11 Aquamid®
Summary and Key Features
• Aquamid® is a homogeneous polyacrylamide hydrogel (PAAG) containing no microparticles that is used as a tissue filler with a long-lasting effect
• PAAG belongs to the most commonly used permanent fillers worldwide
• It is best used for facial volumizing and deep structural augmentation
• Effective treatment must be performed using aseptic technique with injections subcutaneously into the deep layers of tissue in a retrograde fan-like manner
• PAAG has an immediate filling effect, adding volume; it does not depend on foreign-body reaction
• Aquamid® has been shown to have favorable physical–chemical interaction between PAAG and surrounding tissue, being accessible to water and solutes, including benzyl penicillin
Product
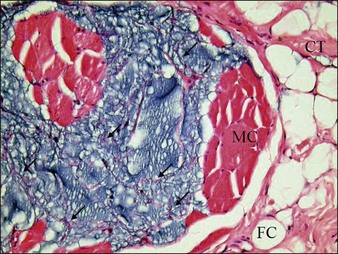
Studies by Bello and colleagues and by De Cassias Novaes & Berg have shown that this PAAG does not migrate within the tissue after subcutaneous injection because of its large molecular size and high cohesive properties. Christensen found that cells grew well in the Aquamid® gel, which allows vessel ingrowth from adjacent tissue. It is kept in place by means of a fibrous capsule (endoprosthesis) that develops after being injected in the subcutaneous tissue. These capsules are surrounded by fibroblasts and macrophages (Fig. 11.1). Another study by Zarini and colleagues emphasized the importance of the microstructure and porosity of hydrogels in relation to their functions and interactions with surrounding medium and tissue. Hydrogels could behave as an accessible foreign body that creates the appearance of infections that are untreatable. PAAG can exchange both physiological and non-physiological constituents very efficiently with the surrounding medium. In the context of hydrogels as tissue fillers, Brahm et al considered the ability of water and solutes (including an antibiotic) to cross between the hydrogel and the surrounding tissue to be attractive.
Technique / treatment
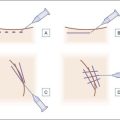
As with all permanent fillers the appropriate injection technique and the judicious use of the filler in the proper location and in the right plane of tissue are fundamental for success. Injections of PAAG should be conducted using aseptic technique. The injecting physician should wear sterile gloves, and the face of the patient has to be carefully cleaned with an antiseptic treatment. PAAG is injected subcutaneously with a 27-gauge needle in a retrograde, fan-like manner by injecting the gel while withdrawing the needle (Fig. 11.2). It should not be injected in large boluses in single sessions, but rather should be administered with a multi-line injection technique of small droplets. A series of small deposits facilitates tissue integration and vascularization of the implanted hydrogel, minimizing the risk of migration and other late-onset complications such as biofilm-assisted infection. PAAG has to be injected strictly subcutaneously; care should be taken when retracing the needle not to inject into the dermis and not to overcorrect. A light massage of the tissue after injection will help to obtain even distribution of the gel (Figs 11.3 and 11.4). If necessary touch-up injections may be spaced apart by several weeks. Although PAAG should not be thought of as a reversible tissue filler, it may be possible to remove the implant through a small incision under local anesthesia without damaging surrounding tissue. Complete or partial removal is possible because PAAG remains soft after injection.
Abenavoli F, Servili A, Corelli R. Aquamid: where is the reality? (letter). Plastic and Reconstructive Surgery. 2008;122:32e–33e.
Alijotas-Reig J, Garcia-Gimenez V, Miro-Mur F, et al. Delayed immune-mediated adverse effects related to polyacrylamide dermal fillers: clinical findings, management and follow-up. Dermatologic Surgery. 2009;35:360–366.
Bello G, Jackson IT, Keskin M, et al. The use of polyacrylamide gel in soft tissue augmentation: an experimental assessment. Plastic and Reconstructive Surgery. 2007;119:1326–1336.
Bjarnsholt T, Tolker-Nielsen T, Givskov M, et al. Detection of bacteria by FISH in PAAG culture-negative soft tissue filler lesions. Dermatologic Surgery. 2009;35:1620–1624.
Brahm J, Lessel R, Ditley S, et al. Flux of selected body fluid constituents and benzylpenicillin in polyacrylamide hydrogel (PAAG). Journal of Tissue Engineering and Regenerative Medicine. 2011;10:1–10.
Christensen L. Normal and pathologic tissue reactions to soft tissue gel fillers. Dermatologic Surgery. 2007;33:S168–S175.
Christensen LH, Breiting VB, Aasted A, et al. Long-term effects of polyacrylamide hydrogel on human breast tissue. Plastic and Reconstructive Surgery. 2003;111:1883–1890.
De Cassias Novaes W, Berg A. Experiences with a new nonbiodegradable hydrogel (Aquamid): a pilot study. Aesthetic Plastic Surgery. 2003;27:376–380.
Liu HL, Cheung WY. Complications of polyacrylamide hydrogel (PAAG) injection in facial augmentation. Journal of Plastic, Reconstructive and Aesthetic Surgery. 2010;63:e9–e12.
Narins RS, Schmidt R. Polyacrylamide hydrogel differences: getting rid of the confusion. Journal of Drugs in Dermatology. 2011;10:1370–1375.
Narins RS, Coleman WP, 3rd., Rohrich R, et al. 12-Month controlled study in the United States of the safety and efficacy of a permanent 2,5 % polyacrylamide hydrogel soft-tissue filler. Dermatologic Surgery. 2010;36(suppl 3):1819–1829.
Pallua N, Wolter TP. A 5 year assessment of safety and aesthetic results after facial soft tissue augmentation with polyacrylamide hydrogel (aquamid); a prospective multicenter study of 251 patients. Plastic and Reconstructive Surgery. 2010;125:1797–1804.
Von Buelow S, Pallua N. Efficacy and safety of polyacrylamide hydrogel for facial soft tissue augmentation in a 2 year follow up: a prospective multicenter study for evaluation of safety and aesthetic results in 101 patients. Plastic and Reconstructive Surgery. 2006;118:S85–S91.
Von Buelow S, von Heimburg D, Pallua N. Efficacy and safety of polyacrylamide hydrogel for facial soft tissue augmentation. Plastic and Reconstructive Surgery. 2005;116:1137–1146.
Wolter TP, Pallua N. A 5-year assessment of safety and aesthetic results after facial soft-tissue augmentation with polyacrylamide hydrogel (Aquamid): a prospective multicenter study of 251 patients. Plastic and Reconstructive Surgery. 2010;125:1797–1804.
Wolters M, Lampe H. Prospective multicenter study for evaluation of safety, efficacy, and esthetic results of cross-linked polyacrylamide hydrogel in 81 patients. Dermatologic Surgery. 2009;35:S338–S343.
Zarini E, Supino R, Pratesi G, et al. Biocompatibility and tissue interactions of a new filler material for medical use. Plastic and Reconstructive Surgery. 2004;114:934–942.