CHAPTER 39 Arthroscopy in the Throwing Athlete
INTRODUCTION
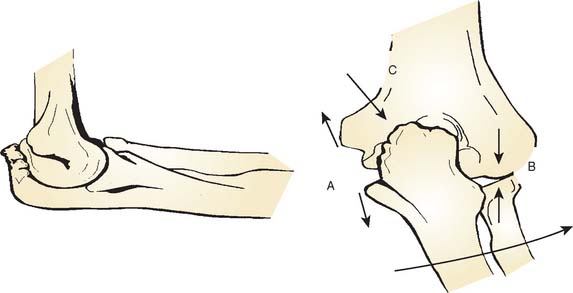
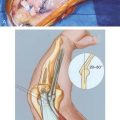
On the medial side, the repetitive tensile forces challenge the ultimate strength of the UCL, creating the well known injury risk to the ligament. Patients who develop valgus instability and continue to throw may initiate and exacerbate pathology in the posterior and lateral aspects of the elbow. In the posterior compartment, throwing repeatedly drives the olecranon into the olecranon fossa. The combination of valgus and extension forces creates shear forces on the medial aspect of the olecranon tip and olecranon fossa may cause injury and development of osteophytes (Fig. 39-1). This constellation of injuries has earned the term “valgus extension overload syndrome.” The relationship between the posterior compartment of the elbow and the UCL is evident in a series of professional baseball players who underwent olecranon débridement. Twenty-five percent of these athletes developed valgus instability and eventually required UCL reconstruction. This observation suggests that both the olecranon and the UCL contribute to valgus stability, and the authors believed that they may have missed UCL injuries in many of these patients. A cadaver study supports this theory and has demonstrated that UCL injury results in contact pressure alterations in the posterior compartment that explains the formation of osteophytes on the posteromedial olecranon.1 Another cadaver biomechanical study demonstrated that sequential partial resection of the posteromedial aspect of the olecranon increases elbow valgus angulation.14 Another cadaver study confirmed that strain in the UCL is increased with increasing posteromedial olecranon resection beyond 3 mm.15 These last two studies conclude that overaggressive olecranon resection used to treat posteromedial impingement puts the UCL at risk for injury. In summary, patients with posteromedial impingement pain from valgus extension overload should be critically evaluated for suspected concomitant UCL injuries and avoid overaggressive olecranon resection.
VALGUS EXTENSION OVERLOAD
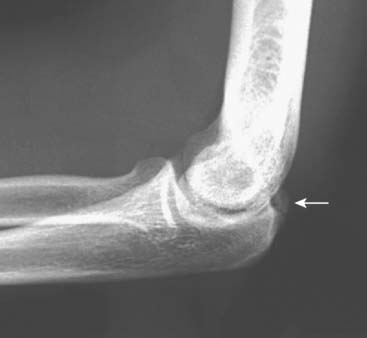
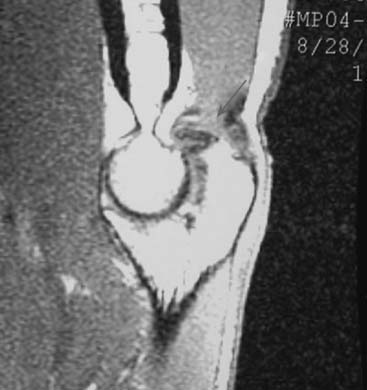
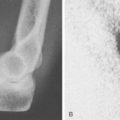
Patients report posteromedial elbow pain that occurs during the deceleration phase of throwing as the elbow reaches terminal extension. Patients also report limited extension, which results from impinging posterior osteophytes or locking and catching resulting from loose bodies. Physical examination demonstrates crepitus and tenderness over the posteromedial olecranon, and pain is reproduced when forcing the elbow into extension. Valgus stress testing, milking maneuver, and moving valgus stress tests are important to assess the status of the UCL. Anteroposterior, lateral, oblique, and axillary views of the elbow may reveal posteromedial olecranon osteophytes and/or loose bodies (Fig. 39-2). Magnetic resonance imaging (MRI) can further assess osteophytes and soft tissues (Fig. 39-3). Computed tomography with two-dimensional reconstruction and three-dimensional surface rendering may best visualize the bony pathology in addition to MRI.
Surgical treatment is indicated for those patients who maintain symptoms of posteromedial impingement despite nonoperative management. A relative contraindication to performing isolated olecranon débridement is the presence of UCL insufficiency. UCL insufficiency may become symptomatic following posteromedial decompression. Therefore, careful history, physical examination, and advanced imaging must be performed to avoid missed UCL injuries or valgus instability.
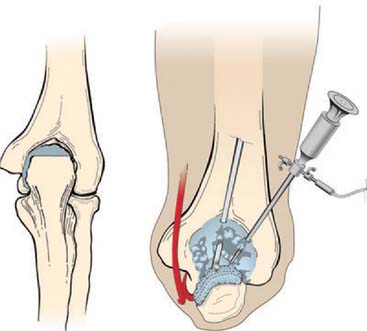
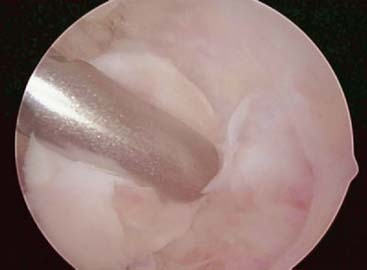
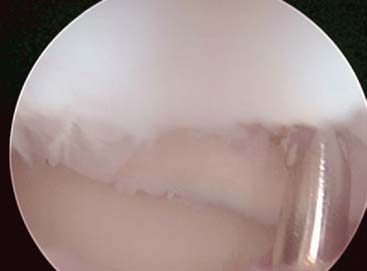
Evaluation of the posterior compartment uses direct posterior and posterolateral portals. Diagnostic arthroscopy evaluates presence of osteophytes on the posteromedial aspect of the olecranon, loose bodies, and any evidence of chondromalacia. Figure 39-4 illustrates removal of the olecranon osteophyte with a small osteotome placed at the margin of the osteophyte and normal olecranon (Fig. 39-5A). The motorized burr introduced through either the direct posterior portal or posterolateral portal may further contour the olecranon tip, as shown in Figure 39-5B. A lateral radiograph may be obtained intraoperatively to ensure adequate bone removal and that no bone debris remains in the soft tissues surrounding the elbow. It is important to remove only the osteophyte and not normal olecranon to prevent increased strain on the UCL during valgus loading.15 Once the osteophyte has been removed from the olecranon, the trochlea and olecranon fossa should be examined for osteophytes or kissing lesions, which should also be removed. It is important to recognize that the osteophytes occur on both the ulna and the humerus. Some nonthrowing athletes have a major component of direct extension overload without significant valgus stress. For these patients, the risk of developing UCL insufficiency following posterior decompression is much less compared with that of the throwing athletes and, therefore, débridement can be more aggressive, if necessary.
OSTEOCHONDRITIS DISSECANS
Lesions of the capitellum in young athletes must be differentiated as either Panner’s disease or osteochondritis dissecans (OCD). Panner’s disease is an osteochondrosis of the capitellum, which affects individuals younger than 10 years of age.24 Radiographic features include fissuring, irregularity, and fragmentation of the capitellum. Panner’s disease is usually self-limited and resolves spontaneously with radiographic reossification. In adolescent athletes, OCD is believed to be caused by repetitive radiocapitellar joint compression forces that result in cartilage and bone damage.10,13,25,28 Patients with capitellar OCD typically have a history of repetitive activity, such as throwing or gymnastics. Symptoms include lateral elbow pain associated with loss of motion. Locking and catching suggests the presence of intra-articular loose bodies. Physical examination most commonly demonstrates a 15 to 20 degree flexion contracture with crepitus and tenderness localized to the radiocapitellar joint. Plain radiographs demonstrate fragmented subchondral bone with lucencies and irregular ossification of the capitellum. Loose bodies may also be seen in the elbow joint on plain radiographs. MRI further delineates the avascular segment and loose bodies (Fig. 39-6).
Capitellar OCD lesions have been classified based on status and stability of the overlying cartilage.4,9 Initial treatment for all lesions consists of activity modification, avoidance of throwing or other related sports, use of nonsteroidal anti-inflammatory drugs, and occasionally a short period of bracing for acute symptoms. For lesions that do not demonstrate detachment or frank loose bodies, throwing and sports are restricted for 4 weeks. Physical therapy is instituted during this time to regain strength and motion. Following return of strength and motion, a progressive throwing program with proper pitching mechanics instruction is begun. Three to four months is typically required to achieve return to preinjury performance. Surgery is indicated for patients with stable intact lesion in whom nonoperative treatment has failed. Surgical treatment includes diagnostic arthroscopy confirming the stability of the overlying cartilage, followed by drilling of the lesion with a 2-mm diameter smooth pin.
For patients with unstable or detached lesions, sur-gery is indicated immediately, particularly if mechanical symptoms exist. Diagnostic arthroscopy is performed and the overlying cartilage is assessed. Loose bodies are removed and the cartilage is contoured to a stable rim using shavers and curettes. Antegrade drilling of the lesion is then performed with perforations separated by 2 to 3 mm to introduce marrow elements and create a fibrocartilage healing response. Figures 39-6 to 39-9 depict an MRI scan with a displaced OCD lesion treated with loose body removal and drilling.
Mosaicplasty is a technique of multiple osteochondral autograft transfer and is an alternative for large OCD lesions of the capitellum. Osteochondral transfer was first introduced as a treatment in the knee and has demonstrated good results.5,11 Advantages of mosaicplasty include ready availability of donor cartilage-bone plugs, ability to cover defects of varying size, and use of native hyaline cartilage containing active, mature chondrocytes. Theoretically, the native articular cartilage used for grafting will function superior to fibrocartilage, which has been shown to have inferior biomechanical properties. For mosaicplasty treatment of the capitellum, a case report and two clinical series using an open approach have reported good results.12,23,31
All-arthroscopic mosaicplasty has been performed by the senior author (NSE) and is indicated for large capitellar lesions that allow the radial head to engage the lesion as observed during arthroscopy. These lesions typically involve the lateral aspect of the capitellum with loss of the lateral buttress to the radial head articulation and occupy greater than 50% of the overall capitellar articular surface. Advanced stages of OCD that involve degenerative changes of the radial head and gross deformity of the capitellum are relative contraindications to mosaicplasty.
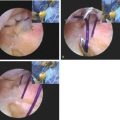
The surgical technique involves initial standard diagnostic arthroscopy and removal of loose bodies. Visualization of the OCD lesion is then achieved through the midlateral portal. A working portal is created adjacent and slightly lateral to the midlateral portal. The lesion is prepared by shaving loose fragments of cartilage to subchondral bone and establishing healthy stable cartilage borders. The size of the recipient site is determined with a calibrated probe or sizing guides (Arthrex, Inc., Naples, FL) and is typically either 4 or 6 mm. Next, the elbow is positioned in 90 to 100 degrees of flexion and a spinal needle is introduced for an exact perpendicular approach to the lesion. This needle entrance is 3 to 4 cm distal from the midlateral portal and penetrates the anconeus. The recipient site is then drilled creating the desired diameter tunnel according to the osteochondral grafting instrumentation used (Fig. 39-10).

FIGURE 39-10 Recipient tunnel for osteochondral autograft drilled.
(From Ahmad, C. S., and ElAttrache, N. S.: Mosaicplasty for capitellar osteochondritis dissecans. In Yamaguchi, K., O’Driscoll, S., King, G., and McKee, M. [eds.]: Advanced Reconstruction: Elbow. Rosemont, IL, American Academy of Orthopaedic Surgeons, 2007, p. 182.)
The donor osteochondral graft is then arthroscopically harvested from the intercondylar notch of the knee. The osteochondral graft is introduced into the recipient site using the harvester/inserter instrumentation and impacted flush with the surrounding cartilage (Fig. 39-11). Care must be taken to avoid either proud or recessed plugs that will create local increased stress and early cartilage degeneration. It is better to error with the plug slightly recessed than proud.17,18 It is also critical to harvest the donor plug and drill the recipient site exactly perpendicular to the surface to avoid a graft that is nonconforming to the surrounding cartilage. Nonconforming grafts have proud regions and opposite side recessed regions relative to the surrounding cartilage that may result in early cartilage degeneration.17 The process of osteochondral grafting is repeated until the lesion is adequately replaced (Figs. 39-12 and 39-13). If the entire lesion is not resurfaced, the remaining uncovered areas are treated with drilling. Typically two to three plugs are used. Synthetic graft substitutes are alternative sources to fill the recipient sites on the capitellum (OsteoBiologics, Inc., San Antonio, TX). Such substitute plugs are composed of polylactide-do-glycolide copolymer and are available in multiple diameters. These graft substitutes are currently under investigation for the treatment of capitellar OCD.

FIGURE 39-11 First osteochondral autograft in place and flush with surrounding articular surface.
(From Ahmad, C. S., and ElAttrache, N. S.: Mosaicplasty for capitellar osteochondritis dissecans. In Yamaguchi, K., O’Driscoll, S., King, G., and McKee, M. [eds.]: Advanced Reconstruction: Elbow. Rosemont, IL, American Academy of Orthopaedic Surgeons, 2007 p. 182.)

FIGURE 39-12 Recipient tunnel for second osteochondral autograft drilled.
(From Ahmad, C. S., and ElAttrache, N. S.: Mosaicplasty for capitellar osteochondritis dissecans. In Yamaguchi, K., O’Driscoll, S., King, G., and McKee, M. [eds.]: Advanced Reconstruction: Elbow. Rosemont, IL, American Academy of Orthopaedic Surgeons, 2007, p. 182.)

FIGURE 39-13 Completed mosaicplasty with osteochondral graft plugs indicated.
(From Ahmad, C. S., and ElAttrache, N. S.: Mosaicplasty for capitellar osteochondritis dissecans. In Yamaguchi, K., O’Driscoll, S., King, G., amd McKee, M. [eds.]: Advanced Reconstruction: Elbow. Rosemont, IL, American Academy of Orthopaedic Surgeons, 2007, p. 182.)
Several long-term follow-up studies of patients with capitellar OCD indicate impairment of elbow function. Bauer et al.3 reported a 50% incidence of restricted motion, exertional pain, and radiographic degenerative disease at an average follow-up of 23 years. Similar findings have been observed in other long term follow-up studies.22,29 Ability to return to competitive sports has been variable in some shorter term follow-up studies,20,30 with other studies demonstrating more favorable return to competitive athletics.4,6–8,19,21,26
RADIOCAPITELLAR PLICA
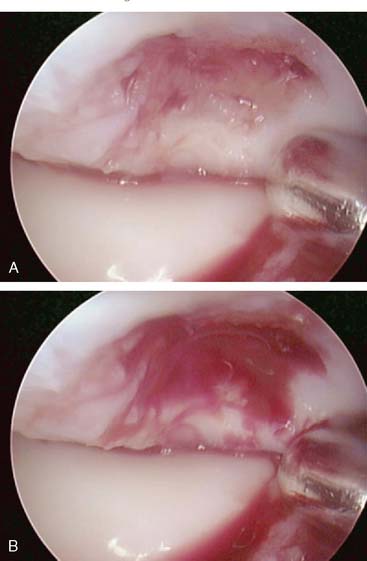
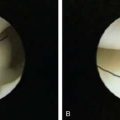
Lateral synovial plica in the elbow has been identified as a cause of lateral elbow pain most often observed in throwers and golfers. Patients report posterolateral elbow pain associated with clicking or catching. Examination with forced flexion with the forearm pronated reproduces symptoms. Diagnostic arthroscopy demonstrates thickened, hypertrophic lateral synovial plicae, often with adjacent synovitis and capsular inflammation. Some patients also have chondromalacia involving the capitellum and/or the radial head. Arthroscopic removal is depicted in Figure 39-14 and has a high success rate in relieving symptoms.2,16,27
1 Ahmad C.S., Park M.C., Elattrache N.S. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am. J. Sports Med. 2004;32:1607.
2 Antuna S.A., O’Driscoll S.W. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17:491.
3 Bauer M., Jonsson K., Josefsson P.O., Linden B. Osteochondritis dissecans of the elbow. A long-term follow-up study. Clin. Orthop. Relat. Res. 1992;284:156.
4 Baumgarten T.E., Andrews J.R., Satterwhite Y.E. The arthroscopic classification and treatment of osteochondritis dissecans of the capitellum. Am. J. Sports Med. 1998;26:520.
5 Bobic V. Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction: a preliminary clinical study. Knee Surg. Sports Traumatol. Arthrosc. 1996;3:262.
6 Bojanic I., Ivkovic A., Boric I. Arthroscopy and microfracture technique in the treatment of osteochondritis dissecans of the humeral capitellum: report of three adolescent gymnasts. Knee Surg. Sports Traumatol. Arthrosc. 2005;14:491.
7 Brownlow H.C., O’Connor-Read L.M., Perko M. Arthroscopic treatment of osteochondritis dissecans of the capitellum. Knee Surg. Sports Traumatol. Arthrosc. 2006;14:198.
8 Byrd J.W., Jones K.S. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am. J. Sports Med. 2002;30:474.
9 Difelice G., Meunier M., Paletta G.J. Elbow injury in the adolescent athlete. In: Altchek D., Andrews J., editors. The Athlete’s Elbow. Philadelphia: Lippincott Williams & Wilkins; 2001:231.
10 Douglas G., Rang M. The role of trauma in the pathogenesis of the osteochondroses. Clin. Orthop. Relat. Res. 1981;158:28.
11 Hangody L., Kish G., Karpati Z., Szerb I., Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg. Sports Traumatol. Arthrosc. 1997;5:262.
12 Iwasaki N., Kato H., Ishikawa J., Saitoh S., Minami A. Autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans in teenaged patients. Am. J. Sports Med. 2006;34:1233.
13 Jackson D.W., Silvino N., Reiman P. Osteochondritis in the female gymnast’s elbow. Arthroscopy. 1989;5:129.
14 Kamineni S., Hirahara H., Pomianowski S., Neale P.G., O’Driscoll S.W., ElAttrache N., An K.N., Morrey B.F. Partial posteromedial olecranon resection: a kinematic study. J. Bone Joint Surg. Am. 2003;85-A:1005.
15 Kamineni S., ElAttrache N.S., O’Driscoll S.W., Ahmad C.S., Hirohara H., Neale P.G., An K.N., Morrey B.F. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J. Bone Joint Surg. Am. 2004;86-A:2424.
16 Kim D.H., Gambardella R.A., Elattrache N.S., Yocum L.A., Jobe F.W. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am. J. Sports Med. 2006;34:438.
17 Koh J.L., Kowalski A., Lautenschlager E. The effect of angled osteochondral grafting on contact pressure: a biomechanical study. Am. J. Sports Med. 2006;34:116.
18 Koh J.L., Wirsing K., Lautenschlager E., Zhang L.O. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am. J. Sports Med. 2004;32:317.
19 Krijnen M.R., Lim L., Willems W.J. Arthroscopic treatment of osteochondritis dissecans of the capitellum: Report of 5 female athletes. Arthroscopy. 2003;19:210.
20 Maffulli N., Chan D., Aldridge M.J. Overuse injuries of the olecranon in young gymnasts. J. Bone Joint Surg. Br. 1992;74:305.
21 McManama G.B.Jr., Micheli L.J., Berry M.V., Sohn R.S. The surgical treatment of osteochondritis of the capitellum. Am. J. Sports Med. 1985;13:11.
22 Mitsunaga M.M., Adishian D.A., Bianco A.J.Jr. Osteochondritis dissecans of the capitellum. J. Trauma. 1982;22:53.
23 Nakagawa Y., Matsusue Y., Ikeda N., Asada Y., Nakamura T. Osteochondral grafting and arthroplasty for end-stage osteochondritis dissecans of the capitellum. A case report and review of the literature. Am. J. Sports Med. 2001;29:650.
24 Panner H. An affection of the capitulum humeri resembling Calvé-Perthes’ Disease of the hip. Acta Radiol. 1927;8:617.
25 Ruch D.S., Poehling G.G. Arthroscopic treatment of Panner’s disease. Clin. Sports Med. 1991;10:629.
26 Ruch D.S., Cory J.W., Poehling G.G. The arthroscopic management of osteochondritis dissecans of the adolescent elbow. Arthroscopy. 1998;14(8):797-803.
27 Ruch D.S., Papadonikolakis A., Campolattaro R.M. The posterolateral plica: a cause of refractory lateral elbow pain. J. Shoulder Elbow Surg. 2006;15:367.
28 Singer K.M., Roy S.P. Osteochondrosis of the humeral capitellum. Am. J. Sports Med. 1984;12:351.
29 Takahara M., Ogino T., Sasaki I., Kato H., Minami A., Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin. Orthop. Relat. Res. 1999;363:108.
30 Tivnon M.C., Anzel S.H., Waugh T.R. Surgical management of osteochondritis dissecans of the capitellum. Am. J. Sports Med. 1976;4:121.
31 Yamamoto Y., Ishibashi Y., Tsuda E., Sato H., Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am. J. Sports Med. 2006;34:714.