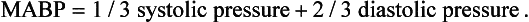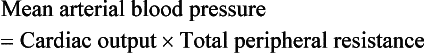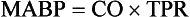ARTERIAL BLOOD PRESSURE
Introduction
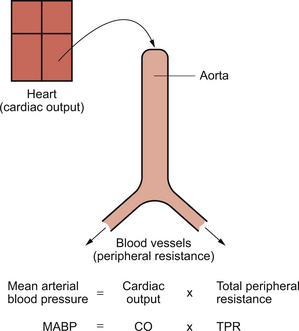
A simple model of the arterial circulation is shown in Figure 10.1. Blood pressure can be regulated by changes in either cardiac output or the peripheral resistance.
Arterial blood pressure provides the driving force to perfuse the tissues of the body with blood. This driving force is dissipated as blood moves round the circulation and there is a continuous fall in pressure between the arteries and the right atrium (see Fig. 9.1). Mean aortic pressure is typically about 95 mm Hg whereas right atrial pressure is about 0–5 mm Hg. Although the output of the heart is phasic, i.e. blood enters the circulatory system during cardiac systole and this ceases when the heart refills during diastole, circulation of blood around all the tissues of the body is continuous. This is driven by the head of pressure maintained in the arterial tree (see page 86). Blood pressure in the arteries is pulsatile but by the time blood reaches the capillaries, where the important functions of the circulatory system take place, the pressure fluctuations have been damped out (see Fig. 9.1).
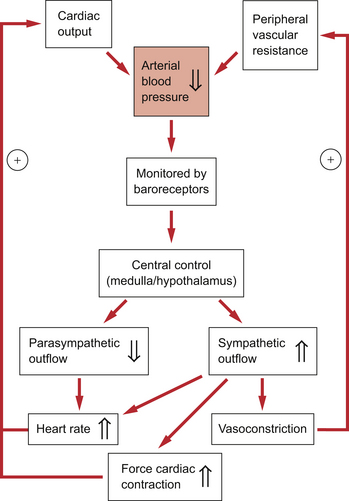
It is important that arterial pressure is maintained quite constant, as a fall in pressure below a critical level leads to underperfusion of the brain and the subject faints (see Chapter 9). A sustained rise in arterial blood pressure—hypertension—leads to the pathological changes in blood vessels described later in this chapter. Moment to moment fluctuations in arterial blood pressure are minimized by the baroreceptor reflex. This reflex is summarized in Figure 10.2.
An outline of a case history of a patient with hypertension is shown in Case 10.1:1.
The factors determining cardiac output have been discussed in Chapter 4. The essential features are that the heart has an intrinsic contractile rate which can be modified by the parasympathetic (vagus) nerve to slow the heart or by the sympathetic nerves to increase heart rate. The force of contraction of the heart and, therefore, the stroke volume is determined by a combination of preload effects (blood volume, venous return, venous tone), contractility effects (changes in myocyte [Ca++] produced physiologically by sympathetic nerve stimulation) and afterload effects (mainly opposition to opening the aortic valve produced by the arterial blood pressure).
The factors determining peripheral blood vessel resistance to flow have been discussed in Chapter 9. Some of the mechanisms (metabolite, endothelial and local hormone effects) ensure an appropriate distribution of flow within tissues to match the local metabolic activity. Adjustment of the overall resistance to maintain arterial blood pressure at a relatively constant level is achieved by the autonomic nerve supply to peripheral blood vessels. This has the fundamental characteristics that it is sympathetic in origin, using noradrenaline (norepinephrine) as the main neurotransmitter which, acting primarily through α1-receptors, leads to vasoconstriction (see Chapter 9). Although there are parts of the circulation where one or other of these characteristics does not apply, these are very limited and specialized aspects of vascular control and are not major factors determining the total peripheral resistance.
Arterial baroreceptors
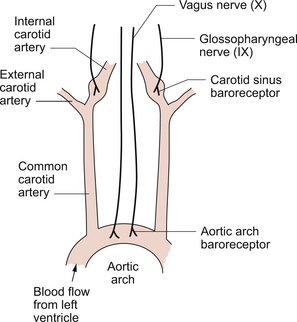
Anatomical location of baroreceptors
The location of the main arterial baroreceptors is shown in Figure 10.3.
Information from these two groups of baroreceptors enters the brain at the level of the medulla.
Central control of the cardiovascular system
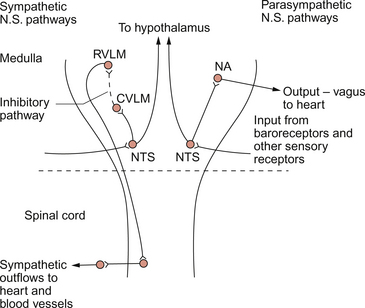
The initial processing of information from the baroreceptors occurs in the nucleus tractus solitarius (NTS) in the medulla. The NTS has connections to the regions of the medulla which organize the outflow to the two divisions of the autonomic nervous system. The cell bodies of the preganglionic parasympathetic nerves, which slow heart rate, are located in the nucleus ambiguus and the dorsal motor nucleus. There are also neural connections from the NTS to the rostral ventrolateral medulla, a region of the brainstem controlling the sympathetic outflow to the heart (increases rate) and to the peripheral blood vessels (mainly leading to vasoconstriction). Figure 10.4 summarizes the main pathways in the medulla involved in the control of arterial blood pressure.
The cerebellum is involved in the coordination of muscle movement in exercise and is also involved in the circulatory responses to exercise. Aspects of this are discussed in Chapter 13.
The baroreceptor reflex
A flow chart summarizing the baroreceptor reflex for the regulation of arterial blood pressure is shown in Figure 10.2. The responses to a fall in blood pressure are illustrated. The reflex response is an increase in heart rate, an increase in the force of cardiac contraction and an increase in peripheral vasoconstriction, all of which will help to restore blood pressure to normal levels. It has been said that regulation of heart rate is the primary role of the baroreceptor reflex and that responses leading to vasoconstriction and venoconstriction are of secondary importance.
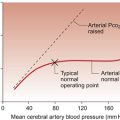
Baroreceptor reflex resetting occurs during the long-term rises in blood pressure known as hypertension and also during the changes in blood pressure which occur with increasing age. The site of these adaptive changes is in the central control mechanisms. The changes in baroreceptor function are a consequence of raised blood pressure rather than a primary cause of hypertension. Sensitivity of the baroreceptor mechanism may decrease when there are changes in the arterial wall compliance which sometimes occur as a consequence of atherosclerotic changes (see Chapter 8).
Cardiopulmonary reflexes
Groups of receptors located at the junction of the atria and the great veins effectively monitor blood volume. Approximately two thirds of blood volume is contained in the veins and changes in this volume will alter the stretch in the wall of the veins. This stretch is detected by myelinated vagal afferent nerves. In the control of blood volume this information is supplemented by an input from the arterial baroreceptors and by hormonally mediated changes in kidney function. An increase in blood volume leads to decreased secretion of antidiuretic hormone (ADH) and therefore a diuresis. Concurrently a decrease in renin secretion from the kidney leads to a decrease in aldosterone mediated sodium retention (see Chapter 9). An increase in the secretion of atrial natriuretic peptide (ANP), released in response to increased atrial stretch, also contributes to a diuretic and natriuretic response. All of these endocrine responses to increased blood volume help to return blood volume to normal. There is also a neurally mediated component to the response. Increase in blood volume leads to reflex inhibition of sympathetic vasoconstriction within the kidney. The resulting vasodilatation leads to an increase in glomerular filtration rate (GFR) and contributes to the diuretic response. These hormonal mechanisms which regulate kidney function effectively control blood volume and therefore play a major role in the long-term control of arterial blood pressure (BP). The mechanisms for acute control of BP (primarily the baroreceptor reflex) are superimposed on this background.
Stretch receptors at the vena cava–right atrium junction provide the sensory component of the Bainbridge reflex. A rapid infusion of saline, and consequent stretch of the great veins, leads to a reflex increase in heart rate. This reflex would help to shift blood from a congested venous system through to the arterial side of the circulation. The reflex may be important in the early stages of exercise when the skeletal muscle pump first produces an increase in venous return (see Chapter 13).
Measurement of arterial blood pressure
The first recorded measurement of arterial blood pressure was made in 1773 by the Reverend Stephen Hales. The measurement was made by direct cannulation of an artery in a horse. Although derivatives of this approach are still used in, for example, intensive care units or for experimental studies, direct artery cannulation is certainly not the basis for routine blood pressure measurements. Historically much effort was devoted to finding non-invasive methods. In 1896 the Italian physician Riva-Rocci produced a sphygmomanometer with an inflatable cuff. This heralded the modern method for blood pressure measurement, the ausculatory method developed by the Russian surgeon Korotkoff in 1905.
The fundamental physical principle used is the transition from laminar flow to turbulent blood flow in an artery. Laminar flow (see Chapter 8) is essentially silent but turbulent flow sets up vibrations in the blood vessel wall which can be heard using a stethoscope placed over the artery. These flow murmurs are known as Korotkoff sounds. The conditions for the development of turbulence are that it is more likely to develop when the flow velocity is high and when the tube has a large diameter.
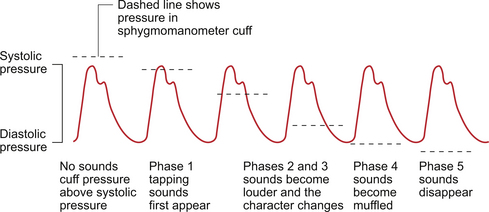
During blood pressure measurement, the sphygmomanometer cuff is placed over an artery, usually the brachial artery in an upper arm, and inflated. When the pressure within the cuff exceeds the pressure in the artery, the blood vessel is compressed and no blood flow occurs (Fig. 10.5). The peak of the arterial pressure wave is called the systolic pressure. If the pressure in the cuff is allowed to fall slowly, eventually the point is reached at which the cuff pressure is slightly below the systolic pressure. At this peak of the pressure wave, the blood will flow, at increased velocity, through the still greatly narrowed section of blood vessel and emerge into the uncompressed section of downstream blood vessel. This is a situation in which turbulence will develop and Korotkoff sounds will be generated. Systolic pressure is recorded as the cuff pressure at which Korotkoff sounds are first heard (phase 1).
As the pressure in the cuff is allowed to fall further the vessel will only be completely occluded for progressively shorter periods of time (Fig. 10.5). Although Korotkoff sounds are still produced, the character of the sounds changes (phases 2 and 3). The problem now becomes to record the diastolic pressure, the trough pressure reached in the arterial tree. This pressure is reached just before the aortic valve reopens and a further volume of blood enters the arteries. The best correlation with direct (arterial cannulation) methods for blood pressure measurement is when the Korotkoff sounds become muffled (phase 4). This is often very difficult to discern, especially in an environment such as a hospital ward where there may be considerable background noise. Disappearance of the Korotkoff sounds (phase 5) is routinely used even though it occurs slightly below the true diastolic pressure. In some situations, for example sometimes in pregnancy when blood viscosity is reduced, the Korotkoff sounds do not disappear and so phase 4 (muffling) must be used to record diastolic pressure.
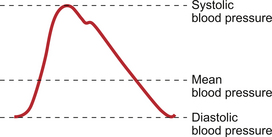
Mean arterial blood pressure is often calculated. Because of the shape of the arterial pressure wave (Fig. 10.6) mean pressure is not the same as the average of the systolic and diastolic pressures. The mean pressure through the duration of the arterial pressure wave is weighted more towards the diastolic pressure. A convenient way to calculate mean arterial blood pressure (MABP) is therefore:
This is the same as the alternative formula:
Normal arterial pressure
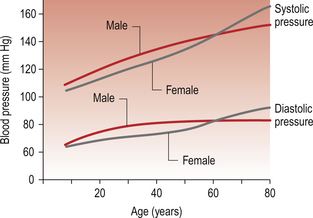
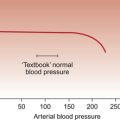
A typical ‘normal’ blood pressure measurement is often quoted as 120/80 mm Hg (systolic/diastolic). While this value is appropriate for the textbook subject (male, 20–25 years old weighing 70 kg), it is not appropriate for the entire population. There is firstly a gender-related difference. Considering large population groups, blood pressures in premenopausal females are a little (5–10 mm Hg) lower than in males. There are also changes in blood pressure with age with lower than ‘textbook’ values in children (see Chapter 12) and higher pressures in older people (Fig. 10.7).
Hypertension
Mechanisms of hypertension
There is evidence that essential hypertension may be associated with a reduction in the number of nephrons within the kidneys. How precisely this links to peripheral vasoconstriction is not entirely clear. A possibility is that reduced renal function leads to a failure to adequately excrete a sodium load. This could trigger the release of a hypothalamic natriuretic peptide which has a cardiac glycoside structure. This is variously described as a digoxin-like or ouabain-like glycoside. As with the action of digoxin on the heart (see Chapter 4), the hormone inhibits Na+/K+-ATPase. In the smooth muscle cell membrane there is also a Na+/Ca++ passive exchange co-transporter (see Chapter 9). This is driven by the [Na+] gradient across the membrane, with Na+ moving into the cell and Ca++ moving out. Inhibition of the Na+/K+-ATPase would lead to increased intracellular [Na+] and hence reduced Ca++ extrusion from the cell. Such an action on vascular smooth muscle would raise intracellular [Ca++], promote contraction and hence raise the peripheral resistance. Other popular mechanisms for the pathogenesis of hypertension include some aspect of overactivity of the renin-angiotensin system or the sympathetic nervous system. No one mechanism can currently explain all of the experimental data. Essential hypertension in any one individual may eventually prove to be the cumulative effect of a number of genetic, lifestyle and environmental factors.
Pathological consequences of raised arterial pressure
Worldwide, hypertension is estimated to cause 7.1 million premature deaths per year. Longstanding essential hypertension results in left ventricular hypertrophy. In this ‘pressure-overload’ effect on the heart additional sarcomeres are added in parallel to the original sarcomeres (see Chapter 2). However hypertrophy may progress to congestive failure with dilatation of the heart. This ‘volume overload’ means additional sarcomeres are added in series. Changes in the architecture of the heart which involve both the myocytes and the extracellular matrix are referred to as remodelling (see Chapter 6).
Hypertension is a risk factor for the development of atherosclerosis (see Chapter 9) and will accelerate its development in large arterial blood vessels. However, the structure of the lesions produced is the same as in normotensive subjects. In addition there are changes in the vascular wall which are selective responses to hypertension. These include thickening of the media layer of muscular arteries as a result of smooth muscle hyperplasia and collagen deposition. The latter changes occur in small arteries and arterioles rather than the large vessels affected by atherosclerosis (Fig. 10.8).
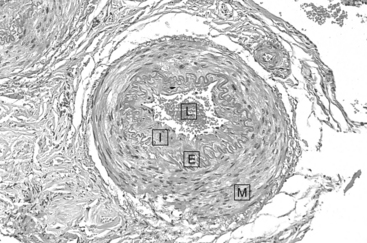
Fig. 10.8 Structure of a small artery from a patient with benign hypertension. The smooth muscle in the media (M) has undergone hypertrophy. The intima (I) and the elastic lamina (E) have become thickened. The lumen (L) is reduced in size. Source: Stevens & Lowe 2000.
Other vascular disorders associated with hypertension include intracerebral haemorrhage, dissecting aneurysm of the aorta and subarachnoid haemorrhage due to the rupture of berry aneurysms (see Chapter 8). Globally, data suggest that about 62% of cerebrovascular disease and 49% of ischaemic heart disease can be attributed to inadequate management of hypertension. However, in patients with hypertension 80% of strokes are caused by intra-arterial thrombosis or embolism from the heart or large arteries. Intuitively one might expect hypertension to be linked to haemorrhagic events as a cause of stroke, but in fact this represents only 20% of the total.
Endothelial dysfunction is characteristic of hypertension and also many other disease mechanisms associated with altered vascular function. The changes in the endothelium appear to be a response to hypertension rather than a cause of hypertension linked to reduced production of vasodilator mediators (nitric oxide or prostacyclin) or increased production of a vasoconstrictor (endothelins).
Treatment of hypertension
A considerable range of drugs is available to reduce blood pressure and a detailed discussion of these is outside the scope of this book. Individually each drug will only produce fairly modest (5–15 mm Hg) reductions in blood pressure and so combinations of different classes of drugs may be necessary to achieve adequate blood pressure control. Common strategies include the use of beta-blockers and/or thiazide diuretics as first line treatments. More recently, calcium channel blocking drugs (also called calcium antagonists) have been added to this list and angiotensin converting enzyme inhibitors (ACEI) (see Chapter 9) also have their advocates as first line drugs. Brief further details of all these groups of drugs is shown below.
Beta-blockers, e.g. atenolol (β1 selective) propranolol (β1/β2 non-selective)
Beta-blockers have a number of pharmacological actions. Reductions in heart rate and myocardial contractility through actions on β1 receptors lead to a decrease in cardiac output (see Chapter 4). Blockade of juxtaglomerular cell β receptors in the afferent arteriole of the kidney suppresses the secretion of renin. In addition to their role as antihypertensive agents, beta-blockers have other beneficial effects in relation to heart disease (see Chapter 6). β-adrenoceptor blocking drugs have a wide range of side effects. These include tiredness, exercise limitation, dizziness, nausea, anorexia and bradycardia. In patients with asthma the use of a β1 selective drug is preferable as a non-selective β1/β2 blocker may contribute to bronchoconstriction.
Calcium channel blocking drugs, e.g. nifedipine, verapamil and diltiazem
These drugs block the entry of Ca++ ions into voltage gated (L-type) calcium channels in vascular smooth muscle (see Chapter 9) and hence reduce the peripheral resistance. Nifedipine and structurally related drugs are mainly used in hypertension management. As these calcium channels exist in a number of tissues there are predictable side effects. They may include headache (cerebral vasodilatation), and constipation due to effects on gut wall smooth muscle. Some calcium channel blocking drugs also have a negative inotropic action on the heart (see Chapter 4).
Angiotensin converting enzyme inhibitors (ACEI)
These compounds, together with the more recently developed angiotensin receptor blockers (ARB), have been discussed in Chapter 9. In addition to their blood pressure lowering vasodilator effects and their mild diuretic actions, they have beneficial effects in relation to the prevention of fibrotic changes in the heart and slowing the progression of renal disease (renoprotective actions).
Other antihypertensive drugs
Imidazoline I1 receptors are concentrated in the medulla of the brain in areas concerned with the central regulation of arterial blood pressure (see page 116). Moxonidine is a drug which blocks imidazoline I1 receptors and hence decreases sympathetic outflow and increases vagal tone.
The centrally acting α2-adrenoceptor agonists such as methyldopa and clonidine now have only limited use as blood pressure lowering drugs. They reduce sympathetic outflow by actions in the medulla, particularly on the nucleus tractus solitarius (NTS) (see p. 116).
Hydrostatic pressure in the circulation
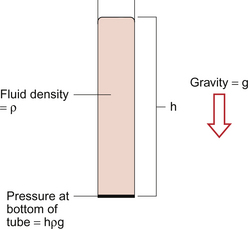
We also need to consider a second type of pressure, often referred to as a ‘hydrostatic’ pressure, generated simply by the height of a column of blood. This is illustrated in Figure 10.9. Pressure at the bottom of a tube containing a fluid depends on the height of the column of fluid (h), the density of the fluid (ρ) and gravity (g). Pressure = hρg.
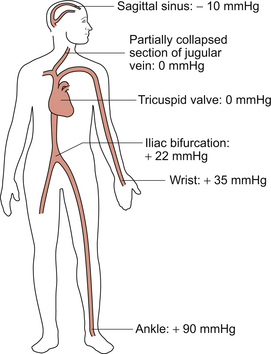
The circulatory system also has tubes containing a fluid and the hydrostatic fluid pressure generated is illustrated in Figure 10.10. Thus, in an upright person, the actual pressure in an artery in the ankle will be the sum of the ‘dynamic’ pressure (mean approximately 95 mm Hg) generated by the pumping action of the heart and the ‘hydrostatic’ pressure (about 90 mm Hg) produced by the height of the column of blood from the level of the tricuspid valve in the heart (the notional zero point) to the feet. This gives a total ankle artery pressure of about 185 mm Hg in an upright person. Note: the density of mercury is 13.6 times the density of water. Thus, as the convention is to express blood pressures in units of mm Hg, a column of 13.6 mm of blood will exert a pressure of 1 mm Hg. (This assumes that the density of blood is the same as the density of water.)
On the venous side of the circulation, consideration of the impact of the hydrostatic pressure gradient is a little more complicated. If we imagine a person suspended by a harness under the armpits, so that their feet are off the floor and there is no need for skeletal muscle contraction in the legs, hydrostatic pressure in the veins of the feet would be similar to the arterial side of the circulation. However, skeletal muscle contraction in the legs compresses the veins, an important aspect of maintaining venous return to the heart (see Chapter 4). The column of blood in the venous system is therefore separated into shorter lengths and so, during walking, the effective hydrostatic pressure in the veins of the ankle might only be about 25 mm Hg.
In an upright person, if the hydrostatic pressure at the right atrium (tricuspid valve) is zero (i.e. the same as atmospheric pressure), pressure in the jugular vein, which is above the heart, is negative. The jugular vein is therefore partially collapsed and has an oval cross-section in the normal upright person. It is only when pressure rises in the jugular, as in heart failure (see Chapter 6), that the jugular vein becomes visible. This is the basis for using the height of the column of blood inflating the jugular vein as an index of right atrial pressure. Pressure in the sagittal sinus (just under the top of the cranium) in an upright normal person is about −10 mm Hg.
As the hydrostatic pressure in the veins depends on the effect of gravity (hydrostatic pressure = hρg), there has been considerable interest in the effects of higher gravitational forces on the circulatory system. This is particularly important in the context of space flight and for the pilots of fighter aircraft making fast changes in direction. In a normal ‘inside’ turn there will be an increased gravitational force towards the feet. Venous pooling can be prevented in this case by using a G-suit, a tightly fitting garment which opposes expansion of the lower half of the body. Although these considerations of space travel and fighter planes may not affect the majority of the population, a considerable number of people do try bungee jumping. In this case, during the initial dive head-first, the hydrostatic pressure in the lower half of the body will increase with a corresponding decrease in the upper part of the body. Blood volume will be pooled in the lower half of the body and venous return to the heart will fall. As the major baroreceptors are located in the upper part of the body in the carotid sinus and aortic arch (see section on arterial baroreceptors in this chapter), this will contribute to the already excitement induced fast heart rate. However, once the fall is increasingly restrained by the elastic properties of the ‘ropes’, the person will rapidly decelerate but the hydrostatic pressure in the top half of the body will increase as the blood moves up the circulatory system. This will tend to bring about a rapid fall in heart rate.
Beevers, G., Lip, G. Y. H., O’Brien, E. ABC of Hypertension, fifth ed. London: BMJ Books; 2007.
Donnelly, R., London, N. J. M. ABC of Arterial and Venous Disease, second ed. London: BMJ Books; 2009.
ESH/ESC Hypertension Guidelines Committee. Practice guidelines for primary care physicians. J. Hypertens.. 2003; 21:1779–1786.
Field, M., Pollock, C., Harris, D. The Renal System, second ed. Edinburgh: Churchill Livingstone; 2010.
Gallagher, P. J. Cardiovascular system. In Underwood J. C. E., Cross S. S., eds. : General and Systematic Pathology, fifth ed., Edinburgh: Churchill Livingstone, 2009.
JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in practice, 2005. Heart 91 (Suppl. V): v1–v52.
Levick, J. R. An Introduction to Cardiovascular Physiology, fifth ed. London: Arnold; 2009.
Marcia, G., Mark, A. L. Arterial baroreflexes in humans. In: Shepherd J. T., Abboud F. M., eds. Handbook of Physiology. Bethesda: American Physiological Society, 1983.
Sagawa, K. Baroreflex control of systemic arterial pressure and vascular bed. In: Shepherd J. T., Abboud F. M., eds. Handbook of Physiology. Bethesda: American Physiological Society, 1983.
Stevens, A., Lowe, J. Pathology, second ed. Edinburgh: Mosby; 2000.
Waller, D. G., Renwick, A. G., Hillier, K. Medical Pharmacology and Therapeutics, third ed. Edinburgh: WB Saunders; 2009.
Williams, B., Poulter, N. R., Brown, M. J., et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV) summary. BMJ. 2004; 328:634–640.
World Health Organization (WHO). International Society of Hypertension (ISH) statement on management of hypertension. J. Hypertens.. 2003; 21:1983–1992.