Chapter 26 Arachnoid, Suprasellar, and Rathke’s Cleft Cysts
Most of the intracranial cystic lesions are related to neoplasms, bacterial or parasitic infections, or loss of tissue due to malformation, infarction, or injury, including that resulting from surgical resection of brain tissue. These topics are discussed in other chapters of this book; however, an additional group of cystic intracranial lesions is encountered in neurosurgical practice, and three of these lesions are discussed in this chapter: the arachnoid, suprasellar, and Rathke’s cleft cysts. Of particular interest is that the management of these lesions continues to evolve with the development of endoscopic neurosurgical techniques.1–12
Intracranial Arachnoid Cysts
Arachnoid cysts are intra-arachnoid benign cystic lesions filled with cerebrospinal fluid (CSF).13 According to Cohen and Perneczky,2 in 1831 Bright was the first to describe the intra-arachnoid location of intracranial arachnoid cysts. These lesions are probably developmental in origin and become symptomatic either because of their progressive enlargement or because of hemorrhage into the cyst. The enlargement of arachnoid cysts has been discussed controversially and is at this point in time a matter for discussion. There are various hypotheses to explain the growth of arachnoid cysts:
1. Active fluid secretion from the cyst wall14,15
2. Fluid accumulation caused by an osmotic pressure gradient16
3. Pumping of CSF through a persistent communication between the cyst and the arachnoid space due to vascular pulsation17
4. The so-called slit-valve mechanism, which is described later1,18,19
Arachnoid cysts occur throughout the neuraxis, and generally, no communication is demonstrable between the cyst and the subarachnoid space, although occasionally during surgery an arachnoid cyst is observed being filled through an apparent one-way valve.20,21 Arachnoid cysts may be asymptomatic throughout life, and rarely, they may spontaneously regress;22–24 however, if they do become symptomatic, the progression results from compression on the underlying brain, overlying bone, or both. There is an ongoing discussion whether space-occupying asymptomatic cysts should be operated on to prevent a hindrance to normal brain development and function.25–27 Recently, Di Rocco28 commented on this. He discussed whether past failures in the management of this lesion have allowed to individuate reliable criteria to distinguish those subjects who may benefit of the surgical management from those who should be only observed. He emphasized that there are no clear-cut indications for operative treatment of Sylvian fissure arachnoid cysts, and the absence of defined criteria for postoperative success. In his conclusion, he argued that in many instances, surgical indications are offered based on preferences rather than objective evaluation of individual patients.
If the indication for surgery is questionable, intracranial pressure (ICP) monitoring should be performed to prove ICP elevation or pathologic pressure waves.4,29 The clinical symptoms resulting from these arachnoid cysts depend greatly on their location—whether over the sylvian fissure; over the cerebral convexity; in the interhemispheric region; in the sella and suprasellar region; around the optic nerve, the quadrigeminal plate, or the cerebellopontine angle; in the region of the clivus; over the cerebellar vermis or cerebellar hemisphere; or within the lateral or fourth ventricle.30–36 Arachnoid cysts have also been described extending across the region of the foramen magnum from the posterior cranial fossa into the upper cervical spine posterolateral to the spinal cord.37–39 The midline lesions often lead to an obstruction of the CSF flow and result in focal symptoms and raised ICP.40–43 There is a continuing discussion about whether intracranial arachnoid cysts are related to a specific seizure type and electroencephalographic focus.44
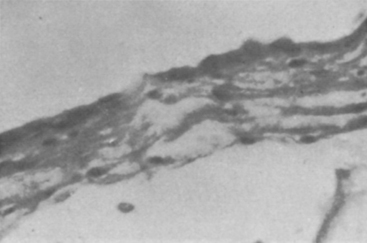
The arachnoid cyst wall is histologically indistinguishable from normal arachnoidal membrane. Moderate thickening of the arachnoid and an increase in connective tissue are common (Fig. 26-1).45 Ultrastructural studies confirm the similarity of the cyst membrane with the normal meningeal counterpart, including the cell-cell connections and the occurrence of basal laminal structures. Quantitative differences in the contribution of the single components have been found both between different cysts and between cysts and normal arachnoid.
In a recently published microsurgical and endoscopic anatomical study, Inoue and colleagues46 claimed that there are two types of arachnoid membranes: outer and inner. The outer arachnoidal membrane surrounds the whole brain, and the inner membranes divide the subarachnoid space into cisterns. Twelve inner arachnoid membranes and 9 cisterns were identified in this study. A special kind of arachnoid cyst formation consists of a luminal epithelial layer connected with a glial sheet, followed peripherally by a thin connective tissue covering (Fig. 26-2.).
The glial nature of parts of the cyst lining can be shown by glial fibrillary acidic protein staining. Some authors have called these cysts “glioependymal.”47
In many cases, arachnoidal cysts are incidental findings noted on CT scanning or MRI of the head performed for a reason unrelated to the cyst. Such patients are informed about the radiographic finding; provided with a copy of the study so they can present it to a physician at a later date, if necessary; and followed up annually. In approximately 15% of middle fossa arachnoid cysts, an asymptomatic lesion may become symptomatic as a result of bleeding in association with the cyst and raised ICP. This event may occur after minor head trauma.48–50
In a report of 6 cases of subdural hematoma occurring in 18 patients with previously asymptomatic middle cranial fossa arachnoid cysts, Rogers and colleagues51 recommended a cystoperitoneal shunt to treat the cyst after evacuation of the hematoma. Auer and co-workers52 also recommended evacuation of the hematoma and the cyst’s wall in one procedure. Handa and associates53 reported on the two-stage removal of bilateral cysts and hematomas. They recommend that the hematomas be drained initially through burr holes and that 3 months later the cyst wall be resected and a cystoperitoneal shunt inserted. Mori and colleagues as well propose a two-step procedure. Hematoma evacuation is adequate at first operation. If the preoperative symptoms persist, additional arachnoid cyst surgery should be considered.54 Markakis and colleagues55 also recommend a two-stage approach to the management of large arachnoidal cysts, beginning with a shunt procedure, which is followed several weeks later by the resection of the cyst wall and a ventriculostomy. Another treatment option is to evacuate the hematoma through an endoscopic burr hole approach and perform a cystostomy to the CSF space in one procedure.56 Interestingly, successful treatment of middle fossa arachnoid, cysts, which means reduction of cyst size, may not reduce the risk of posttraumatic injury hemorrhage as demonstrated by Spacca and co-workers in a series of 40 patients operated with endoscopic fenestration. Four of these patients experienced a post-traumatic intracystic bleeding after surgery.57
Cerebral convexity cysts occurring in adults present as seizures, headache, raised ICP, and, sometimes, marked reactive thickening of the overlying skull with erosion of the inner table. These cases can be managed by the wide excision of the membranes and the establishment of communication between the cyst interior and the CSF of the subarachnoid space. The same approach has been used in the treatment of symptomatic interhemispheric arachnoid cysts and cysts in the region of the quadrigeminal plate that produce aqueductal obstruction that leads to hydrocephalus. Before the advent of MRI, arachnoid cysts of the cerebellopontine angle often presented a diagnostic dilemma that required differentiation from other mass lesions located in the cerebellopontine angle.31 Cysts of the cerebellopontine angle may mimic other lesions in this location and may cause hearing loss and cerebellar signs. These cysts may present as intermittent downbeat nystagmus with an associated hydrocephalus or as vague symptoms, including hearing loss and disequilibrium, contralateral trigeminal neuralgia, or hemifacial spasm.58–61
Surgical options for the management of symptomatic arachnoid cysts include the endoscopic resection of the cyst wall with opening of the membranes, which establishes communication with the hemispheric or ventricular CSF pathways. Levy and co-workers described their results using the microsurgical keyhole approach for middle fossa arachnoid cysts.62 This procedure can be performed with minimal morbidity via minicraniotomy. Compared with an endoscopic approach, better control of hemostasis can be obtained. The operative time and length of hospital stay were not excessively increased. Other options are stereotactic cyst aspiration,63–65 shunt drainage or drainage of the lesion through a burr hole,66 craniotomy with resection or marsupialization of the cyst walls,67 and craniectomy and ventriculostomy of the cyst.24,68 In a cooperative European study of the management of arachnoid cysts in children, total excision or marsupialization emerged as the first-choice surgical procedure, and shunting procedures were often applied to cysts located in deeper locations. Among the 285 patients, from birth to 15 years of age, there was a resultant reduction of the size of the cyst in approximately two thirds of the cases, and in 18%, the cyst had disappeared completely on follow-up CT scanning.69 Another study of the relative merits of different approaches to the management of arachnoid cysts in children is based on an analysis of 40 children treated between 1978 and 1989 at the University of California, San Francisco. Of 15 patients with cysts that were treated initially by fenestration alone, 67% showed no clinical or radiographic improvement and subsequently required cyst-peritoneal or ventriculoperitoneal shunting. All of these patients improved postoperatively, although shunt revision was required in approximately one third of cases as a result of the recurrence of a cyst. These authors concluded that, irrespective of the location of the lesion, cyst-peritoneal or cyst-ventriculoperitoneal shunting is the treatment of choice.25 However, there is considerable risk of overdrainage with posterior fossa overcrowding and acquired Chiari I malformation, or hindbrain herniation.70
Several groups reported about their results in neuroendoscopic treatment of arachnoid cysts. In a prospective study, Schroeder and coworkers10 treated seven consecutive patients with symptomatic arachnoid cysts in different locations endoscopically. The authors performed cystocisternostomies and ventriculocystostomies via burr holes with the aid of a universal neuroendoscopic system. Symptoms were relieved in five patients and improved in one patient, whereas the size of the cyst decreased in six patients. Although the follow-up period was short (15 to 30 months), the authors recommend neuroendoscopic treatment of arachnoid cyst as the first therapy of choice. The second study was conducted by Hopf and co-workers.6 They evaluated 24 patients with intracranial arachnoid cysts that were treated endoscopically. Their surgical strategy was to create broad communication between the cyst and the subarachnoid space. Various techniques were used: endoscopic fenestration (10 cases), endoscopic controlled microsurgery (5 cases), and endoscopy-assisted microsurgery (9 cases). In all patients sufficient fenestration of the cysts could be achieved, with a favorable outcome in 17 patients. Operative complications included infection (3 patients), bleeding into the cyst (1 patient), and subdural fluid collections (4 patients). The authors conclude that different endoscopic techniques do provide sufficient treatment of selected arachnoid cysts. Recent advances in neurosurgical techniques and neuroendoscopy continue to favor cyst fenestration over shunt insertion as the method of choice for initial cyst decompression.71
Case Report 1: Arachnoid Cyst
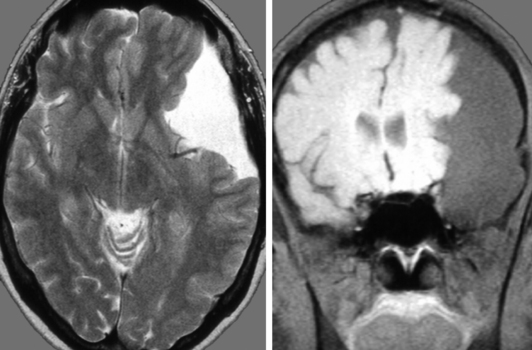
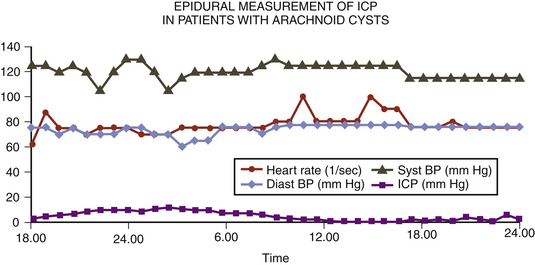
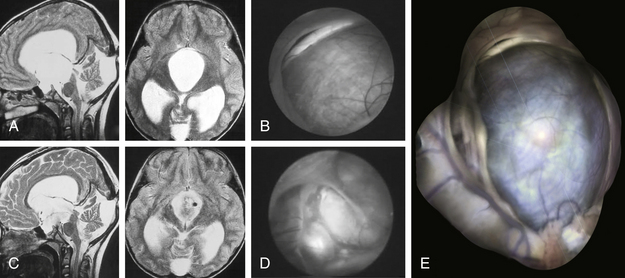
A 14-year-old boy was admitted with aggressive attitude, loss of motivation, headache, and nausea. The neurologic examination was normal. An electroencephalogram showed a left-sided reduction of activity in the frontotemporal region. Magnetic resonance imaging (MRI) demonstrated a large left, frontotemporal arachnoid cyst with a slight mass effect (Fig. 26-3). Epidural ICP measurement during a period of 24 hours revealed normal values (Fig. 26-4). We concluded that the cyst was not related to the patient’s symptoms, and no further intervention was performed. Long-term follow-up examinations showed that the patient was in good neurologic condition with normal age-related capacity.
Case Report 2: Arachnoid Cyst
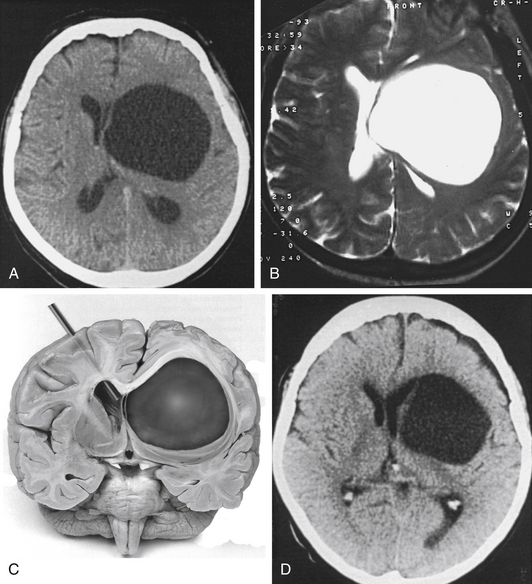
A 72-year-old woman suffered from headache, stupor, and right hemiparesis. A large left hemispheric cystic process with a midline shift was diagnosed by computed tomography (CT) and MRI examination (Fig. 26-5A and B). Using three-dimensional (3D) stereotactic trajectory and target-point calculation, the cyst membrane was approached endoscopically through a right frontal burr hole. The gray membrane was opened by radiofrequency coagulation, and biopsies were taken (Fig. 26-5C). The cyst contained CSF-like fluid. There was no evidence of tumor or other pathology. The histopathologic diagnosis was that of an epithelial cyst. The cyst was opened endoscopically to the left lateral ventricle. After surgery, the CT scan showed that the midline shift was greatly reduced. The left frontal horn was enlarged again (Fig. 26-5D). A few days after the intervention, the patient was alert and she was discharged with only a slight hemiparesis.
Case Report 3: Arachnoid Cyst and Chronic Subdural Hematoma
A 12-year-old boy had been hit on the head by a hockey stick. He suffered from headaches, and 2 weeks later his consciousness was impaired. MRI examination showed a left chronic subdural hematoma (Fig. 26-6A) related to a temporal arachnoid cyst (Fig. 26-6B). The subdural hematoma was drained through a silicone catheter. On CT examination, the subdural hematoma was greatly reduced with no signs of raised ICP or mass effect (Fig. 26-6C). We decided to leave the arachnoid cyst without further operative intervention. The patient did not develop complications during the postoperative course. Over a follow-up period of 9 years, the arachnoid cyst has remained.
Case Report 4: Arachnoid Cyst and Postoperative Chronic Subdural Hematoma
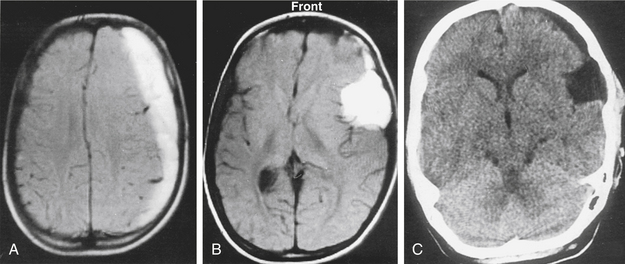
This female patient suffered from headache, vertigo, and partial oculomotor palsy. CT demonstrated a left-sided temporal arachnoid cyst (Fig. 26-7A). Endoscopic cystocisternostomy was performed. Postoperative CT examination showed reduction of the cyst size (Fig. 26-7B), and symptoms improved. However, in the follow-up a subdural fluid collection with mass effect developed (Fig. 26-7C and D). This subdural hematoma was evacuated successfully via burr hole drainage as seen in control CT examination (Fig. 26-7E).
Neuroendoscopic Instrumentation and Operative Technique in Treatment of Arachnoid Cysts
Endoscopes
Various rigid and flexible endoscopes are available to perform cystostomy, cystoventriculostomy, or cystocisternoventriculostomy (Fig. 26-8A and B). The advantages of rigid-lens scopes are the brilliant and bright pictorial quality and the guidance via a predetermined direct trajectory. Angled rigid scopes are used together with microscopes and neuronavigational devices in endoscopy-assisted microsurgery.72 They offer the possibility of looking around corners. Flexible neuroscopes have the advantages of steerability and maneuverability, which make inspection and interventions on multifocal and multiseptated cystic lesions easier.73
Guidance
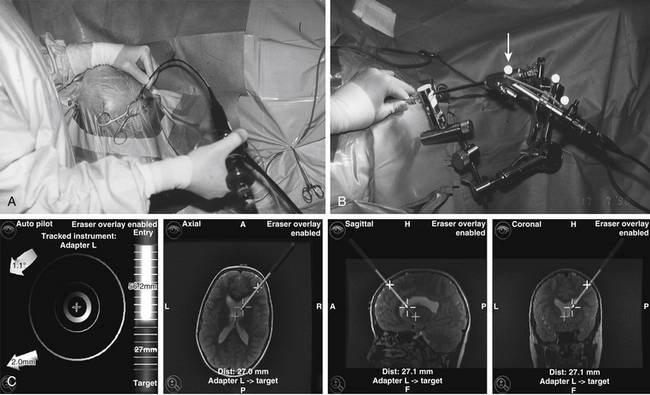
Frame-based stereotactic guidance provides high accuracy and ensures orientation but could be time consuming and restricts endoscope movements. Standard neuronavigation systems provide image guidance, which is interactive and precise.74 These systems can be applied in a freehand mode or in combination with holding and guiding devices (Fig. 26-9A to C).
Instruments
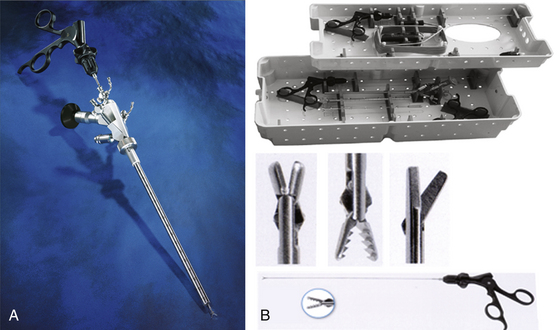
Various instruments are available for endoscopic interventions on arachnoid cysts. Microforceps and scissors are helpful to open and resect cyst membranes (Fig. 26-10A and B). Newly developed bipolar microforceps make it possible to dissect membranes and perform hemostasis using a single instrument (Fig. 26-11).75
In many cases, it is advisable to open the arachnoid cysts using electrosurgical devices. In cooperation with Erbe Company, we have developed a bipolar cutting and coagulation microprobe, which is controlled automatically by an electrosurgical unit. The regulated energy release avoids thermal damage to vulnerable structures. Safe hemostasis is ensured by pinpoint accuracy and effective coagulation of small vessels. The cutting depth is freely adjustable up to 3 mm. The cutting needle can be retracted into the endoscope’s working channel. The probes are available for both rigid and flexible endoscopes with diameters of between 0.9 and 1.5 mm (Fig. 26-12).76 Balloon catheters (Fogarty, double balloon) are very useful to enlarge the stomas in a nontraumatic fashion.
Imaging
Intraoperative digital dynamic subtraction cystography (cystoventriculography) is performed routinely to show the communication of the arachnoid cyst to the adjoining CSF compartments and the restoration of normal CSF flow. After the endoscopic intervention postoperative electrocardiogram-gated dynamic MRI examination demonstrates the normalized CSF flow under real-time conditions. In a recently published study Hoffmann and colleagues77 could show 90% accuracy in diagnosis of communication between arachnoid cysts and neighboring CSF spaces using cine-mode MR imaging. Algin and coworkers78 compared phase-contrast (PC) cine MRI versus MR cisternography to evaluate the communication between intraventricular arachnoid cysts and neighboring cerebrospinal fluid spaces. They found that PCMRI is an effective method for evaluating noncommunicating arachnoid cysts. The results should be confirmed with MR cisternography as suspected jet flow is depicted.
Case Report 5: Pineal Cyst
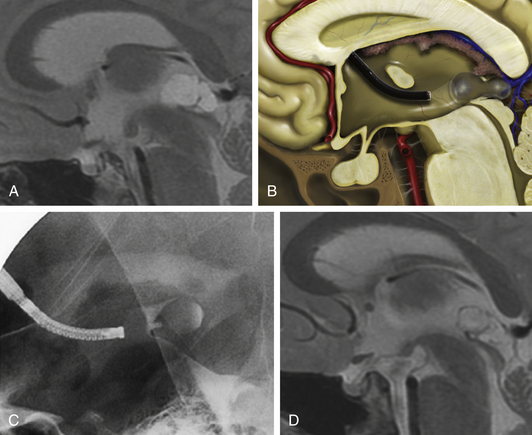
A 38-year-old female suffered from vertigo, headache, and intermittent loss of consciousness. MRI revealed an obstructive hydrocephalus due to a sylvian aqueduct obstruction caused by a cystic lesion in the posterior part of the third ventricle (Fig. 26-13A). Cystoventriculostomy was performed using the flexible, steerable endoscope (Fig. 26-13B). Fluoroscopy was used for intraoperative control (Fig. 26-13C). Postoperative MRI shows a cyst-collapse and re-establishment of CSF flow through the aqueduct (Fig. 26-13D). Fig. 26-14 shows the intraoperative findings during the operative procedure. By moving the flexible endoscope nearly the whole dorsal part of the third ventricle can be explored, as shown in Fig. 26-14A.
Suprasellar Cysts
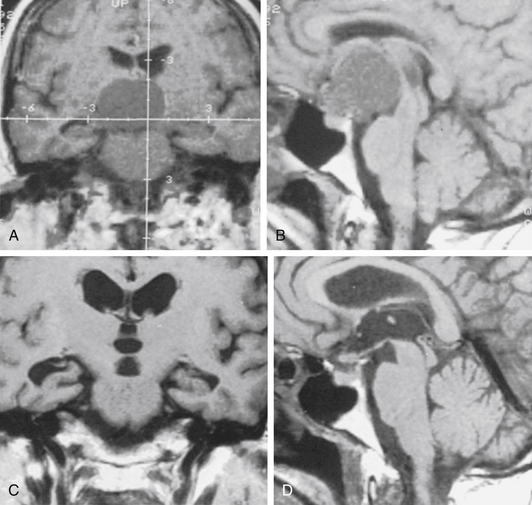
Suprasellar cysts are arachnoid cysts that occur in the sella region and become symptomatic as locally expanding lesions. Arachnoid cysts in relation to the sella turcica represent 10% of all cases.79 The term suprasellar cyst, formerly a synonym for craniopharyngioma, designates a small group of lesions, usually congenital, with a thin, even transparent wall that is filled with clear, colorless, or light-yellow fluid. The congenital effect from these cysts, which constitute fewer than 1% of all intracranial mass lesions, was severe enough to cause symptoms in the first 2 decades in 46 of the 54 reports collected by Hoffman and co-workers.80 The lesion evolves as a consequence of prevention of CSF circulation into the chiasmatic cistern or laterally from the interpeduncular cistern beneath the hypothalamus and behind the pituitary stalk and optic chiasm. The presence of CSF from below the pontine cistern then pushes the hypothalamic floor upward and thins it greatly, so that above the arachnoid dome are only at most a few glial and ependymal cells, as described by Harrison81 in three of four cases. In many of the cases, thin, even transparent, connective tissue is the only lining to the cyst. As do arachnoid cysts in other locations, suprasellar arachnoid cysts may enlarge over time. This change could be effected by active secretion of the membrane82 or from ectopic choroid-like structures14 or osmotic pressure gradients.16 Another theory for the enlargement of suprasellar arachnoid cysts is based on endoscopic and cine-mode evidence of a slit valve,1,19,20 formed by an arachnoid membrane around the basilar artery. This valve is supposed to open and close with arterial pulsations and lead to an inflow of CSF into the cyst forced by a pressure gradient.
By the time that the diagnosis is made, much or all of the third ventricle is usually filled by the cyst, causing an obstruction of one or both foramina of Monro, lateral ventricular dilatation, and a huge head. Indeed, the dome of the cyst is usually much higher than that shown in Fig. 26-15, lying just beneath the corpus callosum.
One possible mechanism for this block is excessive development of an arachnoidal curtain that extends from the posterior hypothalamus to the dorsum sellae below, originally described by Key and Retzius.83 Its presence, confirmed by Lillequist84 and by Fox and Al-Mefty,85 becomes a menace when such curtain and the arachnoid lateral to it become imperforate. Another mechanism of pathogenesis, proposed by Starkman and coworkers,86 is that intra-arachnoidal spaces in the embryo persist and expand exclusively within the arachnoid. This event was demonstrated incidentally at autopsy in a careful dissection of an intact suprasellar cyst by Krawchenko and Collins.13 Most suprasellar arachnoid cysts occur in children, with a male prevalence.2 The clinical picture often includes, in addition to hydrocephalus with a big head and ataxia, disturbed visual acuity and fields due to forward and upward displacement of the chiasm, and hypopituitarism due to pressure in the hypothalamus and pituitary stalk. These symptoms caused by a suprasellar cystic lesion had been first described by Pieter Pauw, a Dutch anatomist, in the 16th century.87
A constant forward and backward nodding of the head and neck, the bobble-head doll syndrome, is an inconstant sign.88–90 Hagebeuck and coworkers91 describe this syndrome in a 4-year-old boy, which resolved completely 3 years after endoscopic cystoventriculostomy. An unusual symptom is precocious puberty.92 Headache may occur in older patients. On CT scans, the cyst has the density of CSF; its wall shows neither enhancement nor calcification and is often mistaken for a dilated third ventricle. According to Cohen and Perneczky,2 suprasellar arachnoid cysts appear as midline round or oval hypodense lesions adjacent to the dilated frontal horns. They have a typical “Mickey Mouse” configuration on axial CT scans or MRI. Prenatal diagnosis is possible using antenatal ultrasound combined with antenatal MRI.93 Effective surgical treatment, which would seem to require simply making a big opening between the cyst and a normal CSF compartment, proves to be surprisingly difficult. Various combinations have been tried, including the transfrontal removal of the lower anterior wall of the cyst beneath the chiasm, a transcorticoventricular or transcallosal approach to remove much of the dome, the insertion of catheters between the cyst and ventricle or chiasmatic cistern, and the insertion of shunts from the lateral ventricles. Any one of these operations alone has a poor chance of sustained success. Agreement seems to be converging on the insertion of a combination of shunts from the lateral ventricles to the peritoneal cavity, with either a transcallosal route to remove the cystic dome, which was used with sustained success by Hoffman and coworkers in five cases,71 or subfrontal removal of the anterior cyst wall. This latter tactic was successful in two cases from Gonzalez and colleagues,94 three cases from Raimondi and colleagues,95 and two cases from Murali and Epstein.96 However, the lower opening closed, and symptoms recurred in one case of each of the last two groups and in one of Hoffman and co-workers’ cases. That shunts from the ventricles alone may not suffice was demonstrated in a case from Murali and Epstein, in a child in whom a neonatal shunt kept the ventricles small but who also required opening of a suprasellar cyst to control bilateral visual loss 9 years later. Ventriculoperitoneal shunting alone was satisfactory in Raimondi’s fourth case. Each of the traditional approaches has been associated with a high rate of recurrence of cysts.2
Upcoming neuroendoscopic techniques seem to solve some of the major surgical problems in treatment of suprasellar arachnoid cysts. Pierre-Khan and associates89 were the first to publish their results after performing endoscopic ventriculocystostomy of suprasellar arachnoid cysts. They used a monopolar electroprobe to create the wide stoma between the cyst and the ventricular cistern (ventriculocystostomy). In contrast to them, Caemaert and associates1 prefer to use the neodymium:yttrium aluminum garnet (Nd:YAG) laser to open the cyst to the ventricular system and the basal cisterns (ventriculocystcisternostomy). None of the patients had postoperative complications or need for a secondary shunting procedure. Additional authors have published similar successful results.2,19,97–100 Gangemi and associates11 did an extensive literature review and described the postoperative follow-up of five patients with suprasellar arachnoid cysts at their own institution after endoscopic fenestration. They found among the reviewed cases, a rate of cure or improvement of 90% (92 of 102 patients including their own cases) after endoscopy and 81% (60 among 74 cases) after other surgical procedures. The results of this study suggest that endoscopic ventricle-cyst-cisternostomy is the best treatment for suprasellar arachnoid cysts, because it is less invasive, provides the best results, and avoids shunt dependency in most cases.
Case Report 6: Suprasellar Arachnoid Cyst
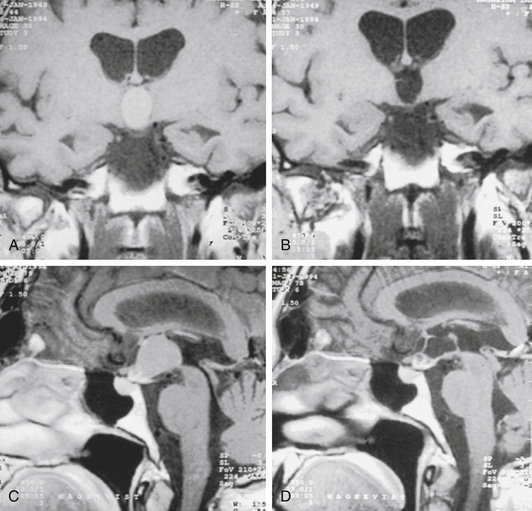
An 8-year-old girl presented with signs of raised ICP, ataxia, and a cognitive disorder. MRI showed a hydrocephalus caused by a large suprasellar cyst with brain stem compression (Fig. 26-16A). The girl was operated on with the neuroendoscopic technique using the frontal transventricular burr-hole approach. The cyst bulged into the foramen of Monro (Fig. 26-16B). After cystocisternostomy the pituitary stalk was clearly seen (Fig. 26-16D). Six days after the intervention, the girl’s clinical condition was good and she was discharged. A control MRI, taken 1 year after the intervention, shows that the cystic lesion has reduced greatly in volume, and free CSF communication existed between the ventricular system and the basal cisterns, which is documented by a postoperative MRI scan with typical flow-void signal in T2-weighted sequences (Fig. 26-16C). The girl still has no neurologic signs.
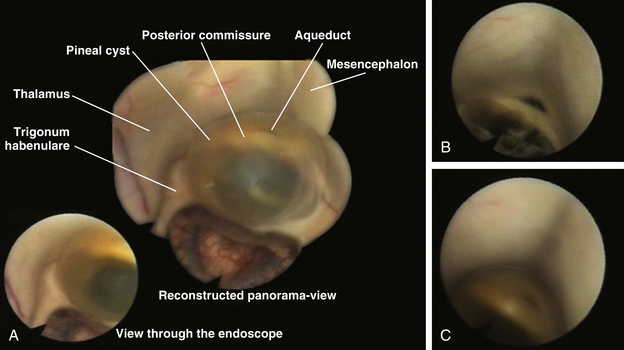
Figure 26-16E was reconstructed from different endoscopic photographs to show the entire range of the endoscopic views while moving the endoscope during the procedure.
Neuroendoscopic Technique in Treatment of Suprasellar Arachnoid Cysts
Preoperatively, it is advisable to plan the operative approach whether stereotactically or by neuronavigation. Through a frontal precoronal burr-hole approach, the surgeon enters the frontal horn of the lateral ventricle with the endoscope.74 The cyst dome is typically bulging through the foramen of Monro. Ventriculocystostomy is performed using microscissors and microforceps as well as the bipolar coagulation and cutting device. It is advisable to create a large stoma (10–15 mm in diameter). After inspection of the parasellar region, it is absolutely necessary to open the membrane of Lillequist (cystocisternostomy), which forms the inferior wall of the cyst toward the prepontine cistern, using the basilar artery as a landmark. Our bipolar coagulation and cutting electrodes prove to be the safe instruments to make bloodless openings and avoid thermal effects to the surrounding nerve tissue.75,76
Because many patients are severely impaired by the time of initial diagnosis, prompt aggressive effort and close follow-ups are required. In rare cases prepontine arachnoid cysts can disappear spontaneously as described by Dodd101 and Thomas.102
Case Report 7: Low-Grade Astrocytoma
A 62-year-old woman was admitted with a progressive bitemporal hemianopia and agitated psychosis (Karnofsky score of 30%). On MRI, a large cystic suprasellar space-occupying lesion with a blockage of foramen of Monro (Fig. 26-17A and B) was noted. Endoscopic stereotactic cyst evacuation was performed as an emergency procedure. The cystic lesion was reached through a right frontal burr hole. The gray membrane that bulged into the foramen of Monro was coagulated and was opened by microscissors. The sticky yellow contents of the cyst were aspirated. The remaining cyst membrane was vaporized using a laser. The histopathologic diagnosis was of a low-grade astrocytoma. Visual loss and the psychological disturbances of the patient normalized immediately after the procedure. A postoperative MRI showed that the cystic process was totally evacuated (Figs. 26-17C and D). The patient was discharged 12 days after the procedure (Karnofsky score of 90%).
Rathke’s Cleft Cysts
Rathke’s pouch, the superiorly directed evagination from the stomodeum of the 4-week-old human embryo, becomes obliterated at all but its cranial portion by the seventh week of gestation. The anterior wall of the remaining small cavity, “the pituitary pouch,” develops into the anterior lobe of the pituitary gland, and its posterior wall proliferates much less to become the pars intermedia of the gland. At autopsy, Shanklin103 found that a residual lumen between a portion of these two structures persisted in 22 of 100 normal pituitary glands. Small asymptomatic, fluid-containing cysts were found in 13 of these 22 specimens. Such cysts of Rathke’s cleft were recorded in 26% of routine autopsy series in five publications.104–107 Infrequently, these cysts enlarge enough to produce symptoms. These residual clefts of Rathke’s pouch are usually lined with cuboidal or columnar epithelial cells, which are often ciliated and include mucin-secreting goblet cells that stain positively by the periodic acid-Schiff method. Stratified or pseudostratified squamous epithelium may also be present and may rest on a collagenous connective tissue stroma.
By November 1989, Voelker and co-workers108 had collected a total of 155 histologically confirmed symptomatic cases from the world literature, including their own 8 cases. The increased recognition of the disorder is evident from the total of only 35 cases found in the literature in 1977 by Yoshida and associates.109 The Rathke’s cleft cyst was both intrasellar and suprasellar in 90 patients, intrasellar in 22, suprasellar in 15, and intrasphenoidal in 1. The cyst capsule varies in thickness and can be any color. Common colors of the more watery fluids are yellow, blue, or green, at times with cholesterol crystals. The content of the cyst may vary from watery or serous (in 15 cases) to mucoid, gelatinous, caseous, or motor-oil-like consistency, to white and creamy. This last appearance may be suggestive of pus. The content of one of the cysts was so tough as to require a rongeur for removal. Although in these series reported with only a few patients and a limited range of abnormal appearances may be described in CT scans and MRI,110 the extreme differences in the cystic content of protein and other chemicals are matched by similar variation in the scans, as pointed out by many authors.111–117 However, the size and location of the lesion were delineated in approximately 100% of the MRI studies and 90% of the CT scans. Image features such as a sellar epicenter, smooth contour, absence of calcification, absence of internal enhancement, and homogeneous attenuation or signal intensity within the lesion suggest the diagnosis of a Rathke’s cleft cyst.118–120 Choi et al.121 determined the differential magnetic resonance image (MRI) features of pituitary adenoma, craniopharyngioma and Rathke’s cleft cyst involving both intrasellar and suprasellar regions in 64 patients. A snowman shape and solid characteristics, and homogeneous enhancement of the solid portion were more common in pituitary adenomas. A superior lobulated shape and third ventricle compression by superior tumor extension were more common in carniopharyngiomas. Finally, an ovoid shape, a small tumor volume, cystic characteristics, and no or thin cyst wall enhancement were typical in Rathke cleft cyst. The statistically accuracies of diagnosis were as follows: 92.1% in pituitary adenoma, 92.3% in craniopharyngioma, and 92.2% overall. Kunii et al.122 described the value of single-shot, fast spin–echo, diffusion-weighted MR imaging to differentiate Rathke’s cleft cysts from other cystic lesions. Several regions of interest (ROIs) for apparent diffusion coefficient (ADC) measurements were identified in the fluid component of the lesions.
Kleinschmidt and associates123 proposed a new pathognomonic MR feature—the posterior ledge sign—of Rathke’s cleft cysts. Ross and colleagues111 stated that the diagnosis can be made at operation after the cystic cavity is irrigated. The lining of Rathke’s cleft cyst is smooth and transparent; that of a craniopharyngiomatous or a pituitary adenomatous cyst is lined at least at some point by tumor.
Case Report 8: Rathke’s Cleft Cyst
A 28-year-old patient suffering from a secondary amenorrhea was admitted to our department. An MRI scan showed a cystic lesion growing up from the sellar region and bulging into the third ventricle (Fig. 26-18A). We decided to perform primarily an endoscopic cyst evacuation using the frontal transventricular burr hole approach. The cyst was totally evacuated, and the cyst membrane was partly resected (Fig. 26-18B). The histopathologic diagnosis of the cyst membrane and its contents was ambiguous; some cells showed characteristics of craniopharyngioma cells, whereas others seemed to be of epithelial origin. Crystalloid and amorphic material was found in the contents of the cyst. The established histopathologic diagnosis was that of Rathke’s cleft cyst; the differential diagnosis was a craniopharyngioma.
Clinical Data of Rathke’s Cleft Cyst
Fager and Carter,124 who had the earliest reported series of five living patients, found no solid abnormal tissue other than the thin wall in any of them. Visual fields and acuity, grossly abnormal in four of the patients preoperatively, improved greatly after the operation. The authors did not remove the cyst wall completely, but no symptoms recurred in any of the patients, who were followed up for as long as 9 years. They, therefore, regard total excision of the wall as unnecessary, concluding that a less radical approach suffices for these purely cystic intrapituitary or parapituitary lesions containing milky or mucoid fluid.
The following data are taken mainly from the reviews by Voelker and associates.108 The female-to-male ratio was greater than 2:1, and patients ranged in age from 4 to 78 years, with a mean age of 38 years and the highest frequency in the sixth decade. The preoperative duration of symptoms was from 3 days to 18 years, with an average of 34.9 months. Clinical presentation of patients is characterized by the triad: pituitary dysfunction, visual impairment, and headache.116,125 The most common symptoms were those caused by pituitary hypofunction. Dwarfism occurred in more than 70% of those younger than 18 years of age. Of the 37 patients with amenorrhea-galactorrhea, 14 had also a pituitary adenoma. Hyperprolactinemia might have occurred whether or not the amenorrhea-galactorrhea syndrome was present. Also precocious puberty in a 16-month-old child has been described by Acharya et al.126 This child was suffering from breast enlargement, height increase, and an increase of growth velocity. Her vaginal mucosa was estrogenized. Treatment with leuprolide resulted in normalization of her growth rate and regression of the breast development; the vaginal mucosa became unestrogenized. Half of the patients had visual field defects, and about one fourth had decreased visual acuity. Almost half of the patients had headaches, which were often frontal headaches. Nausea and vomiting were noted in only 18 patients. Bouts of aseptic meningitis, though infrequent, should be recognized and are discussed later. A few patients described vertigo, diplopia, lethargy, or syncope. Intermittent episodes of fever in only six cases were, at least in the patient of Van Hilten and colleagues,127 an unusual symptom of hypothalamic involvement. Attacks of fever at approximately 2-week intervals sometimes woke her at night or occurred randomly. They lasted for 6 hours, during which she had a rectal temperature of 39°C, followed by 3 hours of excessive sweating and a gradual return of temperature to normal. This picture proved to result from a 20 by 20 by 20 mm suprasellar cyst extending up to the foramina of Monro and causing dilatation of both lateral ventricles and increased ICP. After lasting drainage of the cyst (three operations), all symptoms virtually disappeared.
Surgical excision of the cyst (usually partial) was carried out in 137 of the 155 patients, the remaining 18 cysts having been found at necropsy. The approach was by craniotomy in 60 cysts and via the sphenoid sinus in 59 cysts. Ross and coworkers111 used the trans-sphenoidal route in 40 of 43 patients. The three patients with suprasellar lesions involving the pituitary stalk had transcranial approaches. The 10 patients of Midha and coworkers128 were all operated on trans-sphenoidally, as were the 28 patients of El Mahdy and Powell.116 This route was selected in only three of Voelker and co-workers’ eight patients, all of whom had suprasellar extensions from the main intrasellar mass.108 Operative mortality was zero in each of these four series. The recurrence rate after a craniotomy is approximately twice as high as that following the trans-sphenoidal route. Furthermore, if the cyst wall is removed only partially at craniotomy, the material secreted by the remnant may provoke an aseptic meningeal reaction. However, headache is not satisfactorily controlled by the partial removal of cyst wall advocated by the neurosurgeon Ross and colleagues.111 Of their 32 patients whose preoperative symptoms included headaches, 21 obtained relief. Of 14 patients in whom headache was the only preoperative symptom, the headache persisted after operation in 7 patients. No correlation existed between the size of the lesion and the incidence of headache or the likelihood of disappearance of the headache after operation. The two patients with the largest lesions (23 and 25 mm) did not have a headache. However, of the 17 patients who had preoperative hyperprolactinemia, only 3 have continued to have prolactin levels above normal after operation. No data have been collated on the results on these scores after craniotomies.
Ross and co-workers111 advocate the extremely conservative simple drainage of the cyst trans-sphenoidally, “accompanied by biopsy of the cyst wall, when this is possible without entering the subarachnoid space or damaging normal structures.” In fact, they took no operative pathologic specimen to confirm the diagnosis in 17 of their 40 patients. El Mahdy and Powell116 performed a partial excision of the cyst wall and drainage of the contents into the sphenoid sinus. In seven patients with an intraoperative CSF leakage, they used fascia lata or fat grafts.
Midha and associates128 obtained biopsies of the cyst wall in eight patients and removed all of it in two. The preoperative symptoms and signs in all 10 had disappeared, except those related to pituitary dysfunction and in 3 patients with visual problems. Although Ross and colleagues111 followed up their patients for a mean of 68 months, with the longest follow-up at 126 months, they have seen only one symptomatic recurrence.
Totally benign behavior is far from invariable, however. One Japanese patient presented with acute adrenal insufficiency, which capped the hypopituitarism secondary to the intrasellar Rathke’s cleft cyst.129 There are two reports of an acute hemorrhage into a Rathke’s cleft cyst.130,131 Another report is of hemosiderin deposits in the calcified epithelium of a Rathke’s cleft cyst,132 which emerged with an abrupt onset of severe headache and gave rise to an enhancing intrasellar and suprasellar mass. The mass was successfully removed trans-sphenoidally. One of Yoshida and associates’ patients had a small nodule on her cyst wall that was scraped out with a curet.109 Only part of the cyst wall was removed, and a serious recurrence took place within 1 year. Raskind and coworkers’ patient, whose cyst contained a clear, colorless fluid, experienced a recurrence requiring repeat surgery 26 years later.133 The patient reported by Berry and Schlezinger134 had only a fragment of the cyst wall removed at the first craniotomy. At a major recurrence 37 months later, the cyst was three times the original size but contained the same clear, colorless mucoid fluid. The recurrences described by Yoshida and colleagues,109 Iraci and colleagues,135 and Matsushima and colleagues136 were in patients with solid components to their lesions that contained stratified epithelium. In Matsushima’s case, although the cyst was filled with the typical mucinous, pus-like material and some of its lining comprised ciliated, mucin-containing columnar cells, most of it consisted of stratified squamous epithelium. This last component determined the outcome, namely death from recurrent tumor 20 months after its subtotal removal. Clearly, the solid portion of the tumor more than the appearance of the cystic fluid determines the prognosis. Two patients of Marcincin and Gennarelli137 and one of Rout and colleagues138 experienced recurrences in 4 months to 2 years after trans-sphenoidal evacuation of the cysts, even though the cyst fluid and wall were typical of pure Rathke’s cleft lesions. A permanent visual loss ensued in one of the patients. The lesion was approached intracranially at recurrence in the second patient, and the entire cyst wall was removed. Two more recurrences after craniotomy have been reported by Yamamoto and co-workers139 and by Leech and Olafson,140 and four more have been reported after trans-sphenoidal approaches by Roux and co-workers,141 Midha and co-workers,128 Ross and coworkers,111 and El Mahdy and Powell.116 Surprisingly, Mukherjee and associates142 describe a re-expansion rate of 33% in 12 patients with Rathke’s cleft cysts during a follow-up time from 1 to 168 months (median of 30 months). They propose to evaluate the role of radiotherapy for recurrent symptomatic tumors. Raper and Besser143 reported 5 recurrences out of 12 patients in an Australian population and conclude that the relatively high rate of recurrence may indicate a link between this pathology and craniopharyngioma. In a large series of patients with symptomatic Rathke cleft cysts (118 patients), who underwent trans-sphenoidal resection and participated in a long postoperative follow-up (1984–1995, at least 5 years), Aho and coworkers144 found that recurrence was statistically associated with the use of a fat or fascial graft for closure and the presence of squamous metaplasia in the cyst wall. The extent of resection of the cyst wall was not associated with an increased rate of recurrence. They conclude, that the high recurrence rate (18%) supports the theory that a relationship exists between symptomatic Rathke cleft cysts and craniopharyngioma.
As the reports have accumulated, it has become clear that many patients have transitional lesions or even highly unusual accompanying lesions. Russell and Rubenstein145 were among the first to point this out, describing in two patients dumbbell cysts, the intrasellar portion of which was lined by a single layer of ciliated epithelium that changed abruptly at the diaphragma sellae to the squamous epithelium characteristic of a craniopharyngioma for the suprasellar portion. In a case reported by Yoshida and associates,109 there was a tumor nodule with an inner lining of columnar cells that covered many layers of stratified squamous epithelium. Tajika and coworkers146 found some areas of stratified epithelium in two of their three patients with Rathke’s cleft cysts; in one of the two patients, cholesterol crystals, calcification, and brown fluid were present. Conversely, some ciliated, combined with columnar, cell areas were found in two other patients with histologic findings otherwise typical of a craniopharyngioma. This underlines the assumption that Rathke’s cleft cysts may originate from squamous cell rests along the craniopharyngeal canal, resulting in a spectrum of cystic lesions in this area ranging from simple Rathke’s cleft cysts to complex craniopharyngiomas.116,147
Goodrich and coworkers148 described a suprasellar soft necrotic tumor rather than a fluid-containing tumor that contained many ciliated cuboidal or columnar cells typical of the lesion under discussion; however, other parts of the tumor included masses of squamous epithelium. This assumption was underlined by a recently published case of Sato et al.149 They suggest that the basal cells of Rathke cleft cyst transform to papillary type craniopharyngioma after squamous metaplasia, explaining the presence of cilia and goblet cells. Recently Okada and colleagues150 described another case of ciliated craniopharyngioma developing from a Rathke cleft cyst.
Another Rathke’s cleft cyst was reported that had characteristics of an epidermoid cyst.151 Harrison and associates152 suggested that Rathke’s cleft cysts, epithelial cysts, epidermoid cysts, dermoid cysts, and craniopharyngiomas represent a continuum of ectodermally derived epithelial cystic epithelial lesions. Other groups described the association of pituitary adenomas with the typical histologic form of Rathke’s cleft cyst.153–159 The more solid tissue there is associated with the cyst, the more likely it is to include a chronic inflammatory process or the stratified epithelium of a craniopharyngioma or of the glandular tissue of a pituitary adenoma. These tissues are likely to show enhancement in a scan. In rare cases the association of a craniopharyngioma with a gonadotroph adenoma and of a Rathke cleft cyst with a corticotroph adenoma is possible.160
These lesions are also more likely to have a major or completely suprasellar portion. Yuge and coworkers161 have added two more exclusively suprasellar Rathke’s cleft cysts to the 15 cysts collected by Voelker and coworkers.108 In the patients from Miyagi and co-workers,155 the lesions extended into the third ventricle. The patients in each of two publications161,162 actually presented with hypothalamic tumors, one as a noncystic hypothalamic mass. This patient had an X, X, Y karyotype in all cells (Klinefelter’s syndrome). These larger tumors were not totally removed. In the case from Itoh and Usui,163 the subepithelial tissue consisted of normal pituitary gland. Wenger and colleagues added in 2001 another case of an entirely suprasellar Rathke’s cleft cyst.164 The Rathke’s cleft cyst reported by Onda and colleagues165 was associated with an arachnoid cyst; the cyst reported by Ikeda and colleagues166 was associated with an eosinophil (acromegalic) adenoma. Arita and coworkers167 described a case of Cushing’s disease accompanied by Rathke’s cleft cyst, and Ersahin and associates168 presented a case of Rathke’s cleft cyst with diabetes insipidus. Rathke’s cleft cysts should be kept in mind as a potential cause for the syndrome of inappropriate secretion of antidiuretic hormone and adrenal insuffiency.169 Two papers have reported chronic (granulomatous) hypophysitis related to Rathke’s cleft cyst.170,171
Cannova and associates172 found a granulomatous sarcoidotic lesion of the hypothalamic-pituitary region associated with a Rathke’s cleft cyst. More distant accompanying lesions have been described by Koshiyama and colleagues173 in the form of Hashimoto’s thyroiditis with diabetes insipidus and by Kim and colleagues174 as a maldevelopmental mass with absence of pituitary gland, a rudimentary prosencephalon, and two other cysts—one a pigmented epithelial cyst (possibly a rudimentary eye) and the other a dorsal ependymal cyst, plus several other congenital abnormalities.
A single case report was published of a large suprasellar tumor extending downward to attach to the anterior wall of the pituitary gland. The cyst wall of the tumor was consistent with Rathke’s cleft cyst in many places, but both its immunohistochemical and ultrastructural features were indistinguishable from colloid cysts of the third ventricle.175 The cyst wall was totally removed via a subfrontal approach, and the authors achieved an excellent clinical result. Graziani and associates176 assumed a common embryologic origin of suprasellar neurenteric cysts, the Rathke’s cleft cyst, and the colloid cyst.
The reports of recurrences after mere evacuation of cysts have provided support for those who, from the first, included readily removable cyst wall as part of the surgical objective. In a 1984 publication (when high-resolution CT scanning was available), Shimoji and coworkers177 noted enhanced capsules around low-density cysts in all three of their patients. They, therefore, elected to remove “as much as possible of the capsule” in all three and achieved good clinical results. Specimens from all three patients showed a histologic pattern typical of Rathke’s cleft cyst, with the addition of squamous epithelium in the third case. Swanson and coworkers157 also used the presence of capsular enhancement at CT scanning of an intrasellar mass to guide them to a transfrontal excision of the cyst wall. Nonfunctional pituitary adenomatous cells constituted a part of that wall; therefore, they gave the patient a course of radiation therapy. This patient represented the sole reported case thus treated. The trans-sphenoidal route has been favored for most cases, especially when the suprasellar portion shows minimal enhancement. This approach offers an effective means of achieving complete cyst drainage for lesions requiring surgery. Fenestration and aspiration of the cyst are usually sufficient to achieve total resolution of symptoms and signs caused by RCC. Clinical symptoms such as headaches improve in the majority of patients after surgery, however hormonal disturbances can persist.178 Madhoc and colleagues179 favor the endoscopic endonasal approach for resection of Rathke cleft cysts. The authors retrospectively reviewed a series of 35 patients after endoscopic endonasal resection at the University of Pittsburgh between 1998 to 2008. Neither were there any intraoperative complications, postoperative CSF leaks, or new neurological deficits. The average hospital stay was 1.8 days. They concluded that the endoscopic endonasal approach is safe and effective in the treatment of Rathke cleft cysts. These positive results were confirmed by Cavallo and coworkers, who operated on a series of 76 consecutive patients with sellar lesions using the endoscopic endonasal approach. They performed endoscopic exploration of the sellar cavity during trans-sphenoidal surgery and recommended to use this technique to achieve maximal and safe tumor removal.180
In our experience with nontumorous cystic midline lesions, involving more than 100 patients, it is not necessary to resect the whole cyst membrane to prevent a recurrence. As an example, we have operated on 36 colloid cysts of the third ventricle in neuroendoscopic technique using the frontal burr hole approach. The cysts were evacuated and the membranes were resected subtotally. During a follow-up period from 6 months to 10 years, there were two recurrences which have been operated in a second endoscopic intervention.181
Case Report 9: Colloid Cyst
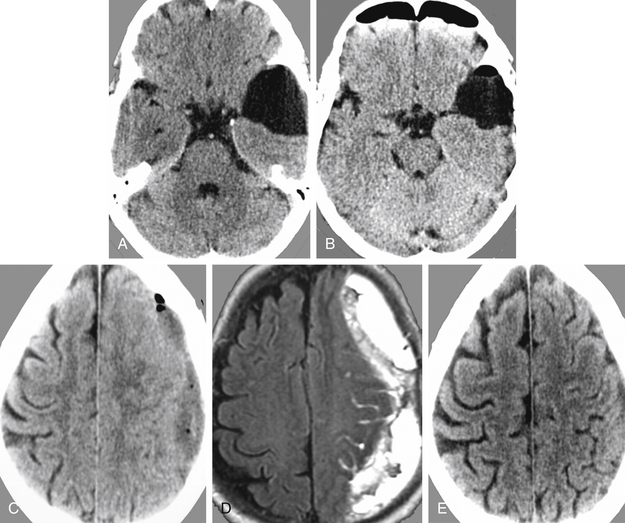
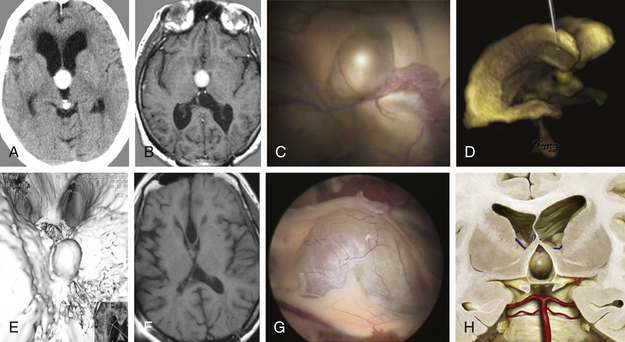
For a 41-year-old woman had chronic headache and gait disturbances, CT and MRI including 3D reconstructions revealed a cystic lesion in the third ventricle with obstructive hydrocephalus (Fig. 26-19A to F). Through a right frontal burr hole, an endoscopic cyst perforation and evacuation of the colloidal material was performed (Fig. 26-19D). The histopathologic diagnosis was a colloid cyst. The cyst wall was shrunk using the bipolar microelectrode. Membranous material adherent to the ventricular ependyma was left in situ. This was confirmed by a postoperative MRI examination (Fig. 26-19F). Seven days after the intervention, the patient was discharged without any new neurologic deficit. Five years after the operation, the patient showed no clinical or radiologic signs of cyst recurrence. Figures G and H show the typical endoscopic and illustrated macroscopic aspects of colloid cysts.
Intrasellar or Suprasellar Abscess
Gomez Perun and associates182 stated in 1981 that 50 intrasellar abscesses had been reported; usually, the infection was propagated from a neighboring air or vascular sinus. The uninfected content of Rathke’s cleft cyst may be a thick, white, or yellowish pus-like fluid that was mistaken for pus by the authors. Typically, the cellular reaction in the CSF is predominantly lymphocytic, and culture results of the “pus” and CSF are repeatedly negative. The patient is reacting to a “foreign body” that he himself has secreted, as described in the following section.183
Bouts of Chemical Meningitis: The Syndrome of the Toxin-Leaking Central Nervous System Cyst
An important point emerges from collation of the data from scattered individual case reports of patients with curious repeated febrile episodes, often with CSF pleocytosis. A culture was obtained from an organism in only one case. Attacks of recurrent chemical febrile meningitis in a craniopharyngioma, presumably from leakage of keratin or cholesterol, are a rare but well-authenticated occurrence.184
In the first such case of Rathke’s cleft cyst, reported as an abscess by Obenchain and Becker,185 the patient had five episodes in 3 years of severe headaches, nausea, vomiting, general malaise, and fever, all leading to hospital admissions but resolving spontaneously a few days later. Finally, blurring of vision in her inferior temporal quadrants led to the diagnosis of her intrasellar and minimally suprasellar mass, from which, via a subfrontal route, 2 ml of “purulent” fluid was aspirated. Staphylococcus epidermidis was cultured from this fluid, and the patient was given penicillin, isoniazid, and ethambutol for 1 month. Then, via the trans-sphenoidal route, 3 ml of “pus” was aspirated, and the capsule was removed. Histologically, the wall was fibrous and lined by columnar epithelium with chronic inflammatory cells. The patient’s recovery was excellent and sustained. The absence of acute inflammatory cells and the spontaneously subsiding brief attacks that had occurred for 3 years cast doubt on the role of the staphylococci.
In the next reported case,186 similar recurrent brief episodes occurred. These episodes each lasted only 2 or 3 days and were characterized by intense supraorbital pain, fever to 39°C or 40°C, and about 50 clear-cut temporal lobe seizures a day. These seizures each lasted for several seconds and occurred mainly during the bouts of fever. These mysterious episodes continued for 10 years, during which time the patient’s weight increased from 50 to 72 kg. Although the patient did not develop nuchal rigidity in the attacks, a lumbar puncture finally performed in 1977 revealed a largely lymphocytic pleocytosis and a normal protein level. Demonstration of an infratemporal quadrantanopia was followed by pneumography, which revealed a suprasellar mass. At a subfrontal exposure, thick, pus-like fluid was removed from a subchiasmatic cyst, the capsule of which was largely removed. The cyst wall was heavily vascularized and infiltrated with inflammatory cells but lined with the typical ciliated columnar and cuboidal epithelium. The patient had also developed the symptoms and laboratory findings of deficient thyroid-stimulating hormone, adrenocorticotropic hormone, and luteinizing hormone-releasing hormone. The febrile episodes involving a headache stopped at once after operation, and the patient gradually made a full recovery. The seizures continued to require phenobarbital and valproic acid for control.
The following year, Verkijk and Bots151 described a patient in whom meningeal reactions developed after surgery on a cyst; these reactions resolved spontaneously in 9 months. In the case reported by Steinberg and coworkers,187 the initial symptoms pointed to a pituitary origin because of defects in visual acuity and fields, but then over the next 2 years, the patient was in the hospital numerous times with bouts of severe headache, nausea, vomiting, confusion, stiff neck, decreased visual acuity, and ataxic gait. On different occasions, the CSF showed pleocytosis, an increased protein level, and elevated pressure. All culture results were always negative. The episodes either resolved spontaneously or disappeared promptly after increases in the dosage of dexamethasone. Decreases in this dosage were followed by a recurrence of the symptoms. The sella was found to be enlarged and to contain a mass without suprasellar extension; the sella was, however, partially empty, with its anterior portion filling with air. These findings were apparently considered to be incidental. The lateral and third ventricles became increasingly dilated, and egress of contrast was delayed through the aqueduct and out of the fourth ventricle. The hydrocephalus was treated by ventriculoatrial shunt in 1972. Intracranial obstruction of the shunt required two revisions; each time, the symptoms promptly resolved. The patient died 1 year later of pneumonia. At autopsy, the Rathke’s cleft cyst occupying the entire sella was filled with thick, yellow-green fluid. The cyst epithelium was largely ciliated and columnar, squamous in one area and keratinized in others. The leptomeninges adjacent to the chiasm and third ventricle were “moderately fibrotic with mild chronic inflammation.”
Episodes of severe meningeal symptoms and signs, also with completely negative culture results, characterized a patient reported on by Gomez Perun and coworkers.182 One episode in May 1977 was especially severe. When a suprasellar lesion was demonstrated and explored 6 months later, the cyst contained a thick, white fluid, which had an intense inflammatory reaction, and numerous vessels in the cyst wall, along with a lining of ciliated columnar epithelium. Also, an extensive frontal basal arachnoiditis was present that was not noted in the other cases. The “pus” was sterile, and the patient made a prompt recovery but soon regressed, with similar episodes of aseptic meningitis. Despite three more operations, he too died; no organism was ever grown.
The patient reported on by Sonntag and coworkers188 had only two episodes of a lymphocytic aseptic meningeal reaction before his intrasellar-suprasellar mass was subfrontally exposed. Seven milliliters of “pus” were aspirated, and the cyst wall was subtotally removed. The aspirate was sterile, but rare gram-negative rods were seen, and chloramphenicol was administered for 1 week. The symptoms recurred in 1 month, leading to trans-sphenoidal drainage of 5 ml of “pus” and “extensive removal of its wall.” Culture results from this fluid were also negative. Therapy with chloramphenicol was continued for 1 month, and the patient had remained well for almost 2 years as of this writing.
Shimoji and associates177 described three clear-cut cases of chemical meningitis. In the first, brief episodes of headache, nausea, vomiting, and fever were accompanied by enough eye signs to point to the sellar region and its cyst. At craniotomy, the “abscess-like viscous fluid” was sterile. As much cyst wall as possible was removed, and an excellent result persisted 2 years later. In the second case, a 52-year-old woman, four episodes of aseptic meningitis were required before a trans-sphenoidal evacuation of “milky, abscess-like viscous fluid” occurred and “as much as possible” of the cyst wall was removed. She, too, made an excellent recovery, which continued at 2 years. Both patients were also given 3000 cGy of 60Co radiation. In the third case, an intermittent fever to 38°C on hospitalization rose to 39°C after pneumoencephalography; the patient, a 42-year-old woman, had meningeal signs and a CSF pleocytosis of 456 neutrophils and 74 lymphocytes. No growth occurred on culture. A week of antibiotic therapy brought no change, but 30 mg/day of prednisolone given in addition to the antibiotics dropped the temperature to normal the next day. The yellowish white gelatinous cyst fluid and, “as far as possible,” its capsule, were removed via craniotomy. The periodic acid-Schiff-positive stratified squamous epithelium was heavily infiltrated with inflammatory cells. Good recovery persisted at 28 months. Thick, yellow, pus-like material and a cyst wall accompanied by squamous epithelium and thick connective tissue infiltrated with inflammatory cells were described in two other reports.138,189
One more clear-cut case of a foreign body reaction to the content of Rathke’s cleft cyst was reported by Albini and associates.190 The 19-year-old woman developed headache, galactorrhea, and a few months later, amenorrhea. Bitemporal hemianopia and deficiencies in thyroid-stimulating hormone, adrenocorticotropic hormone, luteinizing hormone, and luteinizing hormone-releasing hormone were identified. There had never been any episodes of fever or other suggestion of an aseptic meningeal reaction. The sella was enlarged, and while in the hospital, the patient developed a partial right third nerve paresis along with polydipsia and polyuria. At right pterional craniotomy, a large cyst seen on CT scan arising from the sella turcica was emptied of its thick, white fluid, and the cyst wall was insofar as possible removed. An excellent clinical result was obtained. The cyst wall was lined by the ciliated columnar and squamous epithelium of a Rathke’s cleft cyst. The rest of the specimen was infiltrated with lymphocytes, plasmacytes, and multinucleated giant cells among islands of preserved pituitary tissue. No organisms were ever demonstrated. Apparently, the cystic fluid never seeped into the subarachnoid space. At 3-year follow-up, the patient was free of headache and mass observed by CT. Bognàr and coworkers191 reported on two more patients with symptomatic histologically typical Rathke’s cleft cysts and no preoperative episodes, which suggested aseptic meningitis. In both of them, the cyst contents were removed via the sphenoid sinus. Inflammatory cells and bacteria were recognized in the operative specimen, but technical problems were said to have prevented identification of organisms. Antibiotics were used, and one patient recovered uneventfully. In the other patient, trans-sphenoidal partial removal was followed by worsening in 2 weeks, leading to a frontolateral craniotomy, which likewise did not eliminate all of the abnormal tissue. Staphylococcus aureus and Streptococcus pyogenes grown from the specimen at the second operation were treated by antibiotics, but they failed to avert death on the ninth postoperative day. This sequence suggests that the cyst content facilitated the bacterial growth seen only after the second operation. Because of the diagnosis of infection, neither of these patients was given large doses of corticosteroids. Both patients may have been reacting primarily to the chemicals in the cyst fluid. In 2008, Schittenhelm and colleagues192 reported on a lymphocytic hypophysitis related to a ruptured Rathke’s cleft cyst in a 45-year-old woman who initially presented with headache and temporary double vision followed by amenorrhea. The strongest inflammatory reaction was observed at the site of cyst integrity disrupture, suggesting that high protein levels from the rupture of Rathke’s cleft cyst might have triggered a lymphocytic hypophisitis.
At least four reports have been published of spontaneous rupture of cystic craniopharyngiomas into the subarachnoid space that gave rise to a major but sterile meningeal reaction. This reaction was not different from those associated with Rathke’s cleft cyst in that a predominantly neutrophilic, rather than lymphocytic, reaction occurred in severe cases.177,193,194 Another case with spontaneous intraventricular rupture of a craniopharyngioma cyst was described in 2000 by Kulkarni and colleagues. The rupture resulted in an acute neurologic deterioration with consecutive bilateral optic nerve atrophy due to chemical meningitis. The patient was treated with ventricular drainage, steroids, and anticonvulsants. CSF showed high cholesterol and 1-lactate dehydrogenase levels. The diagnosis of craniopharyngioma was subsequently verified histologically.195 Intracranial epidermoid tumors196,197 and dermoid cysts198,199 also rarely show this behavior as reported by several authors.
In conclusion, Rathke’s cleft cysts tend to occur mainly within the sella; their precise extent can now be determined by modern CT and MRI scanning. Most of these cysts are best approached trans-sphenoidally; however, approximately 10% that are wholly suprasellar should be removed via craniotomy. Whether trans-sphenoidal aspiration of the cyst and biopsy suffice, as urged by Ross and colleagues,111 the endoscopic transventricular approach with cyst opening and aspiration proposed by Hellwig and colleagues,74 or whether removal of as much cyst wall as seems safe is preferable is currently unclear. The neurosurgeons who favor a more aggressive stance will need to show that they achieve better relief of symptoms such as significant headache as well as fewer recurrences. The suprasellar component may need to be removed to the full extent dictated by safety because a repeat craniotomy is probably more hazardous than a second trans-sphenoidal approach.
Case Report 10: Cystic Craniopharyngioma
A 44-year-old patient had two febrile episodes with headache and opisthotonos 2 years before admission. Repeated CSF punctures revealed a slight pleocytosis. The first MRI scan showed no evidence of an intracranial space-occupying lesion. Later, it was suggested that these symptoms were the result of aseptic meningitis after spontaneous perforation of a cystic craniopharyngioma. An MRI scan that was performed 3 months after the second febrile attack showed a cystic process in the anterior part of the third ventricle growing up from the suprasellar region (Fig. 26-20A and C). The cyst was approached by 3D stereotactic calculation under direct endoscopic control. The cyst wall was coagulated using bipolar radiofrequency, and the cyst was opened using microscissors. The cyst contained a thick yellow fluid. The cyst was emptied, and the capsule was coagulated. The postoperative follow-up was uneventful. On MRI, the residual tumor membrane was visible (Fig. 26-20B and D). The patient was discharged 12 days after the intervention without neurologic symptoms or psychological disorder (Karnofsky score of 100%). The patient decided to undergo gamma knife treatment for the remaining tumor capsule.
Aho C.J., Zelman V., Couldwell W.R., et al. Surgical outcomes in 118 patients with Rathke cleft cyst. J Neurosurg. 2005;102(2):189-193.
Algin O., Hakyemez B., Gokalp G., et al. Phase-contrast cine MRI versus MR cisternography on the evaluation of the communication between intraventricular arachnoid cysts and neighbouring cerebrospinal fluid spaces. Neuroradiology. 2009;51(5):305-312.
Cavallo L.M., Prevedello D., Esposito F., et al. The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev. 2008;31(1):55-64.
Choi S.H., Kwon B.J., Na D.G., et al. Pituitary adenoma, craniopharyngioma, and Rathke cleft cyst involving both intrasellar and suprasellar regions: differentiation using MRI. Clin Radiol. 2007;62(5):453-462.
Di Rocco C. Sylvian fissure arachnoid cysts: we do operate on them but should it be done? Childs Nerv Syst. 2010;26:173-175.
Ersahin Y., Kesikci H., Rüksen M., et al. Endoscopic treatment of suprasellar arachnoid cysts. Childs Nerv Syst. 2008;24(9):1013-1020.
Gangemi M., Coletta G., Magro F., et al. Suprasellar arachnoid cysts— endoscopy versus microsurgical cyst excision and shunting. Br J Neurosurg. 2007;21(3):276-280.
Grunert P., Gaab M.R., Hellwig D., et al. German neuroendoscopy above the skull base. Neurosurg Focus. 2009;27(3):E7.
Hellwig D., Bauer B.L., Schulte D.M., et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2008;62(6 Suppl 3):1101-1109.
Hellwig D., Haag R., Bartel V., et al. Application of new electrosurgical devices and probes in endoscopic neurosurgery. Neurol Res. 1999;21:67-72.
Hellwig D., Riegel T. Stereotactic endoscopic treatment of brain abscess. In: Jimenez D.F., editor. Intracranial Endoscopic Neurosurgery. Park Ridge, IL: AANS Publications Committee; 1998:199-207.
Hellwig D., Riegel T., Bertalanffy H. Neuroendoscopic techniques in treatment of intracranial lesions. Minim Invasive Ther Allied Technol. 1998;7:123-135.
Inoue K., Seker A., Osawa S., et al. Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cistern. Neurosurgery. 2009;65(4):644-664.
Karavitaki N., Scheithauer B.W., Watt J., et al. Collision lesions of the sella: co-existence of craniopharyngioma with gonadotroph adenoma and Rathke’s cleft cyst with corticotroph adenoma. Pituitary. 2008;11(3):317-323.
Martinez-Lage J.F., Ruiz-Espejo A.M., Almagro M.J., et al. CSF overdrainage in shunted intracranial arachnoid cysts: a series and review. Childs Nerv Syst. 2009;25(9):1061-1069.
Okada T., Fujitsu K., Miyahara K., et al. Ciliated craniopharyngioma-case report and pathological study. Acta Neurochir. 2009. (Epub ahead of print)
Pradilla G., Jallo G. Arachnoid cysts: case series and review of the literature. Neurosurg Focus. 2007. 15:22(2):E7
Raper D.M., Besser M. Clinical features, management and recurrence of symptomatic Rathke’s cleft cyst. J Clin Neurosci. 16(3), 2009. 385.9
Riegel T., Freudenstein D., Alberti O., et al. Novel multipurpose instrument for endoscopic neurosurgery. Neurosurgery. 2002;51(1):270-274.
Tamburrini G., D’Angelo L., Paternoster G., et al. Endoscopic management of intra and periventricular CSF cysts. Childs Nerv Syst. 2007;23(6):645-651.
Thomas B.P., Pearson M.M., Wushensky C.A. Active spontaneous decompression of a suprasellar-prepontine arachnoid cyst detected with routine magnetic resonance imaging. Case report. J Neurosurg Pediatr. 2009;3(1):70-72.
Tirakotai W., Bozinov O., Sure U., et al. The evolution of stereotactic guidance in neuroendoscopy. Childs Nerv Syst. 2004;20:790-795.
Tirakotai W., Hellwig D., Bertalanffy H., et al. The role of neuroendoscopy in the management of solid or solid-cystic intra- and periventricular tumours. Childs Nerv Syst. 2007;23(6):653-658.
Zada G., Ditty B., McNatt S.A., et al. Surgical treatment of Rathke cleft cysts in children. Neurosurgery. 2009;64(6):1132-1137.
1. Caemaert J., Abdulah J., Calliauw L., et al. Endoscopic treatment of suprasellar arachnoid cysts. Acta Neurochir (Wien). 1992;119:68-73.
2. Cohen A., Perneczky A. Endoscopy and the management of third ventricular lesions. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:922-927.
3. Hellwig D., Bauer B.L., List-Hellwig E. Stereotactic endoscopic interventions in cystic brain lesions. Acta Neurochir Suppl. 1995;64:59-63.
4. Fitzpatrick M.O., Barlow P. Endoscopic treatment of prepontine arachnoid cysts. Br J Neurosurg. 2001;15:234-238.
5. Grotenhuis J.A. The use of endoscopes during surgery of the suprasellar region. In: Hellwig D., Bauer B.L. Minimally Invasive Techniques for Neurosurgery. Heidelberg: Springer-Verlag; 1998:107-110.
6. Hopf N.J., Resch K.D.M., Ringel K., et al. Endoscopic management of intracranial arachnoid cysts. In: Hellwig D., Bauer B.L. Minimally Invasive Techniques for Neurosurgery. Heidelberg: Springer-Verlag; 1998:111-119.
7. Kamikawa S., Inui A., Tamaki N., et al. Application of flexible neuroendoscopes to intracerebroventricular arachnoid cysts in children: use of videoscopes. Minim Invasive Neurosurg. 2001;44:186-189.
8. Kirollos R.W., Javadpour M., May P., et al. Endoscopic treatment of suprasellar and third ventricle-related arachnoid cysts. Childs Nerv Syst. 2001;17:713-718.
9. Schroeder H.W., Gaab M.R., Niendorf W.R. Neuroendoscopic approach to arachnoid cysts. J Neurosurg. 1996;85:293-298.
10. Tamburrini G., D’Angelo L., Paternoster G., et al. Endoscopic management of intra and periventricular CSF cysts. Childs Nerv Syst. 2007;23(6):645-651.
11. Gangemi M., Coletta G., Magro F., et al. Suprasellar arachnoid cysts—endoscopy versus microsurgical cyst excision and shunting. Br J Neurosurg. 2007;21(3):276-280.
12. Tirakotai W., Hellwig D., Bertalanffy H., et al. The role of neuroendoscopy in the management of solid or solid-cystic intra- and periventricular tumours. Childs Nerv Syst. 2007;23(6):653-658.
13. Krawchenko J., Collins G.H. Pathology of an arachnoid cyst: case report. J Neurosurg. 1979;50:224-228.
14. Go K.G., Houthoff H.J., Blaauw E.H., et al. Arachnoid cysts of the sylvian fissure: evidence of fluid secretion. J Neurosurg. 1984;60:803-813.
15. Little J., Gomez M., MacCarty C. Infratentorial arachnoid cysts. J Neurosurg. 1973;39:380-386.
16. Hanieh A., Simpson D.A. North JB: Arachnoid cysts: a critical review of 41 cases. Childs Nerv Syst. 1988;4:92-96.
17. Williams D., Gutkelch A.N. Why do central arachnoid pouches expand? J Neurol Neurosurg Psychiatry. 1974;37:1085-1092.
18. Santamarta D., Aguas J., Ferrer E. The natural history of arachnoid cysts: endoscopic and cine-mode MRI evidence of a slit-valve mechanism. Minim Invasive Neurosurg. 1995;38:133-137.
19. Schroeder H.W., Gaab M.R. Endoscopic observation of a slit-valve mechanism in a suprasellar prepontine arachnoid cyst: case report. Neurosurgery. 1997;40:198-200.
20. Santamarta D., Morales F., Sierra J.M., et al. Arachnoid cysts: entrapped collections of cerebrospinal fluid variably communicating with subarachnoid space. Minim Invasive Neurosurg. 2001;44:128-134.
21. Hornig G.W., Zervas N.T. Slit defect of the diaphragma sellae with valve effect: observation of a “slit valve”. Neurosurgery. 1992;30:265-267.
22. Weber R., Voit T., Lumenta C., Lenard H.G. Spontaneous regression of a temporal arachnoid cyst. Childs Nerv Syst. 1991;7:414-415.
23. Pandey P., Tripathy M., Chandra P.S., et al. Spontaneous decompression of a posterior fossa arachnoid cyst. Pediatr Neurosurg. 2001;35:162-163.
24. Thomas B.P., Pearson M.M., Wushensky C.A. Active spontaneous decompression of a suprasellar-prepontine arachnoid cyst detected with routine magnetic resonance imaging. Case report. J Neurosurg Pediatr. 2009;3(1):70-72.
25. Ciricillo S.F., Cogen P.H., Harsh G.R., et al. Intracranial arachnoid cysts in children: a comparison of the effects of fenestration and shunting. J Neurosurg. 1991;74:230-235.
26. Hund-Georgiadis M., Yves Von Cramon D., Kruggel F., et al. Do quiescent arachnoid cysts alter functional organization? A fMRI and morphometric study. Neurology. 2002;59:1935-1939.
27. Sgouros S., Chapman S. Congenital middle fossa arachnoid cysts may cause global brain ischaemia: a study with 99Tc-hexamethylpropyleneamineoxime single-photon emission computerized tomography scans. Pediatr Neurosurg. 2001;35:188-194.
28. Di Rocco C. Sylvian fissure arachnoid cysts: we do operate on them but should it be done? Childs Nerv Syst. 2010;26:173-175.
29. Di Rocco C., Tamburrine G., Caldarelli M., et al. Prolonged ICP monitoring in sylvian arachnoid cysts. Surg Neurol. 2003;60:211-218.
30. Akor C., Wojno T.H., Newman N.J. Arachnoid cyst of the optic nerve: report of two cases and review of literature. Ophthal Plast Reconstr Surg. 2003;19:466-469.
31. Brooks M.L., Mayer D.P., Staloff R.T., et al. Intracanalicular arachnoid cyst mimicking acoustic neuroma: cT and MRI. Comput Med Imaging Graph. 1992;16:283-285.
32. Floris R., Pastore F.S., Silvestrini M., et al. Supracerebellar arachnoid cyst and reversible tonsillar herniation: magnetic resonance imaging and pathophysiological considerations. Neuroradiology. 1992;34:404-406.
33. O’Reilly R.C., Hallinan E.K. Posterior fossa arachnoid cysts can mimic Meniere’s disease. Am J Otolaryngol. 2003;24:420-425.
34. Ottaviani F., Neglia C.B., Scotti A., et al. Arachnoid cyst of the posterior cranial fossa causing sensorineural hearing loss and tinnitus: a case report. Eur Arch Otorhinolaryngol. 2002;259:306-308.
35. Turgut M., Ozcan O.E., Onol B. case report and review of the literature: arachnoid cyst of the fourth ventricle presenting as a syndrome of normal pressure hydrocephalus. J Neurosurg Sci. 1992;36:55-57.
36. Yamasaki F., Kodama Y., Hotta T., et al. Interhemispheric arachnoid cyst in the elderly: case report and review of the literature. Surg Neurol. 2003;59:68-74.
37. Bhatia S., Thakur R.C., Devi B.I., et al. Craniospinal intradural arachnoid cyst. Postgrad Med J. 1992;68:829-830.
38. Price S.J., David K.M., O’Donovan D.G., et al. Arachnoid cyst of the craniocervical junction: case report. Neurosurgery. 2001;49:212-215.
39. Arunkumar M.J., Korah I., Chandy M.J. Dynamic CSF flow study in the pathophysiology of syringomyelia associated with arachnoid cysts of the posterior fossa. Br J Neurosurg. 1998;12:33-36.
40. Punzo A., Conforti R., Martiniello D., et al. Surgical indications for intracranial arachnoid cysts. Neurochirurgia. 1992;35:35-42.
41. Pagni C.A., Canavero S., Vinci V. Left trochlear nerve palsy, unique symptom of an arachnoid cyst of the quadrigeminal plate: case report. Acta Neurochir (Wien). 1990;105:147-149.
42. Kurokawa Y., Sohma T., Tsuchita H., et al. A case of intraventricular arachnoid cyst: how should it be treated? Childs Nerv Syst. 1990;6:365-367.
43. Mazurkiewicz-Beldzinska M., Dilling-Ostrowska E. Presentation of intracranial arachnoid cysts in children: correlation between localization and clinical symptoms. Med Sci Monit. 2002;8:462-465.
44. Yalcin A.D., Oncel C., Kaymaz A., et al. Evidence against association between arachnoid cysts and epilepsy. Epilepsy Res. 2002;49:255-260.
45. Miyagami M., Tsunokawa T. Histological and ultrastructural findings of benign intracranial cysts. Noshuyo Byori. 1993;10:151-160.
46. Inoue K., Seker A., Osawa S., et al. Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cistern. Neurosurgery. 2009;65(4):644-664.
47. Friede R.L., Yasargil M.G. Supratentorial intracerebral epithelial (ependymal) cysts: review, case reports and fine structure. J Neurol Neurosurg Psychiatry. 1977;40:127-137.
48. Servadei F., Vergoni G., Frattarelli M., et al. Arachnoid cyst of middle cranial fossa and ipsilateral subdural haematoma: diagnostic and therapeutic implications in three cases. Br J Neurosurg. 1993;7:249-253.
49. Gelabert-Gonzalez M., Fernandez-Villa J., Cutrin-Prieto J., et al. Arachnoid cyst rupture with subdural hygroma: report of three cases and literature review. Childs Nerv Syst. 2002;18:609-613.
50. Bilginer B, Onal MB, Oguz KK, et al. Arachnoid cyst associated with subdural hematoma: report of three cases and review of the literature. Childs Nerv Syst. 25(1): 119–124.
51. Rogers M.A., Klug G.L., Siu K.H. Middle fossa arachnoid cysts in association with subdural haematomas: a review and recommendations for management. Br J Neurosurg. 1981;4:497-502.
52. Auer L., Gallhofer B., Ladurner G., et al. Diagnosis and treatment of middle cranial fossa arachnoid cysts and subdural hematoma. J Neurosurg. 1981;54:366-369.
53. Handa J., Okamato K., Sato M. Arachnoid cysts of the middle cranial fossa: report of bilateral cysts in siblings. Surg Neurol. 1981;10:127-130.
54. Mori K., Yamamoto T., Horinaka N., et al. Arachnoid cyst is a risk factor for chronic subdural hematoma in juveniles: twelve cases of chronic subdural hematoma associated with arachnoid cyst. J Neurotrauma. 2002;19:1017-1027.
55. Markakis E., Heyer R., Stoeppler L., et al. Die Apoplexie der perisylvischen Region. Neurochirurgia. 1979;22:211-220.
56. Hellwig D., Riegel T. Endoscopic evacuation of intracerebral and septated chronic subdural hematomas. In: Jimenez D., editor. Endoscopic Intracranial Neurosurgery. Park Ridge, IL: AANS Publications Committee; 1998:185-197.
57. Spacca B., Kandasamy J., Mallucci C.L., et al. Endoscopic treatment of middle fossa arachnoid cysts: a series of 40 patients treated endoscopically in two centres. Childs Nerv Syst. 2009;26(2):163-172.
58. Chan T., Logan P., Eustace P. Intermittent downbeat nystagmus secondary to vermian arachnoid cyst with associated obstructive hydrocephalus. J Clin Neuroophthalmol. 1991;11:293-296.
59. Haberkamp T.J., Monsell E.M., House W.F., et al. Diagnosis and treatment of arachnoid cysts of the posterior fossa. Otolaryngol Head Neck Surg. 1990;103:610-614.
60. Babu R., Murali R. Arachnoid cyst of the cerebellopontine angle manifesting as contralateral trigeminal neuralgia: case report. Neurosurgery. 1991;28:886-887.
61. Higashi S., Yamashita J., Yamamoto Y., et al. Hemifacial spasm associated with a cerebellopontine angle arachnoid cyst. Surg Neurol. 1992;37:289-292.
62. Levy M.L., Wang M., Aryan H.E., et al. Microsurgical keyhole approach for middle fossa arachnoid cyst fenestration. Neurosurgery. 2003;53:1138-1144.
63. D’Angelo V., Gorgoglione L., Catapano G. Treatment of symptomatic intracranial arachnoid cysts by stereotactic cyst-ventricular shunting. Stereotact Funct Neurosurg. 1999;72:62-69.
64. Iacono R.P., Labadie E.L., Johnstone S.J., et al. Symptomatic arachnoid cyst at the clivus drained stereotactically through the vertex. Neurosurgery. 1990;27:130-133.
65. Pell M.F., Thomas D.G. The management of intratentorial arachnoid cysts by CT-directed stereotactic aspiration. Br J Neurosurg. 1991;5:399-403.
66. Germano A., Caruso G., Caffo M., et al. The treatment of large supratentorial arachnoid cysts in infants with cyst-peritoneal shunting and Hakim programmable valve. Childs Nerv Syst. 2003;19:166-173.
67. Tamburrini G., Caldarelli M., Massimi L., et al. Subdural hygroma: an unwanted result of Sylvian arachnoid cyst marsupialization. Childs Nerv Syst. 2003;19:159-165.
68. Lange M., Oeckler R., Beck O.J. Surgical treatment of patients with midline arachnoid cysts. Neurosurg Rev. 1990;3:35-39.
69. Oberbauer R.W., Haase J., Pucher R. Arachnoid cysts in children: a European co-operative study. Childs Nerv Syst. 1992;8:281-286.
70. Martinez-Lage J.F., Ruiz-Espejo A.M., Almagro M.J., et al. CSF overdrainage in shunted intracranial arachnoid cysts: a series and review. Childs Nerv Syst. 2009;25(9):1061-1069.
71. Pradilla G., Jallo G. Arachnoid cysts: case series and review of the literature. Neurosurg Focus. 2007:E7. 15:22(2)
72. Grunert P., Gaab M.R., Hellwig D., et al. German neuroendoscopy above the skull base. Neurosurg Focus. 2009;27(3):E7.
73. Tirakotai W., Bozinov O., Sure U., et al. The evolution of stereotactic guidance in neuroendoscopy. Childs Nerv Syst. 2004;20:790-795.
74. Hellwig D., Riegel T., Bertalanffy H. Neuroendoscopic techniques in treatment of intracranial lesions. Minim Invasive Ther Allied Technol. 1998;7:123-135.
75. Riegel T., Freudenstein D., Alberti O., et al. Novel multipurpose instrument for endoscopic neurosurgery. Neurosurgery. 2002;51(1):270-274.
76. Hellwig D., Haag R., Bartel V., et al. Application of new electrosurgical devices and probes in endoscopic neurosurgery. Neurol Res. 1999;21:67-72.
77. Hoffmann K.T., Hosten N., Meyer B.U., et al. CSF flow studies of intracranial cysts and cyst-like lesions achieved using reversed fast imaging with steady-state precession MR sequences. Am J Neuroradiol. 2000;21:493-502.
78. Algin O., Hakyemez B., Gokalp G., et al. Phase-contrast cine MRI versus MR cisternography on the evaluation of the communication between intraventricular arachnoid cysts and neighbouring cerebrospinal fluid spaces. Neuroradiology. 2009;51(5):305-312.
79. Rengachary S.S., Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981;40:61-83.
80. Hoffman H.J., Hendrick E.B., Humphreys R.P., et al. Investigation and management of suprasellar arachnoid cysts. J Neurosurg. 1982;57:597-602.
81. Harrison M.J.G. Cerebral arachnoid cysts in children. J Neurol Neurosurg Psychiatry. 1971;34:316-323.
82. Dei-Anang K., Voth D. Cerebral arachnoid cysts: a lesion of the child’s brain. Neurosurg Rev. 1989;12:59-62.
83. Key A., Retzius G., Studien in der Anatomie des Nervensystems und des Bindegewebes, Stockholm, PA Norsted & Soner, 1875 :vol 1 plate III
84. Lillequist B. The anatomy of the subarachnoid cisterns. Acta Radiol. 1956;48:61-71.
85. Fox J.L., Al-Mefty O. Suprasellar arachnoid cysts: an extension of the membrane of Lillequist. Neurosurgery. 1980;7:615-618.
86. Starkman S.P., Brown T.C., Linell E.A. Cerebral arachnoid cysts. J Neuropathol Exp Neurol. 1958;17:484-500.
87. Kivela T., Pelkonen R., Oja M., Heiskanen O. Diabetes insipidus and blindness caused by a suprasellar tumor: Pieter Pauw’s observations from the 16th century. JAMA. 1998;279:48-50.
88. Benton J., Nellhaus G., Huttenlocher P., et al. The bobble head doll syndrome: report of a unique truncal tremor associated with third ventricular cyst and hydrocephalus in children. Neurology. 1966;16:725-729.
89. Pierre-Khan A., Capelle L., Brauner R., et al. Presentation and management of suprasellar arachnoid cysts. J Neurosurg. 1990;73:355-359.
90. Desai K.I., Nadkarni T.D., Muzumdar D., et al. Suprasellar arachnoid cyst presenting with bobble-head doll movements: a report of 3 cases. Neurol India. 2003;51:407-409.
91. Hagebeuck E.E., Lkoet A., Grotenhuis J.A., et al. Bobble-head doll syndrome successfully treated with an endoscopic ventriculocystocisternostomy. Case report and review of the literature. J Neurosurg. 2005;103(suppl 3):253-259.
92. Starzyk J., Kwiatkowski S., Urbanowicz W., et al. Suprasellar arachnoidal cyst as a cause of precocious puberty—report of three patients and literature overview. J Pediatr Endocrinol Metab. 2003;16:447-455.
93. Golash A., Mitchell G., Malllucci C., et al. Prenatal diagnosis of suprasellar arachnoid cyst and postnatal endoscopic treatment. Childs Nerv Syst. 2001;17:739-742.
94. Gonzalez C.A., Villarejo F.J., Blazquez M.G., et al. Suprasellar arachnoid cysts in children: report of three cases. Acta Neurochir (Wien). 1982;60:281-296.
95. Raimondi A.J., Shimoji T., Gutierrez F.A. Suprasellar cysts: surgical treatment and results. Childs Nerv Syst. 1980;7:57-72.
96. Murali R., Epstein F. Diagnosis and treatment of suprasellar arachnoid cyst. J Neurosurg. 1979;50:515-518.
97. Dhooge C., Govaert P., Martens F., et al. Transventricular endoscopic investigation and treatment of suprasellar arachnoid cysts. Neuropediatrics. 1992;23:245-247.
98. Decq P., Brugieres P., Le Guerinel C., et al. Percutaneous endoscopic treatment of suprasellar arachnoid cysts: ventriculocystostomy or ventriculocisternostomy? [technical note]. J Neurosurg. 1996;84:696-701.
99. Nakamura Y., Mizukawa K., Yamamoto K., et al. Endoscopic treatment for a huge neonatal prepontine-suprasellar arachnoid cyst: a case report. Pediatr Neurosurg. 2001;35:220-222.
100. Ersahin Y., Kesikci H., Rüksen M., et al. Endoscopic treatment of suprasellar arachnoid cysts. Childs Nerv Syst. 2008;24(9):1013-1020.
101. Dodd R.L., Barnes P.D., Huhn S.L. Spontaneous resolution of a prepontine arachnoid cyst: case report and review of the literature. Pediatr Neurosurg. 2002;37:152-157.
102. Thomas B.P., Pearson M.M., Wushensky C.A. Active spontaneous decompression of a suprasellar-prepontine arachnoid cyst detected with routine magnetic resonance imaging. Case report. J Neurosurg Pediatr. 2009;3(1):70-72.
103. Shanklin W.M. The incidence and distribution of cilia in the human pituitary with a description of microfollicular cysts derived from Rathke’s cleft. Acta Anat (Basel). 1951;11:361-382.
104. Bayoumi M.L. Rathke’s cleft and its cysts. Edinb Med J. 1948;55:745-749.
105. Gillman T. The incidence of ciliated epithelium and mucous cells in the normal Bantu pituitary. S Afr Med J. 1940;5:30-40.
106. McGrath P. Cysts of sella and pharyngeal hypophyses. Pathology. 1971;3:123-131.
107. Rasmussen A.T. Ciliated epithelium and mucous-secreting cells in the human hypophysis. Anat Rec. 1929;41:273-283.
108. Voelker J.L., Campbell R.L., Muller J. Clinical, radiographic and pathological features of symptomatic Rathke’s cleft cysts. J Neurosurg. 1991;74:535-544.
109. Yoshida J., Kobayashi T., Kageyama N., et al. Symptomatic Rathke’s cleft cyst: morphological study with light and electron microscopy and tissue culture. J Neurosurg. 1977;47:451-458.
110. Megdiche-Bazarbacha H., Ben Hammouda K.B., Aicha A.B., et al. Intrasphenoidal Rathke cleft cyst. Am J Neuroradiol. 2006;27:1098-1100.
111. Ross D.A., Norman D., Wilson C.B. Radiologic characteristics and results of surgical management of Rathke’s cysts in 43 patients. Neurosurgery. 1993;30:173-179.
112. Asari S., Ito T., Tsuchida S., et al. MR appearance and cyst content of Rathke cleft cysts. J Comput Assist Tomogr. 1990;14:532-535.
113. Christophe C., Flamant-Durand J., Hanquinet S., et al. MRI in seven cases of Rathke’s cleft cyst in infants and children. Pediatr Radiol. 1993;23:79-82.
114. Ito H., Nishizaka T., Kajiwara K., et al. Pituitary nonadenomatous tumor (Rathke’s cleft cyst). Nippon Rinsho. 1993;51:2711-2715.
115. Nakasu Y., Isozumi T., Nakasu S., et al. Rathke’s cleft cyst: computed tomographic scan and magnetic resonance imaging. Acta Neurochir (Wien). 1990;103:99-104.
116. El Mahdy W.E., Powell M. Transsphenoidal management of 28 symptomatic Rathke’s cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery. 1998;42:7-16.
117. Israel Z.H., Yacoub M., Gomori J.M., et al. Rathke’s cleft cyst abscess. Pediatr Neurosurg. 2000;33:159-161.
118. Hsu Y.J., Chau R., Yang S.S., et al. Rathke’s cleft cyst presenting with hyponatremia and transient central diabetis insipidus. Acta Neurol Scand. 2003;107:382-385.
119. Oka H., Kawano N., Suwa T., et al. Radiological study of symptomatic Rathke’s cleft cysts. Neurosurgery. 1994;35:632-636.
120. Naylor M.F., Scheithauer B.W., Forbes G.S., et al. Rathke cleft cyst: CT, MR, and pathology of 23 cases. J Comput Assist Tomogr. 1995;19:853-859.
121. Choi S.H., Kwon B.J., Na D.G., et al. Pituitary adenoma, craniopharyngioma, and Rathke cleft cyst involving both intrasellar and suprasellar regions: differentiation using MRI. Clin Radiol. 2007;62(5):453-462.
122. Kunii N., Abe T., Tanioka D., et al. Rathke’s cleft cysts; differentiation from other cystic lesions in the pituitary fossa by use of sigle-shot fast spin-echo diffusion-weighted MR-imaging. Acta Neurochir. 2007;149(8):759-769.
123. Kleinschmidt Demasters B.K., Lillehei K.O. Steaaars JC: The pathologic, surgical, and MR spectrum of Rathke cleft cysts. Surg Neurol. 1995;44:19-26.
124. Fager C.A., Carter H. Intrasellar epithelial cysts. J Neurosurg. 1966;24:77-81.
125. Isono M., Kamida T., Kobayashi H., et al. Clinical features of symptomatic Rathke’s cleft cyst [comment]. Clin Neurol Neurosurg. 2001;103:96-100.
126. Acharya S.V., Gopal R.A., Menon P.S., et al. Precocious puberty due to Rathke cleft cyst in a child. Endocr Pract. 2009;15(2):134-137.
127. Van Hilten B.J., Roos R.A.C., de Bakker H.M., et al. Periodic fever: an unusual manifestation of a recurrent Rathke’s cleft. J Neurol Neurosurg Psychiatry. 1990;43:533.
128. Midha R., Jay V., Smyth H.S. Transsphenoidal management of Rathke’s cleft cysts. Surg Neurol. 1991;35:441-454.
129. Tanigawa K., Yamashita S., Namba H., et al. Acute adrenal insufficiency due to symptomatic Rathke’s cleft cyst. Intern Med. 1992;31:467-469.
130. Onesti S.T., Wisniewski T., Post K.D. Pituitary hemorrhage into a Rathke’s cleft cyst. Neurosurgery. 1990;27:644-646.
131. Pawar S.J., Sharma R.R., Lad S.D., et al. Rathke’s cleft cyst presenting as pituitary apoplexy. J Clin Neurosci. 2002;9:76-79.
132. Wagle V.G. Hemorrhage into Rathke’s cleft cyst. Neurosurgery. 1991;28:335.
133. Raskind R., Brown H.A., Mathis J. Recurrent cyst of the pituitary: 26-year follow-up from first decompression. J Neurosurg. 1968;28:595-599.
134. Berry R.G., Schlezinger N.S. Rathke cleft cysts. Arch Neurol. 1959;1:48-58.
135. Iraci G., Girodano R., Gerosa M., et al. Ocular involvement in recurrent cyst of Rathke’s cleft: case report. Ann Ophthalmol. 1979;11:94-98.
136. Matsushima T., Fukui M., Ohta M., et al. Ciliated and goblet cells in craniopharyngioma: light and electron microscopic studies at surgery and autopsy. Acta Neuropathol (Berl). 1980;50:199-205.
137. Marcincin R.P., Gennarelli T.A. Recurrence of symptomatic pituitary cysts following transsphenoidal drainage. Surg Neurol. 1982;18:448-451.
138. Rout D.L., Das L., Rao V.R.K., et al. Symptomatic Rathke’s cleft cysts. Surg Neurol. 1983;19:42-45.
139. Yamamoto M., Takara E., Imanaga H., et al. Rathke’s cleft cyst: report of two cases. Neurol Surg. 1984;12:609-616.
140. Leech R.W., Olafson R.A. Epithelial cysts of the neuraxis: presentation of three cases and a review of the origins and classification. Arch Pathol Lab Med. 1977;101:196-202.
141. Roux F.X., Constans J.P., Monsaingeon V., et al. Symptomatic Rathke’s cleft cysts: clinical and therapeutic data. Neurochirurgia (Stuttg). 1988;31:18-20.
142. Mukherjee J.J., Islam N., Kaltsas G., et al. Clinical, radiological and pathological features of patients with Rathke’s cleft cysts: tumors that may recur. J Clin Endocrinol Metab. 1997;82:2357-2362.
143. Raper D.M., Besser M. Clinical features, management and recurrence of symptomatic Rathke’s cleft cyst. J Clin Neurosci. 16(3), 2009. 385.9
144. Aho C.J., Zelman V., Couldwell W.R., et al. Surgical outcomes in 118 patients with Rathke cleft cyst. J Neurosurg. 2005;102(2):189-193.
145. Russell D.S., Rubinstein L.J. Pathology of Tumors of the Nervous System, 3rd ed. Baltimore: Williams & Wilkins; 1971.
146. Tajika Y., Kubo O., Kamiya M., et al. Clinicopathological features of 5 cases of pituitary cyst including Rathke’s cleft cyst. Neurol Surg. 1982;10:1055-1064.
147. Ikeda H., Yoshimoto T. Clinicopathological study of Rathke’s cleft cysts. Clin Neuropathol. 2002;21:82-91.
148. Goodrich J.T., Post K.D., Duffy P. Ciliated craniopharyngioma. Surg Neurol. 1985;24:105-111.
149. Sato K., Oka H., Utsuki S., et al. Ciliated craniopharyngioma may arise from Rathke cleft cyst. Clin Neuropathol. 2006;25(1):25-28.
150. Okada T., Fujitsu K., Miyahara K., et al. Ciliated craniopharyngioma—case report and pathological study. Acta Neurochir. 2010;152(2):303-306. discussion 307
151. Verkijk A., Bots G.T. An intrasellar cyst with both Rathke’s cleft and epidermoid characteristics. Acta Neurochir (Wien). 1980;51:203-207.
152. Harrison M.J., Morgello S., Post K.D. Epithelial cystic lesions of the sellar and parasellar region: a continuum of ectodermal derivates? J Neurosurg. 1994;80:1018-1025.
153. Hiyama H., Kubo O., Yato S., et al. A case of pituitary adenoma combined with Rathke’s cleft cysts. Neurol Surg. 1986;14:435-440.
154. Matsumori K., Okuda T., Nakayama K., et al. A case of calcified prolactinoma combined with Rathke’s cleft cysts. Neurol Surg. 1984;12:833-838.
155. Miyagi A., Iwasaki M., Shibuya T., et al. Pituitary adenoma combined with Rathke’s cleft cyst—case report. Neurol Med Chir (Tokyo). 1993;33:643-650.
156. Nishio S., Mizuno J., Barrow D.L., et al. Pituitary tumors composed of adenohypophysial adenoma and Rathke’s cleft cyst elements: a clinicopathological study. Neurosurgery. 1987;21:371-377.
157. Swanson S.E., Chandler W.F., Latack J., et al. Symptomatic Rathke’s cleft with pituitary adenoma: case report. Neurosurgery. 1985;17:657-659.
158. Troukedes K.M., Walfish P.G., Holgate R.C., et al. Sellar enlargement with hyperprolactinemia and a Rathke’s pouch cyst. JAMA. 1978;240:471-473.
159. Sumida M., Migita K., Tominaga A., et al. Concomitant pituitary adenoma and Rathke’s cleft cyst. Neuroradiology. 2001;43:755-759.
160. Karavitaki N., Scheithauer B.W., Watt J., et al. Collision lesions of the sella: co-existence of craniopharyngioma with gonadotroph adenoma and Rathke’s cleft cyst with corticotroph adenoma. Pituitary. 2008;11(3):317-323.
161. Yuge T., Shigemori M., Tokutomi T., et al. Entirely suprasellar symptomatic Rathke’s cleft cyst. No Shinkei Geka. 1991;19:273-278.
162. Wenzel M., Salcman M., Kristt D.A., et al. Pituitary hyposecretion and hypersecretion produced by a Rathke’s cleft cyst presenting as a non-cystic hypothalamic mass. Neurosurgery. 1989;24:424-428.
163. Itoh J., Usui K. An entirely suprasellar symptomatic Rathke’s cleft cyst: case report. Neurosurgery. 1992;30:581-585.
164. Wenger M., Simko M., Markwalder R., et al. An entirely suprasellar Rathke’s cleft cyst: case report and review of the literature. J Clin Neurosci. 2001;8:564-567.
165. Onda K., Tanaka R., Takeda N., et al. Symptomatic Rathke cleft cyst simulating arachnoid cyst: case report. Neurol Med Chir (Tokyo). 1989;29:1039-1043.
166. Ikeda H., Yoshimoto T., Katakura R. A case of Rathke’s cleft cyst within a pituitary adenoma presenting with acromegaly: do “transitional cell tumors of the pituitary gland” really exist? Acta Neuropathol (Berl). 1992;83:211-215.
167. Arita K., Uozumi T., Takechi A., et al. A case of Cushing’s disease accompanied by Rathke’s cleft cyst: the usefulness of cavernous sinus sampling in the localization of microadenoma. Surg Neurol. 1994;42:112-116.
168. Ersahin Y., Ozdamar N., Demirtas E., Mutluer S. A case of Rathke’s cleft cyst presenting with diabetis insipidus. Clin Neurol Neurosurg. 1995;97:317-320.
169. Iwai H., Ohno Y., Hoshiro M., et al. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) and adrenal insufficiency induced by Rathke’s cleft cyst: a case report. Endocr J. 2000;47:393-399.
170. Wearne M.J., Barber P.C., Johnson A.P. Symptomatic Rathke’s cleft cyst with hypophysitis. Br J Neurosurg. 1995;9:799-803.
171. Roncaroli F., Bacci A., Frank G., Calbucci F. Granulomatous hypophysitis caused by a ruptured intrasellar Rathke’s cleft cyst: report of a case and review of the literature. Neurosurgery. 1998;43:141-149.
172. Cannova S., Romano C., Buffa R., Faglia G. Granulomatous sarcoidotic lesion of hypothalamic-pituitary region associated with Rathke’s cleft cyst. J Endocrinol Invest. 1997;20:77-81.
173. Koshiyama H., Kato Y., Masutani H., et al. A case of Rathke’s cleft cyst associated with diabetes insipidus and Hashimoto’s thyroiditis. Jpn J Med Sci Biol. 1989;28:406-409.
174. Kim T.S., Cho S., Dickson D.W. Aprosencephaly: review of the literature and a report of a case with cerebellar hypoplasia, pigmented epithelial cyst and Rathke’s cleft cyst. Acta Neuropathol (Berl). 1990;79:424-431.
175. Wolfsohn A.L., Lach B., Benoit B.G. Suprasellar xanthomatous Rathke’s cleft cyst. Surg Neurol. 1992;38:106-109.
176. Graziani N., Dufour H., Figarella Branger D., et al. Do the suprasellar neurenteric cyst, the Rathke’s cleft cyst and the colloid cyst constitute a same entity? Acta Neurochir (Wien). 1995;13:174-180.
177. Shimoji T., Shinohara A., Shimizu A., et al. Rathke cleft cysts. Surg Neurol. 1984;21:295-310.
178. Zada G., Ditty B., McNatt S.A., et al. Surgical treatment of Rathke cleft cysts in children. Neurosurgery. 2009;64(6):1132-1137.
179. Madhok R., Prevedello D.M., Gardner P., et al. Endoscopic endonasal resection of Rathke cleft cysts: clinical outcomes and surgical nuances. J Neurosurg. 2010;112:1333-1339.
180. Cavallo L.M., Prevedello D., Esposito F., et al. The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev. 2008;31(1):55-64.
181. Hellwig D., Bauer B.L., Schulte D.M., et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2008;62(6 Suppl 3):1101-1109.
182. Gomez Perun J., Eiras J. Carcavilla LI: Abcés intrasellaire au sein d’un kyste de la poche de Rathke. Neurochirurgie. 1981;27:201-205.
183. Hellwig D., Riegel T. Stereotactic endoscopic treatment of brain abscess. In: Jimenez D.F., editor. Intracranial Endoscopic Neurosurgery. Park Ridge, IL: AANS Publications Committee; 1998:199-207.
184. Martin J.B. Case records of the Massachusetts General Hospital: case 17—1980. N Engl J Med. 1980;302:1015-1023.
185. Obenchain T.G., Becker D.P. Head bobbing associated with a cyst of the third ventricle. J Neurosurg. 1972;37:457-459.
186. Menault F., Sabouraud O., Javalet A., et al. Kyste de la fente de Rathke. Rev Otoneuroophthalmol. 1979;51:383-390.
187. Steinberg G.K., Koenig G.H., Golden J.B. Symptomatic Rathke’s cleft cysts. J Neurosurg. 1982;56:290-295.
188. Sonntag V.K.H., Lenge K.L., Balis M.S., et al. Surgical treatment of an abscess in a Rathke’s cleft cyst. Surg Neurol. 1983;20:152-156.
189. Okamoto S., Handa H., Yamashita J., et al. Computed tomography in intra and suprasellar epithelial cysts (symptomatic Rathke cleft cysts). Am J Neuroradiol. 1985;6:515-519.
190. Albini C.H., MacGillivray M.H., Fisher J.E., et al. Triad of hypopituitarism, granulomatous hypophysitis and ruptured Rathke’s cleft cyst. Neurosurgery. 1988;22:133-136.
191. Bognàr L., Szeifert G.T., Fedoresàk I., et al. Abscess formation in Rathke’s cleft cyst. Acta Neurochir (Wien). 1992;117:70-72.
192. Schittenhelm J., Beschorner R., Psaras T., et al. Rathke’s cleft cyst rupture as a potential event of a secondary perifocal lymphocytic hypophysitis: proposal of an unusal pathogenetic event and review of the literature. Neurosurg Rev. 2008;31(2):157-163.
193. Russell R.W.R., Pennybacker J.B. Craniopharyngioma in the elderly. J Neurol Neurosurg Psychiatry. 1978;24:13.
194. Patrick B.S., Smith R.R., Bailey T.O. Aseptic meningitis due to spontaneous rupture of craniopharyngioma cyst: case report. J Neurosurg. 1974;41:387-390.
195. Kulkarni V., Daniel R.T., Pranatartiharan R. Spontaneous intraventricular rupture of craniopharyngioma cyst. Surg Neurol. 2000;54:249-253.
196. De Klerk D.J.J., Spence J. Chemical meningitis with intracranial tumours. S Afr Med J. 1974;48:131-135.
197. Schwartz J.F., Balentine J.D. Recurrent meningitis due to an intracranial epidermoid. Neurology. 1978;28:124-129.
198. Cantu R.N., Kjellberg R.N., Moses J.M., et al. Aseptic meningitis due to dermoid tumor: case report and confirming test. Neurochirurgia (Stuttg). 1968;11:94-98.
199. Stendel R., Pietila T.A., Lehmann K., et al. Ruptured intracranial dermoid cysts. Surg Neurol. 2002;57:391-398.