CHAPTER 102 Arachnoiditis and Epidural Fibrosis
Spinal arachnoiditis is a nonspecific inflammatory process of the arachnoid layer of the spinal cord or cauda equina. Arachnoiditis was first described by Victor Horsley in 1909.1 Since Horsley, numerous authors have described it with a variety of terms including: chronic spinal arachnoiditis, adhesive spinal arachnoiditis, meningitis serosa circumscripta spinalis, chronic spinal meningitis, spinal meningitides with radiculomyelopathy, lumbar adhesive arachnoiditis, spinal arachnoiditis, spinal fibrosis, and lumbosacral adhesive arachnoiditis. Furthermore, on the basis of specific radiographic or pathologic findings, arachnoiditis can be termed arachnoiditis ossificans, calcific arachnoiditis, or pachymeningitis.1
Anatomy
The arachnoid mater is an avascular membrane that lies between two vascularized membranes, the pia mater and dura mater. The arachnoid is attached to the underlying pia by numerous arachnoid trabeculae, creating a space between the arachnoid and the pia.2 This space, or potential space in some instances, transmits arterioles and is referred to as the subarachnoid space. The arachnoid is composed of layers of squamous cells held together by a network of connective tissue. The arachnoid contains intercellular pores, which allow for the passage of molecules.3
Pathogenesis
A chronic infection or irritation can cause the arachnoid membrane to become thickened and adherent to both the overlying dura mater and the subjacent pia mater.4 The pia-arachnoid carries the blood vessels to the spinal cord and this layer contains mesenchymal cells. In 1951 Smolik and Nash recognized that when the outer arachnoid layer is injured, both the blood vessels and mesenchymal cells lend themselves to extensive proliferation. The ensuing reaction between the pia-arachnoid and the dura mater leads to obliterative arachnoiditis.5
When the arachnoid membrane is exposed to an insult, an inflammatory response ensues, characterized by fibrinous exudates, neovascularization, and a relative paucity of inflammatory cellular exudates.6,7 Vascular occlusive changes can lead to spinal cord ischemia.4,8–11 The small perforating blood vessels that supply the portions of the white matter may be obliterated with resultant necrosis and cavitation of the spinal cord parenchyma.8,9,11 In addition to ischemia, blockage of venous return from the spinal cord or occlusion of cerebrospinal fluid (CSF) pathways may occur.8
The stages of progressive inflammation of the arachnoid that occur in lumbosacral arachnoiditis were described by Burton. The initial stage, radiculitis, consists of an inflamed pia-arachnoid with associated hyperemia and swelling of the nerve roots. The second stage, arachnoiditis, is characterized by fibroblast proliferation and collagen deposition. During this stage, nerve root swelling decreases and the nerve roots adhere to each other and to the pia-arachnoid. The final stage, adhesive arachnoiditis, is the resolution of the inflammatory process and is characterized by dense collagen deposition. There is marked proliferation of the pia-arachnoid, as well as complete nerve root encapsulation, hypoxemia, and progressive atrophy.12 For reasons that are not fully understood, the adhesions occur preferentially on the dorsal segments.1 The exact time course of these three phases has not been elucidated. Furthermore, it is not known how the specific causative insult for the development of arachnoiditis might affect the time course of each of the three phases.
Yamagami and colleagues13 postulated that the pathologic changes in arachnoiditis may be secondary to diminished nutritional supply. They found that, in an experimental rat model, the development of arachnoiditis and neural degeneration directly corresponded to the magnitude of extradural inflammation and wound healing processes that occurred after laminectomy, with or without foreign bodies. Furthermore, adhesions of the arachnoid cause the nerve roots to lump together and, in doing so, these nerve roots are isolated from contact with the CSF, with resultant nutritional compromise.13
Etiology
In the first half of the 20th century, arachnoiditis was most often attributed to infectious causes.8 Furthermore, arachnoiditis had been described mainly in the cervical and thoracic regions.1 Since the 1950s, there has been a trend toward a higher incidence of arachnoiditis of noninfectious origin affecting the lumbar region.1,8 The precise causes of spinal arachnoiditis are not clear, and likewise, the incidence and prevalence of spinal arachnoiditis in the general population is unknown.8
As stated previously, arachnoiditis was mainly of infectious origin in the first half of the 20th century. Syphilis, tuberculosis, and gonorrhea were the most prevalent causes.1,14 Less common infectious causes include parasitic diseases and viral meningitis.15,16 These infectious causes are important to differentiate from noninfectious causes of arachnoiditis because, in most cases, effective treatment is available for arachnoiditis of infectious origin. However, despite adequate treatment of the causative agent, scarring of the arachnoid membrane may lead to permanent damage.
There are a number of important noninfectious etiologies of arachnoiditis. In the 1940s, blood in the CSF following subarachnoid hemorrhage or surgery became the most prevalent cause of arachnoiditis.1 Spinal arachnoiditis following subarachnoid hemorrhage continues to be common and is usually treated in a conservative fashion.17 The breakdown products of hemoglobin form free radicals, and it has been postulated that these cause damage to nerves.18,19 In experiments on dogs, it has been shown that injecting blood breakdown products into the subarachnoid space causes more meningeal inflammation than does the injection of fresh blood.18 Cases of patients who have received epidural blood patches have given controversial results. Digiovanni and colleagues20 described that the placement of an autologous blood patch into the epidural space produced no more inflammation than a standard lumbar puncture. Other authors, though, have described cases where an epidural blood patch had allegedly been responsible for arachnoiditis.21 Abouleish and colleagues22 described 118 cases of epidural blood patches over a 2-year period. This group found 19 cases of axial back pain, two cases of radiculopathy, and no cases of arachnoiditis.22
Oil-based contrast media have been historically important causes of arachnoiditis. Iophendylate (Myodil, Pantopaque) is an oil-based contrast medium used in diagnostic myelograms. It was first used in the United States, in 1944, and its usage continued for 40 years. In Sweden iophendylate was banned from clinical use, in 1948, secondary to animal studies that identified it as a causative agent for arachnoiditis.23 The incidence of arachnoiditis after the use of iophendylate is dose dependent and is quoted as 1%.24 Iophendylate has a long half-life, so it is usually removed from the thecal space, by aspiration, at the conclusion of the myelogram.8 Often, this removal process is not entirely successful, and in fact, incomplete removal of the contrast dye may produce further trauma and cause bleeding into the CSF.4
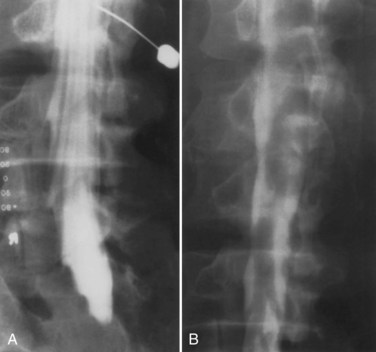
Guyer and colleagues25 listed the following factors as influencing the development of arachnoiditis after myelography: the type of contrast agent used (the risk is greater with the oil-based medium than with the water-soluble medium and greater with the ionic medium than with the nonionic medium), the dosage of contrast medium, and the observation time after myelography (Fig. 102–1).
FIGURE 102–1 Myelogram with oil-based medium demonstrating the marked lack of filling of nerve roots of adhesive arachnoiditis.
The use of intrathecal medications, either steroids or anesthetic agents, has been implicated as a cause of arachnoiditis. Intrathecal injection of corticosteroids was previously used for multiple sclerosis.8 Epidural injection of corticosteroids for back pain is a common practice. One of the most commonly used agents is methylprednisolone acetate (MPA), which has been reported to cause arachnoiditis.26–28 MPA is suspended in polyethylene glycol, which can cause arachnoiditis.26–28 Furthermore, MPA is known to easily cross the intrathecal space, thus causing arachnoiditis.28 Animal studies, though, have not shown MPA to cause significant meningeal inflammation after epidural injections.29–31
The use of intrathecal bupivacaine, with or without epinephrine, has also been reported to cause arachnoiditis. Boiardi and colleagues32 described several cases of arachnoiditis after administration of bupivacaine with epinephrine. Gemma and colleagues33 described a case of arachnoiditis after intrathecal administration of bupivacaine without epinephrine. It is unclear in these cases whether the arachnoiditis was triggered by the bupivacaine or other preservatives. Furthermore, it is unclear whether epinephrine plays a role in the pathogenesis of arachnoiditis.
A history of spinal surgery is a risk factor for arachnoiditis.8 In particular, some investigators have specifically stated that surgery for a herniated intervertebral disc may lead to arachnoiditis.5,7,25 Carroll and Wiesel34 showed that a postoperative pain-free interval lasting between 1 and 6 months, followed by the gradual onset of leg pain, increases the likelihood that some scar tissue is responsible for the symptoms. Smolik and Nash5 showed that simple dural retraction for the visualization of a ruptured intervertebral disc may trigger arachnoiditis. Haughton and colleagues35 showed that, in monkeys, the nucleus pulposus of an intervertebral disc was able to cause focal arachnoiditis.
Clinical Features
The diagnosis of arachnoiditis requires a detailed medical history and physical examination, as well as a review of confirmatory radiographic imaging studies. In obtaining a medical history from a patient with arachnoiditis, the clinician should seek three major characteristics of the pain. Pain of arachnoiditis is typically described as a burning pain that is constant and worsened by activity.12 The pain of arachnoiditis may be located in the back or the lower limbs or both. The symptoms of arachnoiditis can vary from nonspecific back pain to radiculopathy and myelopathy.36 Intractable pain that occurs secondary to arachnoiditis has a poorly localized pain pattern that is diffuse. In many patients, arachnoiditis is asymptomatic and is discovered as an incidental radiographic finding.37 The pain symptoms of chronic arachnoiditis may be similar to those of other chronic pain syndromes such as Complex Regional Pain Syndrome. The exact relationship of these pain syndromes has not been fully elucidated.
The physical examination findings in patients with arachnoiditis have been reviewed in two large clinical series. Burton followed 100 patients with arachnoiditis and found little motor weakness to be present. These patients were commonly found to have a positive straight leg raise sign, a tender sciatic notch, limited range of motion of the trunk, and paravertebral muscle spasms.12 Guyer and colleagues25 followed 51 patients over more than 10 years and found that a decreased range of motion of the trunk was the most common finding on physical examination. In cases of chronic arachnoiditis with resultant syrinx formation, physical examination findings of syringomyelia are present. These include dissociative sensory loss and variable long tract signs.8
Radiographic Features
On myelography, two distinct patterns of radiographic arachnoiditis can be differentiated. In type I arachnoiditis, there is pure adhesion of the nerve roots to the meninges with a homogeneous contrast pattern. No nerve root shadows are seen, and there is a rounded shortening of the nerve root pocket. In type II arachnoiditis, some proliferation is added inside the dural sac, which may be localized or diffuse.38 The filling defects, narrowing, shortening, or occlusion of the spinal canal are also seen in this type of arachnoiditis. In early arachnoiditis, there is central nerve root clumping and thickening. As the arachnoiditis progresses, the nerve roots become adherent peripherally to the thecal sac and the terminal thecal sac appears “sleeveless” where the nerve roots do not fill it out in the normal pattern.8 This finding can cause the thecal sac to appear empty.
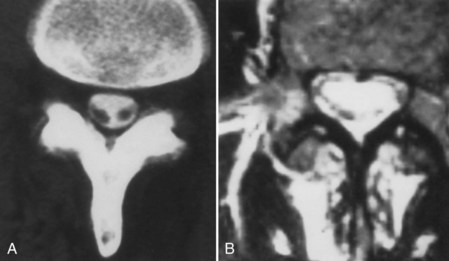
On magnetic resonance imaging (MRI), one of three patterns is commonly found.39 The first pattern is characterized by conglomerations of nerve roots, which are located centrally within the thecal sac. The second pattern is characterized by nerve roots that are clumped and attached peripherally to the meninges (Fig. 102–2). This appearance is similar to the empty sac appearance of myelography. The third pattern demonstrates increased soft tissue signal within the thecal sac with central obliteration of the subarachnoid space (Fig. 102–3).
At times, computed tomography (CT) or MRI reveals calcification or ossification of the spinal arachnoid in an entity called arachnoiditis ossificans. There are several subtypes of spinal arachnoiditis ossificans based on imaging characteristics. Type I has a semicircular arrangement, Type II is circular, and Type III demonstrates englobing of the caudal fibers.40–42
Spinal Epidural Fibrosis
Spinal epidural fibrosis is an entity observed after spine surgery, which contributes to up to 14% of cases of failed back syndrome. Spinal epidural fibrosis is caused when fibroblasts from damaged paraspinal muscles enter the vertebral canal and proliferate, forming extensive epidural scarring.43 This entity has been most typically described after cases of discectomy, whether open or percutaneous, as well as cases of implantation of spinal stimulating electrodes.43,44
Treatment
In a rat model of spinal epidural fibrosis, the administration of tissue plasminogen activator helped to prevent postlaminectomy epidural fibrosis. The presence of arachnoiditis was also less in the treatment group (P = 0.01).45 Lee and colleagues46 showed that, in a rat model, the administration of 0.1 mg/mL of mitomycin C reduced epidural fibrosis after lumbar laminectomy. This group made macroscopic, histologic, as well as MRI evaluations of the animals.46 Epidural scarring was significantly reduced and dural adhesions were absent, while wound healing was not affected.
In a dog postlaminectomy model, it has been shown that a single fraction of 700 cGy external beam radiation helped to prevent epidural fibrosis and arachnoiditis. The authors demonstrated statistically significant reductions in the extent of fibrosis and density of fibroblasts. MRI confirmation of the efficaciousness of the therapy was also demonstrated.47
A recent study in humans aimed to evaluate the role of epidural steroids in preventing epidural fibrosis. Eighty-five of 178 patients received epidural steroids following discectomy. Patients were followed for 1 year and were assessed by questionnaires containing the pain scale. Application of epidural steroids resulted in less pain on the first and third days after surgery, resulted in shorter hospital stays, but did not prevent failed back syndrome or prevent epidural scar formation.48
The role of surgery in the treatment of arachnoiditis and epidural fibrosis is controversial. Surgical procedures that have been used to treat arachnoiditis include spinal fusion procedures, decompressive spinal procedures without fusion, neuroablative procedures, and implantation of spinal cord stimulators.8
A substantial body of literature exists that suggests open surgical procedures are not useful in the treatment of arachnoiditis. Carroll and Wiesel34 found that no open surgical technique could eliminate the pathologic scar or significantly reduce the pain of arachnoiditis. Grahame and colleagues37 also found that open surgical procedures had little or no effect on the long-term course of arachnoiditis.
Some groups argue for aggressive open surgical intervention for arachnoiditis and spinal epidural fibrosis. Shikata and colleagues49 compared microlysis for arachnoiditis with and without spinal fusion. They found significant improvement in the clinical results when fusion was performed.
Spinal cord stimulation has been shown to have some benefit in patients with arachnoiditis. North and colleagues50–54 have shown that, with proper patient selection, spinal cord stimulation can be a successful therapy. This group used temporary percutaneous electrodes as a screening technique before implantation of a permanent stimulator. A minimum of 50% pain relief with temporary electrodes over a 2- to 3-day course, as well as evidence of improved activity level and stable or decreased use of analgesics, was deemed satisfactory pain relief.50–54
Recent work has focused on minimally invasive techniques to treat arachnoiditis and spinal epidural fibrosis. A number of endoscopic techniques for adhesiolysis and promotion of CSF flow pathways have been developed with promising results.55,56 Manchikanti and colleagues57 demonstrated in a recent randomized controlled trial of spinal endoscopic adhesiolysis in chronic, refractory, low back pain and lower extremity pain that adhesiolysis with targeted delivery of local anesthesia and steroids is a successful technique in the treatment of arachnoiditis. This study demonstrated significant improvement in pain in 48% of subjects at 1-year follow-up.57
Summary
Key Points
1 Burton CV. Lumbosacral arachnoiditis. Spine. 1978;3:24-30.
2 Delamarter RB, Ross JS, Masaryk TJ, et al. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine. 1990;15:304-310.
3 Guyer DW, Wiltse LL, Eskay ML, Guyer BH. The long range prognosis of arachnoiditis. Spine. 1989;12:1332-1341.
4 North RB, Kidd DH, Zahurak M, et al. Spinal cord stimulation for chronic, intractable pain: Experience over two decades. Neurosurgery. 2007;32:384-394.
5 Smolik EA, Nash FP. Lumbar spinal arachnoiditis: A complication of the intervertebral disc operation. Ann Surg. 1951;133:490-495.
A classic description of arachnoiditis as a complication of discectomy is provided.
1 Rice I, Wee MYK, Thompson K. Obstetric epidurals and chronic adhesive arachnoiditis. Br J Anaesth. 2004;92:109-120.
2 April EW. Clinical Anatomy, 3rd Ed. Philadelphia: Lippincott Williams & Wilkins; 1997:pp 149-151.
3 Shantha TR, Evans JA. The relationship of epidural anesthesia to neural membranes and arachnoid villi. Anesthesiology. 1972;37:543-557.
4 Ransford AO, Harries BJ. Localised arachnoiditis complicating lumbar disc lesions. J Bone Joint Surg. 1972;54:656-665.
5 Smolik EA, Nash FP. Lumbar spinal arachnoiditis: A complication of the intervertebral disc operation. Ann Surg. 1951;133:490-495.
6 Delamarter RB, Ross JS, Masaryk TJ, et al. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine. 1990;15:304-310.
7 Reigel DH, Bazmi G, Shih S-R, Marquardt MD. A pilot investigation of poloxamer 407 for the prevention of leptomeningeal adhesions in the rabbit. Pediatr Neurosurg. 1993;19:250-255.
8 Heary RF, Northrup BE, Barolat G. Arachnoiditis. In: Benzel EC, editor. Spine Surgery. 2nd Ed. Philadelphia: Elsevier; 2005:2004-2012.
9 Mackay RP. Chronic adhesive spinal arachnoiditis. JAMA. 1939;112:802.
10 McLaurin RL, Bailey OT, Schurr PH, Ingraham FD. Myelomalacia and multiple cavitations of spinal cord secondary to adhesive arachnoiditis. Arch Pathol. 1954;57:138-146.
11 Sklar EML, Quencer RM, Green BA, Montalvo BM, Post MJ. Complications of epidural anesthesia: MR appearance of abnormalities. Radiology. 1991;181:549-554.
12 Burton CV. Lumbosacral arachnoiditis. Spine. 1978;3(1):24-30.
13 Yamagami T, Matsui H, Tsuji H, et al. Effects of laminectomy and retained extradural foreign body on cauda equina adhesions. Spine. 1993;18:1774-1781.
14 Poon TL, Ho WS, Pang KY, Wong CK. Tuberculous meningitis with spinal tuberculous arachnoiditis. Hong Kong Med J. 2003;9:59-61.
15 Hoffman GS. Spinal arachnoiditis. What is the clinical spectrum? Spine. 1983;8:538-540.
16 Jackson A, Isherwood I. Does degenerative disease of the lumbar spine cause arachnoiditis? A magnetic resonance study and review of the literature. Br J Radiol. 1994;67(801):840-847.
17 Kok AJ, Verhagen WI, Bartels RH, et al. Spinal arachnoiditis following subarachnoid haemorrhage: report of two cases and review of the literature. Acta Neurochir. 2000;142:795-799.
18 Jackson IJ. Aseptic hemogenic meningitis. Arch Neurol Psych. 1949;62:572-589.
19 Renk H. Neurological complications of central nerve blocks. Acta Anaesthesiol Scand. 1995;39:859-868.
20 Digiovanni AJ, Galbert MW, Wahle WM. Epidural injection of autologous blood for postlumbar-puncture headache II: Additional clinical experiences and laboratory investigation. Anesth Analg. 1972;51:226-232.
21 Aldrete JA, Brown T. Intrathecal hematoma and arachnoiditis after prophylactic blood patch through a catheter. Anesth Analg. 1997;84:228-236.
22 Abouleish E, De la Vega S, Bledinger I, Tio T-O. Long-term follow-up of epidural blood patch. Anesth Analg. 1975;54:459-463.
23 Burton CV. Adhesive arachnoiditis. In: Youmans JR, editor. Neurological Surgery. 3rd Ed. Philadelphia: WB Saunders; 1990:2856-2863.
24 Shaw M, Russell JA, Grossart KW. The changing pattern of spinal arachnoiditis. J Neurol Neurosurg Psych. 1978;41:97-107.
25 Guyer DW, Wiltse LL, Eskay ML, Guyer BH. The long range prognosis of arachnoiditis. Spine. 1989;12:1332-1341.
26 Berg G, Hammar M, Moller-Nielsen J, et al. Low back pain during pregnancy. Obstet Gynecol. 1988;71:71-75.
27 Bernat JL. Intraspinal steroid therapy. Neurology. 1981;31:168-170.
28 Nelson DA. Dangers from methylprednisolone acetate therapy by intraspinal injection. Arch Neurol. 1988;45:804-806.
29 Abram S, Marasala M, Yaksh T. Analgesic and neurotoxic effects of intrathecal corticosteroids in rats. Anesthesiology. 1994;81:149-162.
30 Cicala RS, Turner R, Moran E, et al. Methylprednisolone acetate does not cause inflammatory changes in the epidural space. Anesthesiology. 1990;72:556-558.
31 Delany TJ, Rowlingson JC, Carron H, Butler A. Epidural steroid effects on nerves and meninges. Anesth Analg. 1980;59:610-614.
32 Boiardi A, Sghirlanzoni A, La Mantia L, et al. Diffuse arachnoiditis following epidural analgesia. J Neurol. 1983;230:253-257.
33 Gemma M, Bricchi M, Grisoli M, et al. Neurologic symptoms after epidural anesthesia. Report of three cases. Acta Anaesthesiol Scand. 1994;38:742-743.
34 Carroll SE, Wiesel SW. Neurologic complications and lumbar laminectomy: A standardized approach to the multiply-operated lumbar spine. Clin Orthop. 1992;284:14-23.
35 Haughton VM, Nguyen CM, Ho K-C. The etiology of focal spinal arachnoiditis: An experimental study. Spine. 1993;18:1193-1198.
36 Smith AS, Blaser SI. Infectious and inflammatory processes of the spine. Radiol Clin North Am. 1991;29:809-827.
37 Grahame R, Clark B, Watson M, Polkey C. Toward a rational therapeutic strategy for arachnoiditis. A possible role for d-penicillamine. Spine. 1991;16:172-175.
38 Jorgensen J, Hansen PH, Steenskov V, Oveson N. A clinical and radiological study of chronic lower spinal arachnoiditis. Neuroradiology. 1975;9:139-144.
39 Ross JS, Masaryk TJ, Modic MT, et al. MR imaging of lumbar arachnoiditis. Am J Roentgenol. 1987;149:1025-1032.
40 Chan CC, Lau PY, Sun LK, Lo SS. Arachnoiditis ossificans. Hong Kong Med J. 2009;15:146-148.
41 Domenicucci M, Ramieri A, Passacantilli E, et al. Spinal arachnoiditis ossificans: report of three cases. Neurosurgery. 2004;55:985.
42 Papavlasopoulos F, Stranjalis G, Kouyialis AT, et al. Arachnoiditis ossificans with progressive syringomyelia and spinal arachnoid cyst. J Clin Neurosci. 2007;14:572-576.
43 Smuck M, Benny B, Han A, Levin J. Epidural fibrosis following percutaneous disc decompression with coblation technology. Pain Physician. 2007;10:691-696.
44 Reynolds AF, Shetter AG. Scarring around cervical stimulating electrode. Neurosurgery. 1983;13:63-65.
45 Kemaloglu S, Ozkan U, Yilmaz F, et al. Prevention of spinal epidural fibrosis by recombinant tissue plasminogen activator in rats. Spinal Cord. 2003;41:427-431.
46 Lee JY, Stenzel W, Impekoven P, et al. The effect of mitomycin C in reducing epidural fibrosis after lumbar laminectomy in rats. J Neurosurg Spine. 2006;5:53-60.
47 Gerszten PC, Moossy JJ, Flickinger JC, et al. Inhibition of peridural fibrosis after laminectomy using low-dose external beam radiation in a dog model. Neurosurgery. 2000;46(6):1478-1485.
48 Hackel M, Masopust V, Bojar M, et al. The epidural steroids in the prevention of epidural fibrosis: MRI and clinical findings. Neuro Endocronol Lett. 2009;30:51-55.
49 Shikata J, Yamamuro T, Iida H, Sugimoto M. Surgical treatment for symptomatic spinal adhesive arachnoiditis. Spine. 1989;14(8):870-875.
50 North RB, Campbell JN, James CS, et al. Failed back surgery syndrome: 5-year follow up in 102 patients undergoing repeated operation. Neurosurgery. 1991;28(5):685-690.
51 North RB, Ewend MG, Lawton MT, et al. Failed back surgery syndrome: 5 year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28:692-699.
52 North RB, Kidd DH, Piantadosi S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochir Suppl. 1995;64:106-108.
53 North RB, Kidd DH, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back syndrome: a cost effectiveness and cost utility analysis based on a randomized controlled trial. Neurosurgery. 2007;61:361-368.
54 North RB, Kidd DH, Zahurak M, et al. Spinal cord stimulation for chronic, intractable pain: Experience over two decades. Neurosurgery. 1993;32:384-394.
55 Warnke JP, Mourgela S. Adhesive lumbar arachnoiditis. Endoscopic subarachnoepidurostomy as a new treatment. Nervenarzt. 2007;78:1182-1187.
56 Warnke JP, Mourgela S. Endoscopic treatment of lumbar arachnoiditis. Minim Invasive Neurosurg. 2007;50:1-6.
57 Manchikanti L, Boswell MV, Rivera JJ, et al. A randomized, controlled trial of spinal endoscopic adhesiolysis in chronic refractory low back and lower extremity pain. BMC Anesthesiol. 2005;5:10.