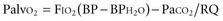8 Arterial Hypoxemia
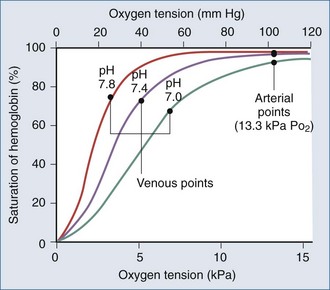
Arterial hypoxemia is defined as a partial pressure of oxygen in arterial blood (PaO2) less than 80 mm Hg while breathing room air. The PaO2 represents the amount of oxygen in physical solution, whereas the oxygen saturation represents the fractional amount of oxyhemoglobin relative to total hemoglobin concentration. Oxygen saturation varies with the PaO2 in a nonlinear relationship and is affected by temperature, partial pressure of carbon dioxide in arterial blood (PaCO2), pH, and 2,3-diphosphoglycerate concentration (Figure 8-1).
Falsely low saturations can be recorded if there is a poor waveform or if light absorption is decreased by dark blue or black nail polish. Patients with methemoglobinemia can have a falsely low oxygen saturation, whereas patients with carboxyhemoglobinemia can have a falsely elevated oxygen saturation, because the pulse oximeter cannot differentiate carboxyhemoglobin from oxyhemoglobin.1 Finally, because the oxygen-hemoglobin dissociation curve is affected by temperature, pH, partial pressure of carbon dioxide (PCO2), and 2,3-diphosphoglycerate concentration, patients can have a higher or lower saturation for a given PaO2.
Etiologies for hypoxemia are best understood if approached from a physiologic point of view rather than by referring to a list of possible differential diagnoses. Simply stated, hypoxemia results from an imbalance between pulmonary ventilation and pulmonary capillary blood flow.2
 Reduced Alveolar Oxygenation
Reduced Alveolar Oxygenation
Alveolar oxygenation is defined by the equation:
where FIO2 is the concentration of inspired oxygen, BP is the barometric pressure, BPH2O is the partial pressure of water, and RQ is the respiratory quotient. The respiratory quotient represents the amount of oxygen consumed relative to the amount of carbon dioxide produced when nutrients are metabolized. RQ is generally assumed to be 0.8. Under normal conditions, where the FIO2 is 21%, BP is 760 mm Hg, BPH2O is 47 mm Hg, and PaCO2 is 40 mm Hg, the Palvo2 = 0.21(760 − 47) − 40/0.8 = 100 mm Hg. According to the equation, several factors may contribute to lower alveolar oxygenation. One is a reduction in barometric pressure, causing hypobaric hypoxemia that affects those climbing at high altitudes.3 The second factor is an increase in PaCO2, which can be explained by the relationship: PaCO2 = carbon dioxide production/respiratory rate (tidal volume − dead space). Accordingly, the PaCO2 increases with either an increase in production or a decrease in alveolar ventilation. Alveolar ventilation represents that portion of the minute ventilation undergoing blood-gas exchange and is represented by the product of respiratory rate and tidal volume minus dead space. Medications such as narcotics and sedatives that reduce the respiratory rate, and processes such as neuromotor weakness that reduce tidal volume, are common causes of hypercarbia.
 Ventilation/Perfusion Mismatch
Ventilation/Perfusion Mismatch
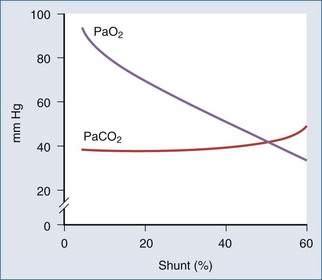
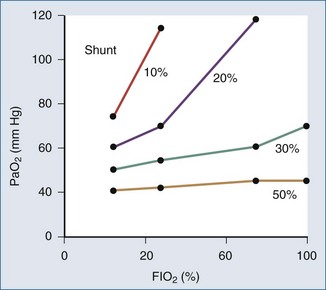
The portion of cardiac output that does not participate in gas exchange is called the shunt fraction. The normal shunt fraction is approximately 3%, and this small amount of shunt is due to the bronchial arterial circulation. When alveoli are not ventilated, such as occurs with pulmonary edema, pneumonia, or atelectasis, the shunt fraction increases. As the shunt fraction increases, PaO2 decreases (Figure 8-2), and there is a blunted response to increasing the FIO2.When the shunt fraction is above 50%, there is little response to increasing FIO2 (Figure 8-3).
 Alveolar-Arterial Partial Pressure of Oxygen Gradient
Alveolar-Arterial Partial Pressure of Oxygen Gradient
The difference between the alveolar PO2 and the arterial PO2 (i.e., the A-a gradient) often is used to estimate the extent of pulmonary pathophysiology and to rule out hypoxemia due to low alveolar PO2 as the cause of arterial hypoxemia.4,5 A patient with a reduced alveolar PO2 (e.g., secondary to breathing room air at high altitude) would have a normal A-a gradient, whereas a patient with ventilation/perfusion mismatching would have a widened A-a gradient. A patient with a PaO2 of 48 mm Hg and a PaCO2 of 80 mm Hg would have an alveolar PO2 on room air of 50 mm Hg; the normal A-a gradient of 2 mm Hg is consistent with reduced alveolar PO2, and causes of hypercarbia need to be ruled out and reversed.
The A-a gradient increases with age or increasing FIO2, making it an unreliable predictor of the degree of pulmonary dysfunction.5,6 The PaO2 : FIO2 ratio also correlates with shunt fraction but is influenced by increasing FIO2.4 The arterial-to-alveolar ratio is not influenced by FIO2.6
 Reduced Mixed Venous Oxygen
Reduced Mixed Venous Oxygen
A final contribution to hypoxemia may be a reduced mixed venous oxygen content (CmvO2) or saturation. In patients with normal lung function, reducing CmvO2 has little influence on arterial oxygenation; however, in patients with a significant shunt fraction, reducing CmvO2 contributes to arterial hypoxemia.7 In patients with a widened A-a gradient and abnormally low CmvO2, oxygenation can be improved by increasing venous saturation either by increasing oxygen delivery (increased hemoglobin concentration or cardiac output or both) or reducing oxygen consumption (e.g., induction of hypothermia or using neuromuscular blocking agents).
1 Welch JP, DeCesare MS, Hess B. Pulse oximetry: instrumentation and clinical applications. Respir Care. 1990;35(6):584-597.
2 Dantzger DR. Pulmonary gas exchange. In: Dantzger DR, editor. Cardiopulmonary Critical Care. 2nd ed. Philadelphia: WB Saunders; 1991:25-43.
3 Grocott M, Martin DS, Levett D, et al. Arterial blood gases and oxygen content in climbers on mount everest. N Engl J Med. 2009;360:140-149.
4 Covelli HD, Nessan VJ, Tuttle WK. Oxygen derived variables in acute respiratory failure. Crit Care Med. 1983;11:646-649.
5 Harris EA, Kenyon AM, Nisbet HD, et al. The normal alveolar arterial oxygen tension gradient in main. Clin Sci. 1974;46:89-104.
6 Guilbert R, Kreighly JF. The arterial/alveolar oxygen tension ratio: an index of gas exchange applicable to varying inspired oxygen concentrations. Am Rev Respir Dis. 1974;109:142-145.
7 Rossaint R, Hahn SM, Pappert D, et al. Influence of mixed venous PO2 and inspired oxygen fraction on intrapulmonary shunt in patients with severe ARDS. J Appl Physiol. 1995;78:1531-1536.