Chapter 49 Are Bone Substitutes Useful in the Treatment and Prevention of Nonunions and in the Management of Subchondral Voids?
Bone substitutes can be considered in two circumstances: (1) as an agent for the prevention and treatment of nonunions, and (2) as a “void filler” to maintain joint or metaphyseal alignment, usually in a periarticular or intra-articular fracture.
Periarticular fractures are common injuries that result from indirect coronal and/or direct axial compressive forces. The usual surgical guidelines for treating depressed articular fractures with joint instability include anatomic reduction, re-establishment of the long bone alignment, subchondral bone grafting to support the articular cartilage, and stable internal fixation.1 In this situation, a substance that withstands compressive forces and provides structural support is of primary importance, with osteoinductive properties being secondary.
OPTIONS
Cancellous bone graft retrieved from an iliac crest donor site has been the traditional substance of choice used both to promote healing and to fill voids and provide structural support. This procedure involves making a separate incision over the crest to obtain the graft, which can cause morbidity such as pain, nerve injury, arterial injury, and infection.2,3 Allograft bone can be used to avoid the morbidity associated with autograft harvesting; however, allograft bone acts mainly as an osteoconductive substance, and there is a risk for disease transmission. In recent years, new bone alternatives have become commercially available to act as “substitute” bone graft. These consist of various forms of ceramics such as tricalcium phosphate and hydroxyapatite.4–7
Evidence
Based on the method that Bajammal and colleagues8 used for their recent meta-analysis, we primarily identified articles with the following features: (1) the target population was skeletally mature patients with a fracture of a bone of the appendicular skeleton; (2) the intervention was the use of calcium phosphate bone cement, calcium sulfate bone cement, or a recombinant human bone morphogenetic protein (rhBMP) compared with alternative or no treatment in the management of these fractures; (3) the outcome measure was either functional (pain or impairment) or radiographic (fracture healing or subsidence) outcome, or infection rate; and (4) the study was a published or unpublished randomized, controlled trial. We secondarily also included other less rigorous studies in this review because of the paucity of articles with Level I evidence.
Osteoconductive Bone Graft Substitutes
Allograft Bone.
Allograft bone harvested from living or deceased donors as cancellous, or corticocancellous chips, has a wide application as an osteoconductive filler for metaphyseal defects typically at the proximal and distal end of the tibia (Table 49-1). McKee and coworkers9 report on a case series of six humeral nonunions treated with a combination of compression plate fixation and cortical onlay grafts. All six nonunions had united at a median of 3.4 months. Hornicek and coauthors10 report a series of nine humeral nonunions treated in a similar fashion; union was achieved in all patients at an average of 2.9 months. Haddad and researchers11 report on a retrospective case series of 40 patients using cortical onlay strut grafts together with well-fixed prosthetic femoral stems; 39 of the 40 patients united. Herrera and investigators12 report on distal radial fractures treated with external fixation and internal fixation together with cancellous bone grafts and concluded that allograft was a useful adjunct for treatment of unstable distal radial fractures. An article that prospectively compared autologous graft versus allograft, delivered at the Orthopaedic Trauma Association (OTA) in 2005, demonstrated that allograft was not equal to autograft. However, autograft did have associated donor morbidity.13 No Level I evidence supports corticocancellous allografts in reconstructive trauma surgery, but Level II and IV evidence does exist, as noted earlier.
Calcium Phosphate Synthetic Substitutes.
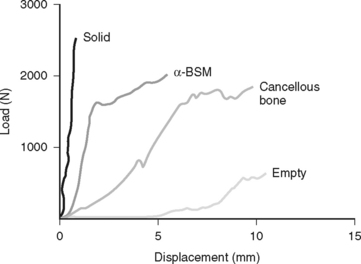
The calcium phosphate synthetic substitutes have been investigated as devices by the FDA and by industry over the last 8 years. Initial studies were done with critical defects in rats, sheep, and dogs. Subsequent biomechanical studies suggested that bioabsorbable calcium phosphate paste (α-BSM) in tibias was stronger than cancellous bone graft used to repair periarticular fractures14 (Fig. 49-1). Trenholm and coworkers14 illustrate that the initial stiffness was significantly better in the α-BSM group (calcium phosphate cement) as compared with autograft under a 1000-newton load. Russell and investigators1 in a prospective, randomized, multicenter trial compared the treatment of subarticular bone defects in tibial plateau fractures with conventional autogenous iliac bone graft (AIBG) with α-BSM. One hundred nineteen acute closed tibial plateau fractures were prospectively enrolled, and randomization occurred at surgery with a 2:1 ration of the α-BSM to autogenous bone graft. Russell and investigators1 found both a significant increase in graft-related adverse effects on the AIBG group, and a statistically significantly greater rate of articular subsidence in the 3- to 12-month period in the AIBG group (Fig. 49-2). Thus, Level I evidence supports the use of bioabsorbable calcium phosphate material, such as α-BSM, as the treatment of choice for subarticular defects in tibial plateau fractures.
Three of these six studies in the meta-analysis by Bajammal and colleagues8 report significantly improved functional outcome scores with the use of calcium phosphate versus autograft, and in evaluating them separately, Chapman and researchers15 found a difference in functional repair in favor of the calcium phosphate group. Cassidy and investigators16 found significant greater scores representing better function in bodily pain, role physical, role emotional, social function, and mental health subdomains of the 36-Item Short Form Health Survey (SF-36). Zimmermann and colleagues17 found significantly better DASH (disability of the arm, shoulder, and hand) scores in distal radius fractures for the calcium phosphate group. Six of the studies also reported loss-of-reduction outcome, and calcium phosphate significantly reduced the incidence of loss of fracture reduction compared with controls.1,15,16,18–20 Thus, loss of fracture alignment was 48% less likely when calcium phosphate was used. For every 17 patients treated with calcium phosphate, one loss of fracture reduction could be prevented.8
Calcium Sulfate Synthetic Substitutes
Evidence for the use of calcium sulfate is extremely poor. In a study done by Petruskevicius and coworkers21 looking at OsteoSet (Wright Medical Technology, Arlington, TN) on bone healing and tibial defect in humans, OsteoSet was compared with no bone graft in the substitution of anterior cruciate ligament repairs. Computed tomographic scans of the defect were taken on the first day after the operation, at 6 weeks, 3 months, and 6 months. No difference was found in the amount of bone in the defect in the OsteoSet and control groups, and indeed, in the control group (no bone graft or pellets), the bone volume increased from 6 weeks to 3 months. A study looking at the use of calcium sulfate in nonunions, presented at the OTA in 2004, demonstrated no improvement in bone healing, an increased infection rate, and increased wound drainage.22 The authors’ conclusion was to suggest calcium sulfate should not be used in the treatment of nonunions. Despite excellent efforts, there is no Level I or II evidence that the healing is enhanced, and indeed, healing may be worse in periarticular injuries or nonunions with the addition of calcium sulfate.
With the increasing popularity of calcium sulfate, there have been some cases of severe inflammatory response particularly in tumor cases. It has been hypothesized that the rapid absorption of the calcium sulfate pellets into a calcium-rich fluid stimulates inflammation.23
Demineralized Bone Matrix
No studies have been reported in which the investigators carefully evaluated the osteoconductive properties of allograft bone per se. Demineralized bone matrix (DBM) is produced by acid extraction of allograft that contains type I collagen, noncollagenous proteins, and osteoconductive growth factors. Tiedeman and coauthors24 report on an uncontrolled case series of 48 patients in whom DBM had been used in conjunction with bone marrow for the treatment of skeletal injuries. Because there was no control group, the role of DBM in the 30 patients who had healing remains unknown. One prospective, controlled study showed equivalent rates of spinal fusion between sides in patients who had been treated with autograft on one side and a 2:1 ratio composite of Grafton DBM gel and autograft on the other, suggesting the potential use of Grafton DBM as a bone graft extender in spine fusions.25 Only anecdotal information is available regarding similar applications in diaphyseal and metaphyseal bone injuries.
Osteoinductive Bone Graft Substitutes
Human Bone Morphogenetic Protein.
The two most widely studied osteoinductive bone substitutes are rhBMP-2 and rhBMP-7. The use of bone morphogenetic protein (BMP) to improve the healing of open tibial shaft fractures has been the focus of several prospective clinical studies. Swiontkowski and investigators26 analyzed two prospective, clinical, randomized studies that recruited a total of 510 patients with open tibial fractures. They were randomized to receive the control treatment, intramedullary nail fixation, and routine soft-tissue management versus treatment with an absorbable collagen sponge impregnated with one of two concentrations of rhBMP-2. Fifty-nine trauma centers in 12 countries participated, and the patients were followed for 12 months after surgery. Two subgroups were analyzed: (1) 131 patients with Gustilo–Anderson type IIIA or IIIB open tibial fractures not treated with internal fixation, and (2) 113 patients treated with reamed intramedullary nailing. The first subgroup demonstrated significant improvements in the rhBMP-2 group with fewer bone grafting procedures and fewer patients requiring invasive secondary interventions. The subgroup analysis of fractures treated with reamed, intramedullary nailing demonstrated no significant difference between the control and the rhBMP-2 groups. Both groups illustrated a trend in reduction of the infection rate in those patients who received rhBMP-2. Therefore, the following conclusion can be reached: If intramedullary nailing is not utilized, the addition of rhBMP-2 to open tibial fractures can significantly reduce the frequency of bone grafting procedures and other secondary interventions. There seems to be no improvement in outcome when IM nailing is used.
Govender and coauthors27 also presented evidence on a prospective, randomized, controlled, single-blind study with 450 patients presenting with open tibial fractures. They were randomized to receive either intramedullary nail fixation and routine soft-tissue management or the same care plus rhBMP-2. A trend suggested better healing and faster bone growth in the rhBMP-2 group that did not reach statistical significance, and the final nonunion rate was similar between the two groups.
McKee and coworkers28 evaluated rhBMP-7 (also known as OP-1) by randomizing 122 patients with 124 open tibial shaft fractures. All patients received an initial débridement plus open reduction and internal fixation with a locked intramuscular nail within 48 hours of injury. At the time of secondary closure, the patients either received no additional treatment, except for wound closure, or wound closure with the addition of OP-1. They were followed up for 1 year. The results did not demonstrate any difference in union rate. The number of nonunions was not decreased with the addition of rhBMP-7. However, the amount of bone created was greater with the treatment group, and the time to healing was reduced, but the study was underpowered and did not reach statistical significance. In addition to the trend to improve bone healing, there was also a corresponding improvement in patient function, with 80% of the OP-1 group having no pain or mild pain with activity at 12 months after injury compared with 65% of the control group (P = 0.04).
Clinical Application of Autologous Bone Marrow.
Bone marrow aspirate has been utilized as another way of applying connective tissue progenitors intraoperatively to enhance bone growth and repair. Garg and coworkers29 and Healey and researchers30 were able to show low-level evidence of improvement with bone marrow aspirate. Goel and coauthors31 report that they used bone marrow injections with the use of local anesthesia for patients who are on waiting lists for open repair of a nonunion. They used this procedure in an attempt to provide a low-cost alternative and claimed success in 15 of 20 patients. Hernigou and colleagues32 report on 60 patients with noninfected nonunions who had undergone bone marrow aspiration of both iliac crests followed by injection at the nonunion site. Results showed union in 53 of 60 patients with positive correlation between the volume of mineralized callous at 4 months and the number and concentration of colony-forming units. This study provided the best evidence (Level III) for the use of autologous bone marrow. An article presented at the 2006 OTA annual meeting in Phoenix demonstrated no benefit of autologous bone marrow in a randomized, controlled study.33 Currently, no level I or II evidence documents the effectiveness of bone marrow for the enhancement of bone healing.
Use of Platelet-Rich Plasma and Related Peripheral Blood Concentrations.
Several strategies for platelet concentration delivery have been developed on the basis of the assumption that delivery of a concentrated amount of platelets would contribute to the early stages of bone repair, thus initiating a fracture healing cascade.34,35 However, to date, indications are based only on longitudinal case series, multiple case reports, and abstracts documenting the effects of platelet gels and concentrates.36 Therefore, at this time, the use of platelet-rich plasma and related peripheral blood concentrates seem to function best as a physiologic carrier for other graft materials.
AREAS OF UNCERTAINTY
If bone substitutes provide equivalent results to bone autograft, is the increased cost justified to avoid the morbidity associated with autogenous bone harvesting? Jones56 presents data to suggest that in severe open tibia fractures the addition of BMP might be cost saving depending on the payor model; however, there are no prospective costing data available to address this issue.
Autogenous iliac graft procurement requires a second surgical procedure with loss of tissues from the body, the induction of pain at a previously uninjured site, and presents the possibility of iatrogenic infection.37,38 Younger and Chapman3 document the relative rates of major and minor complications after an autogenous bone graft harvest procedure. Goulet and coauthors39 report the functional disturbances from the harvest of autogenous iliac bone. Autogenous graft is not a homogenous material; there are significant differences in the hosts, and this problem is exacerbated in osteoporotic donors. Thus, osteoporotic autograft is less useful; however, it is unknown whether bone substitutes are as effective in the setting of an elderly osteoporotic individual as they might be in a younger host with more active osteoprogenitor cells that can respond to the potential osteoinductive properties of a substitute, or that can populate the scaffold provided by an osteoconductive bone substitute.
Acknowledgments
Treatment of Established Nonunions
The treatment of nonunions is always difficult. Finkemeier and Chapman46 in 2002 achieved a 97% union rate in diaphyseal nonunions by following the usual protocol, as noted earlier, without the use of rhBMPs. To date, the power in the studies performed has not been able to demonstrate a significant difference between the usual treatment (débridement, stabilization, and bone graft) and those treated in the usual way plus the addition of an rhBMP. The trend for improved healing in the rhBMP group has been documented, but the cause of nonunions remains multifactorial, and it remains a difficult group to obtain clean Level I evidence.
Management of a Subchondral Void
Calcium phosphate is significantly stronger in compression than in cancellous bone graft,14 and it should be considered the treatment of choice for subchondral void management of tibial plateau fractures. Use in other areas requires additional study. Table 49-2 provides a summary of recommendations.
| RECOMMENDATION | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION |
|---|---|
1 Russell TA, Leighton RK, Bucholz RW, et al: The gold standard in tibial plateau fractures? A prospective multicenter randomized study of AIBG vs. alpha-BSM. Presented at the Orthopaedic Trauma Association Annual Meeting; 2004 Oct 8–10; Hollywood, FL. J Bone Joint Surg 2008 (in press).
2 De Long WGJr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89:649-658.
3 Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192-195.
4 Ladd AL, Pliam NB. Use of bone-graft substitutes in distal radius fractures. J Am Acad Orthop Surg. 1999;7:279-290.
5 Larsson S, Bauer TW. Use of injectable calcium phosphate cement for fracture fixation: A review. Clin Orthop Relat Res. 2002:23-32.
6 Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354-361.
7 Szpalski M, Gunzburg R. Applications of calcium phosphate-based cancellous bone void fillers in trauma surgery. Orthopedics. 2002;25:s601-s609.
8 Bajammal SS, Zlowodski M, Schmitz-Lelwicka AE, et al. The Use of Calcium Phosphate Bone Cement in Fracture Treatment: A Meta-analysis of Randomized Trials. Hamilton, Ontario, Canada: McMaster University, 2007.
9 McKee MD, Miranda MA, Riemer BL, et al. Management of humeral nonunion after the failure of locking intramedullary nails. J Orthop Trauma. 1996;10:492-499.
10 Hornicek FJ, Zych GA, Hutson JJ, et al. Salvage of humeral nonunions with onlay bone plate allograft augmentation. Clin Orthop Relat Res. 2001:203-209.
11 Haddad FS, Duncan CP, Berry DJ, et al. Periprosthetic femoral fractures around well-fixed implants: Use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A:945-950.
12 Herrera M, Chapman CB, Roh M, et al. Treatment of unstable distal radius fractures with cancellous allograft and external fixation. J Hand Surg. 1999;24:1269-1278.
13 Volgas D, Emblom B, Stannard JP, et al: A randomized controlled prospective trial of autologous bone graft versus iliac crest bone graft for nonunions and delayed unions. Presented at the Orthopaedic Trauma Association Annual Meeting; 2004 Oct 8–10; Hollywood, FL.
14 Trenholm A, Landry S, McLaughlin K, et al. Comparative fixation of tibial plateau fractures using a-BSM, a calcium phosphate cement, versus cancellous bone graft. J Orthop Trauma. 2005;19:698-702.
15 Chapman MW, Bucholz R, Cornell C. Treatment of acute fractures with a collagen-calcium phosphate graft material. A randomized clinical trial. J Bone Joint Surg Am. 1997;79:495-502.
16 Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with conventional fixation in distal radial fractures. A randomized study. J Bone Joint Surg Am. 2003;85-A:2127-2137.
17 Zimmermann R, Gabl M, Lutz M, et al. Injectable calcium phosphate bone cement Norian SRS for the treatment of intra-articular compression fractures of the distal radius in osteoporotic women. Arch Orthop Trauma Surg. 2003;123:22-27.
18 Dickson KF, Friedman J, Buchholz JG, et al. The use of BoneSource hydroxyapatite cement for traumatic metaphyseal bone void filling. J Trauma. 2002;53:1103-1108.
19 Larsson S, Berg P, Sagerfors M: Augmentation of tibial plateau fractures with calcium phosphate cement: A randomized study using radiostereometry. Presented at the Orthopaedic Trauma Association Annual Meeting; 2004 Oct 8–10; Hollywood, FL.
20 Sanchez-Sotelo J, Munuera L, Madero R. Treatment of fractures of the distal radius with a remodellable bone cement: A prospective, randomised study using Norian SRS. J Bone Joint Surg Br. 2000;82:856-863.
21 Petruskevicius J, Nielsen S, Kaalund S, et al. No effect of Osteoset, a bone graft substitute, on bone healing in humans: A prospective randomized double-blind study. Acta Orthop Scand. 2002;73:575-578.
22 Ziran BH, Smith WR, Lahti Z, et al: Use of calcium-based demineralized bone matrix (DBM) allograft product for nonunions and posttraumatic reconstruction of the appendicular skeleton: Preliminary results and complications. Presented at the Orthopaedic Trauma Association Annual Meeting; 2004 Oct 8–10; Hollywood, FL.
23 Robinson D, Alk D, Sandbank J, et al. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann Transplant. 1999;4:91-97.
24 Tiedeman JJ, Garvin KL, Kile TA, et al. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995;18:1153-1158.
25 Cammisa FPJr, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: A prospective controlled trial employing a side-by-side comparison in the same patient. Spine. 2004;29:660-666.
26 Swiontkowski MF, Aro HT, Donell S, et al. Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am. 2006;88:1258-1265.
27 Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123-2134.
28 McKee MD, Wild LM, Schemitsch EH, et al. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: Early results of a prospective trial. J Orthop Trauma. 2002;16:622-627.
29 Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand. 1993;64:671-672.
30 Healey JH, Zimmerman PA, McDonnell JM, et al. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop Relat Res. 1990:280-285.
31 Goel A, Sangwan SS, Siwach RC, et al. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury. 2005;36:203-206.
32 Hernigou P, Poignard A, Beaujean F, et al. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430-1437.
33 Watson JT, Quigley KJ, Mudd CD. Percutaneous injection of iliac crest aspirate for the treatment of long bone delayed union and nonunion. Phoenix: AZ, 2006.
34 Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638-646.
35 Slater M, Patava J, Kingham K, et al. Involvement of platelets in stimulating osteogenic activity. J Orthop Res. 1995;13:655-663.
36 Bibbo C, Bono CM, Lin SS. Union rates using autologous platelet concentrate alone and with bone graft in high-risk foot and ankle surgery patients. J Surg Orthop Adv. 2005;14:17-22.
37 Fowler BL, Dall BE, Rowe DE. Complications associated with harvesting autogenous iliac bone graft. Am J Orthop. 1995;24:895-903.
38 Seiler JG3rd, Johnson J. Iliac crest autogenous bone grafting: Donor site complications. J South Orthop Assoc. 2000;9:91-97.
39 Goulet JA, Senunas LE, DeSilva GL, et al. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997:76-81.
40 Cornell CN, Lane JM, Chapman M, et al. Multicenter trial of Collagraft as bone graft substitute. J Orthop Trauma. 1991;5:1-8.
41 Horstmann WG, Verheyen CC, Leemans R. An injectable calcium phosphate cement as a bone-graft substitute in the treatment of displaced lateral tibial plateau fractures. Injury. 2003;34:141-144.
42 Keating JF, Hajducka CL, Harper J. Minimal internal fixation and calcium-phosphate cement in the treatment of fractures of the tibial plateau. A pilot study. J Bone Joint Surg Br. 2003;85:68-73.
43 Yetkinler DN, McClellan RT, Reindel ES, et al. Biomechanical comparison of conventional open reduction and internal fixation versus calcium phosphate cement fixation of a central depressed tibial plateau fracture. J Orthop Trauma. 2001;15:197-206.
44 Knaack D, Goad ME, Aiolova M, et al. Resorbable calcium phosphate bone substitute. J Biomed Mater Res. 1998;43:399-409.
45 Welch RD, Zhang H, Bronson DG. Experimental tibial plateau fractures augmented with calcium phosphate cement or autologous bone graft. J Bone Joint Surg Am. 2003;85-A:222-231.
46 Finkemeier CG, Chapman MW. Treatment of femoral diaphyseal nonunions. Clin Orthop Relat Res. 2002:223-234.
47 Kopylov P, Aspenberg P, Yuan X, et al. Radiostereometric analysis of distal radial fracture displacement during treatment: A randomized study comparing Norian SRS and external fixation in 23 patients. Acta Orthop Scand. 2001;72:57-61.
48 Kopylov P, Runnqvist K, Jonsson K, et al. Norian SRS versus external fixation in redisplaced distal radial fractures. A randomized study in 40 patients. Acta Orthop Scand. 1999;70:1-5.
49 Jeyam M, Andrew JG, Muir LT, et al. Controlled trial of distal radial fractures treated with a resorbable bone mineral substitute. J Hand Surg. 2002;27:146-149.
50 Kopylov P, Adalberth K, Jonsson K, et al. Norian SRS versus functional treatment in redisplaced distal radial fractures: A randomized study in 20 patients. J Hand Surg. 2002;27:538-541.
51 Mattsson P, Larsson S. Stability of internally fixed femoral neck fractures augmented with resorbable cement. A prospective randomized study using radiostereometry. Scand J Surg. 2003;92:215-219.
52 Mattsson P, Larsson S. Unstable trochanteric fractures augmented with calcium phosphate cement. A prospective randomized study using radiostereometry to measure fracture stability. Scand J Surg. 2004;93:223-228.
53 Mattsson P, Alberts A, Dahlberg G, et al. Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures. A prospective, randomised multicentre study. J Bone Joint Surg Br. 2005;87:1203-1209.
54 Mattsson P, Larsson S. Calcium phosphate cement for augmentation did not improve results after internal fixation of displaced femoral neck fractures: A randomized study of 118 patients. Acta Orthop. 2006;77:251-256.
55 Reed J, Le ILD, Buckley RE, et al: A prospective randomized controlled trial of a bioresorbable calcium phosphate paste (a-BSM) in displaced intra-articular calcaneal fractures. Montreal, Quebec, Canada, 2005.
56 Jones AL. Recombinant human bone morphogenetic protein-2 in fracture care. J Orthop Trauma. 2005;19:S23-S25.