CHAPTER 73 Arterial Anatomy of the Pelvis and Lower Extremities
NORMAL ANATOMY OF THE PELVIS
General Anatomic Descriptions
The abdominal aorta divides into the common iliac arteries at approximately the level of L4. The middle sacral artery, usually a very small vessel, arises from the posterior surface of the aortic bifurcation and extends inferiorly along the anterior surface of the sacrum, in the midline, to the distal tip of the coccyx. The length of the common iliac artery is variable. Each common iliac artery divides into an external iliac artery and internal iliac artery (Fig. 73-1).
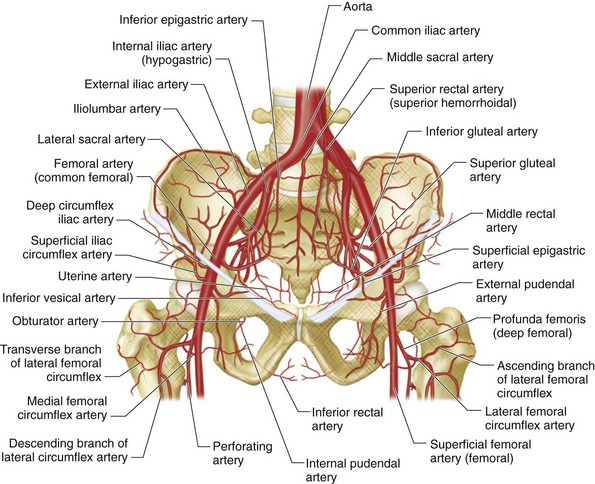
 FIGURE 73-1 Schematic drawing of the pelvic arterial vessels.
FIGURE 73-1 Schematic drawing of the pelvic arterial vessels.
(Adapted from Uflacker R. Atlas of Vascular Anatomy: An Angiographic Approach, 2nd ed. Philadelphia, Lippincott Williams & Wilkins, 2006.)
The external iliac artery has only two branches, the deep circumflex iliac and inferior epigastric arteries. Both arise approximately at the point where the inguinal ligament crosses anterior to the external iliac artery, and both serve as markers on arteriograms for the junction of the external iliac artery and common femoral artery. Some experts consider the inferior epigastric artery to arise from the proximal femoral artery. The deep circumflex iliac artery extends superolaterally from its origin on the lateral aspect of the external iliac artery, and supplies the pelvic side wall. It anastomoses with the iliolumbar and superior gluteal arteries, branches of the internal iliac artery, and with the ascending branch of the lateral circumflex femoral artery, a branch of the profunda femoral artery. The inferior epigastric artery arises from the medial aspect of the external iliac artery and dips inferiorly and medially before turning superiorly to course deep to the rectus abdominis muscle in the anterior abdominal wall (Fig. 73-2). Superiorly, it anastomoses with the superior epigastric artery, a branch of the internal thoracic artery.
The internal iliac artery has anterior and posterior divisions. Branches of the anterior division primarily supply the pelvic viscera, whereas branches of the posterior division supply pelvic bones and muscles (Fig. 73-3A).
Typically, the three branches of the posterior division are the iliolumbar artery, lateral sacral artery, and superior gluteal artery (see Fig. 73-3B). The iliolumbar artery extends laterally and superiorly to divide into two branches that supply the iliacus muscle and ilium (iliac branch) and the psoas major and quadratus lumborum muscles (lumbar branch). The lateral sacral artery extends medially and then superiorly to supply the spinal meninges and the roots of the sacral nerves. The superior gluteal artery, the largest branch of the internal iliac artery, extends laterally, passes above the piriformis muscle through the greater sciatic foramen as it leaves the pelvis posteriorly, and supplies the gluteal muscles, overlying soft tissues and skin. These posterior division branches may, on occasion, arise from the anterior division or from trunks formed by various combinations of internal iliac artery branches (see later).
The three principal branches of the anterior division are the obturator artery, inferior gluteal artery, and internal pudendal artery (Fig. 73-4A). The obturator artery courses along the pelvic brim, bifurcates above the obturator notch, and passes laterally through the obturator foramen to supply muscles of the thigh and ligament of the femoral head. It is usually the most lateral of the anterior division branches. A distal branch of the obturator artery anastomoses with the medial circumflex femoral artery, a branch of the profunda femoral artery. The inferior gluteal artery has a variable intrapelvic course, typically concave laterally. It courses inferiorly, anterior to the piriformis muscle and sacral plexus, extends laterally, and exits the bony pelvis via the greater sciatic notch. It supplies the muscles and skin of the buttock and posterior surface of the thigh. In rare cases, it gives rise to a persistent sciatic artery (see later). The internal pudendal artery courses inferiorly along the anterior surface of the piriformis muscle, lateral to the inferior gluteal artery, and enters the ischiorectal fossa through the lesser sciatic foramen. It has numerous small named branches. It supplies the perineum and external genitalia.
The remaining branches of the anterior division are less prominent (see Fig. 73-1). The superior vesical artery has an inferomedial course until it reaches the lateral aspect of the bladder, at which point it courses along the superior surface of the bladder; its position varies depending on the degree of bladder distention. It supplies up to 80% of the bladder, as well as the distal ureters and, in males, the ductus deferens. The inferior vesical artery has an inferomedial course whose position is not dependent on the degree of bladder distention. It is a small vessel, difficult to appreciate angiographically, which supplies the inferolateral surface of the bladder, the trigone and, in males, the seminal vesicles and prostate. In females, the analogous vessel is sometimes termed the vaginal artery. It supplies the vagina, posteroinferior portions of the bladder, and pelvic part of the urethra. The vaginal artery may be a single vessel or two or three separate arteries. The inferior vesical artery often forms a common trunk with the middle rectal artery. The middle rectal artery may arise from the anterior division, but is frequently a branch of another artery in the internal iliac artery distribution. This small vessel is the most posterior of the anterior division vessels, and descends inferomedially to the ipsilateral side of the middle portion of the rectum. It anastomoses with the superior and inferior rectal arteries to supply the rectum and sometimes inferior vesical–vaginal artery territory. The uterine artery has a characteristic U-shaped course; it descends, turns medially to course along the broad ligament, and then ascends in the parametrium along the lateral border of the uterus. The cervicovaginal artery, which supplies the cervix and vagina, arises near the junction of the medial and ascending portions. The ascending portion is typically convoluted and gives off numerous convoluted branches that extend medially (see Fig. 73-4B). In postpartum women, the uterine artery may extend superiorly, without demonstrating the U-shaped course typical of the nongravid uterus.
Detailed Description of Specific Areas
Normal Variants
Variations of the internal iliac artery and its branches are common. Typically, the internal iliac artery divides into two branches, as noted earlier. However, this arrangement is seen in approximately 60% of cases, and the remaining 40% demonstrate one (10%), three (20%), or four or more (10%) principal trunks. The sequence of branch origins and trunk formation is extremely variable. The obturator artery may arise from the external iliac artery or the inferior epigastric artery. A persistent sciatic artery, which arises from the inferior gluteal artery, is a rare but clinically important variant (Fig. 73-5). Because it is often symptomatic, it is discussed separately later.
Differential Considerations
Arterial bypass grafts for infrarenal aortic or iliac artery disease originate from the axillary artery or the infrarenal aorta and insert in the external iliac, common femoral, or profunda femoral arteries, depending on the site(s) of disease (Fig. 73-6). Common femoral artery to common femoral artery grafting can be performed for unilateral common iliac or external iliac artery disease (Fig. 73-7). Lower extremity atherosclerosis requiring surgical bypass can be treated with a graft originating at the common femoral artery and terminating at the popliteal artery (ideally, superior to the knee joint), posterior tibial artery, or anterior tibial artery, as required (Fig. 73-8).
In the pelvis, a persistent sciatic artery can be clinically important. The sciatic artery normally involutes by the 22-mm embryo stage, with remnants persisting as the proximal portion of the inferior gluteal artery, popliteal artery, and peroneal artery.1 Rarely, the sciatic artery persists, either because of failure of development of the superficial femoral artery or failure of regression of the sciatic artery.2 A persistent sciatic artery is the continuation of the internal iliac artery (see Fig. 73-5), arising from the anterior division distal to the origin of the internal pudendal artery. It follows the course of the inferior gluteal artery through the greater sciatic foramen, where it may accompany the posterior cutaneous nerve or sciatic nerve. It continues inferiorly along the posterior aspect of the adductor magnus and then passes through the popliteal fossa to form the popliteal artery and supply the leg and foot.1 The reported incidence, based on angiographic series, is 0.01% to 0.05%.2 Bilateral persistent sciatic arteries are estimated to occur in about 10% of these individuals.2
Approximately 60% of individuals with a persistent sciatic artery are symptomatic. The most common symptom is lower extremity ischemia.3 Approximately 40% of patients with a persistent sciatic artery develop an aneurysm of the vessel at the level of the greater trochanter, believed to be caused by repetitive microtrauma from the sacrospinal ligament and piriformis muscle during hip flexion and extension.4 These patients may present with thrombosis, distal embolization, or a buttock mass. Symptomatic patients have a risk of limb loss as high as 25%.
On imaging studies in these individuals, the internal iliac artery is larger than the external iliac artery. The persistent sciatic artery is ectatic and courses laterally at the level of the femoral head before turning inferiorly. On oblique views, it can be appreciated as a posterior structure. The external iliac artery and common femoral artery are in their normal locations, but may be small. The superficial femoral artery tapers in the thigh, may bifurcate on the region of the adductor canal, and then disappears. Persistent sciatic arteries are readily appreciated on CT angiography (CTA), which also permits the determination of aneurysm size, despite the possible presence of luminal thrombus.3
NORMAL ANATOMY OF THE LOWER EXTREMITIES
General Anatomic Descriptions
The femoral artery (usually referred to in clinical practice as the common femoral artery) is the continuation of the external iliac artery distal to the inguinal ligament. It is the usual percutaneous access site for catheter angiography. The femoral artery is variable in length. It divides into the profunda femoral artery posterolaterally and the superficial femoral artery anteromedially (Fig. 73-9) and may also give rise to the superficial circumflex iliac artery, external pudendal arteries, and muscular branches, although this is variable.
The profunda femoral artery (deep femoral artery) is the largest branch of the femoral artery and supplies the thigh muscles anteriorly and posteriorly. It normally gives rise to the medial and lateral circumflex femoral arteries and then continues inferiorly as it gives off several perforating branches (see Fig. 73-9).
The superficial femoral artery extends inferiorly to the inferior end of the adductor canal (Hunter’s canal), where it continues as the popliteal artery (Fig. 73-10A). The adductor canal is a fascia-lined compartment bounded by the vastus medialis muscle anteriorly and laterally and the adductor magnus and adductor longus muscles posteriorly, which winds medially and posteriorly around the femur. The junction of the superficial femoral artery and popliteal artery is thus in the distal thigh, and not at the level of the knee joint, as is occasionally—and incorrectly—stated in clinical practice. The superficial femoral artery serves as conduit to the leg and foot. It gives rise to multiple small muscular branches and, just superior to its entrance into the adductor canal, it gives rise to the descending (supreme) genicular artery.
The popliteal artery extends from the inferior aspect of the adductor canal to a point inferior to the knee, where it divides into the tibial and peroneal arteries. Along its course, posterior to the femur and the tibia, it gives off a small medial genicular artery, multiple sural arteries, medial and lateral superior genicular arteries, and medial and lateral inferior genicular arteries (see Fig. 73-10B). The last four genicular arteries wrap around the femur and tibia to form an anastomotic network around the patella. They supply the articular capsule and ligaments of the knee joint. The sural arteries extend inferiorly to supply the calf muscles.
In the leg, the popliteal artery gives off the anterior tibial artery at the level of the inferior edge of the popliteus muscle. The popliteal artery continues as the tibioperoneal trunk, which then divides into the posterior tibial artery and peroneal artery (see Fig. 73-10).
The anterior tibial artery, a major source of supply to the foot, passes anteriorly between the two heads of the tibialis posterior muscle and through the interosseous membrane to the anterior leg. It extends inferiorly, medial to the fibula and continues on to the dorsum of the foot. After it crosses the inferior extensor retinaculum, it becomes the dorsalis pedis artery (Fig. 73-11).
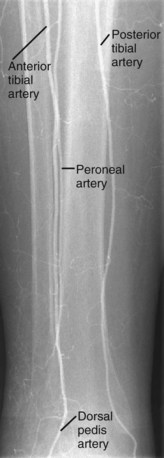
 FIGURE 73-11 Angiogram of the right runoff arteries—anterior tibial, peroneal, and posterior tibial arteries.
FIGURE 73-11 Angiogram of the right runoff arteries—anterior tibial, peroneal, and posterior tibial arteries.
The posterior tibial artery, also a major source of supply to the foot, extends inferiorly in the posterior leg to reach the ankle behind the medial malleolus and continues on to the foot. In a frontal view, the posterior tibial artery is the most medial of the three major calf vessels (see Fig. 73-11).
In the foot (Fig. 73-12), the posterior tibial artery divides into a relatively small medial plantar artery, which runs along the first ray, and a larger lateral plantar artery. These vessels run in the plantar aspect of the foot. The lateral plantar artery runs laterally and distally to the base of the fifth metatarsal and then swings medially to form one side of the plantar arch, which supplies plantar metatarsal and common digital arteries to the forefoot.
Detailed Description of Specific Areas
Normal Variants
Collateral Pathways
The anterior pathway extends across the anterior chest wall and anterior abdominal wall via the internal thoracic artery and intercostal arteries, through the superior and inferior epigastric arteries, to the distal external iliac artery (Fig. 73-13). The posterior pathway extends from the posterior chest wall and posterior abdominal wall to the internal iliac artery via intercostal and lumbar arteries, and then through the iliolumbar and superior gluteal arteries. Both anterior and posterior collateral pathways provide alternate routes for ipsilateral arterial flow from the aorta to the pelvis.
Collateral formation in the thigh is common in patients with calf claudication, because the superficial femoral artery is a common site of atherosclerosis, especially as it exits the adductor canal. Collateral pathways are from the profunda femoral artery branches to the superficial femoral artery and from the superficial femoral artery proximal to the lesion to the superficial femoral artery distal to the lesion via muscular branches (Fig. 73-14).
There are numerous collateral pathways in the region of the knee. The genicular arteries form a rich anastomotic network, which comes into play when there is stenosis or occlusion of the popliteal artery. Collateral flow via the distal branches of the peroneal artery can supply the foot in patients with occlusion of the anterior tibial or posterior tibial artery (Fig. 73-15).
Differential Considerations
Popliteal artery entrapment syndrome (PAES) is caused by a congenital anomaly in the anatomic relationships between the popliteal artery and soft tissue structures of the popliteal fossa, most commonly the medial head of the gastrocnemius. Strenuous athletic activity can cause repetitive compression or microtrauma to the popliteal artery and may result in foot or calf claudication. Symptoms usually develop over time, but acute onset after strenuous exercise has also been described. Ultimately, popliteal artery aneurysm, pseudoaneurysm, atherosclerosis, thrombosis, or thromboembolism may occur. It is a rare disorder, with an estimated prevalence of 0.16%.5
Most patients with popliteal artery entrapment syndrome are young men—active individuals, physically fit, and otherwise in good health.6 Most are athletes or on active military duty. More than 80% are younger than 30 years; fewer than 10% are older than 50 years.7 One or both lower extremities may be affected.
Numerous classification schemes have been proposed, based on the anatomy of the entrapment. Whelan and colleagues8 have described four anatomic variations, and Rich and associates9 added a fifth variant. More recently, the Popliteal Vascular Entrapment Forum has modified the classification to include functional entrapment.10 In type I anatomy, the popliteal artery passes medially to the normally inserted medial head of the gastrocnemius muscle. In type II anatomy, the popliteal artery is in its normal position but passes medial to an abnormal medial head of the gastrocnemius muscle, which inserts too far laterally on the distal femur. In type III anatomy, a normally positioned popliteal artery is either compressed by an aberrant muscle slip of the medial head of the gastrocnemius muscle or passes through the medial head of the gastrocnemius. In type IV anatomy, the posterior aspect of the popliteal artery is compressed by an anomalous fibrous band or by the popliteus muscle. The artery may or may not pass medially to the medial head of the gastrocnemius.
In the study by Rich and coworkers,9 patients who had entrapment by branches of the tibial nerve were included in this group. In type V, the popliteal vein is involved, as well as the popliteal artery. Imaging studies typically demonstrate the artery deviated medially at the level of the knee. Type VI is functional entrapment, with normal anatomy and compression caused by hypertrophied muscles. Other unusual anatomic variants also exist.
Adventitial cystic disease (ACD) is a rare disorder of uncertain cause characterized by mucoid cysts in the adventitia of the arterial wall. The incidence has been estimated as 1 in 1200 cases of claudication.11 The mass effect of the cysts compresses the arterial lumen and compromises flow. ACD has been reported in the popliteal artery in the popliteal fossa, the external iliac artery, and less commonly in the distal brachial, radial and ulnar arteries. It is most commonly seen in men ages 20 to 50 years.10
Popliteal artery ACD is a classic although rare cause of lower extremity claudication. Arterial imaging often demonstrates a smooth tapering stenosis (scimitar sign) or smooth concentric or eccentric stenoses.12 MRI and ultrasound demonstrate mucoid cysts, which are hyperintense on T2-weighted images and of variable intensity on T1-weighted images.10 Treatment with cyst aspiration under ultrasound guidance has been suggested but is not always successful.12,13 Surgical unroofing of the cysts and bypass grafting are more commonly performed.
The popliteal artery is considered aneurysmal when its diameter exceeds 7 mm.14 This vessel is the most common site of aneurysm formation in the peripheral vasculature, accounting for approximately 80% of peripheral arterial aneurysms.15 Popliteal artery aneurysms are usually at least 20 mm in diameter at diagnosis, are bilateral in 50% of cases, and are associated with an aortic aneurysm in 40% of cases.15 They are secondary to atherosclerosis in nearly 90% of patients.16 Other uncommon causes include trauma, infection (mycotic aneurysm), connective tissue disorders (e.g., Marfan syndrome, Ehlers-Danlos syndrome), vasculitis and, rarely, pregnancy.14 Because most cases are caused by atherosclerosis, the patient population is typically older and predominantly male.
Approximately 25% to 45% of popliteal artery aneurysms are asymptomatic at the time of diagnosis.14,16 Patients with symptomatic popliteal artery aneurysms typically present with acute or chronic limb ischemia caused by thrombosis of the aneurysm or distal embolization of thrombotic material.10 These complications will eventually occur in 18% to 75% of patients. Acute limb ischemia has a relatively poor prognosis, with a 15% amputation rate because of occlusion of runoff vessels.15 By contrast, treatment of asymptomatic popliteal aneurysms yields a 5-year graft patency of 80% and 98% limb salvage. Popliteal artery aneurysm rupture is uncommon. Symptomatic patients are treated surgically with exclusion of the aneurysm, thrombectomy, and bypass grafting. If the patient can tolerate an additional period of ischemia during therapy, thrombolysis is highly successful for recanalizing the distal vessels to provide targets for bypass grafting.
Pertinent Imaging Considerations
The arteries of the thigh and knee usually can be evaluated adequately in a frontal view. Imaging of the popliteal artery in the popliteal fossa sometimes requires a lateral view. A frontal view of the leg often demonstrates the distal anterior tibial artery and peroneal artery superimposed. When imaged with CTA or MRA, these vessels should also be evaluated in a lateral view, as should the vessels of the foot. Specific provocative maneuvers have been described to elicit vascular compression in patients with popliteal entrapment.10
KEY POINTS
 Knowledge of the expected and possible variant arterial anatomy of the pelvis and lower extremities is essential for successful CTA and MRA scan prescription and interpretation.
Knowledge of the expected and possible variant arterial anatomy of the pelvis and lower extremities is essential for successful CTA and MRA scan prescription and interpretation.Kadir S. Atlas of Normal and Variant Angiographic Anatomy. Philadelphia: WB Saunders; 1991.
Lippert H, Pabst R. Arterial Variations in Man. Classification and Frequency. Munich: JF Bergmann Verlag; 1985.
Merland J-J, Chiras J. Arteriography of the Pelvis. Diagnostic and Therapeutic Procedures. New York: Springer-Verlag; 1981.
Uflacker R. Atlas of Vascular Anatomy. An Angiographic Approach, 2nd ed. Philadelphia, Lippincott: Williams & Wilkins; 2007.
Valentine RJ, Wind GG. Anatomic Exposures in Vascular Surgery. Philadelphia, Lippincott: Williams & Wilkins; 2003.
1 Mandell VS, Jaques PF, Delany DJ, Oberheu V. Persistent sciatic artery: clinical, embryologic, and angiographic features. AJR Am J Roentgenol. 1985;144:245-249.
2 Kurtoglu Z, Uluutku H. Persistent sciatic vessels associated with an arteriovenous malformation. J Anat. 2001;199(Pt 3):349-351.
3 Jung AY, Lee W, Chung JW, et al. Role of computed tomographic angiography in the detection and comprehensive evaluation of persistent sciatic artery. J Vasc Surg. 2005;42:678-683.
4 Kritsch D, Hutter HP, Hirschl M, Katzenschlager R. Persistent sciatic artery: an uncommon cause of intermittent claudication. Int Angiol. 2006;25:327-329.
5 Bouhoutsos J, Daskalakis E. Muscular abnormalities affecting the popliteal vessels. Br J Surg. 1981;68:501-506.
6 Collins PS, McDonald PT, Lim RC. Popliteal artery entrapment: an evolving syndrome. J Vasc Surg. 1989;10:484-489.
7 Rosset E, Hartung O, Brunet C, et al. Popliteal artery entrapment syndrome: anatomic and embryologic bases, diagnostic and therapeutic considerations following a series of 15 cases with a review of the literature. Surg Radiol Anat. 1995;17:161-169.
8 Whelan TJ, Haimovici H Popliteal artery entrapment syndrome. 2nd ed. Haimovici H, editor. Vascular Surgery: Principles and Techniques, vol 2. 1984. McGraw-Hill. New York. 557-567.
9 Rich NM, Collins GJJr, McDonald PT, et al. Popliteal vascular entrapment. Its increasing interest. Arch Surg. 1979;114:1377-1384.
10 Holden A, Merrilees S, Mitchell N, Hill A. Magnetic resonance imaging of popliteal artery pathologies. Eur J Radiol. 2008;67:159-168.
11 Flanigan DP, Burnham SJ, Goodreau JJ, Bergan J. Summary of cases of adventitial cystic disease of the popliteal artery. Ann Surg. 1979;189:165-175.
12 Cassar K, Engeset J. Cystic adventitial disease: a trap for the unwary. Eur J Vasc Endovasc Surg. 2005;29:93-96.
13 Do DD, Braunschweig M, Baumgartner I, et al. Adventitial cystic disease of the popliteal artery: percutaneous US-guided aspiration. Radiology. 1997;203:743-746.
14 Wright LB, Matchett WJ, Cruz CP, et al. Popliteal artery disease: diagnosis and treatment. Radiographics. 2004;24:467-479.
15 Thompson MM, Bell PR. ABC of arterial and venous disease. Arterial aneurysms. BMJ. 2000;320:1193-1196.
16 Davidovic LB, Lotina SI, Kostic DM, et al. Popliteal artery aneurysms. World J Surg. 1998;22:812-817.

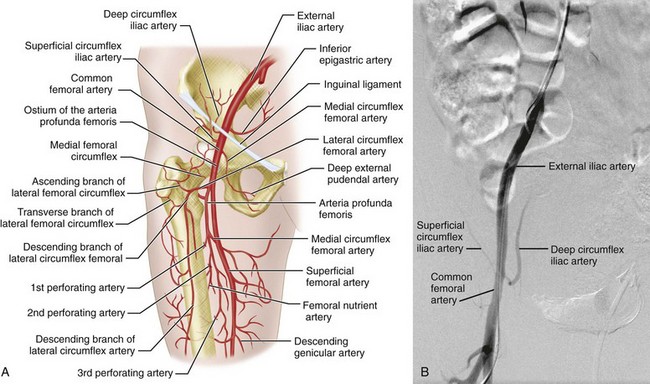
 FIGURE 73-2
FIGURE 73-2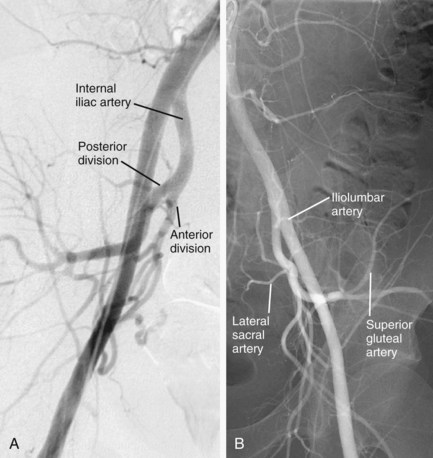
 FIGURE 73-3
FIGURE 73-3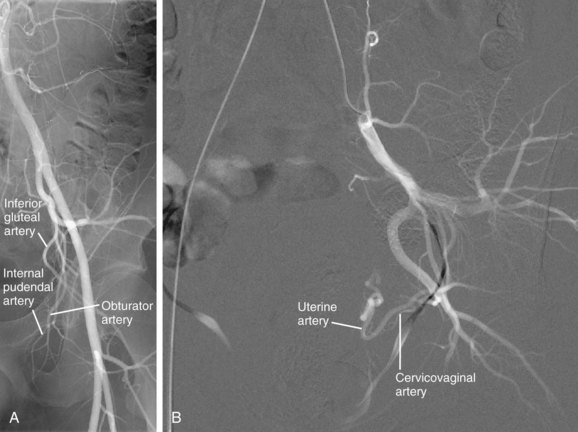
 FIGURE 73-4
FIGURE 73-4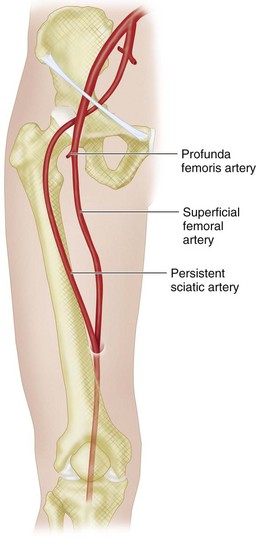
 FIGURE 73-5
FIGURE 73-5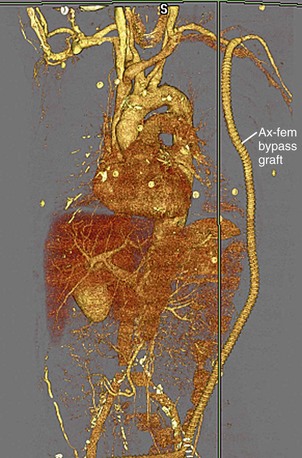
 FIGURE 73-6
FIGURE 73-6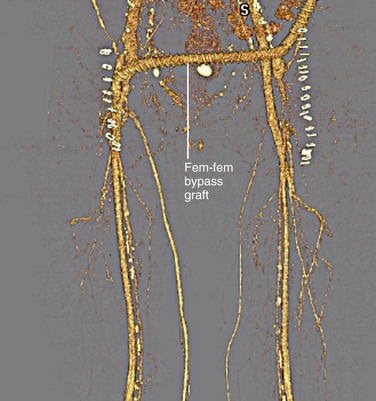
 FIGURE 73-7
FIGURE 73-7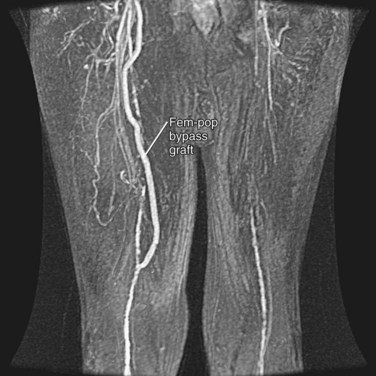
 FIGURE 73-8
FIGURE 73-8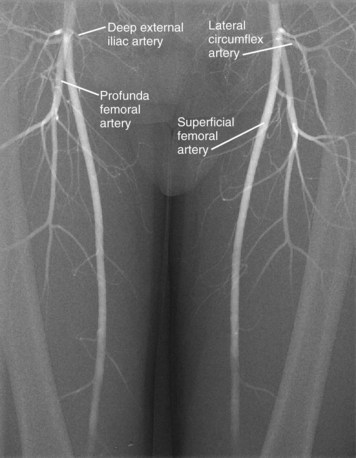
 FIGURE 73-9
FIGURE 73-9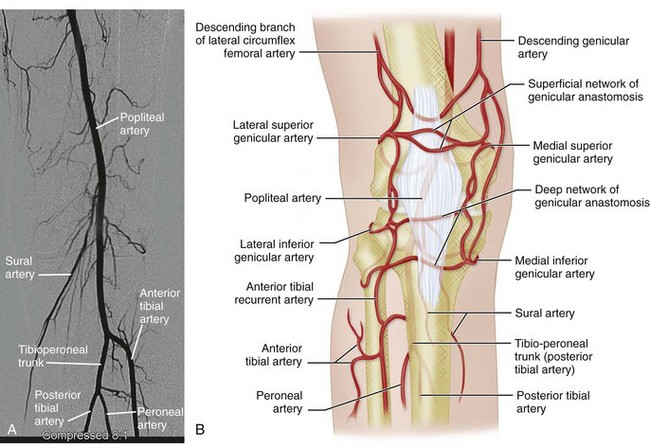
 FIGURE 73-10
FIGURE 73-10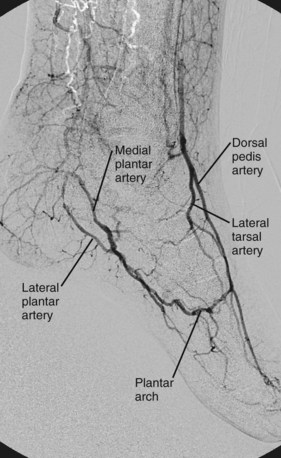
 FIGURE 73-12
FIGURE 73-12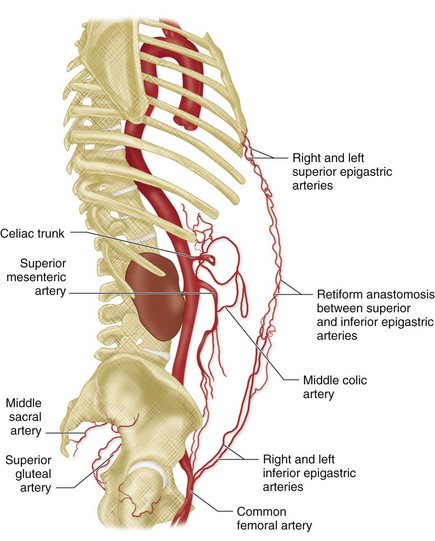
 FIGURE 73-13
FIGURE 73-13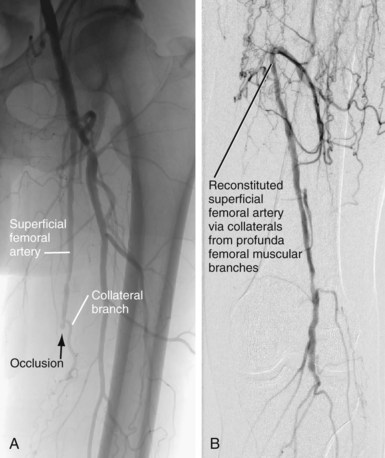
 FIGURE 73-14
FIGURE 73-14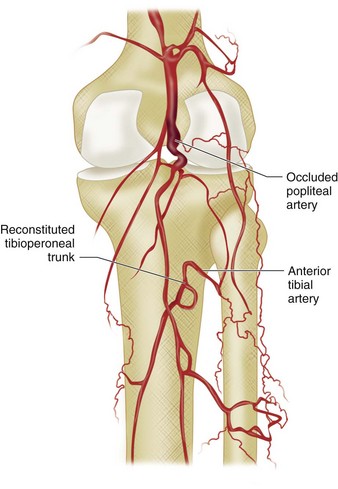
 FIGURE 73-15
FIGURE 73-15

