CHAPTER 15 Arthroscopic Osteochondral Transplantation
Techniques for marrow stimulation, such as abrasion arthroplasty, drilling, and microfracture, produce fibrocartilage rather than native hyaline cartilage. Good short- and midterm results have been reported by various authors. However, inconsistent long-term results have perpetuated the interest in finding alternative methods for treating full-thickness chondral lesions. These include autologous chondrocyte implantation, autologous osteochondral transplantation, fresh osteochondral allografting, and bulk allografting, as well as a whole host of synthetics, scaffolds and first-, second-, and third-generation cell-based technologies.1–4 This chapter will focus on the COR system (DePuy Mitek; Raynham, Mass) for arthroscopic osteochondral transplantation.
ANATOMY
In adults, hyaline cartilage normally has a thickness of roughly 8 to 9 mm. As a person ages, the articular cartilage thins. The presence of pathology can accelerate this eroding process. To produce symptoms, articular cartilage must be thinned by more than 75% before subchondral nerve fibers can detect the change in contact surface pressure. However, overall size of the lesion also plays a role in this. A small chondral defect will not produce symptoms because the stress is distributed to the surrounding joint surface. Only when the cartilage defect becomes large enough, and thin enough will the patient become symptomatic.
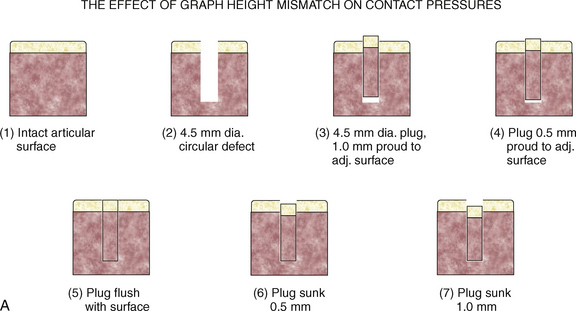
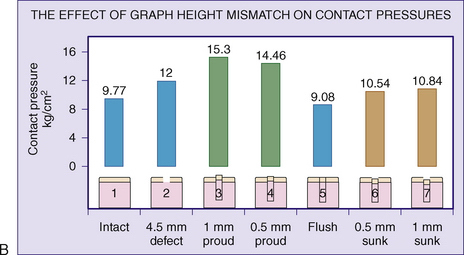
To illustrate this point, Jason and Koh5 have examined the contact pressures across an articular surface with respect to a focal defect and a grafted defect. Various graft height mismatches were modeled (Fig. 15-1), with the results shown in Table 15-1. Not surprisingly, plugs that were flush to their articular surfaces most closely resembled the contact pressure of the normal intact articular surface. However, plugs countersunk by 0.5 to 1.0 mm also closely mimicked the native pressures. Thus, filling a defect to a near-congruent articular surface can reproduce native articular-surfaced pressures. This is the foundation of autogenous osteochondral transplantation.
TABLE 15-1 Effect of Graft Height Mismatch on Contact Pressures
| Condition of Intra-articular Joint Surface | Contact Surface Pressure (kg/cm2) |
|---|---|
| Normal intact surface | 9.77 |
| Open 4.5-mm hole | 12.00 |
| Plug flush to the surface | 9.08 |
| Plug countersunk 0.5 mm to surface | 10.54 |
| Plug countersunk 1.0 mm to surface | 10.84 |
| Plug 0.5 mm proud to the surface | 14.46 |
| Plug 1.0 mm proud to the surface | 15.30 |
Because small chondral defects will not produce symptoms, the goal of osteochondral transplantation is to decrease the size of large defects functionally to become less clinically significant.
PATIENT EVALUATION
History and Physical Examination
A history of an acute injury with a subsequent hemarthrosis can be found in focal chondral and osteochondral injuries. This has been reported to have as high as a 20% incidence in patients with no ligamentous instability. Often, patients can present with mechanical symptoms of locking, catching, pain, and recurrent effusions. Ligamentous and meniscal injury can present additional symptoms and challenges.6–8
TREATMENT
Arthroscopic Technique
Preparation of the Defect Site
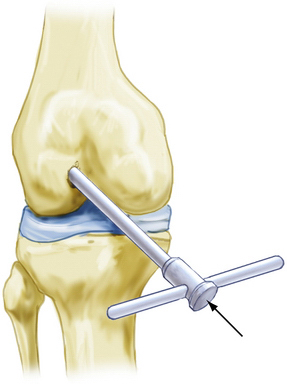
Débride loose fragments away from the cancellous bone bed with an oscillating shaver while avoiding generalized bone bleeding. Keeping in mind the number of grafts to be transplanted, position the Innovasive COR drill near a margin of the defect, as initially scored. Under arthroscopic vision, drill almost perpendicularly until resistance is felt. Observe that the 8-mm drill stop is resting on the stable bone surrounding the repair site.
Harvesting COR Grafts
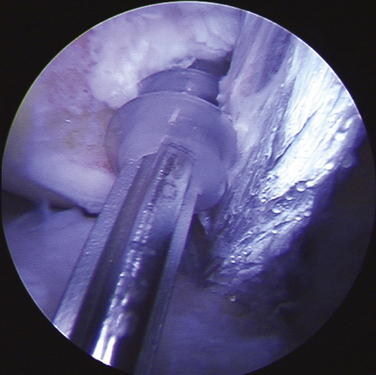
Insert the harvester (T-handled instrument) into the disposable cutter, screwing them together (Fig. 15-2). Advance the plunger through the lumen of the harvester, where it acts like an obturator to minimize soft tissue capture when passing the instruments into the joint. Once the assembly is properly positioned within the joint, replace the plunger with the anvil to minimize loss of fluid while harvesting grafts.
Position the cutter-harvester assembly on the non–weight-bearing donor site selected to provide the graft (Fig. 15-3). Ensure that the end of the cutter is almost perpendicular to the surface prior to taking the donor graft. In the knee, the superior and lateral aspects of the intercondylar notch may provide easiest arthroscopic access.
Cartilage Graft Implantation
Position the graft over the drill hole. Withdraw the plunger from the harvester, removing the spacer ring. Gently reinsert the plunger, carefully pressing the plug to fit in the undersized drill hole, flush with the surrounding bone (Fig. 15-4). Excess prominences may be trimmed with a basinet. Avoid impacting the graft to round off the corners, because this will mushroom the graft and damage the graft’s integrity.
PEARLS& PITFALLS
PEARLS
Recipient Site
PITFALLS
OUTCOMES
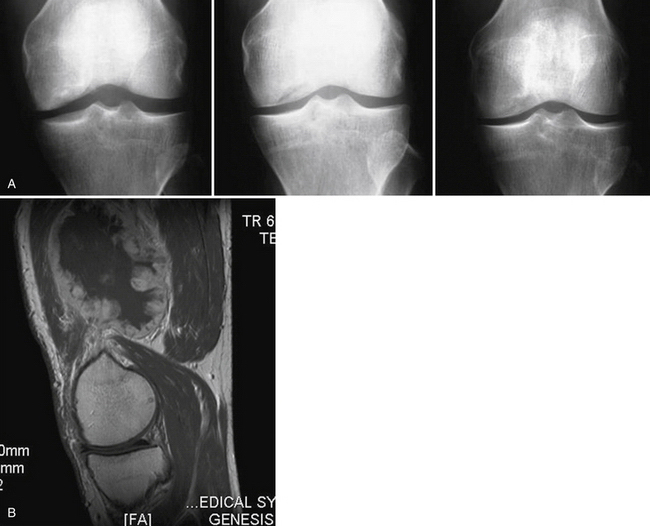
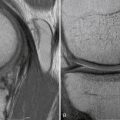
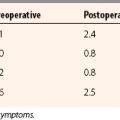
Patient follow-up results have been monitored. In 1999, 3-year results showed 86% with good to excellent results. In 2001, the 5-year results showed 76% with good to excellent results. In 2008, at 13 years, 74% of the patients still had good to excellent results. Repeat x-rays and MRI scans were obtained for asymptomatic patients who agreed to participate in this study (Fig. 15-5). In only one MRI was there a persistent bone graft margin, indicating the possibility of delayed incorporation, at 4 years and 7 months postoperatively. The patient was asymptomatic with a congruent articular surface (Fig. 15-6). The significance of this finding is uncertain, but it may not be related to clinical sequelae.
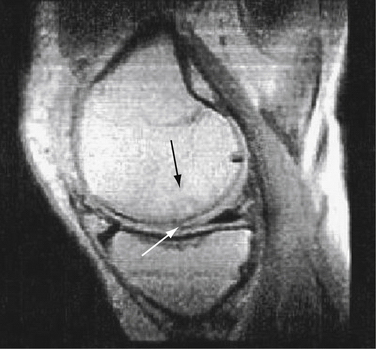
FIGURE 15-6 Persistent bone graft margin (black arrow) with congruency at the articular surface (white arrow).
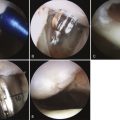
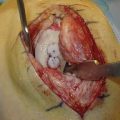
One patient had a 4-cm defect (outside of our recommended defect size), necessitating the transplantation of four graft plugs (Fig. 15-7). In this particular case, the patient was young and wished to undergo osteochondral transplantation as a final effort to temporize the necessity for a knee replacement. The patient was well-informed and accepted a potentially high risk of failure prior to surgery. Ultimately, this patient returned pain-free to playing baseball with his children (see Fig. 15-7C).
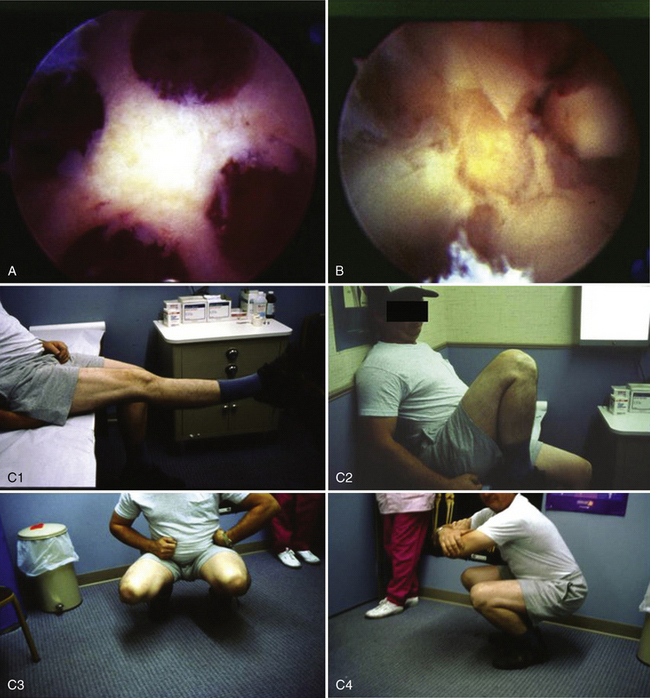
FIGURE 15-7 A, B, Large 4-cm osteochondral defect requiring four grafts. C, Clinical presentation 4 weeks postoperatively. C1, full extension; C2, full flexion; C3, full squat, frontal view; C4, full squat, side view.
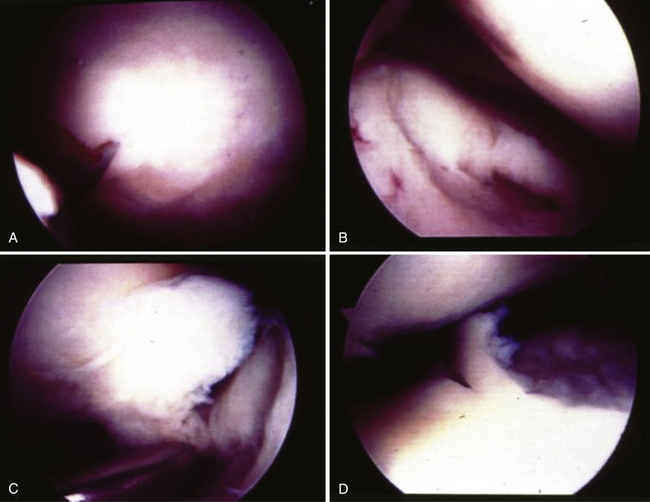
Second-look arthroscopy was performed in one case. This patient had a medial femoral osteochondral transplantation graft in August 1997. Two years later, the patient returned with persistent pain and repeat arthroscopy was performed (Fig. 15-8). On second look, the original graft site showed excellent gross congruity, incorporation, and no signs of failure. A new, full-thickness defect was present in the tibia of the opposite compartment. This patient ultimately had a total knee arthroplasty in 1999.
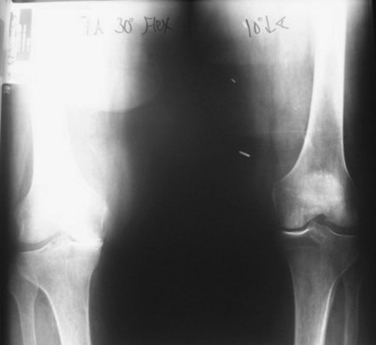
Although difficult to prove, it is possible that osteochondral transplantation may slow the development of arthritis in the absence of mechanical or systemic pathology. Anecdotally, an 85-year -old female patient presented for left total knee arthroplasty. She had received an osteochondral transplant in her right knee 10 years earlier. The comparison x-ray of both knees shown in Figure 15-9 reveals a remarkable difference.
CONCLUSIONS
Full-thickness defects smaller than 1 cm in diameter are not thought to be appropriate for treatment with this technique. They do not seem to be clinically troublesome and do well when left alone. It follows that a smaller defect—only 6 mm in diameter (COR technique donor site)—in an area that is not load bearing and does not affect the patellofemoral articulation should also be well tolerated. These defects fill in without additional bone grafting and are covered with fibrocartilaginous scar, much like the area between donor plugs and the adjacent intact hyaline cartilage.
Overall, experience indicates that the primary goal of osteochondral transplantation is not to reproduce a normal anatomic, physiologic, or biomechanical knee joint surface. The goal of this procedure is simply to decrease the patient’s clinical sequelae regarding the defect being treated. This may be related to decreasing the effective size of the defect, and thus decreasing the biomechanical shear and compressive forces surrounding these defects. This is illustrated by Jason and Koh’s research on force transmissions surrounding peridefect- and periplug-related contact pressures (see Fig. 15-1 and Table 1).5 Because of this phenomenon, this is a forgiving technique and the angle of graft insertion can be 10 to 20 degrees off of perpendicular without clinical consequences. The senior author speculates that the remodeling potential of the narrow fibrocartilage in the inter-graft space may make this possible. This is supported with our second-look arthroscopy findings (Fig. 15-8), and the good to excellent clinical results may be highly related to patient selection.
Arthroscopic osteochondral transplantation is an acceptable option for treating full-thickness osteochondral defects in appropriately selected patients. The arthroscopic technique is straightforward, with a low incidence of complications. The intergraft areas fill in, creating a homogenous appearance. The ability to manage these conditions arthroscopically at the time of discovery offers the advantage of convenience, a single procedure, and cost savings.
1. McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16:196-201.
2. Kerker JT, Leo AJ, Sgaglione NA. Cartilage repair: synthetics and scaffoldsbasic science, surgical techniques, and clinical outcomes. Sports Med Arthrosc. 2008;16:208-216.
3. Sgaglione NA. Biologic approaches to articular cartilage surgery: future trends. Orthop Clin North Am. 2005;36:485-495.
4. Sgaglione N, Miniaci A, Gillogly S, et al. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions of the knee. Arthroscopy. 2002;18:9-32.
5. Jason L, Koh MD. The effect of graft height mismatch on contact pressures following osteochondral grafting. Am J Sports Med. 2004;32:317-320.
6. Areon A, Loken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-215.
7. Hjelle K, Solheim E, Strand T. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-734.
8. Noyes FR, Bassett RW, Grood ES, et al. Arthroscopy in acute traumatic hemarthrosis of the knee. J Bone Joint Surg Am. 1980;62:687-695.
9. Ferkel RD, Zanotti RM, Komenda GA, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36:1750-1762.
10. Sgaglione NA. The future of cartilage restoration. J Knee Surg. 2004;17:235-243.
11. Kon E, Delcogliano M, Filardo G, et al. Second-generation issues in cartilage repair. Sports Med Arthrosc. 2008;16:221-229.
12. Coons DA, Barber FA. Arthroscopic osteochondral autografting. Orthop Clin North Am. 2005;36:447-458.
13. Marcacci M, Kon E, Delcogliano M, et al. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med. 2007;35:2014-2021.
14. Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;(466):952-962.
15. Cain EL, Clancy WG. Treatment algorithm for osteochondral injuries of the knee. Clin Sports Med. 2001;20:321-342.
16. Koulalis D, Di Benedetto P, Citak M, et al. Comparative study of navigated versus freehand osteochondral graft transplantation of the knee. Am J Sports Med. 2009;37:803-807.
17. Hangody L, Vásárhelyi G, Hangody LR, et al. Autologous osteochondral grafting—technique and long-term results. Injury. 2008;39(suppl 1):S32-9.
18. Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
19. Hangody L, Feczkó P, Bartha L, et al. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res. 2001;(391 suppl):S328-S336.
20. Kircher J, Patzer T, Magosch P, et al. Osteochondral autologous transplantation for the treatment of full-thickness cartilage defects of the shoulder: results at nine years. J Bone Joint Surg Br. 2009;91:499-503.
21. Patil S, Butcher W, D’Lima DD, et al. Effect of osteochondral graft insertion forces on chondrocyte viability. Am J Sports Med. 2008;36:1726-1732.
22. Barber FA, Chow JCY, Chow JCC. Autogenous osteochondral transplantation. In: Chow JCY, editor. Advanced Arthroscopy. New York: Springer-Verlag; 2000:573-579.
23. Chow JCY, Hantes ME, Houle JB, Zalavras CG. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2 to 5 year follow-up study. Arthroscopy. 2004;20:681-690.
24. Brittberg M, Lindahl A, Nilsson, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895.
25. Stedman JR, Briggs K, Rodrigo J, et al. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-484.
26. Alford J, Cole B. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295-306.
27. Alford J, Cole B. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443-460.
28. Sgaglione N. The biological treatment of focal articular cartilage lesions in the knee: future trends. Arthroscopy. 2003;19:154-160.
29. Brown W, Potter H. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res. 2004;(422):214-223.
30. Sgaglione N. Decision making and approach to articular cartilage surgery. Sports Med Arthrosc Rev. 2003;11:192-201.
31. O’Driscoll S. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795-1807.
32. Barber A, Chow J. Arthroscopic osteochondral transplantation: histologic results. Arthroscopy. 2001;17:832-835.
33. Horas U, Pelinkovic D, Aigner T. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective comparative trial. J Bone Joint Surg Am. 2003;85:185-192.