, resulting in bloody ascitic fluid—perhaps because of rupture of lymphatics that are under high pressure. Samples from patients with hepatocellular carcinoma are regularly bloody, but only about 10% of samples from patients with peritoneal carcinomatosis are red.2 Although many physicians have the impression that tuberculosis results in bloody ascitic fluid, less than 5% of tuberculous samples are hemorrhagic in my experience.
Ascitic fluid frequently is lipid-laden. Lipid opacifies the fluid. The degree of opalescence of ascitic fluid ranges from slightly cloudy to completely opaque and chylous. Most opaque, milky fluid samples have a triglyceride concentration greater than 200 mg/dL (2.26 mmol/L) and usually greater than 1000 mg/dL (11.30 mmol/L). Fluid that has the appearance of dilute skim milk has a triglyceride concentration between 100 mg/dL (1.13 mmol/L) and 200 mg/dL (2.26 mmol/L). A substantial minority of cirrhotic ascitic fluid samples are neither transparent nor frankly milky. These opalescent samples have slightly elevated triglyceride concentrations ranging from 50 mg/dL (0.56 mmol/L) to 200 mg/dL (2.26 mmol/L).14 The opacity of these fluids does not have the shimmering characteristics of ascitic fluid with an elevated white blood cell count. The lipid usually layers out when a tube of ascitic fluid is placed in the refrigerator for 48 to 72 hours. In contrast with findings in older published reports, most patients with chylous or opalescent ascites have cirrhosis.14,15
Dark-brown fluid with a bilirubin concentration greater than that of serum usually indicates biliary perforation.16 Deeply jaundiced patients have bile-stained ascitic fluid, but the bilirubin level and the degree of pigmentation are visually less than those of the corresponding serum. Pancreatic ascites may be pigmented because of the effect of pancreatic enzymes on red blood cells. The red blood cells may have to be centrifuged before the discolored supernatant is revealed. The degree of pigmentation ranges from tea-colored to jet black, as in pancreatic necrosis (formerly hemorrhagic pancreatitis). Black ascitic fluid also may be found in patients with malignant melanoma.
Tests
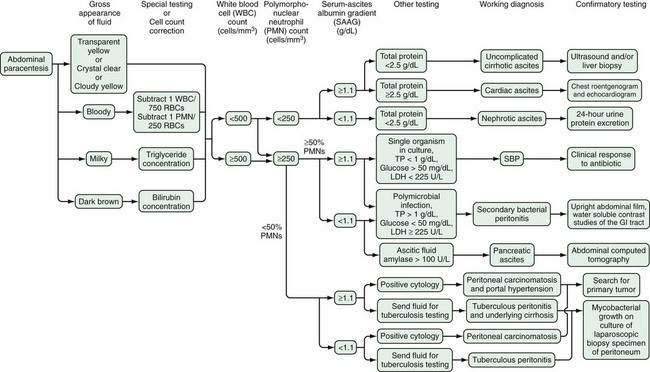
The practice of ordering every available body fluid test on every ascitic fluid specimen is expensive and can be more confusing than helpful, especially when unexpectedly abnormal results are encountered. An algorithm for the analysis of ascitic fluid is shown in Figure 91-2. The basic concept is that screening tests are performed on the initial specimen; additional testing is performed only when necessary as indicated by the results of the screening tests. Further testing may require another paracentesis, but because most specimens consist of ascitic fluid resulting from uncomplicated cirrhosis, no further testing is needed in a majority of cases. Also, because laboratories frequently store the fluid for a few days, additional testing can often be ordered on the stored fluid.
On the basis of cost analysis, tests can be classified as routine, optional, unusual, and unhelpful (Table 91-2).9 The cell count is the single most helpful ascitic fluid test. Only approximately 10 µL of fluid is required for a standard manual hemocytometer count. Therefore, if only one drop of fluid can be obtained, it should be sent for cell count. More fluid is almost always obtainable, however. The fluid should be submitted in an anticoagulant-containing tube (i.e., ethylenediaminetetraacetic acid) to prevent clotting. Because the decision to begin empirical antibiotic treatment of suspected ascitic fluid infection is based largely on the absolute neutrophil count (which should have a turnaround time of a few minutes), rather than the culture (which takes 12 to 48 hours to demonstrate growth), the cell count is more important than the culture in the early detection and treatment of ascitic fluid infection. Even samples from asymptomatic outpatients undergoing therapeutic paracentesis should be sent for a cell and differential count; the information obtained can lead to early, life-saving treatment of bacterial infection.
Cell Count
Surprisingly, ascitic fluid cell counts have not been standardized. Some laboratories count mesothelial cells in addition to white blood cells (WBCs) and label the sum as “nucleated cells.” The usefulness of mesothelial cell counts is not clear. The WBC count in uncomplicated cirrhotic ascites is usually less than 500 cells/mm3 (0.5 × 109/L) (see Fig. 91-2).9,17 During diuresis in patients with cirrhotic ascites, the WBC count can concentrate to more than 1000 cells/mm3 (1.0 × 109/L).17 A diagnosis of diuresis-related elevation of the ascitic fluid WBC count, however, requires that a prediuresis count be available, that normal lymphocytes predominate in the fluid, and that unexplained clinical symptoms or signs (e.g., fever or abdominal pain) be absent.
The upper limit of normal for the absolute PMN count in uncomplicated cirrhotic ascitic fluid is usually stated to be lower than 250/mm3 (0.25 × 109/L).9,17 The short survival of PMNs results in relative stability of the absolute PMN count during diuresis.17 Therefore, the 250 cells/mm3 (0.25 × 109/L) cutoff value remains reliable even after diuresis.
New methods have been developed to estimate the number of ascitic fluid cells.18 Dipsticks can detect an ascitic fluid PMN count greater than 250/mm3 (0.25 × 109/L) in 90 to 120 seconds. Urine-specific dipsticks have been used to date and are not very sensitive.19 What is now needed is an ascitic fluid–specific dipstick.
Any inflammatory process can result in an elevated ascitic fluid WBC count. Spontaneous bacterial peritonitis is the most common cause of inflammation of ascitic fluid and the most common cause of an elevated ascitic WBC count (see later). The total WBC count, as well as the absolute PMN count, is elevated in spontaneous bacterial peritonitis, and PMNs usually account for more than 70% of the total WBC count. Also, in tuberculous peritonitis and peritoneal carcinomatosis, the total ascitic WBC count is frequently elevated, but usually with a predominance of lymphocytes.2
In most instances, bloody ascitic fluid is the result of a slightly traumatic tap. Leakage of blood into the peritoneal cavity leads to an elevated ascitic fluid WBC count. Because neutrophils predominate in blood, the ascitic fluid differential count may be altered by contamination of ascitic fluid with blood. To correct for this, 1 PMN is subtracted from the absolute ascitic fluid PMN count for every 250 red blood cells17 (see Fig. 91-2). If the leakage of blood occurred at a remote time, the PMNs will have lysed, and the corrected PMN count will be a negative number. If the corrected PMN count in a bloody specimen is greater than or equal to 250 cells/mm3 (0.25 × 109/L), the patient must be assumed to be infected.
Exudate/Transudate Classification
Before the 1980s, the ascitic fluid total protein concentration was used to classify ascites as either exudative (greater than 2.5 g/dL [25 g/L]) or transudative (less than 2.5 g/dL [25 g/L]). Unfortunately, this classification does not work well in ascitic fluid, and these terms as applied to ascitic fluid were never carefully defined or validated. Attempts at using combinations of lactate dehydrogenase (LDH) and serum-to–ascitic fluid ratios of LDH and protein also have not been shown to classify ascitic fluid accurately into exudates and transudates.20
Serum-Ascites Albumin Gradient
The serum-ascites albumin gradient (SAAG) has been proved to categorize ascites better than the total protein concentration or other parameters21 (Table 91-3). The SAAG is based on oncotic-hydrostatic balance. Portal hypertension results in an abnormally high hydrostatic pressure gradient between the portal bed and ascitic fluid. A similarly large difference must exist between ascitic fluid and intravascular oncotic forces. Albumin exerts greater oncotic force per gram than that exerted by other proteins. Therefore, the difference between the serum and ascitic fluid albumin concentrations correlates directly with portal pressure.
Table 91-3 Classification of Ascites by Serum-Ascites Albumin Gradient
| HIGH GRADIENT ≥1.1 g/dL (11 g/L) |
LOW GRADIENT <1.1 g/dL (11 g/L) |
|---|---|
| Alcoholic hepatitis | Biliary ascites |
| Budd-Chiari syndrome | Bowel obstruction or infarction |
| Cardiac ascites | Nephrotic syndrome |
| Cirrhosis | Pancreatic ascites |
| Fatty liver of pregnancy | Peritoneal carcinomatosis |
| Fulminant hepatic failure | Postoperative lymphatic leak |
| Massive liver metastases “Mixed” ascites Myxedema |
Serositis in connective tissue diseases |
| Tuberculous peritonitis | |
| Portal vein thrombosis | |
| Sinusoidal obstruction syndrome |
Calculating the SAAG involves measuring the albumin concentration of serum and ascitic fluid specimens and simply subtracting the ascitic fluid value from the serum value. Unless a laboratory error has been made, the serum albumin concentration is always the larger value. The gradient is calculated by subtraction and is not a ratio. If the SAAG is 1.1 g/dL (11 g/L) or greater, the patient can be considered to have portal hypertension with an accuracy of approximately 97%.21 Also, if the serum albumin minus ascitic fluid total protein gradient is 1.1 g/dL (11 g/L) or greater, the patient has portal hypertension because the ascitic fluid albumin concentration cannot be greater than the ascitic fluid total protein concentration. Conversely, if the SAAG is less than 1.1 g/dL (11 g/L), the patient is unlikely to have portal hypertension. The SAAG does not explain the pathogenesis of ascites formation, nor does it explain where the albumin came from—that is, liver or bowel. It simply gives the physician an indirect but accurate index of portal pressure. The accuracy of the test is excellent, even with ascitic fluid infection, diuresis, therapeutic paracentesis, intravenous infusions of albumin, and various causes of liver disease.21
Serum hyperglobulinemia (serum globulin level greater than 5 g/dL [50 g/L]) leads to a high ascitic fluid globulin concentration and can narrow the albumin gradient by contributing to the oncotic forces. A narrowed gradient caused by high serum globulin levels occurs in only approximately 1% of ascitic fluid specimens. To correct the SAAG in the setting of a high serum globulin level, the following formula is used22:
Approximately 5% of patients with ascites have “mixed” ascites (that is, two causes of ascites) (see Table 91-1). Most of these patients have portal hypertension from cirrhosis as well as another cause of ascites, such as tuberculous peritonitis or peritoneal carcinomatosis.21 The albumin gradient is high (1.1 g/dL [11 g/L] or greater) in mixed ascites, as a reflection of the underlying portal hypertension.21
The presence of a high SAAG does not confirm a diagnosis of cirrhosis; it simply indicates the presence of portal hypertension. Many causes of portal hypertension other than cirrhosis are recognized (see Tables 91-1 and 91-3 and Chapter 90). A low SAAG does not confirm a diagnosis of peritoneal carcinomatosis. Although peritoneal carcinomatosis is the most common cause of a low SAAG, other causes exist (see Table 91-3). The SAAG needs to be determined only on the first paracentesis specimen in a given patient; it does not need to be repeated on subsequent specimens, if the first value is definitive. If the first result is borderline (e.g., 1.0 or 1.1 g/dL [10 or 11 g/L]), repeating the paracentesis and analysis usually provides a definitive result. High-albumin-gradient and low-albumin-gradient should replace the modifiers “transudative” and “exudative” in the classification of ascites.21
Culture
In the past, culture methodology for ascitic fluid was based on the notion that most episodes of ascitic fluid infection were polymicrobial with high colony counts, as in surgical peritonitis. The most common bacterial infection of ascitic fluid, spontaneous bacterial peritonitis, is monomicrobial, however, with a low bacterial concentration (median colony count of only 1 organism/mL).23 The older method of culture consisted of inoculation (in the microbiology laboratory) of each of three agar plates and some broth with a few drops of fluid. This method of culturing ascitic fluid, as is used for urine or stool, is predictably insensitive for detecting monomicrobial infections with a low colony count. Spontaneous bacterial peritonitis is more like bacteremia in terms of the number of bacteria present; culturing ascitic fluid as if it were blood has a high yield.23 In fact, the sensitivity of culture in detecting bacterial growth in neutrocytic ascites (i.e., ascitic fluid with a PMN count of 250 cells/mm3 [0.25 × 109/L] or greater) depends on the method of culture used. The older method of culture has been found to detect bacterial growth in approximately 50% of neutrocytic samples, whereas bedside inoculation of blood culture bottles with ascitic fluid detects growth in approximately 80%.9 Multiple prospective studies have demonstrated the superiority of the blood culture bottle method.9 Also, bedside inoculation is superior to delayed laboratory inoculation of blood culture bottles in the laboratory.24 Gene probes are now commercially available for the detection of bacteremia; hopefully, they will also lead to rapid (30-minute) and accurate detection of organisms in ascitic fluid. Culture will continue to be required, however, for assessment of the susceptibility of the organism to antibiotics.
Total Protein
As noted earlier, the antiquated exudate/transudate system of ascitic fluid classification, which is based on ascitic fluid total protein concentration, is problematic. The protein concentration in ascitic fluid in the setting of cirrhosis is determined almost entirely by the serum protein concentration and portal pressure. A patient with cirrhosis and a relatively high serum protein concentration will have a relatively high ascitic fluid protein concentration. Because of this relationship, almost 20% of ascitic samples in patients with cirrhosis will have a protein concentration greater than 2.5 g/dL (25 g/L). The ascitic fluid total protein concentration does not increase during spontaneous bacterial peritonitis; it remains stable before, during, and after infection.25 In fact, patients with the lowest ascitic protein concentrations are the most susceptible to spontaneous peritonitis.26 During a 10-kg diuresis, the ascitic fluid total protein concentration doubles, and 67% of such patients with cirrhotic ascites have a protein concentration greater than 2.5 g/dL (25 g/L) by the end of diuresis.17 In almost one third of patients with malignant ascites, the ascites is caused by massive liver metastases or hepatocellular carcinoma, and the ascitic fluid in these patients has a low protein concentration.2 In cardiac ascites, the ascitic fluid protein concentration is greater than 2.5 g/dL (25 g/L).27
Therefore, the exudate/transudate method of classification of ascites places many patients with cirrhosis and ascites and all patients with cardiac ascites in the exudate category and many patients with malignant ascites and essentially all patients with spontaneously infected ascites in the transudate category. Clearly, this method of classification is not useful. By contrast, the SAAG classifies fluid by the presence or absence of portal hypertension and is much more physiologic and intuitive in nature.21 The albumin gradient classifies cardiac ascites in the high-SAAG category, similar to cirrhotic ascites. The high SAAG of cardiac ascites is presumably the result of high right-sided cardiac pressures. In patients with cardiac ascites, the SAAG may narrow with diuresis; such narrowing does not happen in patients with cirrhosis.
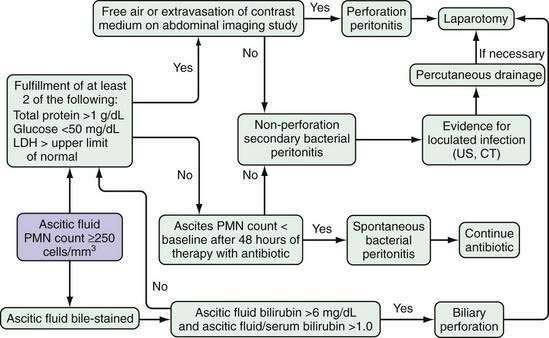
The combination of ascitic fluid total protein, glucose, and LDH is of value in distinguishing spontaneous bacterial peritonitis from intestinal perforation with leakage of gut contents into ascites28 (Fig. 91-3). Patients who have neutrocytic ascitic fluid, in whom the clinical picture suggests bacterial peritonitis (rather than peritoneal carcinomatosis or tuberculous peritonitis) and who meet two of the following three criteria, are likely to have surgical peritonitis and merit immediate radiologic evaluation to determine if intestinal perforation with leakage of intestinal contents into ascites has occurred: total protein greater than 1 g/dL (10 g/L), glucose less than 50 mg/dL (2.8 mmol/L), and LDH greater than the upper limit of normal for serum.28
Glucose
The glucose molecule is small enough to diffuse readily into body fluid cavities. Therefore, the concentration of glucose in ascitic fluid is similar to that in serum, unless glucose is being consumed by ascitic fluid WBCs or bacteria.28 In early spontaneous bacterial peritonitis, the ascitic fluid glucose concentration is similar to that of sterile fluid.25 By contrast, in spontaneous bacterial peritonitis detected late in its course (as well as in the setting of intestinal perforation into ascitic fluid), the ascitic fluid glucose concentration usually drops to 0 mg/dL (0 mmol/L) because of large numbers of stimulated neutrophils and bacteria.28
Lactate Dehydrogenase
The LDH molecule is too large to enter ascitic fluid readily from blood,28 and the ascitic fluid concentration of LDH usually is less than one half of the serum level in uncomplicated cirrhotic ascites. In spontaneous bacterial peritonitis, the ascitic fluid LDH level rises because of the release of LDH from neutrophils, and the ascitic fluid concentration is greater than that of serum. In secondary peritonitis, the LDH level is even higher than that seen in spontaneous bacterial peritonitis and may be several-fold higher than the serum LDH level.28
Amylase
In uncomplicated ascites in the setting of cirrhosis, the ascitic fluid amylase concentration usually is one half that of the serum value, approximately 50 U/L.29 In patients with acute pancreatitis or intestinal perforation (with release of luminal amylase into the ascitic fluid), the fluid amylase concentration is elevated markedly, usually greater than 2000 U/L and approximately five-fold greater than simultaneous serum values.28–30
Gram Stain
Gram stains of body fluids demonstrate bacteria only when more than 10,000 bacteria/mL are present. The median ascitic concentration of bacteria in spontaneous bacterial peritonitis is only 1 organism/mL, similar to the colony count in bacteremia.23 Requesting an ascitic fluid Gram stain to detect bacteria in spontaneous bacterial peritonitis is analogous to requesting a Gram stain of blood to detect bacteremia. Bacteria are detected on Gram stain only with overwhelming infection, as in advanced spontaneous bacterial peritonitis or asplenic pneumococcal sepsis. Gram stain of ascitic fluid is most helpful in the diagnosis of free perforation of the intestine into ascitic fluid. In this setting, sheets of multiple different bacteria are found. Gram stain of the centrifuged sediment of 50 mL of ascites has a sensitivity rate of only 10% for visualizing bacteria in spontaneous bacterial peritonitis.23
Smear and Culture for Tuberculosis
A direct smear of ascitic fluid to detect mycobacteria is almost never positive because of the rarity of tuberculous peritonitis and the low concentration of mycobacteria in ascitic fluid in tuberculous peritonitis.31 The older literature suggests that 1 L of fluid should be cultured. The largest centrifuge tube found in most laboratories, however, has a capacity of 50 mL. In general, only one 50-mL aliquot of fluid is centrifuged, and the pellet is cultured. In contrast to a sensitivity rate of approximately 50% for ascitic fluid mycobacterial culture with optimal processing, laparoscopy with histology and culture of peritoneal biopsies has a sensitivity approaching 100% for detecting tuberculous peritonitis.31 Tuberculous peritonitis can easily be confused with spontaneous bacterial peritonitis because both conditions are associated with abdominal pain and fever, and one half of the patients with tuberculous peritonitis have cirrhosis. A negative bacterial culture and predominance of mononuclear cells in the differential count, however, provide clues to the diagnosis of tuberculous peritonitis. DNA probes are now available to detect mycobacteria and probably will replace older methods of detection.32 Nevertheless, cultures still will be required to determine susceptibility to antimicrobial agents.
Cytologic Examination
In the past, ascites related to malignancy was assumed to be caused only by peritoneal carcinomatosis; massive liver metastases and hepatocellular carcinoma superimposed on cirrhosis were not recognized as causes of malignant ascites. These studies did not compare cytologic examination with a standard diagnostic test, such as autopsy, laparotomy, or laparoscopy, and cytologic study was reported to have a sensitivity of only about 60% in detecting malignant ascites.33 Cytologic studies, however, can be expected to detect malignancy only when tumor cells line the peritoneal cavity and exfoliate into the ascitic fluid—that is, in peritoneal carcinomatosis. Such studies should not be expected to detect tumor when the peritoneum is uninvolved, as in ascites resulting from portal hypertension in patients with hepatocellular carcinoma or massive liver metastases or from lymph node obstruction in patients with malignant lymphoma.2 In one study in which the location and type of tumor that caused ascites were confirmed by a standard test, only approximately two thirds of patients with malignancy-related ascites were found to have peritoneal carcinomatosis, but nearly 100% of patients with peritoneal carcinomatosis were reported to have positive findings on cytologic examination of ascitic fluid; the remaining one third of patients with massive liver metastases, chylous ascites caused by lymphoma, or hepatocellular carcinoma had negative cytologic findings.2 Therefore, the sensitivity of cytology is approximately 100% for detecting peritoneal carcinomatosis but much lower for detecting malignancy-related ascites caused by conditions other than peritoneal carcinomatosis. Cytologic studies should not be falsely positive if performed carefully; I have never encountered a false-positive result.
Because hepatocellular carcinoma rarely metastasizes to the peritoneum, a positive ascitic fluid cytology in a patient with hepatocellular carcinoma is unusual enough to be the subject of a case report.34 Measurement of the serum alpha fetoprotein concentration (which is always higher in serum than in ascitic fluid) may be of value in detecting hepatocellular carcinoma; serum alpha fetoprotein is much more sensitive than ascitic cytology for this purpose.2 In malignancy-related ascites, the fluid may have an elevated PMN count, presumably because dying tumor cells attract neutrophils.2 The elevated PMN count may cause confusion with spontaneous bacterial peritonitis; however, a predominance of lymphocytes in malignancy-related ascites is usual. Flow cytometry and magnetic enrichment of ascitic fluid as an adjunct to cytology may further increase diagnostic accuracy.35
Triglyceride
A triglyceride level should be measured in opalescent or frankly milky ascitic fluid (see Fig. 91-2). By definition, chylous ascites has a triglyceride concentration greater than 200 mg/dL (2.26 mmol/L) and greater than the serum level; usually, the level is greater than 1000 mg/dL (11.30 mmol/L).36 In sterile ascitic fluid specimens in the setting of cirrhosis that are slightly cloudy, without an elevated cell count (i.e., opalescent), the triglyceride concentration is elevated—64 ± 40 mg/dL (0.72 ± 0.45 mmol/L), compared with 18 ± 9 mg/dL (0.20 ± 0.10 mmol/L) for clear ascites in the setting of cirrhosis.14
Bilirubin
The bilirubin concentration should be measured in ascitic fluid that is dark brown. An ascitic fluid bilirubin level greater than 6 mg/dL (102 µmol/L) and greater than the serum level of bilirubin suggests biliary or proximal small intestinal perforation into ascitic fluid.16,28
Tests That Are Seldom Helpful
Tests that have been proposed to be helpful in the analysis of ascitic fluid but shown subsequently to be of no benefit include determination of pH, lactate, fibronectin, and cholesterol. The studies that attempted to validate the value of pH and lactate included small numbers of patients and used suboptimal culture techniques. In the two largest and most recent studies, which did not have some of the deficiencies of the earlier studies, the ascitic fluid pH and lactate were found not to be helpful.37,38 The pH was found to have no impact on decision-making regarding the use of empirical antibiotic therapy.37
Fibronectin and cholesterol have been proposed to be useful in detecting malignant ascites. The basic premise in studies of these markers was that ascitic fluid cytologic examination is insensitive. Unfortunately, the design of the studies was problematic, several subgroups of malignancy-related ascites (e.g., massive liver metastases, hepatocellular carcinoma with cirrhosis) were not considered, and appropriate control groups (e.g., patients with ascites caused by conditions other than cirrhosis or peritoneal carcinomatosis) were not included. Other studies have demonstrated that in patients with massive liver metastases, ascitic fluid fibronectin and cholesterol concentrations are not abnormally elevated.39,40 Therefore, in patients with malignancy-related ascites and negative cytologic findings, these “humoral tests of malignancy” are usually negative. Additionally, patients with high-protein noncirrhotic ascites nearly always have ascitic fibronectin and cholesterol elevations despite the absence of malignancy.2,39,40
Carcinoembryonic antigen (CEA) in ascitic fluid has been proposed as a helpful marker for detecting malignant ascites.41 The study that attempted to validate this proposal, however, was flawed, and more studies, with various subgroups of patients, are required before testing for ascitic fluid CEA can be considered validated.
Measurement of adenosine deaminase has been proposed as a useful test for detecting peritoneal tuberculosis. In the United States, however, where greater than 50% of patients with tuberculous peritonitis have underlying cirrhosis, the adenosine deaminase level has been found to be too insensitive to be helpful.31
DIFFERENTIAL DIAGNOSIS OF ASCITES
Although cirrhosis is the cause of ascites in most patients with ascites evaluated by an internist, a cause other than liver disease is found in approximately 15% of patients (see Table 91-1). Approximately 5% of patients have two causes of ascites, that is, “mixed” ascites.21 Usually, these patients have cirrhosis plus one other cause, such as peritoneal carcinomatosis or tuberculous peritonitis (see Table 91-1). Because tuberculosis is potentially fatal but curable and frequently occurs in cirrhotic patients with preexisting ascites, the physician must not assume that liver disease is the only cause of ascites in a febrile alcoholic patient if the ascitic fluid analysis is atypical. For example, if the ascitic fluid lymphocyte count is unusually high, tuberculous peritonitis may be present. Interpretation of the results of ascitic fluid analysis is difficult in patients with mixed ascites but crucial to accurate diagnosis and treatment. Additionally, liver diseases other than cirrhosis (e.g., alcoholic hepatitis or fulminant hepatic failure) may cause ascites (see Table 91-1).
An algorithm for the differential diagnosis of ascites is shown in Figure 91-2. This proposed strategy is applicable to a majority of patients with ascites, including many with the causes listed in Table 91-1. Not every patient (including patients with rare causes of ascites) can be categorized readily with such an algorithm, however. Many patients with enigmatic ascites eventually are found to have two or even three causes of ascites (e.g., heart failure, cirrhosis caused by NASH, diabetic nephropathy). In these cases, the sum of predisposing factors leads to sodium and water retention, even though each factor alone may not be severe enough to cause fluid overload.
In most patients with ascites, cirrhosis is the cause. This form of ascites, especially when low in protein, is complicated frequently by spontaneous bacterial peritonitis (see later).26 Other forms of ascites are complicated by spontaneous peritonitis so rarely that they are the subjects of case reports or small series.
The intestine can perforate with spillage of contents in patients with ascites of any cause, cirrhosis or otherwise. The ascitic fluid analysis in intestinal perforation is dramatically different from that in spontaneous bacterial peritonitis (see Fig. 91-3).28 Distinguishing spontaneous bacterial peritonitis from surgical peritonitis in a patient with cirrhosis is critical to the patient’s survival; spontaneous bacterial peritonitis is treated with antibiotics alone, whereas surgical peritonitis is treated with antibiotics and emergency surgical intervention (see Chapter 37).
Cancer accounts for fewer than 10% of cases of ascites (see Table 91-1). Not all cases of malignancy-related ascites are caused by peritoneal carcinomatosis; the characteristics of the ascitic fluid and the treatments vary, depending on the pathophysiology of the ascites—for example, peritoneal carcinomatosis versus massive liver metastases2 (Table 91-4; see also “Ascitic Fluid Analysis”).
Table 91-4 Classification of Malignancy-Related Ascites
Congestive heart failure accounts for less than 5% of cases of ascites (see Chapter 83). Cardiac ascites is characterized by a high-albumin gradient, high ascitic fluid protein concentration, and normal blood hematocrit value.27 The gradient may narrow with diuresis, in contrast to cirrhosis. Patients with cardiac ascites often have alcoholic cardiomyopathy, with cardiomegaly on a chest radiograph and four-chamber enlargement of the heart on an echocardiogram. Clinically, heart failure may mimic cirrhosis, including the presence of small nonbleeding esophageal varices and hepatic encephalopathy.42 Ascites in the setting of cirrhosis is characterized by a high albumin gradient, as in cardiac ascites, but a low protein concentration, and patients with cirrhosis and ascites have a lower mean blood hematocrit value of 32%.27 Serum pro-brain-type natriuretic peptide also can be useful in distinguishing cardiac ascites from ascites due to cirrhosis. The median value is 6100 pg/mL in the former but only 166 pg/mL in the latter.43
In the United States, tuberculous peritonitis generally is a disease of Asian and Latin American immigrants to the West Coast, poor African Americans, and the elderly. Tuberculous peritonitis was a rare disease between 1955 and 1985, but it has increased in prevalence since then because of the acquired immunodeficiency syndrome (AIDS).44 Fifty percent of patients with tuberculous peritonitis have underlying cirrhosis (and thus, “mixed” ascites). Although most patients with liver disease are not unusually predisposed to the hepatotoxicity of antituberculosis drugs, they tolerate drug toxicity less well than do patients with a normal liver.45 Underdiagnosis can lead to unnecessary deaths from untreated tuberculosis, whereas overdiagnosis and overtreatment of suspected but unproven tuberculous peritonitis may lead to unnecessary deaths from the hepatotoxicity of isoniazid. If the clinical circumstances (e.g., fever in an immigrant from an area endemic for tuberculosis) and results of the initial ascitic fluid analysis (high lymphocyte count) suggest tuberculosis, strong consideration should be given to an urgent laparoscopy with histologic examination and culture of peritoneal biopsy specimens. If at laparoscopy the peritoneum demonstrates the typical “millet-seed” and “violin-string” appearance, antituberculosis therapy can be started immediately. Blind peritoneal biopsy may be performed in the patient without cirrhosis; however, in a patient with cirrhosis, the predictable presence of peritoneal collateral veins makes blind biopsy potentially hazardous, and laparoscopically guided biopsy is preferable. Suspected tuberculous peritonitis is one of the few remaining indications for diagnostic laparoscopy. Peritoneal coccidioidomycosis can mimic tuberculous peritonitis, including its appearance at laparoscopy, and can occur in patients without AIDS.46
The high sensitivities of cytology for peritoneal carcinomatosis and ultrasound-guided biopsy for focal liver lesions have obviated the need for laparoscopy in detecting tumor, for all practical purposes.2
Pancreatic ascites, an uncommon condition, occurs in patients with clinically obvious severe acute pancreatitis or a history of chronic pancreatitis or pancreatic trauma (see Chapters 58 and 59).29 Ordering an ascitic fluid amylase level on all ascitic fluid samples is unnecessary; the test is indicated only in patients in whom pancreatitis is suspected or the initial ascitic fluid is nondiagnostic (see Table 91-2). Patients with alcohol-related pancreatic ascites may also have underlying alcoholic cirrhosis. Pancreatic ascites frequently is neutrocytic and may also be complicated by bacterial infection. Patients with an ascitic fluid neutrophil count of 250 cells/mm3 (0.25 × 109/L) or greater merit empirical antibiotic coverage, at least until the cause of the elevated neutrophil count is explained.
Nephrogenous ascites is a poorly understood form of ascites that develops in patients undergoing hemodialysis.47 On careful evaluation, most patients with ascites in the setting of hemodialysis are found to have another cause of ascites, usually cirrhosis from alcohol abuse or from hepatitis C. The presence of a second cause of fluid overload explains why these patients have ascites, whereas a majority of patients on dialysis do not.
Although the nephrotic syndrome used to be a common cause of ascites in children, it is rare in adults.48 When it occurs in adults, a second cause of ascites usually is present, just as in nephrogenous ascites.48 The ascitic fluid is usually characterized by a low protein concentration and low SAAG and can be complicated by spontaneous bacterial peritonitis.
In some patients, pathologic accumulation of fluid develops in the peritoneal cavity as a result of leakage from a ruptured viscus (e.g., “bile ascites” from a ruptured gallbladder).16,28 The ascitic fluid analysis is critical to the preoperative diagnosis of this condition (see earlier “Ascitic Fluid Analysis,” and Fig. 91-3).
Chylous ascites develops when intra-abdominal lymphatics containing chyle rupture. The older literature suggests that this form of ascites is caused by a malignancy in nearly 90% of cases.36 By contrast, cirrhosis is the cause of chylous ascites in more than 90% of the patients whom I have encountered (see Table 91-1).15,21 The high lymphatic flow and pressure are presumed to be the cause of lymphatic rupture in patients with cirrhosis. In addition, retroperitoneal surgery and radical pelvic surgery in patients with cancer can transect lymphatics and thereby lead to chylous ascites.
Additional causes of ascites include ambulatory peritoneal dialysis, Budd-Chiari syndrome, myxedema, connective tissue disease, postoperative ascites, and rare entities. With the iatrogenic form of ascites associated with peritoneal dialysis, the patient is usually not under the care of a gastroenterologist. Although Budd-Chiari syndrome is regularly complicated by ascites, hepatic vein thrombosis is rare and accounts for less than 0.1% of cases of ascites (see Chapter 83). Ascites in patients with myxedema appears to be related to heart failure49; treatment of the hypothyroidism cures the fluid retention. Serositis with development of ascites may complicate systemic lupus erythematosus (see Chapter 35).7
Ascites after abdominal surgery (often after cholecystectomy in the setting of asymptomatic gallstones and abnormal liver biochemical test results) is a common mode of presentation of previously undiagnosed cirrhosis.50 Resection of hepatocellular carcinoma in the setting of cirrhosis regularly leads to hepatic decompensation, which all too often starts a downward spiral ending in death.51
Aggressive hormone administration to induce ovulation can lead to ascites from “ovarian hyperstimulation syndrome.”52 Other rare causes of ascites include the POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M component, and skin changes) and hemophagocytic syndrome.53,54 The latter is a rare syndrome that usually occurs in patients with leukemia or lymphoma and can masquerade as decompensated cirrhosis.54 Ascites that recurs or does not resolve after liver transplantation appears to be due to relative hepatic venous outflow obstruction or hepatitis C but frequently is enigmatic.55,56
COMPLICATIONS
ASCITIC FLUID INFECTION, INCLUDING SPONTANEOUS BACTERIAL PERITONITIS
Ascitic fluid infection can be classified into five categories based on ascitic culture results, PMN count, and presence or absence of a surgical source of infection (Table 91-5). An abdominal paracentesis must be performed and ascitic fluid must be analyzed before a confident diagnosis of ascitic fluid infection can be made. A “clinical diagnosis” of infected ascitic fluid without a paracentesis is inadequate.
Table 91-5 Classification of Ascitic Fluid Infection
Classification
Of the three subtypes of spontaneous ascitic fluid infection, the prototype is spontaneous bacterial peritonitis. The diagnosis of spontaneous bacterial peritonitis is made when there is a positive ascitic fluid culture and an elevated ascitic fluid absolute PMN count (i.e., at least 250 cells/mm3 [0.25 × 109/L]) without evidence of an intra-abdominal surgically treatable source of infection.9 When Correia and Conn coined the term “spontaneous bacterial peritonitis” in 1975, their goal was to distinguish this form of infection from surgical peritonitis,57 an important distinction. Therefore, although many patients with spontaneous bacterial peritonitis have a focus of infection (e.g., urinary tract infection or pneumonia), the diagnosis of spontaneous bacterial peritonitis is still appropriate unless the focus requires surgical intervention (e.g., a ruptured viscus). I have not encountered a convincing case of polymicrobial spontaneous bacterial peritonitis; all of the patients presumed to have spontaneous bacterial peritonitis in whom ascitic fluid cultures initially grew more than one organism eventually were found to have surgical peritonitis or an erroneous culture result (e.g., a pathogen plus a contaminant or two colony morphologies of one species of bacteria).
The criteria for a diagnosis of monomicrobial non-neutrocytic bacterascites (MNB) include (1) a positive ascitic fluid culture for a single organism, (2) an ascitic fluid PMN count lower than 250 cells/mm3 (0.25 × 109/L), and (3) no evidence of an intra-abdominal surgically treatable source of infection.58 In the older literature, MNB was either grouped with spontaneous bacterial peritonitis or labeled “asymptomatic bacterascites.” Because many patients with bacterascites have symptoms, the modifier “asymptomatic” seems inappropriate.
Culture-negative neutrocytic ascites (CNNA) is diagnosed when (1) the ascitic fluid culture grows no bacteria, (2) the ascitic fluid PMN count is 250 cells/mm3 (0.25 × 109/L) or greater, (3) no antibiotics have been given (not even a single dose), and (4) no other explanation for an elevated ascitic PMN count (e.g., hemorrhage into ascites, peritoneal carcinomatosis, tuberculosis, or pancreatitis) can be identified.59 This variant of ascitic fluid infection seldom is diagnosed when sensitive culture methods are used.23
Secondary bacterial peritonitis is diagnosed when (1) the ascitic fluid culture is positive (usually for multiple organisms), (2) the PMN count is 250 cells/mm3 (0.25 × 109/L) or greater, and (3) an intra-abdominal surgically treatable primary source of infection (e.g., perforated intestine, perinephric abscess) has been identified.28 The importance of distinguishing this variant from spontaneous bacterial peritonitis is that secondary peritonitis usually requires emergency surgical intervention (see also Chapter 37).
Polymicrobial bacterascites is diagnosed when (1) multiple organisms are seen on Gram stain or cultured from the ascitic fluid and (2) the PMN count is lower than 250 cells/mm3 (0.25 × 109/L).60 This diagnosis should be suspected when the paracentesis is traumatic or unusually difficult because of ileus or when stool or air is aspirated into the paracentesis syringe. Polymicrobial bacterascites is essentially diagnostic of intestinal perforation by the paracentesis needle.
Clinical Setting
Essentially all patients with spontaneous bacterial peritonitis have an elevated serum bilirubin level and abnormal prothrombin time due to advanced cirrhosis.9 Ascites appears to be a prerequisite for the development of spontaneous bacterial peritonitis. The peritonitis is unlikely to precede the development of ascites. Usually, the infection develops when the volume of ascites is at its maximum.
Secondary bacterial peritonitis and polymicrobial bacterascites can develop with ascites of any type. The only prerequisite, in addition to the presence of ascites, is an intra-abdominal surgical source of infection.28 Such an infection can result from penetration of a needle into the bowel during attempted paracentesis.60
Pathogenesis
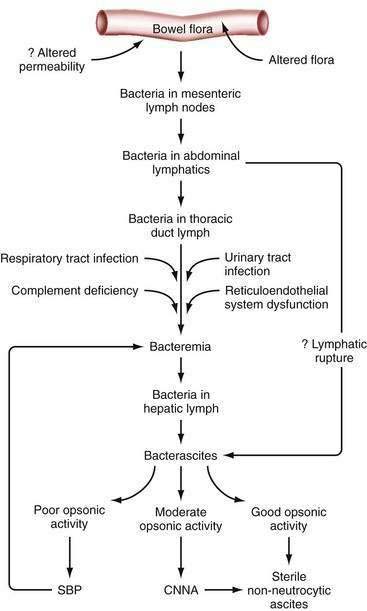
Since the 1990s, the elusive cause of spontaneous bacterial peritonitis has become clearer, and the pathogenesis of spontaneous forms of ascitic fluid infection has been partially elucidated (Fig. 91-4). The body of currently available evidence suggests that the spontaneous forms of ascitic fluid infection are the result of overgrowth of a specific organism in the intestine, “translocation” of that microbe from the intestine to mesenteric lymph nodes, and resulting spontaneous bacteremia and subsequent colonization of susceptible ascitic fluid61–62 (see Fig. 91-4).
When bacteria enter the fluid in the abdomen, by whatever route, a battle ensues between the virulence factors of the organism and the immune defenses of the host.63 The ascitic fluid protein concentration does not change with development of spontaneous infection.25 Low-protein ascitic fluid (e.g., protein content less than 1 g/dL [10 g/L]) is particularly susceptible to spontaneous bacterial peritonitis.26 The endogenous antimicrobial (opsonic) activity of human ascitic fluid correlates directly with the protein concentration of the fluid.62 Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis.64 Patients with detectable ascitic fluid opsonic activity appear to be protected from spontaneous bacterial peritonitis unless they are exposed to a particularly virulent organism (e.g., Salmonella).63,64
Studies in both patients and animals with cirrhosis demonstrate that MNB is common.58,65 Pieces of bacterial DNA are commonly present in serum and ascitic fluid of patients with cirrhosis.66 In both humans and rats, most episodes of bacterascites resolve without antibiotic treatment.58,65 The fluid frequently becomes sterile without an increase in ascitic PMNs. Apparently, the host’s defense mechanisms are able to eradicate the invading bacteria on most occasions. Uncontrolled infection probably develops only when the defenses are weak or the organism is virulent (see Fig. 91-4). Bacterascites probably is more common than spontaneous bacterial peritonitis. Conceivably, ascitic fluid in the setting of cirrhosis is colonized regularly by bacteria, and almost just as regularly, the colonization resolves. The entry of PMNs into the fluid probably signals failure of the peritoneal macrophages to control the infection.67 A majority of episodes of MNB appear to resolve in cirrhotic rats and humans, whereas untreated spontaneous bacterial peritonitis is frequently fatal. In summary, MNB probably represents an early stage of ascitic fluid infection, which can resolve or progress to CNNA or to spontaneous bacterial peritonitis.
Most episodes of CNNA are diagnosed by insensitive culture methods for which numbers of bacteria are insufficient to reach the threshold of detectability.23 Inoculation of ascitic fluid into blood culture bottles can lead to detection of a single organism in the cultured aliquot of fluid, whereas the older method of culture by inoculation of agar plates and broth probably requires at least 100 organisms/mL.23 Even when optimal culture methods are used, however, a small percentage of specimens of neutrocytic ascitic fluid grow no bacteria. A study of rapid sequential paracenteses (before the initiation of antibiotic treatment) in patients with CNNA demonstrated that, in most cases, the PMN count dropped spontaneously and the culture results remained negative in the second specimen.68 When sensitive culture techniques are used, CNNA probably results from (1) previous antibiotic treatment (even one dose), (2) an inadequate volume of fluid inoculated, or (3) spontaneously resolving spontaneous bacterial peritonitis in which the paracentesis is performed after all bacteria have been killed by host defenses but before the PMN count has normalized.
The pathogenesis of secondary bacterial peritonitis is more straightforward than that of spontaneous bacterial peritonitis. When the intestine perforates, billions of bacteria flood into the ascitic fluid. In the absence of a frank perforation, bacteria may cross inflamed tissue planes and enter the fluid. The pathogenesis of polymicrobial bacterascites is also obvious.60 A paracentesis needle enters the bowel, and the bowel contents are released into the ascites.
Symptoms and Signs
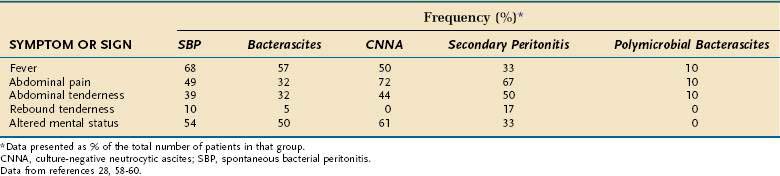
Although 87% of patients with spontaneous bacterial peritonitis are symptomatic at the time the infection is diagnosed, the symptoms and signs of infection are often subtle, such as a slight change in mental status.58 Without prompt paracentesis, the diagnosis and treatment of infected ascites may be delayed, often resulting in the death of the patient. The symptoms and signs manifested in all five variants of ascitic fluid infection are listed in Table 91-6.
Frequency
Since the 1980s, routine paracenteses at the time of hospitalization in patients with ascites have provided data regarding the frequency of ascitic fluid infection. In the 1980s, approximately 10% of patients with ascites were infected at the time of hospital admission; of the subgroup of patients with cirrhosis, about 27% were infected.9 At present, because of measures to prevent spontaneous bacterial peritonitis, the frequency has dropped significantly (see later). Of patients with culture-positive ascitic fluid, about two thirds have neutrocytic ascitic fluid (spontaneous bacterial peritonitis), and one third have MNB.58 The frequency of CNNA depends largely on the culture technique (see earlier). Polymicrobial bacterascites occurs in only 1 in 1000 paracenteses. Secondary bacterial peritonitis is found in only 0% to 2% of patients with ascites at the time of hospital admission.9,28
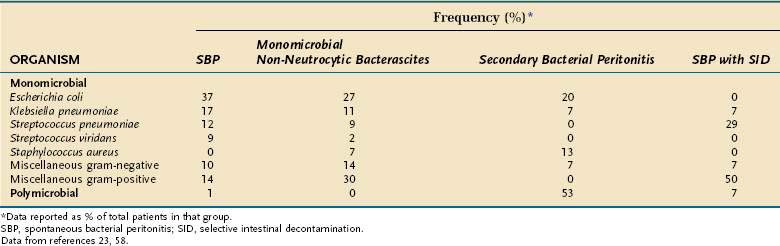
Bacteriology
Escherichia coli, streptococci (mostly pneumococci), and Klebsiella cause most episodes of spontaneous bacterial peritonitis and MNB in patients who are not receiving selective intestinal decontamination (Table 91-7; see later); CNNA is, by definition, culture-negative and polymicrobial bacterascites is, by definition, polymicrobial. The most apparent difference between the spontaneous forms of ascitic fluid infection and the secondary forms (secondary peritonitis and polymicrobial bacterascites) is that the former always are monomicrobial and the latter usually are polymicrobial. Although older papers reported that anaerobic bacteria were present in approximately 6% of cases of spontaneous bacterial peritonitis, the detection of anaerobes probably reflected unrecognized cases of secondary bacterial peritonitis. In more recent series, anaerobes have been found in approximately 1% of cases of spontaneous bacterial peritonitis and MNB.23,58
Selective intestinal decontamination causes a change in the bacteria isolated from patients in whom an ascitic infection develops. Gram-positive organisms are frequently cultured from the ascitic fluid of these patients (see Table 91-7).69
Risk Factors
Patients with cirrhosis are unusually predisposed to bacterial infection because of multiple defects in immune defense. The concept that cirrhosis is a form of acquired immunodeficiency (in the generic sense) is rather new. In a prospective study, a bacterial infection occurred in 34% of 405 patients with cirrhosis at the time of admission to the hospital or during the hospitalization.70 Low ascitic fluid total protein concentrations, as well as the phagocytic (both motile and stationary) dysfunction associated with cirrhosis, are risk factors for bacterial infection.
Paracentesis itself has been proposed as a risk factor for ascitic fluid infection. This theoretical risk has not been substantiated in prospective studies of paracentesis-related complications.10 Spontaneous bacterial peritonitis is statistically more likely to be diagnosed on the first paracentesis than on subsequent taps.10 Needle-induced ascitic fluid infections do not occur unless the bowel is penetrated by the paracentesis needle10,60; fortunately, this occurs in only 1 in 1000 taps. One would expect bacteria of the skin flora such as Staphylococcus aureus to be isolated more frequently if poor paracentesis technique were the cause of many cases of spontaneous bacterial peritonitis; yet skin flora microorganisms are seldom isolated from ascitic fluid when sterile technique is used.23 Iatrogenic peritonitis is most likely to occur when the paracentesis needle enters the bowel during a difficult paracentesis.
Gastrointestinal hemorrhage is an under-recognized risk factor for the development of spontaneous bacteremia and spontaneous bacterial peritonitis. The cumulative probability of infection during a single hospitalization for bleeding is approximately 40%.71 The risk appears to peak 48 hours after the onset of hemorrhage. The high risk of infection probably is mediated by a shock-induced increase in the translocation of bacteria from the intestine to extraintestinal sites. Urinary tract infections also constitute an under-recognized risk factor for spontaneous bacterial peritonitis.72
Diagnosis
Timely diagnosis of ascitic fluid infection requires a high index of suspicion and a low threshold for performing a paracentesis. Clinical deterioration, especially fever or abdominal pain, in a patient with ascites should raise the suspicion of infection and prompt a paracentesis. If the ascitic fluid PMN count is elevated, the working diagnosis is ascitic fluid infection until proved otherwise. Although peritoneal carcinomatosis, pancreatitis, hemorrhage into ascites, and tuberculosis can lead to an elevated ascitic fluid PMN count, most cases of neutrocytic ascites are caused by bacterial infection. A predominance of PMNs in the WBC differential count lends further support for the diagnosis of infection. In patients with peritoneal carcinomatosis, pancreatitis, and tuberculosis, a predominance of PMNs in the ascites would be an uncommon finding. An elevated absolute ascitic fluid PMN count with a predominance of neutrophils in a clinical setting compatible with infection should prompt empirical antibiotic therapy (Table 91-8; see later).
Table 91-8 Indications for Empirical Antibiotic Therapy of Suspected Spontaneous Ascitic Fluid Infection
Although spontaneous bacterial peritonitis is approximately six times as common as surgical peritonitis in a patient with ascites, secondary peritonitis should be considered in any patient with neutrocytic ascites (see also Chapter 37). Clinical symptoms and signs do not distinguish patients with secondary peritonitis from those with spontaneous bacterial peritonitis (see Fig. 91-3).28 Even with free perforation of the colon into ascitic fluid, a classic surgical abdomen does not develop. Peritoneal signs require contact of inflamed visceral and parietal peritoneal surfaces, and such contact does not occur when there is a large volume of fluid separating these surfaces. Intestinal perforation can be suspected and pursued if a specimen of ascites is neutrocytic and meets two of the following three criteria (see Fig. 91-3): (1) total protein greater than 1 g/dL (10 g/L), (2) glucose less than 50 mg/dL (2.8 mmol/L), and (3) LDH greater than the upper limit of normal for serum.28 In the setting of a perforated viscus, cultures of ascitic fluid nearly always disclose multiple organisms, except in gallbladder rupture, which is usually monomicrobial.16 Brown ascitic fluid with a bilirubin concentration that is greater than 6 mg/dL (102 µmol/L) and greater than the serum level is indicative of biliary or proximal small intestinal perforation into ascites.16 An ascitic fluid amylase level that is greater than five-fold that of the serum level also may be indicative of intestinal rupture (but not gallbladder rupture) with the release of luminal amylase.28,29
The initial ascitic fluid analysis is helpful in delineating which patients are likely to have a ruptured viscus (see Fig. 91-3). Within minutes of the detection of neutrocytic ascitic fluid, these patients should undergo imaging studies to confirm and localize the site of rupture. Plain and upright abdominal films and water-soluble contrast studies of the upper and lower intestines or abdominal computed tomography should be obtained. If perforation is documented, emergency surgical intervention is the next step. Timing is crucial; after septic shock occurs, death is nearly certain. Antibiotic therapy without surgical intervention in the treatment of a ruptured viscus is predictably unsuccessful.
In contrast to patients with peritonitis resulting from perforation of a viscus, patients with secondary peritonitis unrelated to perforation tend not to have a diagnostic initial ascitic fluid analysis.28 The need to make the diagnosis of secondary peritonitis in patients without free perforation is less urgent, and there may be time to evaluate the response of the ascitic PMN count and fluid culture to treatment with antibiotics. It is best to repeat the paracentesis to assess the response to treatment after 48 hours of therapy; by 48 hours, the ascitic PMN count will be lower than the pretreatment value and the ascitic culture will be negative in essentially every patient with spontaneous bacterial peritonitis who has been treated with an appropriate antibiotic.28 Before 48 hours of treatment, the ascitic PMN count may rise to a value higher than baseline in either spontaneous bacterial peritonitis or secondary peritonitis.28 The culture remains positive in secondary peritonitis and becomes rapidly negative in spontaneous bacterial peritonitis (see Fig. 91-3).28 Whereas antibiotics alone cannot control secondary peritonitis, medical therapy cures spontaneous bacterial peritonitis rapidly.28
Treatment
Patients with an ascitic fluid PMN count of 250 cells/mm3 (0.25 × 109/L) or greater and a clinical scenario compatible with ascitic fluid infection should receive empirical antibiotic treatment (Table 91-9; see also Table 91-8).9,73 Patients with hemorrhage into the ascitic fluid, peritoneal carcinomatosis, pancreatic ascites, or tuberculous peritonitis may have an elevated ascitic PMN count that is unrelated to spontaneous bacterial peritonitis and usually do not require empirical antibiotic treatment. If they do receive antibiotics, the ascitic PMN count usually fluctuates randomly, in contrast to the dramatic reduction in PMN count typical of spontaneous bacterial peritonitis. If the clinical picture is unclear initially, the physician should err on the side of antibiotic treatment (with a non-nephrotoxic antibiotic). If ascitic fluid cultures are negative, the antibiotic can be stopped after 48 hours. In patients with uninfected neutrocytic ascitic fluid (except those with hemorrhage), lymphocytes usually predominate in the ascitic fluid differential count, in contrast to those with spontaneous bacterial peritonitis, in whom PMNs predominate. In patients with bloody ascitic fluid, a “corrected” PMN count should be calculated (as discussed earlier). Antibiotic therapy is not necessary for patients with bloody fluid unless the corrected ascitic fluid PMN count is 250 cells/mm3 (0.25 × 109/L) or greater.
| DIAGNOSIS | TREATMENT |
|---|---|
| Spontaneous bacterial peritonitis | Five days of intravenous antibiotic to which the organism is highly susceptible (e.g., cefotaxime 2 g every 8 hours empirically followed by more specific therapy after susceptibility results are available) |
| Monomicrobial non-neutrocytic bacterascites | Five days of intravenous antibiotic to which the organism is highly susceptible, if the patient is symptomatic or persistently culture-positive; not all patients with bacterascites require treatment |
| Culture-negative neutrocytic ascites | Five days of intravenous third-generation cephalosporin (e.g., cefotaxime 2 g every 8 hours) |
| Secondary bacterial peritonitis | Surgical intervention plus approximately 2 weeks of intravenous cephalosporin (e.g., cefotaxime 2 g every 8 hours) plus an antianaerobic drug such as metronidazole* |
| Polymicrobial bacterascites |
PMN, polymorphonuclear neutrophil.
* Dose of intravenous metronidazole is 15 mg/kg × 1, then 7.5 mg/kg every 6 hours.
The decision to begin empirical antibiotic treatment in patients with bacterascites must be individualized. Many episodes resolve without treatment58; however, the hospital mortality rate of 32% in patients with MNB is attributable, at least, in part, to infection.58 Therefore, treatment appears to be warranted in many patients. By definition, the ascitic PMN count is lower than 250 cells/mm3 (0.25 × 109/L) in this variant of ascitic fluid infection, and the PMN count cannot be the only parameter on which to base the decision about empirical therapy. Most patients with MNB in whom the colonization does not resolve progress to spontaneous bacterial peritonitis and have symptoms or signs of infection at the time of the paracentesis that documents bacterascites.58 Therefore, patients with cirrhosis and ascites who have convincing symptoms or signs of infection should receive treatment regardless of the ascitic fluid PMN count. Empirical treatment can be discontinued after only two to three days if the culture demonstrates no growth. Asymptomatic patients may not need treatment.58 The paracentesis should be repeated for cell count and culture in patients without clinical evidence of infection, as soon as it is known that the initial culture result is positive. If the PMN count has risen to at least 250/mm3 (0.25 × 109/L) or if symptoms or signs of infection have developed, treatment should be started. Culture results usually are negative in patients without a rise in the ascitic fluid PMN count on repeat paracentesis and without clinical evidence of infection, and these persons do not require treatment58 because colonization has been eradicated by host immune defenses.
The physician will not know initially that the ascitic fluid culture is destined to be negative in a patient with CNNA; therefore, empirical antibiotic treatment should be started. When the preliminary culture demonstrates no growth, it is helpful to repeat the paracentesis after 48 hours of therapy to assess the response of the PMN count to antibiotics. A dramatic decline in PMN count (always below the baseline pretreatment value and frequently a reduction of more than 80%) confirms a response to treatment.28 In such cases, a few more days of therapy is probably warranted. A stable ascitic fluid PMN count, especially with a predominance of lymphocytes and monocytes, suggests a nonbacterial (or mycobacterial) cause of ascitic fluid neutrocytosis, and the fluid should be sent for cytologic examination and mycobacterial culture. Because a negative culture result may be due to insensitive culture techniques, the prevalence of CNNA in a hospital that still uses conventional methods of culture can be reduced by convincing the microbiology laboratory to accept and process ascitic fluid submitted in blood culture bottles.23
Gram stain of the ascitic fluid is most helpful in detecting secondary peritonitis, in which multiple different bacterial forms are seen, but is of little value in guiding the choice of empirical antibiotic treatment for spontaneous ascitic infections. I have found that use of the Gram stain did not help narrow the antibiotic coverage in even 1 patient of approximately 500 with spontaneous bacterial peritonitis. Only approximately 10% of Gram stains demonstrate organisms in spontaneous bacterial peritonitis.23 If a Gram stain indicates secondary peritonitis, coverage of anaerobic flora, in addition to coverage of aerobic and facultative anaerobic flora, is required, as is an emergency search for the source of the infection (see Fig. 91-3; Table 91-9).28 Therefore, a positive Gram stain may lead to broader antibiotic coverage, rather than narrower coverage. Choosing narrow coverage (e.g., penicillin alone) based on a misinterpretation of the significance of the results of the Gram stain may lead to the patient’s death from uncontrolled infection before it becomes apparent that the isolated organism is resistant to the chosen antibiotic.
Until the results of susceptibility testing are available, relatively broad-spectrum antibiotic therapy is warranted in patients with suspected ascitic fluid infection. After sensitivities are known, the spectrum of coverage can usually be narrowed. The antibiotics that have been recommended for empirical treatment have changed over the years. In the late 1970s, the combination of ampicillin and gentamicin was promoted, but this recommendation was not based on susceptibility testing or efficacy data. Subsequently, gentamicin was shown to have an unpredictable volume of distribution in patients with ascites, and the serum creatinine level (and even the creatinine clearance) was found to be a poor index of the glomerular filtration rate in patients with ascites.74 Therefore, determining the appropriate loading and maintenance doses of gentamicin for this patient population is difficult, and no evidence-based guidelines are available for the prescribing physician to follow. In my experience, even if high serum levels are avoided, nephrotoxicity still develops in most cirrhotic patients with ascites who receive an aminoglycoside. One study has documented an adjusted odds ratio of 4.0 for aminoglycosides as a risk factor for renal dysfunction in patients with cirrhosis.75 Evidence that newer aminoglycosides are less nephrotoxic than gentamicin is lacking.
Several antibiotics are now available for the treatment of ascitic fluid infection. Cefotaxime, a third-generation cephalosporin, has been shown in a controlled trial to be superior to ampicillin plus tobramycin for the treatment of spontaneous bacterial peritonitis.76 Fully 98% of causative organisms were susceptible to cefotaxime, which did not result in superinfection or nephrotoxicity.76 Cefotaxime or a similar third-generation cephalosporin appears to be the treatment of choice for suspected spontaneous bacterial peritonitis.9 Anaerobic coverage is not needed, nor is coverage for Pseudomonas or Staphylococcus.23 Cefotaxime, 2 g intravenously every eight hours, has been shown to result in excellent ascitic fluid levels (20-fold killing power after one dose).77 In patients with a serum creatinine level greater than 3 mg/dL, the dosing interval may be extended to 12 hours.77 Neither a loading dose nor an intraperitoneal dose appears to be necessary or appropriate. The clinician should, however, write “first dose STAT” when ordering treatment, to avoid a delay in administration of the life-saving agent.
Other Intravenous Antibiotics
Amoxicillin-clavulanic acid has been shown to be as effective as cefotaxime in a randomized trial but is not available in a parenteral formulation in the United States.78 Other antibiotics have been recommended as well but have been less well studied than has cefotaxime. Some newer drugs have been used to treat spontaneous bacterial peritonitis (without any data on antibiotic penetration into the ascitic fluid) on the basis of their spectrum of coverage and formulary constraints. Infection with organisms that are resistant to the empirical antibiotic or use of drugs that do not enter the ascitic fluid in high enough concentrations to kill the bacteria may lead to the patient’s death.
Intravenous Albumin
Renal impairment occurs in 33% of episodes of spontaneous bacterial peritonitis.79 Spontaneous bacterial peritonitis leads to increased intraperitoneal nitric oxide production, which in turn further increases systemic vasodilatation and promotes renal failure (see Chapter 92).80 Intravenous albumin (1.5 g/kg of body weight at the time the infection is detected and 1.0 g/kg on day three) can increase intravascular volume and, in combination with cefotaxime, has been shown in a large randomized trial to reduce the risk of renal failure and improve survival compared with cefotaxime without albumin.81 Albumin appears to be effective by decreasing vasodilatation.82 A confirmatory randomized trial is needed. Because of the survival advantage, however, the use of intravenous albumin as an adjunct to antibiotic treatment has been recommended.83
Oral Antibiotic Treatment
Oral ofloxacin has been reported in a controlled trial to be as effective as parenteral cefotaxime in the treatment of spontaneous bacterial peritonitis in patients who do not have vomiting, shock, bleeding, or renal failure.84 The dose studied was 400 mg twice daily.84 Another study has demonstrated the efficacy of intravenous ciprofloxacin, 200 mg every 12 hours for 2 days, followed by oral ciprofloxacin, 500 mg every 12 hours for 5 days.85 Because of the possibility of fluoroquinolone resistance in patients receiving fluoroquinolones to prevent spontaneous bacterial peritonitis (see later), however, the empirical use of a fluoroquinolone to treat suspected spontaneous bacterial peritonitis should be avoided.86 Fortunately, bacterial isolates from patients with spontaneous bacterial peritonitis who were receiving fluoroquinolones for prophylaxis of this disorder remain susceptible to cefotaxime.73
Duration of Treatment
Infectious disease subspecialists generally recommend 10 to 14 days of antibiotic therapy for life-threatening infections; however, no data are available to support this duration of treatment in spontaneous ascitic fluid infections. The ascitic fluid culture becomes sterile after one dose of cefotaxime in 86% of patients.28 After 48 hours of therapy, the ascitic fluid PMN count is always less than the pretreatment value in patients with a spontaneous ascitic fluid infection treated with appropriate antibiotics; frequently, an 80% reduction is observed at 48 hours.28 A randomized, controlled trial involving 100 patients has demonstrated that 5 days of treatment is as efficacious as 10 days in patients with spontaneous bacterial peritonitis or CNNA.87 I have been treating spontaneous bacterial peritonitis and CNNA for five days since the late 1980s, with excellent results. The average duration of oral ofloxacin treatment was eight days in the only published trial.84
Follow-up Paracentesis
On the basis of a large database of repeat paracenteses during and after the treatment of spontaneous bacterial peritonitis,28 a follow-up paracentesis does not appear to be needed if the setting (advanced cirrhosis with symptoms and signs of infection), bacterial isolate (monomicrobial with a typical organism), and response to treatment (dramatic reduction in symptoms and signs of infection) are typical.28 Paracentesis should be repeated after 48 hours of treatment if the course is atypical.28
Treatment of Ascitic Fluid Infection Other than Spontaneous Bacterial Peritonitis
Because of the predictable presence of anaerobes, patients with suspected secondary peritonitis require empirical antibiotic coverage that is broader in spectrum than that used for spontaneous bacterial peritonitis. They also require an emergency evaluation to assess the need for surgical intervention (see earlier discussion, and Table 91-8 and Fig. 91-3). Cefotaxime plus metronidazole appears to provide excellent initial empirical therapy of suspected secondary peritonitis.28
Polymicrobial bacterascites (from needle perforation of the bowel) is tolerated relatively well. Peritonitis developed in only 1 in 10 patients with a needle perforation of the intestine with spillage of intestinal contents into ascitic fluid in the one relevant study.60 The single episode of paracentesis-related peritonitis was not fatal. Patients with low-protein ascitic fluid appear to be at most risk for development of a PMN response and clinical peritonitis related to needle perforation of the intestine.60 Most of the patients with a higher ascitic protein concentration (e.g., greater than 1 g/dL [10 g/L]) did not receive antibiotics, yet did well. Many physicians, however, probably would feel uncomfortable in withholding antibiotic treatment if needle perforation is suspected. If a decision to treat is made, anaerobic coverage should be included (e.g., cefotaxime plus metronidazole; see Table 91-9). Whether or not treatment is begun, a follow-up paracentesis is helpful (if it can be performed safely) to monitor the ascitic fluid PMN count and culture results. If a decision was made to defer antibiotic treatment initially and the number of organisms in the ascitic fluid does not decrease or the PMN count rises in the second specimen, antibiotic treatment should be initiated (see Table 91-9).
Prognosis
In the past, 48% to 95% of patients with a spontaneous ascitic fluid infection died during the hospitalization in which the diagnosis was made, despite antibiotic treatment.9,20 The most recent series report the lowest mortality rates (less than 5% if antibiotics are administered in a timely fashion), probably because of earlier detection and treatment of infection, as well as the avoidance of nephrotoxic antibiotics.87 The trial in which cefotaxime plus albumin was studied reported the lowest hospitalization mortality rate yet—10%.81 Even now, however, some patients are cured of their infection but die of liver failure or gastrointestinal bleeding because of the severity of the underlying liver disease. In fact, spontaneous ascitic fluid infection is a good marker of end-stage liver disease and has been proposed as an indication for liver transplantation in a patient who is otherwise a candidate.
To maximize survival, it is important that paracentesis is performed in all patients with ascites at the time of hospitalization, so that infection can be detected and treated promptly. The ascitic fluid cell count should be reviewed as soon as the results are available (approximately 60 minutes), and appropriate treatment should be instituted if indicated. The first dose of antibiotic should be given immediately. Because the “dipstick” test results are available in 90 to 120 seconds, this new tool may speed treatment of spontaneous bacterial peritonitis and improve survival.18
Without surgical intervention, the mortality rate for secondary peritonitis in hospitalized patients with ascites approaches 100%. When secondary peritonitis is diagnosed early and treated with emergency laparotomy, the mortality rate is approximately 50%.28
Prevention
The identification of risk factors for spontaneous bacterial peritonitis (including an ascitic fluid protein concentration less than 1.0 g/dL, variceal hemorrhage, and previous episode of spontaneous bacterial peritonitis) has led to controlled trials of prophylactic antibiotics.26,88–90 Norfloxacin, 400 mg per day orally, has been reported to reduce the risk of spontaneous bacterial peritonitis in inpatients with low-protein ascites and those with previous spontaneous bacterial peritonitis.88,89 Norfloxacin, 400 mg orally twice daily for seven days, helps prevent infection in patients with variceal hemorrhage90 and is cost-effective in preventing recurrent spontaneous bacterial peritonitis.91 More recently, intravenous ceftriaxone 1 g daily for seven days was found to be even more effective than norfloxacin in the setting of gastrointestinal bleeding; this regimen allows administration of antibiotics to patients who are vomiting blood.92 Oral antibiotics select for resistant organisms in the intestinal flora in patients, and in animals these organisms can then cause spontaneous ascitic fluid infection.86,93 Despite this concern, two randomized trials of primary prevention of ascitic fluid infection with prophylactic norfloxacin or ciprofloxacin have demonstrated a survival advantage for the antibiotic-treated patients (Table 91-10).94–96
Table 91-10 Prevention of Spontaneous Bacterial Peritonitis (SBP)
| INDICATION | DRUG | RESULTS |
|---|---|---|
| Prior SBP | Norfloxacin 400 mg orally once daily until death or liver transplantation | 66% Reduction in recurrence |
| Cirrhosis with gastrointestinal hemorrhage | Norfloxacin 400 mg orally twice daily × 7 days | 73% Reduction in infection |
| Ceftriaxone 1 g intravenously/day × 7 days | 67% Reduction in infection compared with norfloxacin | |
| Cirrhosis with ascitic fluid total protein <1.5 g/dL and either Child-Turcotte-Pugh score ≥9 and total bilirubin ≥3 mg/dL, or creatinine ≥1.2 mg/dL, or blood urea nitrogen ≥25 mg/dL, or serum sodium ≤130 mEq/L | Norfloxacin 400 mg/day orally × 1 year |
Data from references 89, 90, 92, 94, 95.
Trimethoprim-sulfamethoxazole has also been shown to prevent spontaneous bacterial peritonitis in an animal model and in patients; in animals survival was increased.97,98 The recommended dose for patients is one double-strength tablet daily.98
Use of parenteral antibiotics to prevent endoscopic sclerotherapy–related or band ligation–related infections in nonbleeding patients does not appear to be warranted, as indicated by a controlled trial.99 Active bleeding, not endoscopic treatment, appears to be the risk factor for ascitic fluid infection. On the other hand, bacterial infection is associated with failure to control variceal hemorrhage.100 This observation provides additional incentive to try to prevent, detect, and treat infections aggressively in this setting to minimize mortality related not only to infection, but also to hemorrhage.
CELLULITIS
Cellulitis of the lower extremities or abdominal wall is a common cause of soft tissue infection in obese patients with edema. One study has documented a 19% cumulative probability of cellulitis during hospitalization of patients with cirrhosis and ascites, compared with only a 4% likelihood of spontaneous bacterial peritonitis.101 Risk factors for cellulitis included obesity (which is increasing in frequency in patients with cirrhosis), homelessness, and greater degree of edema.101 A high index of suspicion and low threshold for treatment with a first-generation cephalosporin or other antibiotic may help decrease morbidity and mortality from uncontrolled cellulitis.
TENSE ASCITES
Some patients with ascites do not seek medical attention until they can no longer breathe or eat comfortably because of the pressure of the intra-abdominal fluid on the diaphragm. Tense ascites requires urgent therapeutic paracentesis (see later). Contrary to folklore, tense ascites can be drained without untoward hemodynamic effects.102 “Total paracentesis,” even more than 22 L, has been demonstrated to be safe.102 In the setting of tense ascites, therapeutic paracentesis improves venous return and hemodynamics; the myth of paracentesis-related hemodynamic catastrophes was based on anecdotal observations in small numbers of patients.
PLEURAL EFFUSIONS
“Sympathetic” pleural effusions are common in patients with cirrhotic ascites. They usually are unilateral and right-sided but occasionally may be bilateral and larger on the right side than on the left. A unilateral left-sided effusion suggests tuberculosis. A large effusion in a patient with cirrhotic ascites is designated hepatic hydrothorax.103 Most carefully studied patients with hepatic hydrothorax have been shown to have a small defect in the right hemidiaphragm. Occasionally, the effusion develops acutely, with sudden onset of shortness of breath as the abdomen decompresses. With large diaphragmatic defects, ascites may be undetectable on clinical examination despite a large pleural effusion.
The most common symptom associated with hepatic hydrothorax is shortness of breath. Infection of the fluid can occur, usually as a result of spontaneous bacterial peritonitis and transmission of bacteria across the diaphragm.104 The analysis of uncomplicated hepatic hydrothorax fluid is similar, but not identical, to that of ascitic fluid because the pleural fluid is subject to hydrostatic pressures different from those that affect the portal bed. The total protein concentration is higher (by approximately 1.0 g/dL [10 g/L]) in the pleural fluid than in ascitic fluid.103
The treatment of hepatic hydrothorax was difficult until the transjugular intrahepatic portosystemic shunt (TIPS) became available (see later).103 The effusions tend to occur in patients who are the least adherent to treatment regimens or in whom ascites is most refractory to therapy. Some authors have recommended chest tube insertion and sclerosing of the pleurae with tetracycline; however, chest tubes inserted to treat hepatic hydrothorax are usually difficult to remove105; moreover, shortness of breath may recur when the tube is clamped, and fluid may leak around the insertion site of the tube. A peritoneovenous shunt (see later) can be considered when the patient with hepatic hydrothorax has large-volume ascites, but the shunt usually clots after a short time. Direct surgical repair of the diaphragmatic defect can be considered, but the patients typically are poor operative candidates. Video thoracoscopic suture of the hole in the diaphragm followed by pleurodesis has been reported to be successful in one patient.106 Sodium restriction plus use of diuretics with intermittent thoracentesis is the safest and most effective first-line therapy of hepatic hydrothorax. TIPS placement has been reported to be successful and constitutes reasonable second-line treatment.103 If the patient is a candidate for liver transplantation, proceeding with a transplantation evaluation may be the best approach.
ABDOMINAL WALL HERNIAS
Abdominal wall hernias are common in patients with ascites. They usually are umbilical or incisional and occasionally inguinal. Up to 20% of patients with cirrhosis and ascites have umbilical hernias at the time of hospitalization.107 Some of these hernias incarcerate or perforate. Because of these potential complications, elective surgical treatment should be considered in a patient with a hernia and ascites. Insertion of mesh should be avoided because of the potential for the mesh to become infected. The ascitic fluid should be medically removed preoperatively because the hernia recurs in 73% of patients who have ascites at the time of hernia repair but in only 14% of those who have no ascitic fluid at the time of repair.108 Nevertheless, hernia repair is not without hazard. Successful laparoscopic repair of a recurrent strangulated umbilical hernia has been described.109 TIPS has also been reported to lead to good control of symptoms and may obviate the need for surgical repair.110 Many transplant surgeons prefer to avoid repair of the hernia or postpone it until the time of liver transplantation. An elastic abdominal binder can be used as a temporizing measure to reduce pain and hernia enlargement.
TREATMENT OF ASCITES
Appropriate treatment of ascites depends on the cause of fluid retention. Accurate determination of the etiology of ascites is crucial. The SAAG is helpful diagnostically and for therapeutic decision-making. Patients with a low SAAG usually do not have portal hypertension and do not respond to salt restriction and diuretics (except for those with nephrotic syndrome). Conversely, patients with a high SAAG have portal hypertension and are usually responsive to these measures.9
LOW-ALBUMIN-GRADIENT ASCITES
Peritoneal carcinomatosis is the most common cause of low-albumin-gradient ascites.2 Peripheral edema in affected patients can be managed with diuretics. By contrast, patients without peripheral edema who receive diuretics lose only intravascular volume, without loss of ascitic fluid. The mainstay of treatment of nonovarian peritoneal carcinomatosis is outpatient therapeutic paracentesis. Patients with peritoneal carcinomatosis usually live only a few months. Patients with ovarian malignancy are an exception to this rule and may exhibit a good response to surgical debulking and chemotherapy.
Ascites caused by tuberculous peritonitis (without cirrhosis) is cured by antituberculosis therapy. Diuretics do not speed weight loss unless the patient has underlying portal hypertension from cirrhosis. Pancreatic ascites may resolve spontaneously, require endoscopic placement of a stent in the pancreatic duct or operative intervention, or respond to treatment with somatostatin.111 A postoperative lymphatic leak from a distal splenorenal shunt or radical lymphadenectomy also may resolve spontaneously but on occasion may require surgical intervention or placement of a peritoneovenous shunt. Chlamydia peritonitis is cured by tetracycline. Ascites caused by lupus serositis may respond to glucocorticoids.7 Dialysis-related ascites may respond to aggressive dialysis.47
HIGH-ALBUMIN-GRADIENT ASCITES
Cirrhosis is the most common cause of liver disease that leads to high-albumin-gradient ascites (see Table 91-1). Many patients with cirrhosis experience multiple insults to the liver, including excessive alcohol use, NASH, and chronic hepatitis C.4 One of the most important steps in treating high-albumin-gradient ascites in a patient with alcoholic liver disease, with or without other causes of liver injury, is to convince the patient to stop drinking alcohol. In a period of months, abstinence from alcohol can result in healing of the reversible component of alcoholic liver disease, and the ascites may resolve or become more responsive to medical therapy. Similarly, patients with other forms of treatable liver disease (e.g., autoimmune hepatitis, hemochromatosis, Wilson disease) should receive specific therapy for these diseases. Occasionally, cirrhosis due to causes other than alcohol is reversible5; however, these diseases are usually less reversible than alcoholic liver disease, and by the time ascites is present, these patients may be better candidates for liver transplantation than for protracted medical therapy.
Hospitalization
Outpatient treatment of patients with small-volume ascites can be attempted initially. However, patients with large-volume ascites and those who are resistant to outpatient treatment usually require hospitalization for definitive diagnosis and management.9 Many of these patients also have gastrointestinal hemorrhage, encephalopathy, infection, or hepatocellular carcinoma. An intensive period of inpatient education and treatment may be required to convince the patient that the prescribed diet and diuretics are actually effective and worth the effort to prevent future hospitalizations.
Fluid Restriction
Indiscriminate restriction of fluid in the treatment of cirrhotic ascites is inappropriate and serves only to alienate patients, nurses, and dietitians; moreover, hypernatremia may result. Sodium restriction, not fluid restriction, results in weight loss; fluid follows sodium passively. The chronic hyponatremia usually seen in patients with cirrhotic ascites is seldom morbid. Attempts to correct hyponatremia rapidly in this setting can lead to more complications than those related to the hyponatremia. Severe hyponatremia (e.g., serum sodium concentration less than 120 mmol/L) does warrant fluid restriction in the patient with cirrhosis and ascites but fortunately occurs in only 1.2% of patients.112 Unless the decline in sodium concentration is rapid, symptoms of hyponatremia usually do not develop in cirrhotic patients until the serum sodium concentration is below 110 mmol/L.
Role of Bed Rest
Although bed rest has traditionally been prescribed, no controlled trials support this practice; bed rest was part of the treatment of heart failure in the past and was extrapolated to the treatment of cirrhosis with ascites without data.113 An upright posture may aggravate the plasma renin elevation found in most cirrhotic patients with ascites and, theoretically, increase renal sodium retention. In all likelihood, however, strict bed rest is unnecessary and may lead to decubitus ulcer formation in emaciated patients.
Urine Sodium Excretion
The 24-hour urinary sodium excretion is a helpful parameter to follow in patients with portal hypertension–related ascites. The completeness of the urine collection can be assessed by measuring the urinary creatinine excretion: Men with cirrhosis should excrete 15 to 20 mg/kg per day of creatinine, and women should excrete 10 to 15 mg/kg per day9; excretion of less creatinine indicates an incomplete collection. Only the 10% to 15% of patients who have significant spontaneous natriuresis can be considered for dietary sodium restriction as sole therapy of ascites (i.e., without diuretics).9 When given a choice, however, most patients would prefer to take some diuretics with more liberal intake of sodium than to take no pills with severe restriction of sodium intake. Contrary to popular belief, most patients, including outpatients, can comply with instructions to collect complete 24-hour urine specimens.
Because urine is the most important route of excretion of sodium in the absence of diarrhea or hyperthermia, and because dietary intake is the only source of nonparenteral sodium, dietary intake and urinary excretion of sodium should be roughly equivalent, if the patient’s weight is stable. Nonurinary sodium losses are less than 10 mmol per day in these patients.114 A suboptimal decline in body weight may be the result of inadequate natriuresis, failure to restrict sodium intake, or both. Monitoring 24-hour urinary sodium excretion and daily weight will clarify the issue. Patients who are adherent to an 88 mmol per day sodium diet and who excrete more than 78 mmol per day of sodium in the urine should lose weight. If the weight is increasing despite urinary losses in excess of 78 mmol per day, one can assume that the patient is consuming more sodium than is prescribed in the diet.
Urine Sodium-to-Potassium Ratio
Although 24-hour urine specimens constitute the diagnostic standard, one study has demonstrated that when a random urine specimen has a sodium concentration greater than the potassium concentration, a 24-hour specimen will reveal sodium excretion greater than 78 mmol per day in approximately 90% of cases.115 Therefore, a random urine sodium-to-potassium concentration ratio greater than 1 predicts that the patient should lose weight if a sodium-restricted diet is followed. Patients who do not lose weight despite a random urine sodium-to-potassium ratio greater than 1 probably are not adherent to the diet.
Avoidance of Urinary Bladder Catheters
Many physicians promptly insert a bladder catheter in hospitalized patients with cirrhosis to monitor urine output accurately. Unfortunately, many of these immunocompromised patients have urinary tract infections on hospital admission,72 and urethral trauma from insertion of the catheter in the setting of cystitis can lead to bacteremia. Prolonged catheterization predictably leads to cystitis and possibly sepsis in these patients. I insert urinary catheters only briefly and only in the intensive care unit setting; these portals of entry for bacteria should be removed as soon as possible. Twenty-four-hour urine specimens can be collected completely without catheters.
Diuretics
Spironolactone is the mainstay of treatment for patients with cirrhosis and ascites but increases natriuresis slowly. Single-agent diuretic therapy with spironolactone requires several days to induce weight loss. Although spironolactone alone has been shown to be superior to furosemide alone in the treatment of cirrhotic ascites,116 I prefer to start spironolactone and furosemide together on the first hospital day in initial doses of 100 mg and 40 mg, respectively, each taken once in the morning.9 Amiloride, 10 mg per day, can be substituted for spironolactone; amiloride is less widely available and more expensive than spironolactone but more rapidly effective, and it does not cause gynecomastia. A new potassium-sparing diuretic, eplerenon, has been used in the treatment of heart failure and does not cause gynecomastia, but studies of its use in cirrhosis are lacking. The half-life of spironolactone is approximately 24 hours in normal control subjects but is markedly prolonged in patients with cirrhosis; almost one month is required to reach a steady state.117 In view of its long half-life, dosing the drug multiple times per day is unnecessary. A loading dose may be appropriate but has not been studied. Single daily doses maximize adherence; 25-, 50-, and 100-mg spironolactone tablets are available generically. Furosemide also should be given once a day.118
If the combination of spironolactone, 100 mg per day (or amiloride, 10 mg per day) and furosemide, 40 mg per day orally, is ineffective in increasing urinary sodium or decreasing body weight, the doses of both drugs should be increased simultaneously, as needed (e.g., spironolactone, 200 mg plus furosemide, 80 mg, then 300 mg plus 120 mg, and finally 400 mg plus 160 mg). In my experience, as well as in a randomized trial, starting both drugs at once speeds the onset of diuresis, whereas slowly increasing the daily dose of spironolactone to 400 mg or even higher before adding furosemide delays diuresis and results in hyperkalemia.119
The 100 : 40 ratio of the daily doses of spironolactone and furosemide usually maintains normokalemia. The ratio of doses can be adjusted to correct abnormal serum potassium levels. Occasionally, an alcoholic patient who has had no recent food intake will have hypokalemia at the time of admission and for a variable interval thereafter. Such a patient should receive spironolactone alone until the serum potassium normalizes; furosemide can then be added. When combined with a sodium-restricted diet in a study of almost 4000 patients, the regimen of spironolactone and furosemide has been demonstrated to achieve successful diuresis in more than 90% of cirrhotic patients.120
Intravenous diuretics cause acute decreases in the glomerular filtration rate in patients with cirrhosis and ascites and generally should be avoided.121 Many patients are given intravenous furosemide when they are hospitalized because of failure of outpatient treatment of ascites in the setting of cirrhosis. The approach of switching from oral to intravenous administration is effective for heart failure, but in patients with cirrhosis, repeated doses of intravenous furosemide regularly lead to azotemia and then to an erroneous diagnosis of hepatorenal syndrome. (The correct diagnosis is diuretic-induced azotemia that resolves when the diuretics are withheld and fluid is administered intravenously.) Some physicians give intravenous albumin with intravenous furosemide, but a randomized crossover study has shown no benefit to albumin in this setting.122 Repeated intravenous dosing of furosemide appears to be too “harsh” for the patient with cirrhosis; oral diuretics are better tolerated.
If rapid weight loss is desired, therapeutic paracentesis should be performed (see later). No limit has been identified for acceptable daily weight loss in patients who have massive edema. As soon as the edema has resolved, a reasonable maximum weight loss is probably 0.5 kg per day.123 Encephalopathy, a serum sodium concentration less than 120 mmol/L despite fluid restriction, and a serum creatinine level greater than 2.0 mg/dL (180 mmol/L) are indications to discontinue diuretics and reassess the patient. Abnormalities in potassium levels almost never prohibit diuretic use because the ratio of the two diuretics can be readjusted. Patients with parenchymal renal disease (e.g., diabetic nephropathy) usually require relatively higher doses of furosemide and lower doses of spironolactone; otherwise, they develop hyperkalemia. Patients in whom complications develop despite a careful attempt at diuretic treatment usually require second-line therapy. Prostaglandin inhibitors (e.g., nonsteroidal anti-inflammatory drugs) should be avoided in patients with cirrhosis and ascites because they inhibit diuresis, may promote renal failure, and may cause gastrointestinal bleeding.124
Reducing the quantity of fluid in the abdomen can improve the patient’s comfort and prevent hepatic hydrothorax and hernias. Also, by concentrating the ascitic fluid, diuresis increases the opsonic activity of ascitic fluid 10-fold and theoretically may be of value in preventing spontaneous ascitic fluid infection.125
Aquaretics
The aquaretics are a new class of drugs that have been used in animals and preliminarily in patients with cirrhosis to increase urinary water excretion and to increase the serum sodium concentration. Patients with mild hyponatremia (serum sodium less than 130 mmol/L) can respond with an increase in the serum sodium level, although dose reductions were common in a randomized trial.126 Whether these drugs will improve severe hyponatremia without causing hypotension awaits further investigation.
REFRACTORY ASCITES
Refractory ascites is defined as ascites unresponsive to a sodium-restricted diet and high-dose diuretic treatment. Refractoriness may manifest as minimal or no weight loss despite diuretics or the development of complications of diuretics.127 Several studies have shown that ascites in the setting of cirrhosis is refractory to standard medical therapy in fewer than 10% of patients.116,120
In the 1960s, portacaval shunts were used to treat refractory ascites, but operative hemorrhagic complications and portosystemic encephalopathy led to abandonment of this approach.113 In Europe in the 1970s, the Paris pump was used to ultrafilter ascitic fluid and reinfuse it intravenously.113 Unfortunately, this approach was complicated by disseminated intravascular coagulation and was abandoned. Viable options for patients refractory to routine medical therapy include liver transplantation, serial therapeutic paracenteses, TIPS, and peritoneovenous shunts (Fig. 91-5).9
Liver Transplantation
Liver transplantation should be considered among the treatment options for patients with cirrhosis and ascites—whether the fluid is diuretic-sensitive or diuretic-refractory (see also Chapter 95). In many areas of the United States, patients with ascites are not offered transplantation until hepatorenal syndrome has developed (see Chapter 92). The 12-month survival rate for patients with ascites refractory to medical therapy is only 32%.128 The survival rate for liver transplantation is much higher.
In patients who are candidates for liver transplantation, procedures that could make transplantation difficult should be avoided. Surgery in the right upper quadrant causes adhesions that become vascularized and difficult to remove during transplant surgery. Even peritoneovenous shunting can lead to the formation of a “cocoon” in the right upper quadrant that can involve the bowel and liver.129
Serial Paracenteses
Therapeutic abdominal paracentesis is one of the oldest medical procedures. In the 1980s, after 2000 years of use, scientific data regarding large-volume paracentesis were reported, and patients were documented to tolerate large-volume paracentesis well, just as patients had in the 1940s and earlier.130 In one large randomized, controlled trial, therapeutic paracentesis plus intravenous infusion of colloid led to fewer minor (asymptomatic) changes in serum electrolyte and creatinine levels than those reported with diuretic therapy; however, no differences in morbidity or mortality rates could be demonstrated.130 Therapeutic paracentesis now appears to be first-line therapy for patients in whom ascites is tense and second-line therapy for cirrhotic patients in whom ascites is refractory to diuretics (see Fig. 91-5).9 The world record for volume of fluid removed at one time appears to be 41 L.131
Colloid Replacement
A controversial issue regarding therapeutic paracentesis is the role of colloid replacement. In one study, patients with tense ascites were randomized to receive intravenous albumin (10 g/L of fluid removed) or no albumin after therapeutic paracentesis.132 More statistically significant (asymptomatic) changes in serum electrolyte, plasma renin, and serum creatinine levels developed in the patients who did not receive albumin than in those who received albumin, but no greater frequency of clinical morbidity or mortality was seen. Although another study has documented that the patients who have a postparacentesis rise in plasma renin levels have a decreased life expectancy compared with those who have stable renin levels, no study has demonstrated a decreased survival rate in patients not given a plasma expander compared with patients given albumin after paracentesis.133 A new phrase, “paracentesis-induced circulatory dysfunction,” has been coined to describe the rise in plasma renin levels after paracentesis.134 Despite the lack of benefit of albumin infusion on survival, the authors of the two studies cited previously recommend routine infusion of albumin after therapeutic paracentesis.132,134 Albumin infusions markedly increase the degradation of albumin, however, and albumin is expensive.135,136 In a study performed in the 1960s, 58% of infused albumin was offset by increased degradation, and a 15% increase in the serum albumin level led to a 39% increase in degradation.135 Increasing the concentration of albumin in cell culture media has been shown to decrease albumin synthesis.137 In view of the cost ($7 to $25/g or $350 to $1250/tap), it is difficult to justify the expense of routine infusions of albumin based on the available data.
The confusion regarding albumin infusion relates, in part, to the design of the relevant studies. In the studies from Barcelona, patients with “tense” ascites could be entered into the trial of albumin versus no albumin, and 31% of these patients were not even receiving diuretics.131 It seems more appropriate to study the population in which chronic paracenteses are really needed, specifically the diuretic-resistant group, rather than all patients with tense ascites.138 Another group of investigators has shown that patients with cirrhosis and diuretic-resistant ascites tolerate a 5-L paracentesis without a change in plasma renin levels.139 My approach to patients with tense ascites is to take off enough fluid (4 to 5 L) to relieve intra-abdominal pressure and then to rely on diuretics to eliminate the remainder. To remove all of the fluid by paracentesis when most of it can be removed with diuretics seems inappropriate, partly because paracentesis removes opsonins, whereas diuresis concentrates opsonins.125 Patients with early cirrhosis and diuretic-sensitive ascites should be managed with diuretics, not large-volume paracentesis; these patients may be more sensitive to paracentesis-related volume depletion than are patients with advanced cirrhosis.140 Chronic therapeutic paracenteses should be reserved for the 10% of patients in whom diuretic treatment fails to relieve the ascites.
Other studies have compared less expensive plasma expanders with albumin. No differences in electrolyte imbalance or clinically relevant complications between the groups have been found.141 In addition, some authors advocate giving one half of the plasma expander immediately after the paracentesis and the other half six hours later.133,141 This approach converts an otherwise simple outpatient procedure into an all-day clinic visit or even a brief hospitalization and seems unwarranted. A colloid that specifically should be avoided is hetastarch, which can accumulate in Kupffer cells and cause portal hypertension in patients without preexisting liver disease.142
Consensus statements, a randomized trial of albumin versus saline in 6997 critically ill patients, and systematic reviews have pointed out some of the hazards of albumin infusion and have recommended against its liberal use.143–145 Until more convincing data involving appropriate groups of patients are available, it seems reasonable to (1) avoid serial large-volume paracenteses in patients with diuretic-sensitive ascites; (2) withhold albumin after taps of 5 L or less; and (3) consider albumin infusion optional after taps of larger volume in patients with diuretic-resistant ascites.9
A small, randomized trial has shown that terlipressin may be equivalent to albumin after therapeutic paracentesis in preventing paracentesis-induced circulatory dysfunction; if this drug receives approval for use in the United States and further studies support its efficacy, terlipressin would be an alternative to albumin.146
Transjugular Intrahepatic Portosystemic Shunt
TIPS is a side-to-side portacaval shunt that is placed by an interventional radiologist (or hepatologist), usually with the use of local anesthesia. TIPS placement was first used for the treatment of refractory variceal bleeding, but it also has been advocated for diuretic-resistant ascites147 (see Chapter 90). TIPS was received with great enthusiasm in the 1990s, similar to the enthusiasm for the peritoneovenous shunt in the 1970s. Just as with peritoneovenous shunting, TIPS was overused until serious complications and suboptimal efficacy were reported. Four large-scale randomized trials in diuretic-resistant patients have demonstrated consistent superiority of TIPS over repeated paracentesis but no survival advantage.148–151 Multiple meta-analyses have been published confirming efficacy but with more hepatic encephalopathy in the TIPS group.152–156 One meta-analysis has demonstrated a trend toward improved survival in the TIPS group.153 Another meta-analysis, which analyzed individual patient data, did show improved transplant-free survival with TIPS.156 Although TIPS dysfunction was common when an uncoated (or uncovered) shunt was used, polytetrafluoroethylene-coated stents have been reported to improve patency and survival when compared with uncoated stents in a nonrandomized study and to improve patency, with no survival advantage, when compared with uncoated stents in a randomized trial.157,158 Also, the four older TIPS trials preceded development and implementation of the Model for End-stage Liver Disease (MELD) score, which predicts 90-day mortality after TIPS placement (see Chapter 90); new trials using the coated stent and selecting patients according to their MELD scores may demonstrate a survival advantage for TIPS compared with repeated taps.
TIPS also is useful in the treatment of hepatic hydrothorax and umbilical hernia.111,118 A direct intrahepatic portosystemic shunt connects the portal vein directly to the inferior vena cava and has applicability in patients with Budd-Chiari syndrome (see Chapter 83).159
Peritoneovenous Shunt
In the mid-1970s, the peritoneovenous shunt was promoted as a new “physiologic” treatment for the management of ascites. Reports of shunt failure, fatal complications following shunt insertion, and randomized trials demonstrating no survival advantage have led to the relegation of this procedure to third-line therapy in patients with cirrhosis and ascites9,120 (see Fig. 91-5). Patients who are not candidates for liver transplantation and who have a scarred abdomen that is not amenable to repeated paracenteses, who are not candidates for a TIPS, or in whom an attempt at TIPS placement has failed make up the small subset of candidates for a peritoneovenous shunt. A randomized trial has shown that even an uncoated TIPS stent has better “assisted patency” than the peritoneovenous shunt.160
Novel Treatments
Novel treatment options for patients with refractory ascites include weekly infusions of intravenous albumin, ascites reinfusion, ultrafiltration, terlipressin infusion (not available in the United States), partial splenic artery embolization, peritoneal-urinary drainage of the fluid using a surgically implanted pump, and percutaneous placement of a peritoneovenous shunt by an interventional radiologist.161–168 More data are needed before these treatments can be advocated.
PROGNOSIS
Cirrhosis complicated by ascites is associated with significant morbidity and mortality, related, in part, to the severe underlying liver disease and, in part, to the ascites per se. In one half of the patients in whom cirrhosis is detected before decompensation (i.e., development of ascites, jaundice, or encephalopathy or gastrointestinal hemorrhage), ascites occurs within 10 years.169 When ascites appears, the expected mortality rate is approximately 50% in just two years.170 With liver transplantation, survival is improved dramatically.
Akriviadis EA, Runyon BA. The value of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127-33. (Ref 28.)
Fernandez J, Navasa M, Garcia-Pagan JC, et al. Effect of intravenous albumin on systemic and hepatic hemodynamics and vasoactive neurohumoral systems in patients with cirrhosis and spontaneous bacterial peritonitis. J Hepatol. 2004;41:384-90. (Ref 82.)
Fernandez J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-24. (Ref 94.)
Fernandez J, Ruiz del Arbol L, Gomez C, et al. Norfloxacin vs. ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049-56. (Ref 92.)
Follo A, Llovet JM, Navasa M, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: Incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495-501. (Ref 79.)
Perez-Ayuso RM, Arroyo V, Planas R, et al. Randomized comparative study of efficacy of furosemide vs. spironolactone in nonazotemic cirrhosis with ascites. Gastroenterology. 1983;84:961-8. (Ref 116.)
Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology. 2004;39:841-56. (Ref 9.)
Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-5. (Ref 23.)
Runyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology. 1988;8:1104-9. (Ref 2.)
Runyon BA, McHutchison JG, Antillon MR, et al. Short-course vs. long-course antibiotic treatment of spontaneous bacterial peritonitis: A randomized controlled trial of 100 patients. Gastroenterology. 1991;100:1737-42. (Ref 87.)
Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-20. (Ref 21.)
Runyon BA, Squier SU, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792-6. (Ref 61.)
Salerno F, Camma C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: A meta-analysis of individual patient data. Gastroenterology. 2007;133:825-34. (Ref 156.)
Salerno F, Merli M, Riggio O, et al. Randomized controlled study of TIPS vs. paracentesis with albumin in cirrhosis with refractory ascites. Hepatology. 2004;40:629-35. (Ref 151.)
Wong C, Holroyd-Leduc J, Thorpe KE, Straus SE. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results? JAMA. 2008;299:1166-78. (Ref 145.)
1. Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver disease: From the patient to the molecule. Hepatology. 2006;43:S121-31.
2. Runyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology. 1988;8:1104-9.
3. Schrier RW. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy. N Engl J Med. 1988;319:1065-72.
4. Hourigan LF, MacDonald GA, Purdie D, et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index. Hepatology. 1999;29:1215-19.
5. Malekzadeh R, Mohamadnejad M, Rakhshani N, et al. Reversibility of cirrhosis in chronic hepatitis B. Clin Gastroenterol Hepatol. 2004;2:34-7.
6. Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: Clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-9.
7. Weinstein PJ, Noyer CM. Rapid onset of massive ascites as the first presentation of systemic lupus erythematosus. Am J Gastroenterol. 2000;95:302-3.
8. Cattau El, Benjamin SB, Knuff TE, et al. The accuracy of the physical exam in the diagnosis of suspected ascites. JAMA. 1982;247:1164-6.
9. Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology. 2004;39:841-56.
10. Runyon BA. Paracentesis of ascitic fluid: A safe procedure. Arch Intern Med. 1986;146:2259-61.
11. Mannucci PM. Abnormal hemostasis tests and bleeding in chronic liver disease: Are they related? No. J Thromb Haemost. 2006;4:721-3.
12. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracenteses. Hepatology. 2004;40:484-8.
13. Sakai H, Sheer TA, Mendler MH, Runyon BA. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-6.
14. Runyon BA, Akriviadis EA, Keyser AJ. The opacity of portal hypertension-related ascites correlates with the fluid’s triglyceride concentration. Am J Clin Pathol. 1991;96:142-3.
15. Rector WG. Spontaneous chylous ascites of cirrhosis. J Clin Gastroenterol. 1984;6:369-72.
16. Runyon BA. Ascitic fluid bilirubin concentration as a key to the diagnosis of choleperitoneum. J Clin Gastroenterol. 1987;9:543-5.
17. Hoefs JC. Increase in ascites WBC and protein concentrations during diuresis in patients with chronic liver disease. Hepatology. 1981;1:249-54.
18. Runyon BA. Strips and tubes: Improving the diagnosis of spontaneous bacterial peritonitis. Hepatology. 2003;37:745-7.
19. Nousbaum J-B, Cadranel J-F, Nahon P, et al. Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology. 2007;45:1275-81.
20. Runyon BA. Approach to the patient with ascites. In: Yamada T, Alpers D, Kaplowitz N, et al, editors. Textbook of gastroenterology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2003:948-72.
21. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-20.
22. Hoefs JC. Globulin correction of the albumin gradient: Correlation with measured serum to ascites colloid osmotic gradient. Hepatology. 1992;16:396-403.
23. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-5.
24. Runyon BA, Antillon MR, Akriviadis EA, et al. Bedside inoculation of blood culture bottles with ascitic fluid is superior to delayed inoculation in the detection of spontaneous bacterial peritonitis. J Clin Microbiol. 1990;8:2811-12.
25. Runyon BA, Hoefs JC. Ascitic fluid analysis before, during, and after spontaneous bacterial peritonitis. Hepatology. 1985;5:257-9.
26. Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91:1343-6.
27. Runyon BA. Cardiac ascites: A characterization. J Clin Gastroenterol. 1988;10:410-12.
28. Akriviadis EA, Runyon BA. The value of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127-33.
29. Runyon BA. Amylase levels in ascitic fluid. J Clin Gastroenterol. 1987;9:172-4.
30. Haas LS, Gates LKJr. The ascites to serum amylase ratio identifies two distinct populations in acute pancreatitis with ascites. Pancreatology. 2002;2:100-3.
31. Hillebrand DJ, Runyon BA, Yasmineh WG, Rynders GP. Ascitic fluid adenosine deaminase insensitivity in detecting tuberculous peritonitis in the United States. Hepatology. 1996;24:1408-12.
32. Altamirano M, Kelly MT, Wong A, et al. Characterization of a DNA probe for detection of Mycobacterium tuberculosis complex in clinical samples by polymerase chain reaction. J Clin Microbiol. 1992;30:2173-6.
33. Motherby H, Nadjari B, Friegel P, et al. Diagnostic accuracy of effusion cytology. Diagn Cytopath. 1999;20:350-7.
34. Chetty R, Learmonth GM, Taylor DA. Giant cell hepatocellular carcinoma. Cytopathology. 1990;1:233-7.
35. Kielhorn E, Schofield K, Rimm DL. Use of magnetic enrichment for detection of carcinoma cells in fluid specimens. Cancer. 2002;94:205-11.
36. Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med. 1982;96:358-64.
37. Runyon BA, Antillon MR. Ascitic fluid pH and lactate: Insensitive and nonspecific tests in detecting ascitic fluid infection. Hepatology. 1991;13:929-35.
38. Albillos A, Cuervas-Mons V, Millan I, et al. Ascitic fluid polymorphonuclear cell count and serum to ascites albumin gradient in the diagnosis of bacterial peritonitis. Gastroenterology. 1990;98:134-40.
39. Runyon BA. Elevated ascitic fluid fibronectin: A non-specific finding. J Hepatol. 1986;3:219-22.
40. Runyon BA. Editorial: Malignancy-related ascites and ascitic fluid “humoral tests of malignancy.”. J Clin Gastroenterol. 1994;18:94-8.
41. Loewenstein MS, Rittgers RA, Feinerman AE, et al. CEA assay of ascites and detection of malignancy. Ann Intern Med. 1978;88:635-8.
42. Arora A, Seth S, Acharya SK, et al. Hepatic coma as a presenting feature of constrictive pericarditis. Am J Gastroenterol. 1993;88:430-2.
43. Sheer TA, Joo E, Runyon BA. Usefulness of serum N-terminal-BNP in distinguishing ascites due to cirrhosis from ascites due to heart failure. J Clin Gastroenterol. 2009.
44. Saab S, Rickman LS, Lyche KD. Ascites and the acquired immunodeficiency syndrome. Medicine. 1996;89:131-5.
45. Wong W-M, Wu P-C, Yuen M-F, et al. Antituberculosis drug-related liver dysfunction in chronic hepatitis B infection. Hepatology. 2000;31:201-6.
46. Weisman IM, Moreno AJ, Parker AL, et al. Gastrointestinal dissemination of coccidioidomycosis. Am J Gastroenterol. 1986;81:589-93.
47. Han S-HB, Reynolds TB, Fong T-L. Nephrogenic ascites: Analysis of 16 cases and review of the literature. Medicine. 1998;77:233-44.
48. Ackerman Z. Ascites in nephrotic syndrome: Incidence, patients’ characteristics and complications. J Clin Gastroenterol. 1996;22:31-4.
49. Mauer K, Manzione NC. Usefulness of the serum-ascites albumin gradient in separating transudative from exudative ascites: Another look. Dig Dis Sci. 1988;33:1208-12.
50. Brown MW, Burk RF. Development of intractable ascites following upper abdominal surgery in patients with cirrhosis. Am J Med. 1986;80:879-83.
51. Ikeda Y, Kanematsu T, Matsumata T, et al. Liver resection and intractable postoperative ascites. Hepatogastroenterology. 1993;40:14-16.
52. Fabregues F, Balasch J, Gines P, et al. Ascites and liver test abnormalities during severe ovarian hyperstimulation syndrome. Am J Gastroenterol. 1999;94:994-9.
53. Case records of the Massachusetts General Hospital. Case 39-1992. A 49-year-old woman with peripheral neuropathy, hepatosplenomegaly, and intermittent abdominal pain. N Engl J Med. 1992;327:1014-21.
54. de Kerguenec C, Hillaire S, Molinie V, et al. Hepatic manifestations of hemophagocytic syndrome: A study of 30 cases. Am J Gastroenterol. 2001;96:852-7.
55. Cirera I, Navasa M, Rimola A, et al. Ascites after liver transplantation. Liver Transpl. 2000;6:157-62.
56. Stewart C, Wertheim J, Olthoff K, et al. Ascites after liver transplantation—a mystery. Liver Transpl. 2004;10:654-60.
57. Correia JP, Conn HO. Spontaneous bacterial peritonitis in cirrhosis: Endemic or epidemic? Med Clin North Am. 1975;59:963-81.
58. Runyon BA. Monomicrobial non-neutrocytic bacterascites: A variant of spontaneous bacterial peritonitis. Hepatology. 1990;12:710-15.
59. Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: A variant of spontaneous bacterial peritonitis. Hepatology. 1984;4:1209-11.
60. Runyon BA, Canawati HN, Hoefs JC. Polymicrobial bacterascites: A unique entity in the spectrum of infected ascitic fluid. Arch Intern Med. 1986;146:2173-5.
61. Runyon BA, Squier SU, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792-6.
62. Guarner C, Runyon BA, Young S, et al. Intestinal bacterial overgrowth and bacterial translocation in an experimental model of cirrhosis in rats. J Hepatol. 1997;26:1372-8.
63. Runyon BA, Morrissey R, Hoefs JC, et al. Opsonic activity of human ascitic fluid: A potentially important protective mechanism against spontaneous bacterial peritonitis. Hepatology. 1985;5:634-7.
64. Runyon BA. Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis. Hepatology. 1988;8:632-5.
65. Runyon BA, Sugano S, Kanel G, et al. A rodent model of cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 1991;100:489-93.
66. Such J, Frances R, Munoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-nonneutrocytic ascites. Hepatology. 2002;36:135-41.
67. Dunn DL, Barke RA, Ewald DC, Simmons RL. Macrophages and translymphatic absorption represent the first line of host defense of the peritoneal cavity. Arch Surg. 1987;122:105-10.
68. McHutchison JG, Runyon BA. Spontaneous bacterial peritonitis. In: Surawicz CM, Owen RL, editors. Gastrointestinal and hepatic infections. Philadelphia: WB Saunders; 1994:455-75.
69. Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-8.
70. Borzio M, Salerno F, Piantoni L, et al. Bacterial infection in patients with advanced cirrhosis: A multicentre prospective study. Dig Liver Dis. 2001;33:41-8.
71. Bernard B, Cadranel J-F, Valla D, et al. Prognostic significance of bacterial infection in bleeding cirrhotic patients: A prospective study. Gastroenterology. 1995;108:1828-34.
72. Cadranel J-P, Denis J, Pauwels A, et al. Prevalence and risk factors of bacteriuria in cirrhotic patients: A prospective case-control multicenter study in 244 patients. J Hepatol. 1999;31:464-8.
73. Rimola A, Garcia-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: A consensus document. J Hepatol. 2000;32:142-53.
74. Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis: Prospective study. Am J Med. 1987;82:945-52.
75. Hampel H, Bynum GD, Zamora E, et al. Risk factors for the development of renal dysfunction in hospitalized patients with cirrhosis. Am J Gastroenterol. 2001;96:2206-10.
76. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-62.
77. Runyon BA, Akriviadis EA, Sattler FR, et al. Ascitic fluid and serum cefotaxime and desacetylcefotaxime levels in patients treated for bacterial peritonitis. Dig Dis Sci. 1991;36:1782-6.
78. Ricart E, Soriano G, Novella MT, et al. Amoxicillin-clavulanic acid versus cefotaxime in the therapy of bacterial infections in cirrhotic patients. J Hepatol. 2000;32:596-602.
79. Follo A, Llovet JM, Navasa M, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: Incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495-501.
80. Such J, Hillebrand DJ, Guarner C, et al. Nitric oxide in ascitic fluid is an independent predictor of renal impairment in patients with cirrhosis and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2004;16:1-7.
81. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-9.
82. Fernandez J, Navasa M, Garcia-Pagan JC, et al. Effect of intravenous albumin on systemic and hepatic hemodynamics and vasoactive neurohumoral systems in patients with cirrhosis and spontaneous bacterial peritonitis. J Hepatol. 2004;41:384-90.
83. Runyon BA. Albumin infusion for spontaneous bacterial peritonitis. Lancet. 1999;354:1838-9.
84. Navasa M, Follo A, Llovet JM, et al. Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology. 1996;111:1011-17.
85. Terg R, Cobas S, Fassio E, et al. Oral ciprofloxacin after a short course of intravenous ciprofloxacin in the treatment of spontaneous bacterial peritonitis: Results of a multicenter, randomized study. J Hepatol. 2000;33:564-9.
86. Aparicio JR, Such J, Pascual S, et al. Development of quinolone-resistant strains of Escherichia coli in stools of patients with cirrhosis undergoing norfloxacin prophylaxis: Clinical consequences. J Hepatol. 1999;31:277-83.
87. Runyon BA, McHutchison JG, Antillon MR, et al. Short-course vs. long-course antibiotic treatment of spontaneous bacterial peritonitis: A randomized controlled trial of 100 patients. Gastroenterology. 1991;100:1737-42.
88. Soriano G, Teixedo M, Guarner C, et al. Selective intestinal decontamination prevents spontaneous bacterial peritonitis. Gastroenterology. 1991;100:477-81.
89. Gines P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716-24.
90. Soriano G, Guarner C, Tomas A, et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology. 1992;103:1267-72.
91. Younossi Z, McHutchison JG, Ganiats TG. An economic analysis of norfloxacin against spontaneous bacterial peritonitis. J Hepatol. 1997;27:295-8.
92. Fernandez J, Ruiz del Arbol L, Gomez C, et al. Norfloxacin vs. ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049-56.
93. Runyon BA, Borzio M, Young S, et al. Effect of selective bowel decontamination with norfloxacin on spontaneous bacterial peritonitis, translocation, and survival in an animal model of cirrhosis. Hepatology. 1995;21:1719-24.
94. Fernandez J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-24.
95. Terg R, Fassio E, Guevara M, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: A randomized, placebo-controlled study. J Hepatol. 2008;48:774-9.
96. Runyon BA. A pill a day can improve survival in patients with advanced cirrhosis. Gastroenterology. 2007;133:1029-31.
97. Guarner C, Runyon BA, Heck M, et al. Effect of long-term trimethoprim-sulfamethoxazole prophylaxis on ascites formation, bacterial translocation, spontaneous bacterial peritonitis and survival in cirrhotic rats. Dig Dis Sci. 1999;44:1957-62.
98. Singh N, Gayowski T, Yu VL, et al. Trimethoprim-sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: A randomized trial. Ann Intern Med. 1995;122:595-8.
99. Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18:290-4.
100. Goulis J, Armonis A, Patch D, et al. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207-12.
101. Rongey CA, Lim NH, Runyon BA. Cellulitis in patients with cirrhosis and edema: An under-recognized complication currently more common than spontaneous bacterial peritonitis. Open Gastroenterol J. 2008;2:24-7.
102. Titó L, Ginès P, Arroyo V, et al. Total paracentesis associated with intravenous albumin management of patients with cirrhosis and ascites. Gastroenterology. 1990;98:146-51.
103. Strauss RM, Boyer TD. Hepatic hydrothorax. Semin Liver Dis. 1997;17:227-32.
104. Xiol X, Castellote J, Baliellas C, et al. Spontaneous bacterial empyema in cirrhotic patients: Analysis of eleven cases. Hepatology. 1990;11:365-70.
105. Runyon BA, Greenblatt M, Ming RHC. Hepatic hydrothorax is a relative contraindication to chest tube insertion. Am J Gastroenterol. 1986;81:566-7.
106. Temes RT, Dacis MS, Follis FM, et al. Videothorascopic treatment of hepatic hydrothorax. Ann Thorac Surg. 1997;64:1468-9.
107. Fagan SP, Awad SS, Berger DH. Management of complicated umbilical hernias in patients with end-stage liver disease and refractory ascites. Surgery. 2004;135:679-82.
108. Runyon BA, Juler GL. Natural history of umbilical hernias in patients with and without ascites. Am J Gastroenterol. 1985;80:38-9.
109. Sarit C, Eliezer A, Mizrahi S. Minimally invasive repair of recurrent strangulated umbilical hernia in cirrhotic patient with refractory ascites. Liver Transpl. 2003;9:621-2.
110. Bajaj JS, Varma RR. TIPS as therapeutic modality for umbilical hernia in patients with advanced liver disease. Liver Transpl. 2004;10:159-60.
111. Oktedalen O, Nygaard K, Osnes M. Somatostatin in the treatment of pancreatic ascites. Gastroenterology. 1990;99:1520-1.
112. Angeli P, Wong F, Watson H, et al. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44:1535-42.
113. Runyon BA. Historical aspects of treatment of patients with cirrhosis and ascites. Semin Liver Dis. 1997;17:163-73.
114. Eisenmenger WJ, Blondheim SH, Bongiovanni AM, et al. Electrolyte studies on patients with cirrhosis of the liver. J Clin Invest. 1950;29:1491-9.
115. Stiehm AJ, Mendler MH, Runyon BA. Detection of diuretic-resistance or diuretic-sensitivity by the spot urine Na/K ratio in 729 specimens from cirrhotics with ascites: Approximately 90% accuracy as compared to 24-hour urine Na excretion [abstract]. Hepatology. 2002;36:222A.
116. Perez-Ayuso RM, Arroyo V, Planas R, et al. Randomized comparative study of efficacy of furosemide vs. spironolactone in nonazotemic cirrhosis with ascites. Gastroenterology. 1983;84:961-8.
117. Sungaila I, Bartle WR, Walker SE, et al. Spironolactone pharmacokinetics and pharmacodynamics in patients with cirrhotic ascites. Gastroenterology. 1992;102:1680-5.
118. Cohn JN. The management of chronic heart failure. N Engl J Med. 1996;335:490-8.
119. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of moderate ascites in nonazotemic patients with cirrhosis. Gut 2009 [Epub ahead of print].
120. Stanley MM, Ochi S, Lee KK, et al. Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. N Engl J Med. 1989;321:1632-8.
121. Daskalopoulos G, Laffi G, Morgan T, et al. Immediate effects of furosemide on renal hemodynamics in chronic liver disease with ascites. Gastroenterology. 1987;2:1859-63.
122. Chalasani N, Gorski JC, Horlander JC, et al. Effects of albumin/furosemide mixtures on responses to furosemide in hypoalbuminemic patients. J Am Soc Nephrol. 2001;12:1010-16.
123. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: The importance of peripheral edema. Gastroenterology. 1986;90:1827-33.
124. Mirouze D, Zipser RD, Reynolds TB. Effect of inhibitors of prostaglandin synthesis on induced diuresis in cirrhosis. Hepatology. 1983;3:50-5.
125. Runyon BA, Antillon MR, Montano AA. Effect of diuresis versus therapeutic paracentesis on ascitic fluid opsonic activity and serum complement. Gastroenterology. 1989;97:158-62.
126. Wong F, Blei AT, Blendis LM, et al. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: A multicenter, randomized, placebo-controlled trial. Hepatology. 2003;3:182-91.
127. Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Heptology. 1996;23:164-76.
128. Planas R, Montoliu S, Balleste B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385-94.
129. Stanley MM, Reyes CV, Greenlee HB, et al. Peritoneal fibrosis in cirrhotics treated with peritoneovenous shunting for ascites. Dig Dis Sci. 1996;41:571-7.
130. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites: Results of a randomized study. Gastroenterology. 1987;93:234-41.
131. Smith GS, Barnard GF. Massive volume paracentesis (up to 41 liters) for the outpatient management of ascites. J Clin Gastroenterol. 1997;25:402-3.
132. Gines P, Tito L, Arroyo V, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493-502.
133. Gines A, Fernandez-Esparrach G, Monescillo A, et al. Randomized trial comparing albumin, dextran 70 and polygeline in cirrhotic patients with ascites treated by paracentesis. Hepatology. 1996;111:1002-10.
134. Ruiz-del-Arbol L, Monescillo A, Jimenez W, et al. Paracentesis-induced circulatory dysfunction: Mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579-86.
135. Rothschild M, Oratz M, Evans C, et al. Alterations in albumin metabolism after serum and albumin infusions. J Clin Invest. 1964;43:1874-80.
136. Wilkinson P, Sherlock S. The effect of repeated albumin infusions in patients with cirrhosis. Lancet. 1962;2:1125-9.
137. Pietrangelo A, Panduro A, Chowdhury JR, et al. Albumin gene expression is down-regulated by albumin or macromolecule infusion in the rat. J Clin Invest. 1992;89:1755-60.
138. Runyon BA. Patient selection is important in studying the impact of large-volume paracentesis on intravascular volume. Am J Gastroenterol. 1996;92:371-3.
139. Peltekian KM, Wong F, Liu PP, et al. Cardiovascular, renal, and neurohumoral responses to single large-volume paracentesis in patients with cirrhosis and diuretic-resistant ascites. Am J Gastroenterol. 1997;92:394-9.
140. Moller S, Bendtsen F, Henriksen JH. Effect of volume expansion on systemic hemodynamics and central and arterial blood volume in cirrhosis. Gastroenterology. 1995;109:1917-25.
141. Planas R, Gines P, Arroyo V, et al. Dextran-70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Gastroenterology. 1990;99:1736-44.
142. Christidis C, Mal F, Ramos J, et al. Worsening of hepatic dysfunction as a consequence of repeated hydroxyethyl starch infusions. J Hepatol. 2001;35:726-32.
143. Vermeulen LC, Ratko TA, Erstad BL, et al. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloids, and crystalloid solutions. Arch Intern Med. 1995;155:373-9.
144. The SAFE Study Investigators: A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-56.
145. Wong C, Holroyd-Leduc J, Thorpe KE, Straus SE. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results? JAMA. 2008;299:1166-78.
146. Singh V, Kumar R, Nain CK, et al. Terlipressin versus albumin in paracentesis-induced circulatory dysfunction in cirrhosos: A randomized trial. J Gastroenterol Hepatol. 2006;21:303-7.
147. Ochs A, Rossle M, Haag K, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995;332:1192-7.
148. Rossle M, Ochs A, Gulberg V, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701-7.
149. Gines P, Uriz J, Calahorra B, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839-47.
150. Sanyal AJ, Genning C, Reddy KR, et al. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;123:634-41.
151. Salerno F, Merli M, Riggio O, et al. Randomized controlled study of TIPS vs. paracentesis with albumin in cirrhosis with refractory ascites. Hepatology. 2004;40:629-35.
152. Saab S, Niets JM, Ly D, et al. TIPS versus paracentesis for patients with refractory ascites may benefit from trans-jugular intrahepatic portosystemic stent-shunts. Cochrane Database Syst Rev 2004; 3:CD004889.
153. D’Amico G, Luca A, Morabito A, et al. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: A meta analysis. Gastroenterology. 2005;129:1282-93.
154. Albillos A, Banares R, Gonzalez M, et al. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005;43:990-6.
155. Mathurin DP, Moreau DS, Moreau R, et al. Transjugular intrahepatic portosystemic shunt in refractory ascites: A meta-analysis. Liver Int. 2005;25:349-56.
156. Salerno F, Camma C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: A meta-analysis of individual patient data. Gastroenterology. 2007;133:825-34.
157. Angermayr B, Cejna M, Koenig F, et al. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stentgrafts versus bare stents. Hepatology. 2003;38:1043-50.
158. Bureau C, Garcia-Pagan JC, Otal P, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: Results of a randomized study. Gastroenterology. 2004;126:469-75.
159. Petersen B. Intravascular ultrasound-guided direct intrahepatic portacaval shunt: Description of technique and technical refinements. J Vasc Interv Radiol. 2003;14:21-32.
160. Rosemurgy AS, Zervos EE, Clark WC, et al. TIPS versus peritoneovenous shunt in the treatment of medically intractable ascites: A prospective randomized trial. Ann Surg. 2004;239:883-91.
161. Trotter J, Pieramici E, Everson GT. Chronic albumin infusions to achieve diuresis in patients with ascites who are not candidates for transjugular intrahepatic portosystemic shunt (TIPS). Dig Dis Sci. 2005;50:1356-60.
162. Romanelli RG, La Villa G, Barletta G, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: An unblended randomized trial. World J Gastroenterol. 2006;12:1403-7.
163. McGill RL, Bakos JR, Marcus RJ. Ascites reinfusion dialysis (ARD) for renal failure with refractory ascites. Clin Nephrol. 2004;62:374-9.
164. Davenport A. Ultrafiltration in diuretic-resistant volume overload in nephrotic syndrome and patients with ascites due to chronic liver disease. Cardiology. 2001;96:190-5.
165. Krag A, Moller S, Henriksen JH, et al. Terlipressin improves renal function in patients with cirrhosis and ascites without hepatorenal syndrome. Hepatology. 2007;46:1863-71.
166. Koconis KG, Singh H, Soares G. Partial splenic embolization in the treatment of patients with portal hypertension: A review of the English language literature. J Vasc Internvent Radiol. 2007;18:463-81.
167. Rozenblit GN, Del Guercio LRM, Rundback JH, et al. Peritoneal-urinary drainage for treatment of refractory ascites: A pilot study. J Vasc Interv Radiol. 1998;9:998-1005.
168. Park JS, Won JY, Park SI, et al. Percutaneous peritoneovenous shunt creation for the treatment of benign and malignant refractory ascites. J Vasc Interv Radiol. 2001;12:1445-8.
169. Gines P, Quintero E, Arroyo V, et al. Compensated cirrhosis: Natural history and prognostic factors. Hepatology. 1987;7:12-18.
170. D’Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31:468-75.