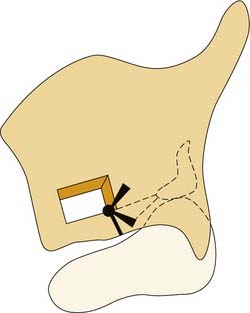
CHAPTER 67 Arytenoid Adduction
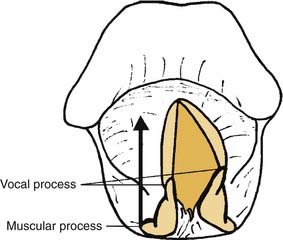
The arytenoid adduction procedure addresses laryngeal incompetence in patients with unilateral laryngeal paralysis.1 It mimics the action of the lateral cricoarytenoid muscle to close the glottis via rotation of the arytenoid cartilage rather than direct displacement of the membranous vocal fold.2 An adduction suture is placed in the muscular process of the arytenoid, which is the origin of the lateral cricoarytenoid muscle. This suture is passed forward through the paraglottic space and secured to the inferior thyroid ala (Fig. 67-1). This anterior traction pulls forward on the muscular process so that the arytenoid rotates. The vocal process, which is orthogonal to the muscular process, moves medially, dragging the membranous vocal fold with it. Arytenoid adduction can be performed in conjunction with type I thyroplasty. Research in animal models indicates that a combination of the two procedures is more effective than either alone.3
Arytenoid adduction is a more invasive procedure than type I thyroplasty and is technically more difficult. It has been reported to carry somewhat greater surgical risks, including airway obstruction, joint dislocation, fistula, and carotid injury.4,5 A 2001 study compared outcomes of type I thyroplasty alone or in combination with arytenoid adduction in 237 patients. The complication rate was 19% in patients undergoing arytenoid adduction versus 14% in those receiving thyroplasty alone. However, the difference in the complication rate was not statistically significant, and the 2 patients who required emergency tracheotomy for airway obstruction had thyroplasty alone.6 Arytenoid adduction does provide better closure of the posterior glottis, and research in animal models has objectively documented that acoustic and aerodynamic results for this procedure are better than for those of thyroplasty alone.7,8 Thus, arytenoid adduction is a valuable component of the therapeutic armamentarium for rehabilitation of patients with laryngeal paralysis, particularly those with significant glottal incompetence.
The clinical impact of laryngeal paralysis varies greatly.9 Some patients with unilateral paralysis are completely asymptomatic. At the other end of the spectrum, some patients are aphonic and have severe problems with aspiration during swallowing. Such severe symptoms result from laryngeal incompetence—the inability of the glottis to close completely. Two key factors that influence glottal closure in patients with laryngeal paralysis are the configuration of the paralyzed vocal fold and the compensatory function of the contralateral fold.
Several theories have been proposed to explain variations in symptoms and vocal fold positions in patients with laryngeal paralysis, ranging from selective damage to abductor fibers10 to adductor action of the cricothyroid muscle (Wagner-Grossman hypothesis).11,12 However, it has been demonstrated that the cricothyroid muscle does not exert an adductor force on the vocal fold.13–15 It is now generally accepted that a medial vocal fold position results from residual or regenerated innervation of laryngeal muscles.16–19 When there is significant innervation of adductor muscles, the paralyzed fold is located near the midline, and compensatory activity of the normal side of the larynx can often close the glottis during phonation. In such cases, aspiration is rare, and hoarseness or breathiness responds well to surgical medialization of the membranous vocal fold performed by injection or thyroplasty. On the other hand, complete flaccid paralysis results in a cadaveric position of the vocal fold with more severe glottal incompetence, often referred to as a posterior gap.
It has long been recognized that vocal fold injection, such as with polytetrafluoroethylene (Teflon), cannot restore glottal competence when there is a large glottal gap.20 There is some controversy regarding the efficacy of Isshiki type I thyroplasty in closing a posterior gap. It has been contended that a posterior extension of the thyroplasty implant can close a posterior gap, based on clinical observation.21 However, other clinical reports as well as experimental studies indicate that arytenoid adduction is much more effective than thyroplasty in closing the posterior glottis.7,8
The concept of a “posterior gap” is not entirely accurate, in that the maximal glottic opening is between the vocal processes. The open glottis actually converges posterior to the vocal processes because lateral displacement of the vocal process involves external rotation of the arytenoid cartilage, not lateral displacement of the arytenoid.22,23 In other words, the angle between the membranous vocal fold and its posterior cartilaginous portion decreases as the vocal fold abducts (Fig. 67-2).24 Thus, the posterior portion of the cartilaginous vocal fold is medial to the vocal process in abduction, and it prevents glottic closure even with vigorous hyperadduction of the normal vocal fold (Fig. 67-3). Procedures that medialize the membranous vocal fold may achieve some medial displacement of the vocal process; however, the force vectors created by such procedures are inadequate to effect significant internal rotation of the arytenoid cartilage.
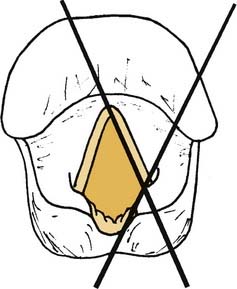
Figure 67-2. Vocal fold angle (X) formed by membranous and cartilaginous portions of the vocal fold.
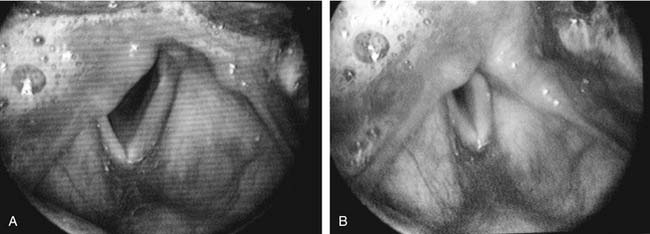
Figure 67-3. A, Apparent vocal fold shortening in laryngeal paralysis. B, Compensatory shortening of the mobile focal fold.
Vocal fold injection and type I thyroplasty do not address differences in the level of vocal folds. The paralyzed vocal fold often lies in a different plane from that of the mobile vocal fold. Although both folds are firmly attached to the anterior commissure, the vocal process may sag on the paralyzed side or lie above the glottic plane, as it does in physiologic abduction. Thus, there can be a significant vertical gap between the edges of the vocal folds, even when they appear to be touching when viewed from above in a two-dimensional image. The paralyzed fold can lie either above or below the glottal plane. In cadaver specimens from subjects who had chronic vocal fold paralysis, the vocal fold has been reported to be caudally displaced with a wide ventricle and shift of the conus elasticus to a horizontal plane.25,26 Other reports describe superior displacement of the paralyzed vocal fold, essentially the same position assumed by an actively abducted vocal fold.2,27
A difference in the level of the vocal folds can often be appreciated with indirect mirror laryngoscopy, which permits binocular vision. In 1932, noting observations with mirror laryngoscopy, New and Childrey28 stated, “In the absence of any innervation from the recurrent nerve fibers, the cord is somewhat relaxed or bowed; it is, therefore, shortened, somewhat narrowed and depressed, lying at a lower level than normal.” In a two-dimensional video image, a level difference is not apparent. What can be perceived is a difference in the apparent length of the vocal folds. Brewer and colleagues29 noted that a paralyzed vocal fold generally appears shorter than its mobile mate. In patients with significant glottal incompetence, the apparent length of a paralyzed vocal fold during inspiration is approximately two thirds that of the mobile side. During phonation, the mobile vocal fold appears to shorten to nearly the same length as its paralyzed mate (see Figure 67-3). Successful arytenoid adduction increases the apparent length of the paralyzed vocal fold.30
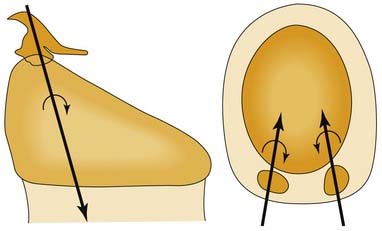
Three-dimensional motion analysis in cadaver larynges indicates that the arytenoid adduction procedure does not actually lengthen the vocal fold but instead moves the vocal process caudally. It is this vertical component of motion that is endoscopically perceived as a length change; the visual image shortens owing to rotation of the vocal fold out of the optical plane.31 In arytenoid adduction, the arytenoid rotates about an oblique helical axis. As the vocal process moves medially, it is displaced caudally as well (Fig. 67-4).32 Conversely, when the posterior cricoarytenoid muscle abducts the vocal fold, the vocal process moves laterally, rostrally, and caudally (Fig. 67-5).23 An abducted vocal fold appears shorter when viewed from above because the vocal process has moved rostrally; the vocal fold slopes upward, out of the plane of the image. In adduction and during arytenoid adduction, the vocal process moves caudally, toward the level of the anterior commissure, so that the vocal fold is parallel to the image plane, and the apparent length is longer.31
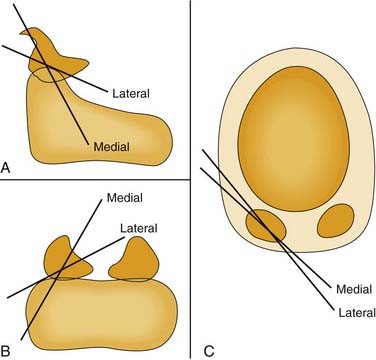
Figure 67-5. Axes of rotation (X) with simulated individual contraction of the two bellies of the posterior cricoarytenoid muscle in cadaver larynges. A, Sagittal view; B, posterior, coronal view; C, axial view. These data, as well as those from the study depicted in Figure 67-4, illustrate the multiaxial nature of the cricoarytenoid joint.
The cricoarytenoid joint is a shallow ball-and-socket, and therefore, it is multiaxial. That is to say, vocal folds do not open and close along a fixed “track,” and the rostrocaudal level of the vocal process is not completely dictated by internal and internal rotation. Cadaver studies of simulated muscle contraction demonstrate that the axis of rotation for the posterior cricoarytenoid is quite different from that for the lateral cricoarytenoid muscle (see Fig. 67-5).23 This means that varying the force of individual muscles enables the rostrocaudal position of the vocal process to be varied independent of its mediolateral position. This is a likely mechanism of compensation by the active vocal fold in patients with laryngeal paralysis.
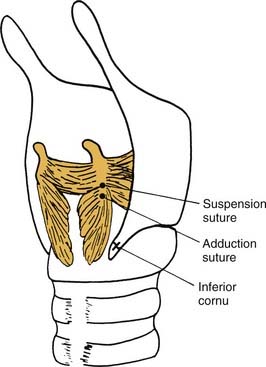
Because of the multiaxial nature of the cricothyroid joint, the vocal process of a paralyzed vocal fold is not necessarily located along the path of physiologic motion. In a normal larynx, the vocal process moves upward as the vocal fold is adducted. But in flaccid paralysis, the pull of the posterior cricoarytenoid muscle is lost, so that the arytenoid may “tip” forward (see Fig. 67-3A). The vector of the pull in the arytenoid adduction procedure is similar to that of the cricoarytenoid muscle, anteriorly and slightly inferiorly. Thus, the procedure cannot correct, and may exacerbate, anterior tip. One approach to correcting the “sagging” arytenoid is a posterior suspension suture, which pulls posteriorly and inferiorly on the vocal process, to “rock” the arytenoid into a position that is nearer than that assumed during normal phonation (Fig. 67-6).33 Another approach to the sagging arytenoid is adduction arytenoidopexy.34 However, adduction arytenoidopexy fixes the arytenoid to the cricoid posteriorly and does not provide a medial vector of force at the vocal process. Thus, the vocal process can be pushed laterally by the adduction force of the normal vocal fold during phonation.
Dysphagia can be a significant problem when laryngeal paralysis is the result of a vagus nerve injury. Pharyngeal propulsion is impaired owing to ipsilateral paralysis of constrictor muscles. But the cricopharyngeal muscle retains tone because it is a continuous muscular sling that receives bilateral innervation. There is inadequate pharyngeal pressure to push the bolus into the esophagus, resulting in stasis of secretions and ingested food in the hypopharynx. In such cases, a cricopharyngeal myotomy can be effective in improving swallowing and reducing aspiration.21 Cricopharyngeal myotomy is easily performed in conjunction with the arytenoid adduction procedure.35
Arytenoid adduction is less effective in patients with long-standing laryngeal paralysis. This limitation does not appear to be due to joint fixation, because the arytenoid can be successfully internally rotated, with objective improvements in maximum phonation time and mean phonatory airflow. But the apparent length, and presumably the vertical position of the arytenoid, is not improved, so compensatory hyperfunction is still required for speech.30 It is possible that contracture and fibrosis of soft tissues, such as muscle and mucosa, prevent correction of the vertical position of the vocal fold. The outcome of the arytenoid adduction procedure is also less favorable in patients with paralysis due to central neural lesions, such as tumor or stroke, because of associated sensory, reflex, and/or cognitive deficits.
Operative Technique
Surgical Procedure
After the thyroplasty window is created, the next objective is to expose the muscular process of the arytenoid. Several approaches have been described. I prefer to dissect lateral to the cervical strap muscles, rotating the larynx away from the opposite side. This rotation displaces the larynx away from the carotid sheath, minimizing risk to those structures. Dissection should “hug” the strap muscles, continuing posteriorly to the inferior constrictor muscle and superiorly to identify the superior cornu of the thyroid cartilage. A sturdy cricoid hook should then be placed on the superior cornu of the thyroid cartilage to rotate the larynx away from the field (Fig. 67-7). Dissection should be continued inferiorly, medial to the thyroid gland, down to the level of the cricoid.
A critical step in the procedure is identification and displacement of the pyriform sinus mucosa, to expose the arytenoid cartilage and to avoid entry into the hypopharynx. The pyriform sinus is separated from the medial surface of the thyroid ala by blunt dissection. Identification of the posterior and inferior limits of the pyriform sinus is facilitated by having the patient utter plosive phrases (such as “puppy”) that generate gentle positive intrapharyngeal pressure. The pressure distends the pyriform sinus and makes its edges apparent. The sac is then reflected superiorly and anteriorly, exposing the posterior cricoarytenoid muscle. The fibers of this muscle are then followed to their convergence and insertion on the muscular process of the arytenoid. In a man with a large thyroid cartilage, adequate exposure may require transaction of the thyrohyoid ligament to permit greater laryngeal rotation. Alternatively, a posterior portion of the thyroid cartilage could be resected, as described by Netterville and colleagues.36 Maragos37 has reported performing arytenoid adduction via a posterior thyroplasty window.
In their original description, Isshiki and colleagues1 recommended transection of the cricothyroid joint as well as opening of the cricoarytenoid joint to locate the muscular process. These steps potentially destabilized the larynx, and most later writers have not found it necessary to compromise these structures.38
The muscular process appears as a white prominence between the attachments of the anterior and posterior bellies of the posterior cricoarytenoid muscle (Fig. 67-8). This is where the adduction suture is placed—a 4-0 permanent suture with a sturdy but small-radius curved needle (I prefer a cardiac valve needle). The muscle tendon is grasped near its insertion, and the needle is passed from back to front through the cartilage process; care should be taken to not injure the pyriform mucosa. The surgeon should then tug the suture while palpating the arytenoid, to assess the “purchase.” If the entire arytenoid does not move with traction on the suture, the stitch was passed through soft tissue rather than the cartilage. The suture is then used to provide traction, to stabilize the arytenoid, and to ensure placement in the cartilage. When a satisfactory suture has been placed, it should be tied down securely, leaving two long ends.
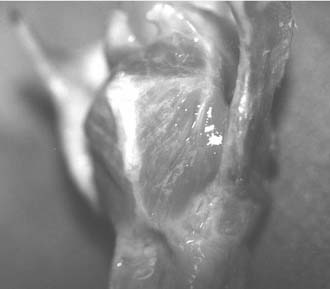
Figure 67-8. Muscular process of the arytenoid cartilage, located at the convergence of posterior cricoarytenoid muscle fibers.
Once adequate rotation is ensured, the second, posterior end of the arytenoid adduction suture is passed forward through the tunnel, bringing it out under the thyroid cartilage rather than through the window. This allows the stitch to be secured by tying of the suture over the inferior thyroid strut, at the posterior inferior corner of the window (Fig. 67-9). Sutures in this position will not be affected by a thyroplasty implant.
If the arytenoid sags forward and the vocal fold is in a lower plane than the normal fold, a posterior suspension suture should be considered. The suspension suture is placed in the arytenoid cartilage a few millimeters superior to the muscular process of the arytenoid, on the ridge that extends from the muscular process to the apex of the cartilage (see Fig. 67-6). The suture is then passed through or around the inferior cornu of the thyroid cartilage, and is tied down to provide inferior traction. The larynx is observed endoscopically while tension is adjusted to rock the larynx posteriorly, without canceling out the adduction achieved by the arytenoid adduction suture.
Postoperative Care
In general, patients can resume oral intake within 1 to 2 hours after surgery. I keep patients overnight in the hospital after the procedure to observe for possible airway obstruction. Bleeding into the paraglottic space is the primary cause of postoperative airway obstruction. Thus, it is imperative to leave an adequate drain, to suppress coughing, and to control blood pressure. Maragos39 has described postoperative airway obstruction due to herniation of pyriform mucosa into the airway. He reports that suture stabilization of pyriform mucosa at the close of the procedure is effective in preventing this complication.
Arytenoid Abduction for Bilateral Paralysis
A variation of arytenoid adduction is arytenoid abduction.40 The procedure is identical to arytenoid adduction, except that the suture through the vocal process is anchored posteriorly rather than anteriorly. This vector of force rotates the larynx externally to abduct the vocal fold. Arytenoid abduction has been described as a surgical option to improve the glottic airway in patients with bilateral laryngeal paralysis. It can be performed in lieu of an emergency tracheotomy in patients with airway distress. Alternatively, it can be performed in patients with a tracheotomy to facilitate decannulation. In contrast to adduction, arytenoid abduction is performed with the patient under general anesthesia. For patients without an existing tracheotomy, an endotracheal tube provides a stable airway. When a tracheotomy is in place, dissection is more difficult, and the surgery is better tolerated with use of general anesthesia.
Crumley RL. Laryngeal synkinesis revisited. Ann Otolaryngol. 2000;109:365-371.
Isshiki N, Tanabe M, Masaki S. Arytenoid adduction for unilateral vocal cord paralysis. Arch Otolaryngol Head Neck Surg. 1978;14:555-558.
Woodson GE. Cricopharyngeal myotomy and arytenoid adduction in the management of combined laryngeal and pharyngeal paralysis. Otolaryngol Head Neck Surg. 1997;117:339-343.
1. Isshiki N, Tanabe M, Masaki S. Arytenoid adduction for unilateral vocal cord paralysis. Arch Otolaryngol Head Neck Surg. 1978;14:555-558.
2. Isshiki N, Ishikawa T. Diagnostic value of tomography in unilateral vocal cord paralysis. Laryngoscope. 1976;86:1573-1578.
3. Noordzij JP, Perrault DF, Woo P. Biomechanics of combined arytenoid adduction and medialization laryngoplasty in an ex vivo canine model. Otolaryngol Head Neck Surg. 1998;119:634-642.
4. Koufman JA, Isaacson G. Laryngoplastic phonosurgery. Otolaryngol Clin North Am. 1991:1151-1173.
5. Weinman EC, Maragos NE. Airway compromise in thyroplasty surgery. Laryngoscope. 2000;110:1082-1085.
6. Abraham MT, Gonen M, Kraus DH. Complications of type I thyroplasty and arytenoid adduction. Laryngoscope. 2001;111:1322-1329.
7. Chhetri DK, Gerratt BR, Kreiman J, et al. Combined arytenoid adduction and laryngeal reinnervation in the treatment of vocal fold paralysis. Laryngoscope. 1999;109:1928-1936.
8. Green DC, Berke GS, Ward PH. Vocal fold medialization by surgical augmentation versus arytenoid adduction in the in vivo canine model. Ann Otol Rhinol Laryngol. 1991;100:280-287.
9. Work WP. Paralysis and paresis of the vocal cords: a statistical review. Arch Otolaryngol. 1941;43:267-280.
10. Semon F. On the proclivity of the abductor fibers of the recurrent laryngeal nerve to become affected sooner than the adductor fibers or even exclusively. Arch Laryngol. 1881;2:197-222.
11. Konrad HR, Rattenborg CC. Combined action of laryngeal muscles. Acta Otolaryngol. 1969;67:646-649.
12. New GB, Childrey JH. Paralysis of the vocal cords: a study of 217 medical cases. Arch Otol. 1932;16:143-159.
13. Woodson GE, Sant’Ambrogio F, Mathew O, et al. Effects of cricothyroid muscle contraction on laryngeal resistance and glottic area. Ann Otol Rhinol Laryngol.. 1989;98:119-124.
14. Amis TC, Brancatisano A, Tully A, et al. Effects of cricothyroid muscle contraction on upper airway flow dynamics in dogs. J Appl Physiol. 1992;72:2329-2335.
15. Koufman JA, Walker FO, Joharji GM. The cricothyroid muscle does not influence vocal fold position in laryngeal paralysis. Laryngoscope. 1995;105:368-372.
16. Hirano M, Nozoe I, Shin T, Maeyama T. Electromyography for laryngeal paralysis. In: Hirano M, Kirchner J, Bless D, editors. Neurolaryngology: Recent Advances. Boston: College-Hill; 1987:232-248.
17. Woodson GE. Configuration of the glottis in laryngeal paralysis II: animal experiments. Laryngoscope. 1993;103:1235-1241.
18. Blitzer A, Jahn AF, Keidar A. Semon’s law revisited: an electromyographic analysis of laryngeal synkinesis. Ann Otol Rhinol Laryngol. 1996;105:764-769.
19. Crumley RL. Laryngeal synkinesis revisited. Ann Otolaryngol. 2000;109:365-371.
20. Dedo HH. Teflon injection of the vocal fold. In: Surgery of the Larynx & Trachea. Philadelphia: Mosby; 1990:25.
21. Montgomery WM, Hilman RE, Varvares MA. Combined thyroplasty type I and inferior constrictor myotomy. Ann Otol Rhinol Laryngol. 1995;103:858-862.
22. Hirano M. Anatomy and behavior of the vocal process. and others. Baer T, Sasaki C, Harris K, editors. Laryngeal Function in Phonation and Respiration. Boston: College-Hill Press, 1987.
23. Bryant NJ, Woodson GE, Kaufman K, et al. Human posterior cricoarytenoid muscle compartments: anatomy and mechanics. Arch Otolaryngol Head Neck Surg. 1996;122:1331-1336.
24. Woodson GE. Configuration of the glottis in laryngeal paralysis I: clinical study. Laryngoscope. 1993;103:1227-1234.
25. Kirchner JA. Atrophy of laryngeal muscles in vagal paralysis. Laryngoscope. 1966;76:1753-1765.
26. Bridger GP. Unilateral laryngeal palsy: a histopathological study. J Laryngol Otol. 1977;91:303-307.
27. Von Leden H, Moore P. The mechanics of the cricoarytenoid joint. Arch Otolaryngol. 1961;73:541-550.
28. New GB, Childrey JH. Paralysis of the vocal cords: a study of 217 medical cases. Arch Otol. 1932;16:143-159.
29. Brewer DW, Woo P, Casper JK, et al. Unilateral recurrent laryngeal nerve paralysis: a re-examination. J Voice. 1991;5:178-185.
30. Woodson GE, Murry T. Glottic configuration after arytenoid adduction. Laryngoscope. 1994;104:965-969.
31. Woodson GE, Rosen CA, Yeung D. Changes in length and spatial orientation of the vocal fold with arytenoid adduction in cadaver larynges. Ann Otol Rhinol Laryngol. 1997;106:552-555.
32. Neuman TR, Hengesteg A, Lepage RP, et al. Three-dimensional motion of the arytenoid adduction procedure in cadaver larynges. Ann Otol Rhinol Laryngol. 1994;103:265-270.
33. Woodson GE, Picerno R, Yeung D, et al. Arytenoid adduction: controlling vertical position. Ann Otol Rhinol Laryngol. 2000;109:360-364.
34. Zeitels SM. Adduction arytenopexy: a new procedure for paralytic dysphonia with implications for implant medialization. Ann Otol Rhinol Laryngol Suppl. 1998;173:2-24.
35. Woodson GE. Cricopharyngeal myotomy and arytenoid adduction in the management of combined laryngeal and pharyngeal paralysis. Otolaryngol Head Neck Surg. 1997;117:339-343.
36. Netterville JL, Stone RE, Luken ES, et al. Silastic medialization and arytenoid adduction: the Vanderbilt experience. A review of 116 phonosurgical procedures. Ann Otol Rhinol Laryngol.. 1993;102:413-424.
37. Maragos NE. The posterior thyroplasty window: anatomical considerations. Laryngoscope. 1999;109:1228-1231.
38. Slavit D, Maragos N. Physiologic assessment of arytenoid adduction. Ann Otol Rhinol Laryngol. 1992;101:321-327.
39. Maragos NE. Piriform sinus stabilization for prevention of postoperative airway obstruction in arytenoid adduction. Presented at: the 124th Annual Meeting of the American Laryngological Association, Nashville, TN, May 3, 2003.
40. Woodson GE, Weiss T. Arytenoid abduction for dynamic rehabilitation of laryngeal paralysis. Ann Otol Rhinol Laryngol. 2007;116:483-490.