Chapter 52 Asbestosis
Inhalational exposure to asbestos produces both malignant and nonmalignant diseases of the chest. The focus of this chapter is on the two major categories of nonmalignant disease—asbestosis and asbestos-related pleural disorders, listed in Table 52-1. These conditions have received a great deal of attention from the scientific and medical communities because of the ubiquitous use of asbestos in modern society and its diverse and pernicious toxicities. Despite major progress in awareness of the issues and in control of exposure, a large burden of asbestos-related disease will continue to accrue as a consequence of ongoing exposure and the characteristic disease latency.
Table 52-1 Nonmalignant Asbestos-Related Diseases
| Condition | Locus of Pathologic Change | Description |
|---|---|---|
| Asbestosis | Parenchyma | Interstitial pulmonary fibrosis |
| Benign nodules | Parenchyma | Lymphoid or fibrotic nodular scars |
| Benign pleural effusion | Pleura | Exudative, transient effusion |
| Pleural plaques | Pleura | Collagenous, hyalinized masses; circumscribed, avascular; usually involving the parietal pleura |
| Diffuse pleural thickening | Pleura | Collagenous, hyalinized masses; diffuse, avascular; involving the parietal and visceral pleura and interlobular space |
| Rounded atelectasis | Combined pleura and parenchyma | Scarring of pleura and adjacent lung tissue, resulting in retraction, entrapment, and local partial collapse of lung |
Epidemiology, Risk Factors, and Pathophysiology
Etiology and Risk Factors
The minerals referred to herein as “asbestos” are a family of naturally occurring, flexible, fibrous hydrous silicates found in soil worldwide. Mined asbestos fibers are categorized as either long and curly (serpentine) or straight and rodlike (amphibole). The serpentine fiber, chrysotile, accounts for most of the commercially used asbestos, favored for its properties of heat resistance, flexibility, and ease of spinning for textiles. Five categories of amphiboles are recognized: crocidolite, amosite, anthophyllite, tremolite, and actinolite. These more rigid fibers are less commonly used but are still pathogenic. All major commercial forms have been associated with development of nonmalignant respiratory disorders and of lung cancer and mesothelioma, as discussed in Chapters 47, 65, and 70.
Asbestosis is the result of either direct or “bystander” exposure to asbestos-containing materials. Major sources of exposure are summarized in Table 52-2. During the first half of the 20th century, high-level exposures to asbestos dust occurred in the manufacturing of asbestos textiles and construction materials and in the construction and ship-building trades. Potential exposures still occur in the construction trades and in the process of asbestos abatement. Although the use of asbestos has been curtailed in many developed nations since the 1970s, in less developed countries this inexpensive but hazardous material continues to be used widely. High cumulative, occupational exposures in these settings are still commonplace.
| Environment | Type of Exposure | Source of Exposure |
|---|---|---|
| Occupational | Asbestos-cement products | Construction industry (sheeting used in roofing and cladding of structural materials, molded into roof tiles, pipes, gutters; filler for wall cracks, cement, joint compound, adhesive, caulking putty) |
| Floor tiling | Filler and reinforcing agent in asphalt flooring, vinyl tile, adhesive | |
| Insulation, fireproofing | Insulators, pipefitters | |
| Construction industry (pipes, boiler covers, ship bulkheads, sprayed on walls and ceilings as fireproofing, soundproofing) | ||
| Textiles | Fireproof textiles used in clothing, blankets | |
| Paper products | Roofing felt, wall coverings, mill board, insulating paper | |
| Friction materials | Brake linings | |
| Rubber, plastic manufacture | Filler in rubber and plastics | |
| Building trades, secondhand exposure | Building maintenance activities, pipefitting, electrical repair, boiler tending and secondhand exposure repair, boiler tending and repair, power station maintenance | |
| Carpenters, plumbers, welders | ||
| Domestic | “Fouling the nest” | Carrying home asbestos in hair and clothes of exposed workers results in exposure to family members |
| Secondhand exposure | Residential remodeling, removal, handling of frayed, friable asbestos in homes can cause environmental exposure | |
| General | Contaminated buildings | Found in low levels in buildings under normal use |
| Elevated exposures from remodeling, renovation, asbestos removal, disturbance of contaminated materials such as acoustic ceiling tiles, vinyl floor tiles, paints, plaster, pipes, boilers, steel beams | ||
| Geologic exposure | Living near asbestos mines or cement factories, or in geographic areas in which naturally occurring asbestos is found in ambient air | |
| Urban environment | Ambient air levels slightly higher in cities, perhaps because of asbestos shed from automotive brakes in denser traffic, and high concentration of industry and construction |
Histopathology and grading
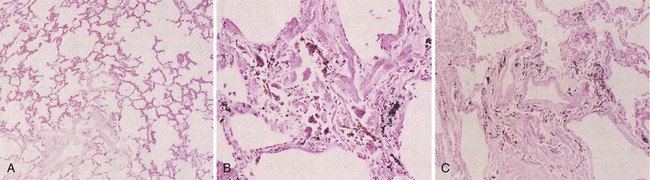
Asbestosis is defined histopathologically as bilateral, diffuse interstitial fibrosis of the lungs caused by the inhalation of asbestos fibers (Figure 52-1). Gray streaks of fibrosis can be seen in the parenchyma along interlobar and interlobular septa. Later, the pleural surface becomes more nodular in appearance, and the parenchyma loses volume and elasticity and forms more fibrotic scars and honeycombing. The gross pathologic changes are most obvious in the lower lung zones bilaterally, with the worst disease nearest to the pleura.
Grade 0—no appreciable peribronchiolar fibrosis, or fibrosis confined to the bronchiolar walls
Grade 1—fibrosis confined to the walls of respiratory bronchioles and the first tier of adjacent alveoli
Grade 2—extension of fibrosis to involve alveolar ducts and/or two or more tiers of alveoli adjacent to the respiratory bronchiole, with sparing of at least some alveoli between adjacent bronchioles
Grade 3—fibrotic thickening of the walls of all alveoli between two or more adjacent respiratory bronchioles
Pathogenesis
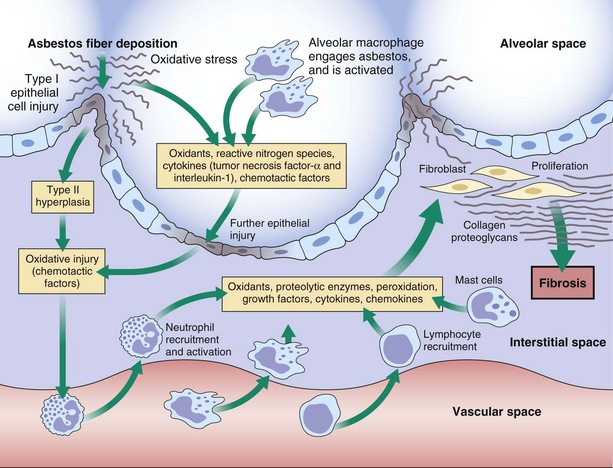
Some of the major events thought to be involved in the pathogenesis of asbestosis are summarized in Figure 52-2. Within minutes after asbestos fibers have been inhaled, a local tissue response is initiated at the bifurcations of terminal bronchioles and alveolar ducts. The first changes occur in epithelial cells and then in alveolar macrophages as they attempt to engulf and are pierced by the fibers. In addition to cell death, which leads to the release of macrophage contents, asbestos-activated macrophages release reactive oxygen species that directly damage the tissue through peroxidation and direct cytotoxicity. Asbestos also can induce toxicity by mechanisms independent of its ability to promote formation of reactive oxygen species.
Clinical Features
Radiologic Findings
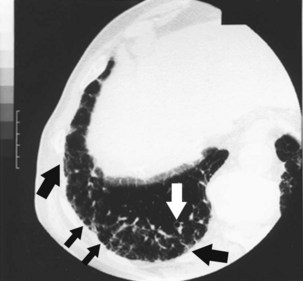
Radiographically, asbestosis typically manifests in the lower lobes with irregular “reticular” markings toward the lung periphery and costophrenic angles (Figure 52-3). Linear opacities that resemble extensions of vascular markings may assume a netlike appearance. In early or less severe disease, the middle and upper lung zones may appear relatively spared. With progression, the linear and irregular opacities thicken and spread to the midlung zones, but rarely to the apex. The International Labour Organization (ILO) International Classification of Radiographs of Pneumoconioses characterizes each type of irregular opacity on the basis of increasing size and thickness as either s, t, or u, and on a scale of profusion (number) of opacities from normal profusion (0/−, 0/0, 0/1) to severe (3/2, 3/3, 3/+) (see Chapter 51, Table 51-2, for classification details). When irregular opacities are seen on the chest radiograph in conjunction with pleural thickening, the radiograph can be considered virtually pathognomonic for asbestosis. However, the chest radiograph lacks sensitivity, because 15% to 18% of symptomatic, biopsy-proven asbestosis cases are associated with a normal appearance on chest radiographs. Focal masses are uncommon except for those caused by rounded atelectasis.
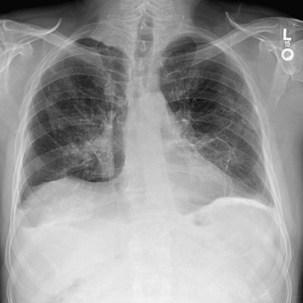
Figure 52-3 Chest radiograph illustrating parenchymal abnormalities in asbestosis. Descriptive designations are from the International Labour Organization (ILO) classification for the radiographic appearance of pneumoconioses (see Chapter 51, Table 51-2). In this case, fine linear opacities of profusion category 1/0 can be seen in the middle and lower lung zones. Calcified pleural plaques are seen along the diaphragm bilaterally.
Computed Tomography
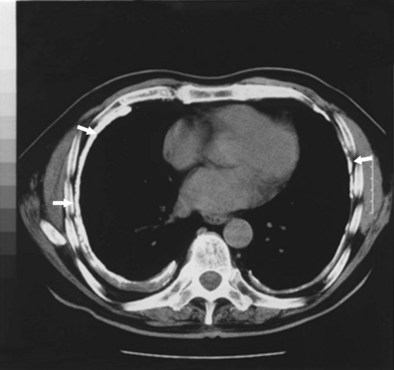
Conventional CT and high-resolution CT (HRCT) are more sensitive and specific than radiography for the diagnosis of pleural disease related to asbestos. HRCT, with use of 1- to 3-mm slices, is superior in sensitivity to plain chest radiography for detection of the fine reticular opacities in this disease. Of asbestos-exposed workers who have normal-appearing chest radiographs, 10% to 30% have HRCT scans suggestive of underlying interstitial disease. Thus, the HRCT scan can prove useful when the clinical index of suspicion for asbestosis is high but the chest radiograph appears normal. The most common HRCT findings in asbestosis are short, peripheral septal lines; subpleural curvilinear lines; ground glass attenuation; peripheral cystic lesions (honeycombing); parenchymal bands adjacent to areas of pleural thickening; and bronchiolar thickening (Figure 52-4). The density of interstitial abnormalities found on HRCT has been shown to correlate with the symptoms and with physiologic and inflammatory indicators of asbestosis, although in general, findings on both the chest radiograph and the HRCT scan demonstrate only a limited correlation with disease severity measured by assessment of lung physiology.
Diagnosis
Treatment
Medical management in cases of asbestosis focuses on the following interventions:
• Supplemental oxygen therapy in the face of hypoxemia or pulmonary hypertension
• Treatment of intercurrent infections
• Treatment of right ventricular failure in advanced disease
• Immunization for influenza virus and pneumococcal infections
• Appropriate medical documentation of the degree of physical impairment and support and referral of the patient to relevant agencies to apply for workers’ compensation benefits if exposure was occupational
• Education on the signs and symptoms of lung cancer and mesothelioma
• Assistance in smoking cessation for current smokers who have asbestosis, to help reduce the risk of lung cancer
• Cessation of ongoing asbestos exposure—always advisable, because it may slow disease progression, as indicated by experimental evidence
Asbestos-Related, Nonmalignant Pleural Disorders
Circumscribed pleural plaques that involve the parietal pleura usually are symmetric and bilateral, most commonly between the fifth and eighth ribs toward the posterolateral aspects of the thorax (Figure 52-5); they also frequently involve the diaphragmatic pleura (Figure 52-6). These lesions remain discrete. Thus, if radiographic evidence of more diffuse thickening is found, either mesothelioma or diffuse pleural thickening must be considered. On histologic examination, pleural plaques are seen to be hyalinized, acellular, avascular masses, and they rarely contain asbestos bodies. They have a tendency to calcify, which can be mistaken for nodular infiltrates on the chest radiograph. Although the ILO classification system has an elaborate section devoted to characterization of pleural abnormalities on the chest radiograph, interreader agreement is relatively low. Because HRCT is more sensitive than chest radiography in the detection of pleural plaques, it helps to determine past asbestos inhalation, because these plaques are pathognomonic for that exposure. Also, HRCT helps to differentiate plaques from extrapleural fat pads. Asbestosis can occur in the absence of pleural disease, and inversely, pleural disease can occur without underlying pulmonary fibrosis, although autopsy studies suggest that when pleural changes are seen, histopathologic features of asbestosis often are evident even if the radiograph is normal in appearance. Pleural plaques rarely, if ever, undergo malignant transformation.
Controversies and Pitfalls
American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med. 2004;170:691–715.
Burgess WA. Asbestos products. In: Burgess WA, ed. Recognition of health hazards in industry. New York: John Wiley & Sons; 1995:443–451.
Craighead JE, Abraham JL, Churg A, et al. The pathology of asbestos-associated disease of the lung and pleural cavities: diagnostic criteria and proposed grading scheme. Arch Pathol Lab Invest. 1982;106:544–595.
Division of Respiratory Disease Studies. National Institute for Occupational Safety and Health (NIOSH) Work-related lung disease surveillance report (DHHS [NIOSH] publication no. 2003–2111). Washington, DC: U.S. Government Printing Office; 2002.
Hillerdal G. Rounded atelectasis: clinical experience with 74 patients. Chest. 1989;9:836–841.
International Labor Office. International classification of radiographs of pneumoconiosis. Geneva, Switzerland: International Labor Organization; 2003.
Kamp DW, Weitzman SA. The molecular basis of asbestos induced lung injury. Thorax. 1999;54:638–652.
Peipins LA, Lewin M, Campolucci S, et al. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environ Health Perspectives. 2003;111:1753–1759.
Roggli VL, Gibbs AR, Attanoos R, et al. Pathology of asbestosis—an update of the diagnostic criteria: report of the Asbestosis Committee of the College of American Pathologists and Pulmonary Pathology Society. Arch Pathol Lab Med. 2010;134:462–480.
Tossavainen A. International expert meeting on new advances in the radiology and screening of asbestos-related diseases. Scand J Work Environ Health. 2000;26:449–454.
Welch LS, Haile E, Dement J, Michaels D. Change in prevalence of asbestos-related disease among sheet metal workers 1986 to 2004. Chest. 2007;131:863–869.
Yamamoto S. Histopathological features of pulmonary asbestosis with particular emphasis on the comparison with those of usual interstitial pneumonia. Osaka City Med J. 1997;43:225–242.
Zhao XH, Jia G, Liu YQ, et al. Association between polymorphisms of DNA repair gene XRCC1 and DNA damage in asbestos-exposed workers. Biomed Environ Sci. 2006;19:232–238.